Abstract
Light detected in the retina modulates several physiological processes including circadian photo-entrainment and pupillary light reflex. Intrinsically photosensitive retinal ganglion cells (ipRGCs) convey rod-cone and melanopsin-driven light input to the brain. Using EEGs and electromyograms, we show that acute light induces sleep in mice during their nocturnal active phase whereas acute dark awakens mice during their diurnal sleep phase. We used retinal mutant mouse lines that lack (i) the ipRGCs, (ii) the photo-transduction pathways of rods and cones, or (iii) the melanopsin protein and showed that the influence of light and dark on sleep requires both rod-cone and melanopsin signaling through ipRGCs and is independent of image formation. We further show that, although acute light pulses overcome circadian and homeostatic drives for sleep, upon repeated light exposures using a 3.5 h/3.5 h light/dark cycle, the circadian and homeostatic drives override the light input. Thus, in addition to their known role in aligning circadian physiology with day and night, ipRGCs also relay light and dark information from both rod-cone and melanopsin-based pathways to modulate sleep and wakefulness.
Keywords: circadian photo-entrainment, intrinsically photosensitive retinal ganglion cell, masking, photoreceptor and opsin
Sleep is controlled by two mechanisms, homeostatic and circadian (1). In the homeostatic mechanism, prolonged wakefulness increases sleep drive, whereas the circadian oscillator partitions sleep within the day-night cycle. For optimal sleep, both the homeostatic and the circadian mechanisms must be synchronized (2). Light is known to affect sleep, predominantly by modulating the phase of the circadian oscillator in a process known as photo-entrainment (3). However, light also affects alertness in humans, indicating a possible direct role for light on the sleep-wake state (4). Furthermore, in albino rats, dark pulses enhance rapid eye movement (REM) amounts (5). Whereas the regulation of sleep by the circadian oscillator is well characterized, there are limited studies on the acute effects of light and dark on sleep or the retinal pathways responsible for relaying these signals to the brain.
In mammals, the eye is the only photoreceptive organ for image-forming and non-image-forming visual functions such as circadian photo-entrainment, pupillary light reflex, and inhibition of melatonin release. Three photoreceptive cell types, rods, cones, and melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs), are responsible for light detection in the retina (6). Rods and cones are essential for the formation of visual images, whereas ipRGCs are necessary for non-image-forming visual functions (7, 8). The light response in ipRGCs originates both from the intrinsic melanopsin photopigment-dependent response and from light signals arising from rods and cones (9, 10). Similar to other RGCs, the presence of light and dark can be signaled by rods and cones to ipRGCs through the ON or OFF pathways (11). The ON pathway is activated in dark-to-light transitions whereas the OFF pathway is activated in light-to-dark transitions. Either the rod-cone photoreceptors or the ipRGCs are sufficient to photo-entrain, constrict the pupil, or drive direct effects of light on behavior (12–18). In rodents, a direct retinal pathway to the ventrolateral preoptic nucleus originating at least in part from ipRGCs provides morphological evidence that sleep may be directly regulated by light (19, 20). It is currently unknown how rods-cones and ipRGCs influence sleep in response to light and dark signals.
Here we show that, in mice, which are nocturnal, light pulses induce sleep and dark pulses induce wakefulness. By using several mutant mouse lines, we show that, similar to circadian photo-entrainment, image formation is not required to modulate sleep. In contrast to circadian photo-entrainment, which can be driven by either the intrinsic photo-responsiveness of ipRGCs or rods-cones relaying signals via the ipRGCs, animals that lack either functional rod-cone or melanopsin-based photoreception exhibit significant deficits in acute light-dependent sleep responses. Thus, rod-cone and melanopsin-based pathways are both necessary for modulating the effects of light and dark on sleep independent of image formation.
Results
Rod-Cone and Melanopsin-Based Photoreception Are Sufficient to Photo-Entrain Sleep-Wake Rhythms.
