Abstract
Leptin, a profibrogenic cytokine plays an important role in the development of non alcoholic steatohepatitis. Leptin also regulates immune responses including macrophage phagocytic activity. Stellate cells are key elements in liver fibrogenesis, and previously we have demonstrated that phagocytosis of apoptotic bodies by stellate cells is profibrogenic. To study the effects of leptin on the phagocytic activity of hepatic stellate cells, we exposed both LX-2 cells and primary stellate cells to leptin, and we have observed increased phagocytic activity. In stellate cells isolated from Zucker (fa/fa) rats, the rate of phagocytosis was significantly decreased. To investigate the mechanism by which leptin induces phagocytosis, we focused on the role of Rho-GTP-ases. We found that leptin induced the PI3K-dependent activation of Rac1, and that NADPH oxidase activation was also implicated in this process. Leptin also induced RhoA activation and translocation to the phagosomes. Expression of the constitutive active Rac1 and RhoA both increased the phagocytic rate, while inhibition of the Rho-dependent kinase decreased the phagocytic activity. In conclusion, we describe a novel role of leptin in the fibrogenic process, the induction of phagocytosis of apoptotic bodies by hepatic stellate cells. The data provide strong evidence of a Rho-GTP-ase mediated regulation of the cytoskeleton during stellate cell phagocytosis. Leptin-mediated phagocytic activity of stellate cells therefore could be an important mechanism responsible for progression of fibrosis in NASH.
Keywords: Leptin, Inflammation, oxidative stress, apoptosis, fibrogenesis, non alcoholic steatohepatitis
Leptin, a 16 kDa peptide, plays a key role in the regulation of body weight (1). It also has an important role in the development of non alcoholic steatohepatitis (NASH), which is characterized by the presence of the metabolic syndrome and progressive steatosis, inflammation and fibrosis in the liver. Hepatic stellate cells (HSC) are key elements of the fibrogenic process. They are normally quiescent and produce only small amounts of extracellular matrix (ECM) components. After activation by cytokines or reactive oxygen species (ROS), they undergo a morphological and functional transition to myofibroblast-like cells with the subsequent production of transforming growth factor-β1 (TGF-β1), and ECM (2). Leptin has been shown to be critical in the development of hepatic fibrosis, as leptin-deficient ob/ob mice do not develop liver fibrosis following CCl4 injury (3). Furthermore, lack of the leptin receptor, Ob-R, prevents fibrogenesis induced by bile duct ligation (BDL) in fa/fa (Zucker) rats (4). Because leptin is essential in the induction of liver fibrosis, it is important to better understand the mechanism(s) by which leptin regulates this process. Although the fibrogenic properties of leptin are postulated to be from the upregulation of procollagen α1(I) (3, 5), mitogenesis and the inhibition of HSC apoptosis (6), the precise mechanism by which leptin promotes liver fibrosis remains undetermined.
Recently, we have shown that HSC phagocytosing apoptotic bodies (AB) from dying hepatocytes undergo activation and a major fibrogenic response with the production of oxidative radicals, as well as the upregulation of procollagen α 1(I) and TGF-β1 gene expression (7). As phagocytosis by HSC is likely to be a common pathway leading to fibrosis, independent of the etiology of the liver disease, factors enhancing the phagocytic process could further trigger activation of HSC and consequently liver fibrogenesis. Leptin-induced phagocytosis requires binding of leptin to the leptin receptor, as leptin replacement in ob/ob mice normalized phagocytic activity (8). These observations led us to postulate that leptin may promote fibrogenesis through enhanced phagocytosis of AB by HSC. How leptin plays a role in phagocytosis is still unclear. Our speculation is that it may involve recruitment and/or activation of cytoskeleton-regulating elements, such as Rho GTP-ases, which play an active role in actin cytoskeleton remodeling, a requisite step in phagocytosis. Interest in the Rho family of small GTP-ases is compounded by a recent work demonstrating that they play an integral role in HSC activation and the fibrogenic process (9).
In the present study we have described a novel profibrogenic activity of leptin; the induction of phagocytosis of AB by HSC. We found that leptin elicits its prophagocytic activity by the regulation of small GTP-binding proteins. Leptin activated Rac1 via the PI3-kinase, and also induced activation and translocation of RhoA to the phagosomes, and subsequent activation of the Rho associated kinase (ROCK). These changes translated into increased phagocytic activity of HSC.
Materials and Methods
Animals
Male Zucker (fa/fa) and wild type rats from Charles River Laboratories Inc. (Wilmington, MA) were used for primary HSC isolation. The animals were housed in facilities approved by the National Institute of Health. All procedures were reviewed and approved by the Animal Welfare Committee of the University of California Davis.
Cell culture and preparation of apoptotic bodies
The human immortalized HSC line LX-2 (kindly provided by Dr SL Friedman, Mount Sinai Medical School, New York) (10) and primary rat HSC isolated from Zucker and wild type rats were used. Primary HSC were isolated from rats as previously described (7), and used 2 days after isolation. The purity of isolated HSC was assessed by the autofluorescence of vitamin A droplets, and was ≥95%. LX-2 cells exhibit typical features of HSC in primary culture, such as expression of desmin and glial acidic fibrillary protein, and responsiveness to TGF-β. HSC were maintained in DMEM supplemented with 5% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin and incubated at 37 °C in a humidified atmosphere with 5% CO2.
For preparation of AB, the human hepatoma cell line HepG2 was used, cultured in MEM and 10% FBS. AB were generated by exposing the cells to ultraviolet (UV) irradiation (100mJ/cm2, 142 seconds), as described preciously (7). AB were then collected 48 hours after the UV irradiation. To generate labeled AB, HepG2 cells were incubated with carboxytetramethyl rhodamine succinimidyl ester (TAMRA) (Invitrogen, Carlsbad, CA) prior to the UV-irradiation, as described previously (7).
Phagocytosis Assay
LX-2 or primary rat HSC were seeded in 6-well dishes at a density of 2.5X105/ml. The cells were then cultured in serum free medium for 16 hours and then treated with the following reagents: leptin (100 ng/ml, R&D Systems Inc. Minneapolis, MN) leptin plus AB, the PI3 kinase inhibitor LY294002 (20 uM, EMD Chemicals Inc. Gibbstown, NJ) plus AB for 6 hours, or the ROCK inhibitor Y27632 (10 uM, Sigma, St-Louis, MO) plus AB. The concentration of leptin used was based on prior rodent studies on liver fibrogenesis (6). TAMRA-labeled AB (approximately 105/ml were added to HSC at 37°C). Intracellular AB were detected by TAMRA labeling after 48 hours, by fluorescent microscopy. The rate of phagocytosis was assessed by dividing the number of TAMRA-positive HSC by the total number of HSC counted.
cDNA Transfection
cDNA encoding constitutive active Rho (RhoQ63L) and constitutive active and dominant-negative Rac (RacQ61L and RacT17N, respectively), originally generated by Dr. J. Silvio Gutkind (National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD) were used. pCMV6-XL6-SOCS3 (Origene, Rockville, Md), RacQ61L, RacT17N, RhoQ63L and control vector were transfected into LX-2 cells (transfection efficiency 60-70%), using the Lipofectamine reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendation.
Rac1- and Rho GTP Pull Down Assays
LX-2 cells were exposed to AB in the presence or absence of LY294002 then an affinity precipitation assay was performed to measure the active GTP-bound Rac1, according to the manufacturer's instructions (Millipore, Billerica, MA). Briefly, the cells were lysed with the buffer MLB containing 10% glycerol and 1mM PMSF and agitated at 4°C for 15 minutes followed by centrifugation at 14,000 × g at 4°C for 5 minutes. The supernatant containing 20 ug of protein was mixed with beads coated with p21-activated kinase containing a Rac-binding domain (p21-binding domain, PBD). The mixture was rotated for 45 minutes at 4°C and the agarose beads were washed and collected by brief centrifugation. The beads were then resuspended in the sample buffer with 50 mM of DTT and separated by SDS-polyacrylamide gel, and Rac1 was detected by a specific antibody provided by the kit. For the Rho-GTP pull down assay, LX-2 cells and HSC from wild-type and Zucker rats were exposed to AB for different times as described before; in the case of LX-2 cells in the presence or absence of the JAK inhibitor AG490 (50 uM, EMD Chemicals Inc., Gibbstown, NY), or LY294002, as above. For the Rho activity assay, the supernatant was collected and mixed with glutathione-agarose beads bound with Rhotekin (Millipore, Billerica, MA), containing a Rho-binding domain (RBD). The mixture was rotated for 45 minutes at 4°C and the agarose beads were washed and collected by centrifugation, then resuspended in the sample buffer and separated by SDS-polyacrylamide gel. The GTP-bound RhoA was detected by a specific antibody provided by the kit.
Western blot analysis
To detect JAK/STAT phosphorylation, LX-2 cells were incubated in serum-free medium then either mock transfected with pCMV6-XL6 or with pCMV6-XL6-SOC3 (Origene, Rockville, Md). They were exposed to AB for 0.5 hour then collected. In brief, the cells were washed with 1 mM Na3VO4/PBS and collected into the lysis buffer containing 50 mM dithiothreitol (DTT), 1 mM Na3VO4, 1 mM NaF (PH 8.0), 5 mM phenylmethylsulfonyl fluoride (PMSF) and 1% SDS. The lysates were centrifuged at 12,000×g for 5min at 4°C. The supernatant was collected and the protein concentration was determined with the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) following the instruction from the manufacturer, and 10-20 μg of protein was separated by SDS-PAGE. The proteins from the gel were then electroblotted (Bio-Rad Mini-Protean II transblot system) onto nitrocellulose paper (Bio-Rad) and blocked by 5% nonfat milk powder in Tris-buffered saline-Tween (20 mM tris-base, 137 mM NaCl, 0.1% Tween 20, pH 7.6), followed by incubation with the appropriate antibodies for 16 hours at 4°C: anti-phospho-JAK1 polyclonal antibody (1:500, EMD-Calbiochem), JAK1 antibody (1:300, Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-STAT3 monoclonal antibody (1:250, Cell Signaling Technology, Danvers, MA), and anti-STAT3 polyclonal antibodies (Santa Cruz, CA).
RhoA Translocation Assay
To determine the membrane-bound (GTP-ase active form) of RhoA, LX-2 cells were serum starved for 16 hours and exposed to leptin or AB. To obtain membrane and cytosolic fractions, the cells were washed and homogenized in a buffer containing 50 mM Tris-HCl, pH 7.4, 1 mM EGTA, 1 mM EDTA, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 5 mM benzamidine HCl, 10 μg/ml soybean trypsin inhibitor, and 1 mM PMSF by ultrasonic polytron. The cell extract was centrifuged at 800 × g for 5 minutes. The supernatant was then collected and further centrifuged at 15,000 × g for 15 minutes at 4°C. The supernatant was centrifugated again at 100,000×g for 1 hour at 4°C, then the supernatant was collected as the cytosolic fraction, and the pellet, as the membrane fraction then resuspended. The protein concentration was measured; and 10 μg of cytosol and 20 μg of membrane fractions were used to perform Western blot analysis for RhoA.