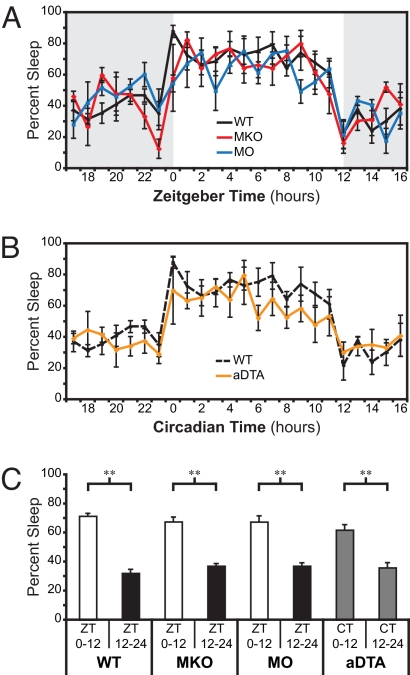
To investigate the effect of circadian photo-entrainment on sleep, we recorded EEGs and electromyograms (EMGs) to determine the sleep state (wake, low-voltage, high frequency EEG with high-amplitude EMG; non-REM [NREM], high-voltage, low frequency EEG with low-amplitude EMG; or REM, prominent theta activity in EEG channels and low EMG) of adult mice under a 12-hour light/12-hour dark cycle. Lights were switched on from ZT0–12 and turned off from ZT12–24. We used mice of a mixed C57Bl6 (B6) and 129 background. Previously, it was shown that the B6 and 129 lines do not differ in their total sleep amounts during either the light or dark periods (21). To determine the contribution of rod-cone photoreception to sleep, we used melanopsin-KO mice (Opn4taulacZ/taulacZ, referred to here as MKO), in which rod-cone light detection is intact and the intrinsic photosensitivity of ipRGCs is eliminated (14). To determine the individual contribution of melanopsin-based photoreception to sleep, we used “melanopsin-only” mice (Gnat1−/−;Cnga3−/−, referred to here as MO), which still retain melanopsin-based photoreception but both rods and cones lack the ability to detect light as a result of mutations in the rod transducin gene (Gnat1) and the cone cyclic nucleotide gated channel gene (Cnga3) (6). On average, WT mice slept 71.5% ± 1.9% of the light portion of the 12:12 light-dark (LD) cycle and 32.2% ± 2.7% of the dark portion (Fig. 1 A and C), confirming that the 12:12 LD cycle photo-entrained sleep rhythms (n = 5; P = 0.001). The amount of sleep was similar to those shown using different strains of mice, indicating that our mixed B6/129 line does not behave differently than either B6 or 129 lines alone (21). Mixed-model ANOVA between the different genotypes shows that MKO and MO mice photo-entrain their sleep-wake rhythms similar to WT animals (WT, n = 5; MKO, n = 4; MO, n = 4; P = 0.18; Fig. 1A). MKO mice slept 67.6% ± 3.2% in the light and 37.1% ± 1.7% in the dark (n = 4, P = 0.002; Fig. 1C), and MO mice slept 67.5% ± 4.1% in the light and 37.1% ± 2.2% in the dark (n = 4; P = 0.02; Fig. 1C), indicating that the total amount of sleep for all genotypes is similar and approaches 50%. Consistent with previous reports that either rod-cone or melanopsin-based photoreception is sufficient to signal light information to the circadian oscillator, these data show that either pathway can also photo-entrain sleep.
Fig. 1.
Rod-cone and melanopsin photoreception entrain sleep rhythms through ipRGCs (A) The percent of time the mice spend sleeping (NREM and REM summed/total time for percentage of sleep) is plotted against the daily time defined as zeitgeber “time-giver” time (ZT). Data binned into 1-hour intervals. WT (n = 5, black), MKO (n = 4, red), and MO (n = 4, blue) mice are all able to confine sleep to the light portion of a 12 h/12 h LD cycle. Gray background indicates lights off; white background indicates lights on. (B) Percent sleep in four aDTA mice (orange) aligned and plotted against circadian time as defined by the start of the sleep phase (CT0). WT from A is plotted for reference (dashed black). (C) Percent sleep in light and dark portions of the day in WT, MKO, and MO animals shows that all three genotypes are able to confine their sleep to the light portion of the day. The aDTA mice are plotted with CT time to show that they segregate sleep and wake to separate portions of the day (**, P < 0.01). All points represent mean ± SEM.
ipRGCs Mediate Circadian Photo-Entrainment of Sleep.
To test whether image-forming pathways influence sleep-wake rhythms independent of circadian photo-entrainment, we used “melanopsin aDTA” mice (7). In these animals, ipRGCs are selectively ablated by specific expression of an attenuated version of the diphtheria toxin A (aDTA) subunit under control of the melanopsin locus (Opn4aDTA/aDTA). We previously found that aDTA mice have normal image formation and intact circadian rhythms, but these rhythms are not aligned to the LD cycle (7). Consistent with the finding that aDTA animals do not photo-entrain their wheel running activity, the sleep rhythms of aDTA mice also free-ran throughout the LD cycle [supporting information (SI) Fig. S1 A and B]. To compare the aDTA sleep rhythms to those of WT animals, we aligned the sleep recordings from the aDTA animals to the start of the sleep phase of each mouse, which was determined by identifying the longest portion of the day with sleep amounts greater than 60% (Fig. 1B). The first 1-hour “bin” was denoted circadian time (i.e., CT 0). WT and aDTA mice were compared by aligning CT0 in aDTA to ZT0 in WT mice. Mixed-model ANOVA showed that aDTA mice had sleep rhythms similar to WT mice (WT, n = 5; aDTA, n = 4; P = 0.75; Fig. 1B). We further compared the amount of sleep during CT0–12 versus CT 12–24 and found that active and inactive phases were present in aDTA, similar to the other mutant mice (aDTA, n = 4; P = 0.006; Fig. 1C). These data therefore indicate that the image pathway, which remains intact in aDTA mice, does not influence photo-entrainment of sleep.
Acute Light-Induction of Sleep Requires Rod-Cone and Melanopsin-Based Photoreception.