Immunohistochemistry
LX-2 cells grown in chamber slides were exposed to TAMRA-labeled AB for 5 hours in the presence or absence of myosin light chain kinase inhibitor ML-7 (5 μM, EMD Chemicals Inc., Gibbstown, NJ,) for 30 minutes. The cells were fixed in 4% paraformaldehyde/PBS for 15 minutes at room temperature then were washed in PBS and blocked with 2% bovine serum albumin (BSA) in PBS for 1 hour followed by incubating with anti-RhoA monoclonal antibody (1:40) for 16 hours at 4°C, (Santa Cruz Biotechnology, Santa Cruz, CA). After washing with PBS, the secondary, Alex Fluor 488-labeled anti-mouse IgG (1:1000) (Invitrogen) was applied. The images were analyzed by fluorescence microscopy.
Results
Leptin increases phagocytosis of apoptotic bodies by HSC
To test whether leptin exposure induces phagocytosis of AB by HSC, and to ascertain that the effect on phagocytosis is leptin receptor-specific, HSC were isolated from wild type and Zucker rats. In HSC from Zucker rats the rate of phagocytosis was significantly lower compared to wild-type HSC (Figure 1). Leptin treatment significantly increased the rate of phagocytosis in HSC from wild-type rats, while no increase was detected in HSC from Zucker rats. This confirms that leptin receptor-mediated signaling is required for the induction of phagocytosis. Next, to elucidate the mechanism responsible for induction of phagocytosis by leptin, we focused on the role of Rho-GTP-ases.
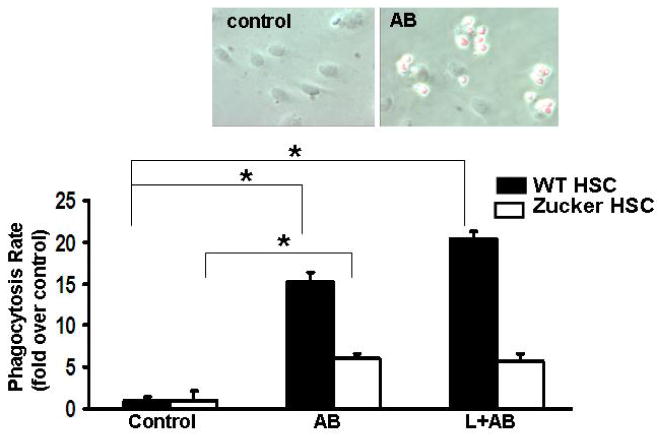
Figure 1.
Primary HSC from wild type and Zucker rats were treated with AB, leptin plus AB. The phagocytosis of wild-type HSC was induced by AB to 15.1-fold (+/-2.41)-fold, and leptin increased this response to 20.3-fold (+/-1.91). HSC from Zucker rats exhibited only a 6.04-fold (+/-1.89) increase in phagocytic activity and leptin did not significantly change the phagocytic rate. Data are expressed as Mean±SE, N=4. *p<0.01. Insert depicts control HSC (wt) and HSC phagocytosing TAMRA-labeled AB.
Leptin induces PI3K–dependent activation of Rac1, and induction of phagocytosis
As Rac1 is a crucial element of the phagocytic machinery (11), first, we focused on the role of this GTP-ase in leptin-induced phagocytosis. Since leptin may mediate its effects on the cytoskeleton via the PI3 kinase, we exposed LX-2 cells to leptin (100 ng/ml) and/or AB, in the presence or absence of the PI3 kinase inhibitor LY294002 (20 uM). As Rac1 is a major subunit of the NADPH oxidase complex, and NADPH oxidase activation occurs during phagocytosis of AB by HSC (7), we also tested if using the NADPH oxidase inhibitor apocynin had an effect on Rac1 GTP-ase activity. Leptin induced activation of Rac1 and this was inhibited by the PI3 kinase inhibitor LY294002 (Figure 2a). Inhibiting NADPH oxidase with apocynin (100 μM), also partially inhibited leptin-induced Rac1 activation, suggesting that Rac1 activation during phagocytosis occurs in concert with NADPH oxidase activation. Since we demonstrated that leptin induces Rac1 GTP-ase activity via the PI3 kinase, next we tested if inhibition of the PI3 kinase has an effect on the phagocytic rate. In leptin-treated LX-2 cells inhibition of PI3 kinase indeed decreased the rate of phagocytosis by 0.28-fold, and inhibiting also the NADPH oxidase by apocynin caused a further decrease by 0.53-fold. This suggests that leptin mediates its effects on phagocytosis via the PI3 kinase-dependent activation of Rac1, and that NADPH oxidase activity is also required for Rac1 induction during phagocytosis (Figure 2b).
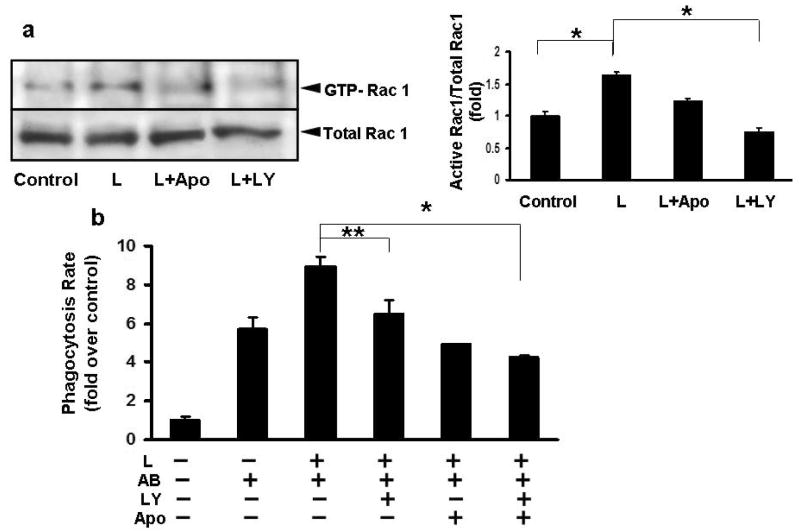
Figure 2. Leptin induces the PI3K-dependent activation of Rac1 and increase in phagocytosis.
a) LX-2 cells were exposed to leptin (100 ng/ml) and/or AB, in the presence or absence of the PI3 kinase inhibitor LY29002 (20 uM), and the NADPH oxidase inhibitor apocynin (100 uM). Western blot and densitometry showed that leptin induced activation of Rac1 and this was inhibited by the PI3 kinase inhibitor LY29002. Inhibiting NADPH oxidase with apocynin, also partially inhibited leptin-induced Rac1 activation. N=4, *p<0.01
b) Inhibition of PI3K decreased the rate of phagocytosis by 0.28-fold (+/-0.09) in leptin-treated LX-2 cells, and inhibiting also NADPH oxidase by apocynin caused a 0.53-fold (+/-0.14) decrease, compared to leptin treated cells. Mean ±SE, N=4, *p<0.01, **p=0.009.
Overexpression of constitutively active Rac1 induces phagocytosis of apoptotic bodies by HSC
Owing to the key role of the Rho family of small GTP-ases in actin remodeling, we next tested the role of this family of proteins in leptin-based phagocytosis by using complementary gain and loss of function approaches. Overexpression of the constitutive active Rac1 (RacQ61L) indeed was sufficient to induce phagocytosis of AB by HSC. LX-2 cells transfected with RacQ61L, engulfed more AB, while transfection of the dominant negative mutant (RacT17N) resulted in a decrease in phagocytosis compared to the RacQ61L-transfected cells but still it was above the baseline level (only empty vector-transfected cells, without AB), suggesting that Rac1 inhibition alone is not sufficient to block phagocytosis of AB (Figure 3). This suggests that there are other signaling pathways induced by phagocytosis, likely involving the activation of RhoA.
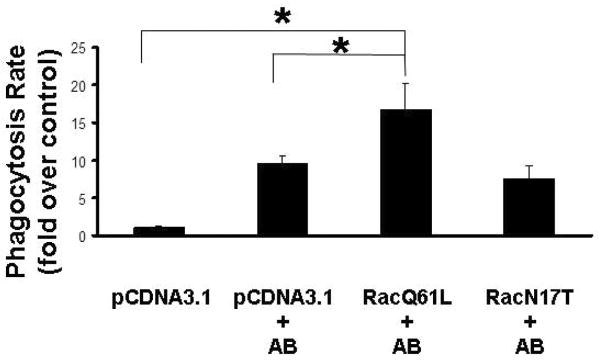
Figure 3. Overexpression of constitutive active Rac1 induces phagocytosis of apoptotic bodies by HSC.
LX-2 cells transfected with Rac1 Q61L, engulfed more apoptotic bodies (1.97-fold, +/-0.17), compared to mock-transfected cells exposed to AB. Transfection of the dominant negative mutant (T17N) resulted in decrease of phagocytosis compared to the Rac1Q61L-transfected cells however, still above the control level (control, only pcDNA3.1-transfected cells). Mean ±SE, N=4, *p<0.01.
Leptin treatment induces RhoA activation in primary HSC phagocytosing AB
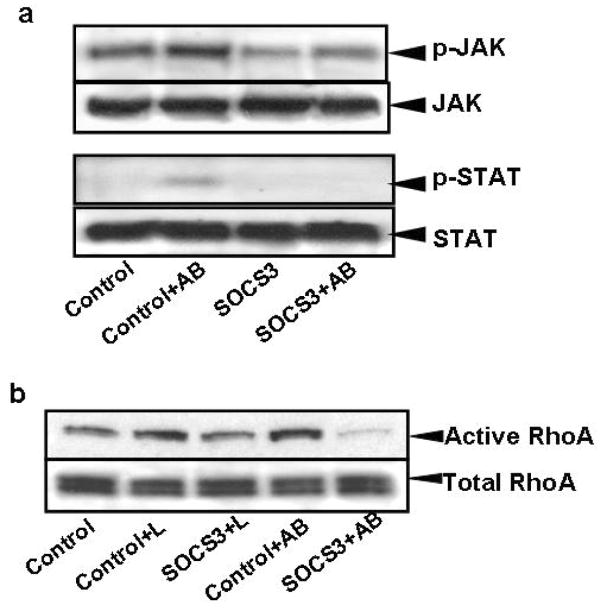
RhoA, another actin-cytoskeleton regulating GTP-ase is also known to be involved in phagocytosis. To study its activation by leptin, pull-down of active GTP-RhoA by the Rhotekin assay was performed. In addition to LX-2 cells, HSC from leptin receptor (Ob-RL) deficient Zucker rats (fa/fa) and wild-type (WT) rats were used to monitor for leptin specificity of RhoA activation. In HSC from WT rats, leptin-induced activation of RhoA, while in HSC from Zucker rats exposed to leptin, no increase in activation of RhoA was detected (Figure 4a). In addition, RhoA GTP-ase activity was dependent on JAK signaling as the JAK inhibitor AG490 inhibited leptin-induced RhoA activity however; inhibition of the PI3 kinase did not affect it (Figure 4b). To confirm that engulfment of AB induced JAK1 and STAT3 phosphorylation, western blot analyses were performed. Transfection with a SOCS3 expressing plasmid both diminished JAK phosphorylation and consequently STAT3 phosphorylation (Figure 5a). To confirm that RhoA activation is indeed reduced by JAK inhibition, RhoA pull-down assay was performed in SOCS3 expressing HSC following exposure to either leptin or AB. We found that both leptin and AB-induced activation of RhoA have decreased in SOCS3 expressing stellate cells, corroborating our previous data (Figure 5b).