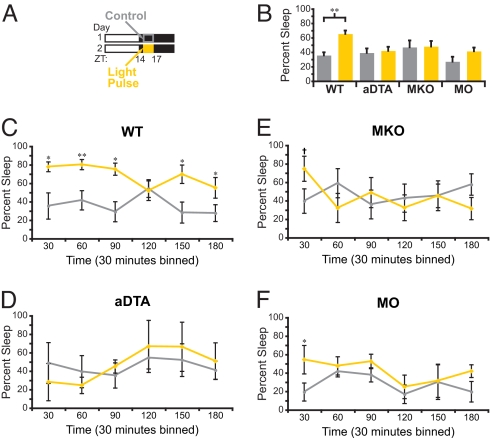
Acute light exposure in the dark phase of the day-night cycle inhibits wheel-running activity in mice, an effect known as masking (22). To test how acute light exposure affects sleep, mice were photo-entrained to a 12:12 LD cycle and then exposed to a 3-h light pulse from ZT14 to ZT17 (Fig. 2A). This is a period when the homeostatic and circadian drives are high for wakefulness. The different mutant mouse lines used (WT, MKO, and MO) have similar baseline sleep at this time (WT, n = 5; MKO, n = 4; MO, n = 4; P = 0.18). In WT animals, the 3-hour light pulse significantly increased the amount of sleep (NREM and REM combined to calculate total sleep) from 35.65% ± 4.4% during the baseline night to 65.36% ± 4.66% during the presentation of the 3-hour light pulse (n = 5; P = 0.006; Fig. 2B). The percentage of sleep during the light pulse is comparable to the percentage of time that mice normally sleep during the day (Fig. 1C and Fig. 2B). This observation indicates that acute light at night is able to induce sleep levels similar to those during the day, possibly by overriding both the homeostatic and circadian drives.
Fig. 2.
Rod-cone and melanopsin photoreception are necessary to sustain induction of sleep by a light pulse (A) A diagram of light paradigm used in B–F. Gray outline demarcates the control period of baseline night and orange box indicates the time of light presentation. For B–F, gray represents the data from control night and yellow represents the data from the light pulse. (B) Changes in total sleep during the light pulse in WT (n = 5), aDTA (n = 3), MKO (n = 5), and MO (n = 4) mice. A significant increase in sleep was observed in only WT animals. (C–F) Data from B subdivided in 30-min bins. (C) WT animals show a sustained sleep induction. (D) aDTA mice do not show any induction of sleep by a light pulse. (E and F) MKO and MO animals show a transient induction of sleep at the beginning of the light pulse (*, P < 0.05; **, P < 0.01; and +, P < 0.05, Student's t test of 30-min bin only). All points represent mean ± SEM.
To uncover the retinal pathways involved in this acute light induction of sleep, we tested MKO, MO, and aDTA mice in a similar manner. When the aDTA animals' free running behavior is accounted for, they show similar sleep amounts to the other mutants between CT14 and CT17 (WT, n = 5; MKO, n = 5; MO, n = 4; and aDTA, n = 3, P = 0.28; Fig. 2B). Because aDTA animals free-run and we are able to accurately estimate their period with wheel running activity (Fig. S1A), we were able to predict their sleep onset days in advance and presented the light pulse only on a day when their sleep cycle aligned with the 12:12 LD cycle (i.e., CT0 occurred at 7 a.m., when the lights turned on). We found that light did not cause an increase in sleep in aDTA mice, demonstrating that the image-forming pathway does not play a role in sleep induction by light (n = 3, P = 0.36; Fig. 2 B and D). Previous reports have shown that light pulses at ZT14 are sufficient to inhibit wheel-running activity in animals that lack either the rod-cone or the melanopsin photoreceptive pathways (15, 18). Surprisingly, similar light exposure failed to induce sleep in either MKO or MO animals (MO, n = 4, P = 0.052; MKO, n = 5, P = 0.79; Fig. 2B). We also examined changes in REM sleep throughout the light pulses and found that REM changed proportionally to total sleep such that there were no significant selective enhancements in REM sleep (Fig. S2A).
To study the changes in total sleep (i.e., NREM and REM combined) as a function of time, we subdivided the data into 30-min bins. In WT animals, light induced significant increases in sleep over the duration of the 3-hour light pulse (n = 5; P = 0.021; Fig. 2C). In contrast, light only transiently promoted sleep in MO animals at the beginning of the pulse (30 min, n = 4; P = 0.02; Fig. 2F). While we did not find overall changes in sleep induction by light in MKO animals, there is mounting evidence in the literature that rods and cones contribute initially to the light response (23). To determine if rods and cones have any influence on the initial response, we analyzed MKO animals for the first 30-min time point by using a Student's pair-wise t test and found that light initially induced a significant increase in sleep (n = 5, P = 0.04; Fig. 2E). This analysis indicates that the intrinsic photo-response of ipRGCs may play a bigger role in inducing sleep than the rod-cone input through the ipRGCs. Finally, we performed t test comparisons between the WT animals and MKO, MO, and aDTA animals for the entire light pulse and found that light induced sleep significantly more in WT mice compared with the other mutant lines (WT, n = 5; MKO, n = 5, P = 0.03; MO, n = 4, P = 0.04; and aDTA, n = 3, P = 0.02; Fig. 2B). These results demonstrate that a sustained effect of light on sleep is dependent on a combination of rod-cone and melanopsin-based photoreception.