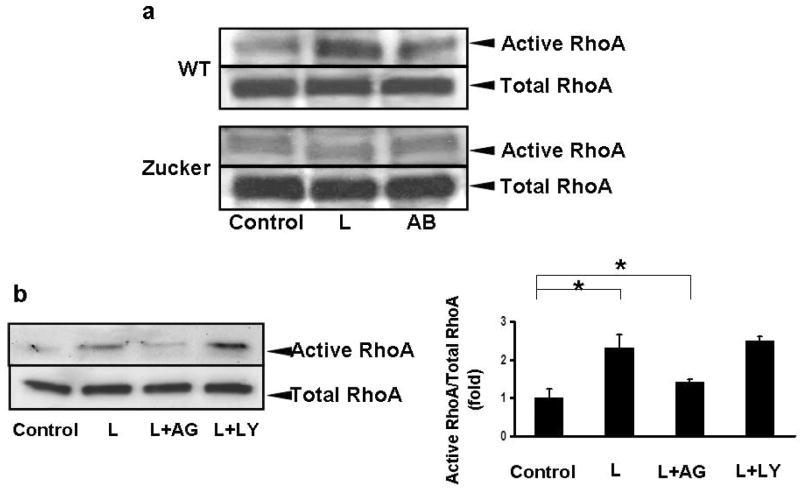
Figure 4. Leptin induces RhoA GTP-ase activity in primary HSC and in LX-2 cells.
a) HSC were isolated from wild type and Zucker rats, cultured for 2 days, and then exposed to leptin (100 ng/ml) or AB. Rho GTP pull down assays and western blot analyses were performed to detect active RhoA. In HSC isolated from wild type rats leptin induced activation of RhoA, while in HSC from Zucker rats exposed to leptin, no increase in activation of RhoA was detected. N=3.
b) LX-2 cells were exposed to leptin in the presence or absence of JAK inhibitor AG490, or PI3 kinase inhibitor LY29002, and RhoA GTPase activity was studied. Western blot and densitometry showed that RhoA GTP-ase activity was dependent on JAK signaling as AG490 inhibited leptin-induced RhoA activity however, inhibition of the PI3K did not affect it. Mean ±SE, N=4, *p<0.01.
Figure 5. Phagocytosis of apoptotic bodies by HSC induces JAK1 and STAT3 phosphorylation and RhoA GTP-ase activity.
A) LX-2 cells were cultured in serum-free medium, mock-transfected (only pCMV-XL6) or transfected with the SOCS3 construct, and 48 hours later exposed to AB for half an hour. Western blot analyses were performed to detect JAK1 and STAT3 phosphorylation. Transfection of LX-2 cells with the SOCS3 construct inhibited JAK1 and consequently STAT3 phosphorylation. B) RhoA pull-down assay was performed in SOCS3 or mock-transfected LX-2 cells after exposure to either leptin or AB. RhoA activity decreased in SOCS3-transfected cells compared to control (mock transfected cells) after leptin, and especially after AB treatment.
Overexpression of constitutive active RhoA induces phagocytosis of apoptotic bodies by HSC
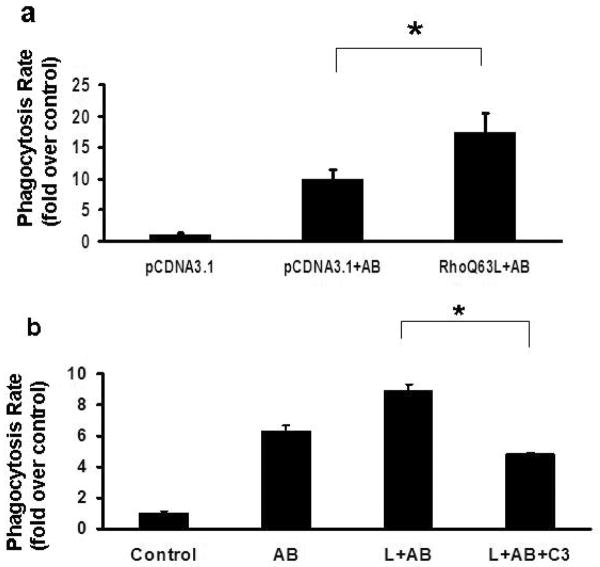
To establish whether expression of the constitutive active RhoA had an effect on the phagocytic rate, we transfected LX-2 cells with RhoQ63L, and exposed them to AB. An increase in phagocytosis was detected, over empty vector-transfected cells exposed to AB (Figure 6a). Treating HSC with C3, which inhibits ADP-ribosylation of RhoA, caused a significant decrease in the engulfment of AB, compared to leptin and AB-exposed cells (Figure 6b). Thus, RhoA in our system is a positive regulator of phagocytosis.
Figure 6. Overexpression of constitutive active RhoA induces phagocytosis of apoptotic bodies by HSC, while inhibition of RhoA GTPase activity decreases it.
a) In LX-2 cells transfected with the constitutively active RhoA (Q63L), an increase in phagocytosis was detected (1.75-fold), compared to empty-vector-transfected cells exposed to AB. Mean ±SE, N=4, *p<0.01
b) HSC were exposed to AB and leptin in the presence or absence of C3, an inhibitor of ADP ribosylation of RhoA. 0.49-fold (+/-0.14) decrease in phagocytosis was detected, compared to leptin and AB-exposed cells. Mean ±SE, N=4, *p<0.01
Leptin treatment and phagocytosis of AB induces RhoA translocation to phagosomes and activation of ROCK
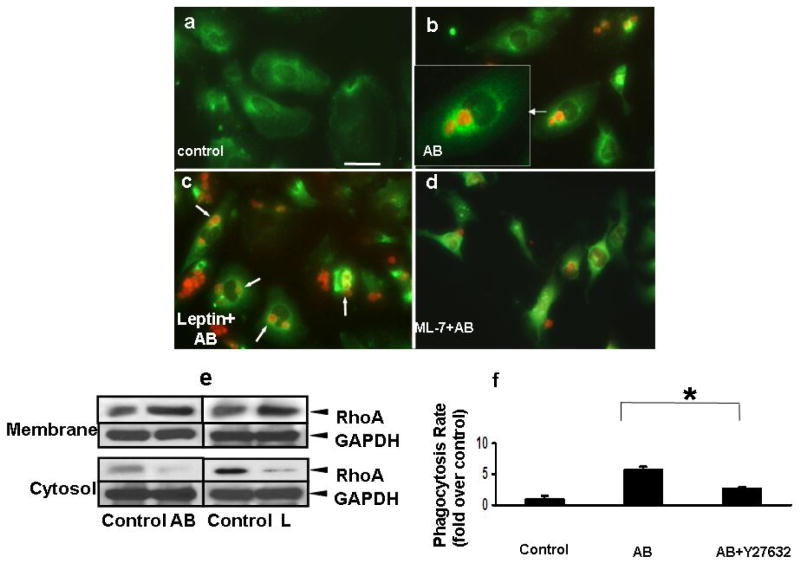
As RhoA is known to play a key role in phagocytosis, next we tested if RhoA translocation to the phagocytic cup occurs upon leptin stimulation. In these experiments LX-2 cells were exposed to TAMRA-labeled AB and immunohistochemistry to detect RhoA was performed. In control cells, RhoA is cytoplasmic (Figure 7a). In HSC phagocytosing AB, RhoA translocates to the phagosomal membrane (Figure 7b, arrow), the AB labeled with TAMRA (insert). Upon leptin stimulation, there is an increase in phagocytosis of AB, and again, RhoA mainly localizes to the phagosomal membrane (Figure 7c). ML-7, an inhibitor of the myosin light chain kinase inhibits the translocation of RhoA to the membrane therefore the signal is cytoplasmic (Figure 7d). To confirm these data, membrane fractions were extracted from leptin, or AB-treated HSC, and Western blot analysis was performed to detect RhoA translocation. Leptin induced translocation of RhoA to the membrane fraction, as did exposure to AB (Figure 7e). This suggests that besides activation of the RhoA GTP-ase, its translocation to the phagocytic cup also occurs during leptin-induced phagocytosis.
Figure 7. Leptin induces translocation of RhoA to phagosomes and ROCK activation.
LX-2 cells were exposed to AB, leptin plus AB in the presence or absence of ML-7 (AB were labeled previously with TAMRA, red). a) Immunofluorescence was performed and RhoA (green) was seen in the cytoplasm of control cells, and b) translocating to the phagosomes (insert) in AB, or c) L+AB treated cells (arrowheads). In the presence of leptin, more RhoA positive phagosomes were seen. d) After treatment of a MLCK inhibitor ML-7, less phagocytosis was observed and RhoA failed to localize to the phagosomal membrane around AB.
e) Western blot assay was performed to assess membrane-bound RhoA. RhoA protein was increased in the membrane fraction and decreased in the cytosol after the exposure to either AB or leptin. GAPDH served as equal loading control. (N=3)
f) Phagocytosing LX-2 cells were exposed to the ROCK inhibitor Y27632 (10uM) prior to exposing them to AB, and this decreased the phagocytic rate by 0.53-fold when compared to that without the inhibitor. Mean ±SE, N=3, * p<0.05
To mechanistically extend the role of RhoA in our model, next we determined if its downstream target ROCK plays a role in the phagocytosis of AB. HSC were exposed to the ROCK inhibitor Y27632, prior to phagocytosis, and this indeed decreased the phagocytic rate by 0.53-fold (Figure 7f), suggesting that RhoA mediates its effects on phagocytosis via ROCK activation.