Acute Dark Exposure Induces Wakefulness and Requires Rod-Cone and Melanopsin-Based Pathways.
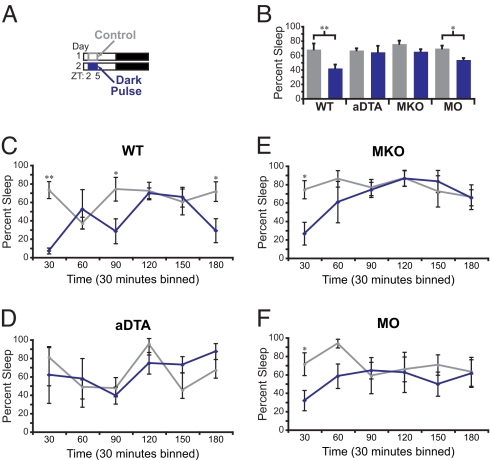
Using wheel-running activity, it has been shown that WT mice do not significantly increase activity during a dark pulse presented during the daytime (24). However, EEG/EMG recording is a direct measure of sleep state and therefore a more accurate representation of behavioral changes. As we found that light induces sleep in mice, we wanted to investigate if, conversely, dark induces wakefulness. We presented a 3-h dark pulse from ZT2 to ZT5, when both the circadian and homeostatic drives are high for the mouse to sleep (Fig. 3A). We found that the baseline sleep time during the day is similar between the mutant lines we used (WT, n = 5; MKO, n = 4; MO, n = 4; and aDTA, n = 3; P = 0.12). A dark pulse induced wakefulness in WT mice, causing a significant reduction in sleep from 68.2% ± 8.1% on the 3-hour period of the control day to 42.1% ± 5.2% during the dark pulse (n = 5, P = 0.006; Fig. 3B). MO mice also showed a significant change in total sleep but with decreased magnitude (69.5% ± 3.8% vs. 53.8% ± 2.5%, n = 4; P = 0.023). However, the same dark pulse failed to produce any significant effect on wakefulness in aDTA and MKO (aDTA, n = 3, P = 0.88; MKO, n = 4, P = 0.21; Fig. 3B). We also analyzed REM sleep and found no significant changes in REM proportions during the dark pulse of any genotype (Fig. S2B). These results indicate that both rod-cone and melanopsin-dependent ipRGC pathways are also required to promote WT levels of wakefulness in response to a dark pulse.
Fig. 3.
A dark pulse induces wakefulness through ipRGCs (A) A diagram of dark exposure used in B–F. Gray outline demarcates the control period of baseline day and the blue box indicates the time of dark presentation. For all panels in B–F, gray represents the data from control day and blue represents the data from the dark pulse. (B) Changes in total sleep during the dark pulse in WT (n = 5), aDTA (n = 3), MKO (n = 4), and MO (n = 4) mice. A significant increase in wakefulness by a dark pulse was observed in WT and MO animals. (C–F) Data from B subdivided in 30-min bins. WT (C), MKO (E), and MO (F) all show a transient induction of wake, whereas aDTA (D) mice do not respond to the dark presentation (*, P < 0.05; **, P < 0.01). All points represent mean ± SEM.
To assess whether any transient responses are present within the dark pulse, we subdivided the data of Fig. 3B into 30-min bins. This analysis revealed a significant initial effect of the dark pulse in inducing wakefulness in WT, MKO, and MO animals (30 min, WT, n = 5, P = 0.006; MKO, n = 4, P = 0.03; and MO, n = 4, P = 0.03; Fig. 3 C, E and F). However, dark did not induce wakefulness in aDTA animals (Fig. 3D), indicating that similar to the light signal, the dark signal is also routed through ipRGCs (Fig. 2D and Fig. 3D). Similar to light pulses, we found by using a Student's t test analysis that the WT animals differed significantly from MKO, MO, and aDTA animals despite the fact that all genotypes induced transient wakefulness significantly (WT, n = 5; MKO, n = 4, P = 0.007; MO, n = 4, P = 0.002; aDTA, n = 3, P = 0.009). The dark signal was efficient in inducing wakefulness in both MO and MKO animals. These results indicate that ipRGCs are conveying a light OFF response from rods-cones, which is the first behavioral evidence for ipRGCs carrying a functional OFF signal. Together, the OFF response from rods-cones and the shutting off of melanopsin signaling causes a larger dark response in WT animals.
Chronic Light Pulses Induce a Differential Effect on Sleep Versus Wheel Running Activity.