Discussion
Phagocytosis of AB by HSC is an important fibrogenic mechanism in the liver (7, 12). We have previously shown that phagocytosis in HSC induces the activation of NADPH oxidase, and upregulation of procollagen α 1(I) and TGF-β expression. The mechanism of HSC activation by phagocytosis is likely to be a common pathway linking chronic liver injury, apoptosis and fibrogenesis in the liver, independent of the cause of the liver disease. Therefore, studying factors that regulate phagocytosis is of great importance, as enhanced phagocytic activity of HSC could lead to accelerated liver fibrogenesis. Leptin has been postulated to play an important role in the phagocytic process, as leptin-deficient ob/ob mice exhibited impaired response to Gram-negative pneumonia (13), and exogenous leptin was shown to upregulate both phagocytosis by macrophages and production of inflammatory cytokines (8). Thus, it was plausible that leptin, one of the major profibrogenic cytokines may play a role in the phagocytic process in HSC, thereby further enhancing their fibrogenic activity. Indeed, we found that leptin induced the phagocytic activity of HSC, and that the phagocytic activity was dependent on signaling through the Ob-R, as HSC from Zucker rats deficient in this receptor, failed to have an increase in the phagocytic rate. Although the increase in the phagocytic activity by leptin at first sight seems to be moderate, induction of different signaling pathways simultaneously could translate into an amplified fibrogenic response (NADPH oxidase activation, procollagen α1 (I) upregulation). The next question is how leptin regulates phagocytosis. As Rho-GTP-ases (Rac and Rho) are known to be important regulators of the phagocytic process (11, 14), we focused on how leptin may modulate their GTP-ase activity. Leptin induces the GTP-ase activity of Rho proteins in migratory cells (15) therefore, we postulated that leptin may stimulate phagocytosis in HSC via activation of the Rho-GTPases. Rac GTP-ases are known to control actin polymerization into lamellipodial and filopodial membrane protrusions (11). Rac1 is ubiquitously expressed and is likely to be the main Rac GTP-ase in non-hematopoietic cells (16). In addition to regulating cytoskeletal function during phagocytosis, Rac GTP-ases are also important elements of the NADPH oxidase complex by participating in the recruitment of the p67phox subunit to the enzyme complex upon activation during phagocytosis (16). Based on these concepts, we first determined whether leptin has an effect on Rac1 GTP-ase activity. We found that Rac1 activity was induced by leptin via the PI3 kinase, and using a PI3 kinase inhibitor decreased the phagocytic rate. In addition, Rac1 activity was also dependent on the NADPH oxidase, as inhibition of the enzyme by apocynin caused decreased GTP-ase activity of Rac1, suggesting that a leptin/NADPH oxidase/Rac1 pathway is also involved in phagocytosis. This is in keeping with a recent report confirming that NADPH oxidase is a downstream mediator of leptin signaling in HSC (17, 18). Apocynin can also directly affect phagocytosis in different systems (albeit at a much higher concentration), where the production of ROS was required for phagocytosis to occur (19). Expressing the constitutive active form of Rac1 in HSC increased phagocytosis of AB, while the dominant negative form decreased it, indicating that Rac1 is indeed a positive regulator of phagocytosis in HSC. The observation that the phagocytic rate was not reduced to the baseline by the dominant negative Rac1 (Figure 3), suggested the existence of additional pathway(s) by which phagocytosis of AB was regulated. To examine this possibility, we studied the role of RhoA. RhoA is known to facilitate the assembly of contractile actomyosin filaments via activation of ROCK (20-22). The substrate of ROCK, myosin light chain phosphatase, is involved in the regulation and assembly of actin-myosin filament bundles during phagocytosis (23). We found that leptin induced GTP-ase activity and also translocation of RhoA to the phagosomes. The expression of the constitutive active RhoA in HSC increased the phagocytic rate, while inhibiting ADP ribosylation with C3 decreased it. In previously published studies, active Rac1 and RhoA played opposite roles in regulating phagocytosis in macrophages, Rac 1 stimulating while RhoA inhibiting it via ROCK (14). However, in our studies ROCK inhibition resulted in a decreased phagocytic rate, which may translate to decreased fibrogenic activity based on our previous study. This is supported by earlier findings where ROCK inhibition decreased HSC activation (24) and was antifibrogenic in the liver and kidney (25, 26). The difference concerning the role of active RhoA on phagocytosis may result also from the fact that HSC are considered to be non-professional phagocytes, and as such, their mechanism of phagocytosis is not well characterized. For instance, RhoA was shown to positively regulate integrin-mediated phagocytosis in macrophages, and inhibition of RhoA by C3 resulted in decreased uptake of serum-opsonized zymosan particles (27, 28). Whether integrins, especially the β2 subclass play a role in phagocytosis by HSC; remains to be determined. It is known, however that integrin-mediated signaling is a crucial element in HSC activation and liver fibrogenesis (29, 30). Thus, it is plausible that phagocytosis-induced signaling events in HSC are integrin-mediated and result in RhoA/ROCK activation, actin cytoskeleton rearrangement and phagocytic cup formation. Interestingly, we also found that RhoA translocated to the phagosomes where it also may play a role in NADPH oxidase-induced superoxide formation during phagocytosis (31).
In conclusion, our data suggest a new model where intact leptin signaling is required to maintain and augment the phagocytic process in HSC, and the clearance of AB during chronic liver injury. It is plausible that leptin deficiency in ob/ob mice could result in a decrease in phagocytosis of apoptotic cells and consequent increase in inflammatory activity in the liver.