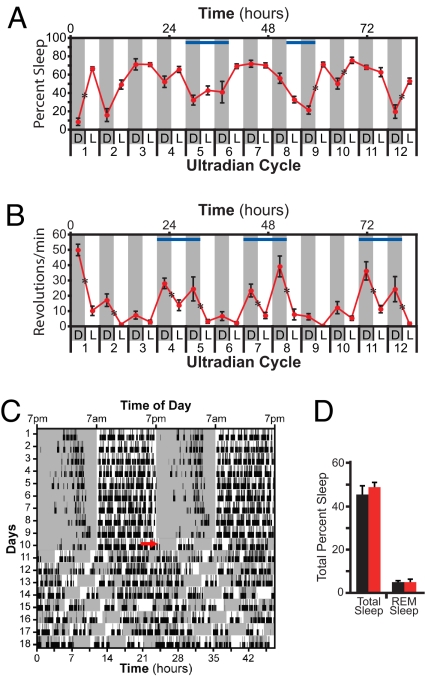
To investigate the effects of repeated exposure to light pulses on sleep independent of circadian photo-entrainment, we exposed WT animals to an ultradian (i.e., short day) 7-hour LD cycle (i.e., 3.5 h/3.5 h LD). WT mice were chosen for this experiment because they showed the only robust sleep induction to the single light pulse, making them the best candidates for determining the effect of chronic exposure. Mice are unable to photo-entrain to this shorter cycle, hence the light and dark portions will fall within all phases of the circadian cycle (25). This repetitive presentation of light and dark allows for the determination of the effects of chronic light pulses on sleep. Light was found to induce sleep in the first ultradian cycle; however, light did not elicit sleep consistently in subsequent cycles (Fig. 4A). In addition, quantification of sleep in the ultradian cycle revealed an obvious circadian rhythm that is slightly longer than 24 h (Fig. 4 A and C). In agreement with the idea that the homeostatic drive is intact under the ultradian cycle, the amount of total sleep (45.3% ± 3.7% vs. 48.6% ± 2.1%; n = 3, P = 0.25) and REM sleep (5.22 ± 0.7 vs. 5.2 ± 1.24; n = 3, P = 0.98) is comparable in the ultradian and 24-h light cycles (Fig. 4D).
Fig. 4.
Chronic light pulses repeatedly inhibit wheel-running activity but are not able to consistently induce sleep. (A and B) WT mice housed under 3.5 h/3.5 h LD ultradian cycles (light, white background; dark, gray background) reveal that light does not consistently induce sleep (A) (n = 4) but does consistently inhibit wheel running activity measured in a second group of animals (B) (n = 10). Lower x axis indicates cycle number and lighting condition. Upper axis depicts hours from the start of cycle 1. Blue bars indicate approximate region of wake maintenance zones in A and phases where the circadian drive to be active is high in B. (C) A representative double plotted diagram of sleep activity for one mouse in 12 h/12 h LD (circadian, d 1–10) and ultradian (d 11–18) paradigms (white background, light; gray background, darkness). Black tick marks represent sleep, and REM sleep is denoted by higher ticks. Red arrow shows the start of the first ultradian cycle. (D) Levels of total sleep and REM sleep are similar between 12/12 LD (black) and ultradian (red) light cycles (n = 3). *, P < 0.05.
Because wheel running is a well established measure of the effect of light on activity, we examined whether the chronic light pulses of the ultradian cycle inhibited activity in a second set of WT mice. Consistent decreases in wheel-running activity were observed upon dark-to-light transition in the ultradian cycle (10 of 12 dark-to-light transitions showed significant decrease, P < 0.05; Fig. 4B), indicating that light is capable of repeatedly inhibiting activity over several days. Even at phases in which the circadian drive for mice to be active is high (Fig. 4B, blue bars), light inhibited the wheel running activity (cycles 4, 5, 7, 8, 11, and 12, Fig. 4B). In contrast, very few dark-to-light transitions showed significant increase in sleep (four of 12 showed significant increases, P < 0.05; Fig. 4A). Light was particularly ineffective at phases in which animals seem to have a wake maintenance zone (Fig. 4A, blue bars). In contrast to the simple binary control of wheel running activity, these results indicate that the threshold for inducing sleep by light is higher than the threshold for inhibiting wheel-running activity, possibly because light has to overcome both circadian and homeostatic mechanisms.
Discussion
The retina measures light and dark information by using rod-cone photoreceptor cells and signals these responses to the brain through ON and OFF retinal ganglion cells (RGCs). RGCs that express the photopigment melanopsin (ipRGCs) are a third type of photoreceptive cell in the mammalian retina. Similar to traditional retinal ganglion cells, ipRGCs also receive ON and OFF light signals from rods and cones (9, 10). In addition to image formation, the retina also signals light and dark for several physiological functions, including circadian photo-entrainment. Here we show in mice that acute light and dark pulses induce sleep and wakefulness, respectively. Furthermore, rod-cone and melanopsin-based photoreception are both necessary to mediate the full effect of light on the sleep state. In addition, the rod-cone signal for sleep and wakefulness is routed although ipRGCs, indicating that image formation does not influence sleep.
Because mice are nocturnal, they confine their sleep mostly to the light portion of the day. Therefore, we chose to expose mice to light pulses shortly after the onset of dark, when the circadian drive for wakefulness is high and the homeostatic drive for sleep is low. To induce sleep at this time, the light signal must overcome two strong intrinsic drives for wakefulness. Our results in WT mice show that an acute light pulse induced sleep to a level comparable to that in the daytime, whereas mice that lack ipRGCs did not respond to any part of the light pulse. This result shows that ipRGCs are necessary to convey the light signal to sleep centers in the brain. Furthermore, neither the rod-cone nor the melanopsin photoreception alone was able to sustain the response for the full 3-hour light pulse. The necessity of both rod-cone and melanopsin photoreception working in concert to mediate acute sleep responses is in contrast to circadian photo-entrainment and light inhibition of wheel running activity, which require only rod-cone or melanopsin photoreception (12–17). The sleep system therefore appears to be more sensitive to the loss of either rod-cone or melanopsin-based photoreception.
We further demonstrated that a dark pulse during the light portion of the day induced wakefulness in WT animals. This response is surprising because dark pulses do not induce wheel running activity (24). We further show that there was no overall effect in animals lacking ipRGCs (i.e., aDTA). Even when the data were analyzed in 30-min bins, the aDTA animals showed no response to any part of the dark pulse, whereas WT mice had significant wake responses throughout the 3-hour pulse. This indicates that ipRGCs convey a dark signal to sleep/wake centers in the brain. It is surprising that melanopsin cells convey a dark signal to induce wakefulness, as the melanopsin cells receive only a weak input from the rod-cone OFF pathway in the retina (11). To determine which photoreceptive pathways contribute to this dark signal, we analyzed the response of MKO and MO mice to the dark pulse. We found that both genotypes had significant transient responses in the first 30 min. This indicates that both rod-cone and melanopsin photoreception contribute to dark detection for induction of wake and that both systems are required to elicit a response beyond the first 30 min. Although WT, MKO, and MO animals all had significant transient induction of wakefulness by a dark pulse (Fig. 3 C, E and F), the magnitude of the WT response over the full 3 hours was greater and significantly different from the other genotypes (Fig. 3B). The stronger WT response indicates that rod-cone and melanopsin systems have to work collectively to signal dark responses to the brain. This indicates not only that ipRGCs signal dark information from rods and cones to the brain, but also that the melanopsin protein is sufficient to detect this information. These results are functional evidence that dark detection and signaling by ipRGCs affects behavior.
A differential effect of light on sleep and wheel running activity was revealed by using the ultradian 3.5 h/3.5 h LD cycle. In this paradigm, light inhibited wheel running activity at nearly all dark-to-light transitions. Whereas light induced sleep in the first ultradian cycle, it failed to induce sleep consistently in subsequent cycles. Thus, light has a weaker effect on sleep than on wheel-running activity. One possible explanation for this difference is that sleep centers such as the ventrolateral preoptic nucleus receive weaker input from the ipRGCs as opposed to circadian centers such as the suprachiasmatic nucleus (20).
Here we present a threshold model explaining how light differentially affects the acute induction of sleep versus the inhibition of wheel running activity. Light detected by rods and cones (i.e., extrinsic) or by melanopsin photo-transduction pathway (i.e., intrinsic) are relayed to the brain via ipRGCs to influence several non-image-forming functions, which include circadian photo-entrainment, pupillary light reflex, masking (7), and acute induction of sleep (Fig. 2) In retina stimulated by light, ipRGCs could be activated by an exclusive extrinsic rod-cone input, only through intrinsic melanopsin input, or a combination of both. Given the higher sensitivity of the dark-adapted rod-cone system to light, one would predict that the initial response of ipRGCs would usually include input from rods-cones. However, the rods and cones adapt to light leading to a decreased input from rods and cones over time. It is worth mentioning that, in electrical recordings, even the melanopsin response shows light adaptation but to a lower extent compared with rods-cones (11).
How do these basic cellular mechanisms contribute to the differential effects of light on these behaviors? For WT animals, the ultradian cycle showed that light induces changes in wheel running activity more readily than sleep, indicating that the threshold for sleep induction is higher than that of wheel running activity. This higher threshold for inducing sleep allowed us to use acute light pulse treatments in different mutant lines to reveal differences in input strength and to show novel interactions between the rod-cone and the melanopsin systems. As expected, in MKO animals, rods-cones increase sleep initially but then the rod-cone system adapts and fails to sustain the response to light. This is similar to results obtained with MKO animals for inhibition of wheel-running activity using a similar light intensity (15). However, light inhibits wheel running for the first 100 min of the 3-h light pulse whereas light induces sleep in only the first 30-min bin (Fig. 2E).
For wheel running activity, MO animals sustain the inhibitory responses to light similar to WT animals (26). However, in agreement with our threshold model, light induces sleep initially in MO animals but fails to sustain the response (Fig. 2F). This is the first behavioral difference between WT and MO at light intensities that activate the intrinsic light response in ipRGCs. Therefore, although the extrinsic and intrinsic light responses are also adapted in WT animals, the combination of signaling through rod-cone and the melanopsin systems could be sufficient for overcoming the threshold for sleep induction for the duration of the light pulse (Fig. S3).
During the submission of this work, a study investigating the effects of light on WT animals, melanopsin-KO animals, and animals that lack rods and cones as a result of degeneration of the outer retina was published (26). There are similar findings between the two studies but also some fundamental differences; especially those concerning the role of rods-cones in sleep induction. First, both studies presented acute light pulses at the dark portion of the 24-h LD cycle. However, the duration and time of presentation of the light pulse differ. We presented a 3 h light pulse 2 hours after the onset of dark (ZT14), when the homeostatic drive for sleep is low. However, Lupi et al. (26) presented a 1-h light pulse starting 4 hours after the onset of dark (ZT16). At ZT16, the animals would have been awake for 2 extra hours and hence their homeostatic drive for sleep would be higher than that at ZT14. A higher homeostatic drive for sleep could cause a reduction in the threshold of sleep induction by light.
Second, for the MKO animals, both studies found no significant difference in sleep induction by light for the duration of the light pulse. However, our 30-min bin analysis revealed that light significantly induces sleep in the MKO animals only initially, indicating that rods-cones contribute significantly to light induction of sleep.
Third, as Lupi et al. (26) used 1-hour light pulses, we “binned” the hour 1 of our 3-h light pulse experiments to make a direct comparison to their results. Even though our 1-h binned data in the MO animals showed significant sleep induction (29.9% ± 9.7% control day to 50.25% ± 15.3% pulsed day; P = 0.0069), in agreement with Lupi et al. (26), there are important differences in the amplitude and the sustenance of the light response. We found that the amount of sleep induction in MO animals was significantly less than that of WT animals for the first hour (P = 0.022), suggesting an important input from rods-cones for sleep induction. In contrast, the results from Lupi et al. (26) showed no differences in sleep induction between their MO and WT animals. The most parsimonious explanation for this discrepancy could be the higher homeostatic drive for sleep when they administered the light pulse at ZT16. Another possibility is the different mutations used to eliminate rod and cone input. We used mutations that functionally eliminate the rod and cone photo-transduction pathways whereas Lupi et al. (26) used mutations that lead to the degeneration of rods and cones. Despite these differences, our 3-h light pulse revealed an inability of light to sustain the induction of sleep in MO animals when compared with WT animals. These results, coupled with the ability of light to initially induce sleep in MKO animals, show that both rods-cones and melanopsin contribute to sleep induction by light.
This study indicates that sleep and wake are modulated by an interplay between the light and dark signaling through the rod-cone and melanopsin-based photoreceptive pathways. Our results show conclusively that sleep is not influenced by conscious visual perception of day and night. The critical role for both the rod-cone and melanopsin systems in mediating the effects of light on sleep imply that humans with deficits in either retinal pathway could be particularly vulnerable to acute effects of light and dark on sleep and wakefulness.
Methods
Animals.
We used adult male mice of a mixed B6 and 129 background. All animal experiments were done according to the institutional regulations of Johns Hopkins University (Baltimore, MD).
Sleep Recordings.
Sleep recordings were done as described (27). In brief, we affixed a 2-channel EEG and 1-channel EMG implant (Pinnacle Technology) into the skull of mice between the ages of 4 and 12 months while under ketamine/xylazine-induced anesthesia. Mice were allowed 10 days to recover in a 12-hour LD cycle before being transferred to the sleep-recording cage. Mice were then tethered with a preamplifier and allowed 3 days to acclimate to the new cage and tether before recordings were started. EEG and EMG were recorded in 10-sec bouts at a frequency of 200 Hz. The high pass filter setting for both EEG channels was set at 0.5 Hz and low pass filtering was set at 40 Hz. EMG signals were high pass filtered at 10 Hz and subjected to a 100 Hz low pass cutoff. Both EEG and EMG signals were amplified ×5,000 and digitized at 14 bits before being sent to the recording software. Signal acquisition was performed using the Sirena acquisition suite (Pinnacle Technology). Sleep state was determined subjectively by a researcher blind to treatment based on frequency and amplitude of EEG and EMG waves using Neuroscore (DSI). Behavioral state was either determined to be wake (low-voltage, high frequency EEG with high-amplitude EMG), NREM (high-voltage, low frequency EEG with low-amplitude EMG), or REM (prominent θ activity in EEG channels and low EMG).
Total sleep shown in Fig. 1 A–C was determined by analyzing 48 consecutive hours of EEG and EMG activity in each mouse and combining 1-hour intervals between days for all genotypes. aDTA animals were aligned to each other for Fig. 1B by finding the longest time of sleep greater than 60%, which was termed the inactive phase. The start of the inactive phase (CT0) was simply the first 1-hour bin of this period.
Light (Fig. 2) and dark (Fig. 3) pulses were given in the background of a 12 h/12 h 1,000 lux LD cycle. Sylvania 23-W Super mini Daylight fluorescent bulbs were used for all light cycles and light pulses in sleep experiments shown in all figures. For WT, MKO, and MO, the 1,000-lux light pulse was administered 2 h after the dark onset (ZT14) and lasted for 3 h. ZT 14–17 on the previous day was used as the control period for the light pulse. The dark pulse was given 2 h after light onset at ZT 2 and also lasted for 3 h. The previous day was similarly used as the control period for the dark pulse.
To perform similar light pulses on aDTA animals, we monitored the free running period of each mouse for days or weeks until the onset of wake (CT12) coincided with lights off (ZT12). The light pulse was then administered at ZT14–17. Thus, we were able to administer the light pulse in a manner that controlled for circadian time and light environment. Similarly, the dark pulse was administered on a day when the onset of sleep (CT0) coincided with lights on (ZT0). The dark pulse was then administered 2 h later from ZT2 to ZT5.
The chronic light pulse environment was simulated by exposing mice to a T7 light cycle (ultradian, 3.5 h; 1,000 lux light; 3.5 h dark) for 8 consecutive days. Sleep recordings were performed in mice for 10 days in a 12 h/12 h LD cycle and then switched to the ultradian cycle and recorded continuously for 8 days. To determine total sleep changes within this cycle, 72 h of consecutive data were scored for three mice and averaged for NREM, REM, total sleep, and wake.
Wheel-Running Activity.
All wheel running activity was performed on male B6/129 hybrid male mice between the ages of 4 and 12 months. Mice were individually housed in cages with a wheel for the duration of the experiment. All lighting conditions were ≈700 lux provided by General Electric Ecolux UltraMax Starcoat T8 fluorescent bulbs. Wheel running activity was monitored with VitalView software (MiniMitter; Respironics) and was analyzed with ClockLab (Actimetrics).
In aDTA mice, wheel running activity (Fig. S1A) was used to measure their free running period on a background of a 12 h/12 h light/dark cycle. These mice were housed with a 9-inch wheel (MiniMitter; Respironics). WT mice in the ultradian (3.5 h/3.5 h LD) cycle were placed in cages with a 4.5-inch running wheel.
Statistical Analysis.
Comparisons of total sleep in WT, MKO, MO, and DTA mice (Fig. 1 A and B) were performed with GraphPad Prism using a mixed model ANOVA. To compare sleep amounts in light and dark periods, Student's t test with Microsoft Excel were used for comparisons between WT and the other mutant lines. For Fig. 2B, Fig. 3B, and Fig. S6 total control versus total pulse time percentages were compared using a Student's t test in Microsoft Excel. In Fig. 2 C–E and Fig. 3 C–E, two-way repeated measures ANOVA with Tukey pos-hoc analysis were performed in SigmaStat to determine overall changes overtime and identify specific periods of change. Total sleep percentage and REM was compared in Fig. 4D using a Student's t test in Microsoft Excel.
Supplementary Material
Acknowledgments.
We would like to sincerely acknowledge Drs. Steven Lockley, Marnie Halpern, Rejji Kuruvilla, and Haiqing Zhao for critical scientific input on the manuscript. We thank the Biostatistics Department at Johns Hopkins University for help with statistics. Finally we would like to thank members of the Mouse Tri-Lab at the biology department at Johns Hopkins University for valuable discussions and advice. This work was supported by a young investigator award from the David and Lucile Packard Foundation (to S.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808312105/DCSupplemental.
References
- 1.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 3.Czeisler CA, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 4.Lockley SW, et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- 5.Benca RM, Gilliland MA, Obermeyer WH. Effects of lighting conditions on sleep and wakefulness in albino Lewis and pigmented Brown Norway rats. Sleep. 1998;21:451–460. doi: 10.1093/sleep/21.5.451. [DOI] [PubMed] [Google Scholar]
- 6.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatori M, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belenky MA, et al. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Leon JA, et al. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur J Neurosci. 2006;24:1117–1123. doi: 10.1111/j.1460-9568.2006.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 13.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 14.Lucas RJ, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 15.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 16.Ruby NF, et al. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 17.Freedman MS, et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 18.Lucas RJ, et al. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 19.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hattar S, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franken P, Malafosse A, Tafti M. Genetic determinants of sleep regulation in inbred mice. Sleep. 1999;22:155–169. [PubMed] [Google Scholar]
- 22.Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol Int. 1999;16:415–429. doi: 10.3109/07420529908998717. [DOI] [PubMed] [Google Scholar]
- 23.Dkhissi-Benyahya O, et al. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron. 2007;53:677–687. doi: 10.1016/j.neuron.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doyle SE, Yoshikawa T, Hillson H, Menaker M. Retinal pathways influence temporal niche. Proc Natl Acad Sci USA. 2008;105:13133–13138. doi: 10.1073/pnas.0801728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redlin U, Mrosovsky N. Masking of locomotor activity in hamsters. J Comp Physiol A. 1999;184:429–437. doi: 10.1007/s003590050342. [DOI] [PubMed] [Google Scholar]
- 26.Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008 doi: 10.1038/nn.2179. in press. [DOI] [PubMed] [Google Scholar]
- 27.Naylor E, et al. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.