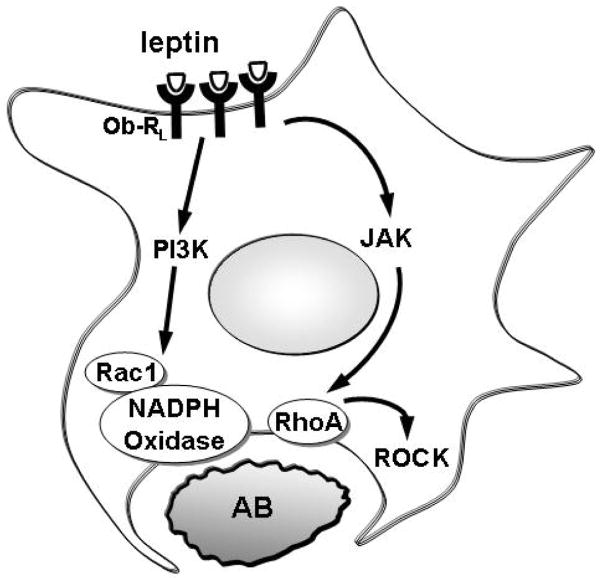
In our study leptin-induced Rac1 and RhoA activation are responsible for the increase in the phagocytic rate (Figure 8). Since previously we have shown that phagocytosis of AB by HSC induces their activation and fibrogenesis (7), further induction of phagocytosis by leptin and activation of Rac1 and RhoA could exacerbate the fibrogenic process. Indeed, a recent study showed that sustained Rac1 activation leads to accelerated liver fibrosis in mice (9). Thus, regulation of Rac1 and RhoA seem to be critical steps in the fibrogenic process. As leptin is a major profibrogenic cytokine, induction of the phagocytic activity by HSC could be an important mechanism of liver fibrosis in NASH.
Figure 8. Mechanisms of leptin-induced phagocytosis in HSC.
Leptin induces Rac1 GTP-ase activity via the PI3 kinase with the involvement of NADPH oxidase. In addition, activation of RhoA GTP-ase via JAK also occurs, and this results in translocation of RhoA to the phagosome, and activation of ROCK.
Acknowledgments
Supported in part by the American Liver Foundation Liver Scholar Award (NJT), the NIH 1 KO8 DK069765 (NJT), and the UC Davis Health System Award (NJT).
References
- 1.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 3.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding X, Saxena NK, Lin S, Xu A, Srinivasan S, Anania FA. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166:1655–1669. doi: 10.1016/S0002-9440(10)62476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Q, Mak KM, Lieber CS. Leptin enhances alpha1(I) collagen gene expression in LX-2 human hepatic stellate cells through JAK-mediated H2O2-dependent MAPK pathways. J Cell Biochem. 2006;97:188–197. doi: 10.1002/jcb.20622. [DOI] [PubMed] [Google Scholar]
- 6.Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. Faseb J. 2004;18:1612–1614. doi: 10.1096/fj.04-1847fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, Torok NJ. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435–443. doi: 10.1002/hep.21093. [DOI] [PubMed] [Google Scholar]
- 8.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, et al. Leptin regulates proinflammatory immune responses. Faseb J. 1998;12:57–65. [PubMed] [Google Scholar]
- 9.Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, Chen W, et al. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology. 2006;44:1267–1277. doi: 10.1002/hep.21375. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niedergang F, Chavrier P. Regulation of phagocytosis by Rho GTPases. Curr Top Microbiol Immunol. 2005;291:43–60. doi: 10.1007/3-540-27511-8_4. [DOI] [PubMed] [Google Scholar]
- 12.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 13.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol. 2002;168:4018–4024. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 14.Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J Biol Chem. 2006;281:8836–8842. doi: 10.1074/jbc.M510972200. [DOI] [PubMed] [Google Scholar]
- 15.Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M, Wymann MP, et al. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. Faseb J. 2000;14:2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- 16.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 17.De Minicis S, Seki E, Schwabe R, Brenner D. NADPH oxidase is required for leptin-induced proliferation and chemokine secretion in hepatic stellate cells. Hepatology. 2006;44:684A. [Google Scholar]
- 18.Dong F, Zhang X, Ren J. Leptin regulates cardiomyocyte contractile function through endothelin-1 receptor-NADPH oxidase pathway. Hypertension. 2006;47:222–229. doi: 10.1161/01.HYP.0000198555.51645.f1. [DOI] [PubMed] [Google Scholar]
- 19.van der Goes A, Brouwer J, Hoekstra K, Roos D, van den Berg TK, Dijkstra CD. Reactive oxygen species are required for the phagocytosis of myelin by macrophages. J Neuroimmunol. 1998;92:67–75. doi: 10.1016/s0165-5728(98)00175-1. [DOI] [PubMed] [Google Scholar]
- 20.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 21.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 22.Yeh BJ, Rutigliano RJ, Deb A, Bar-Sagi D, Lim WA. Rewiring cellular morphology pathways with synthetic guanine nucleotide exchange factors. Nature. 2007 doi: 10.1038/nature05851. [DOI] [PubMed] [Google Scholar]
- 23.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 24.Masamune A, Kikuta K, Satoh M, Satoh K, Shimosegawa T. Rho kinase inhibitors block activation of pancreatic stellate cells. Br J Pharmacol. 2003;140:1292–1302. doi: 10.1038/sj.bjp.0705551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tada S, Iwamoto H, Nakamuta M, Sugimoto R, Enjoji M, Nakashima Y, Nawata H. A selective ROCK inhibitor, Y27632, prevents dimethylnitrosamine-induced hepatic fibrosis in rats. J Hepatol. 2001;34:529–536. doi: 10.1016/s0168-8278(00)00059-3. [DOI] [PubMed] [Google Scholar]
- 26.Moriyama T, Nagatoya K. The Rho-ROCK system as a new therapeutic target for preventing interstitial fibrosis. Drug News Perspect. 2004;17:29–34. doi: 10.1358/dnp.2004.17.1.829023. [DOI] [PubMed] [Google Scholar]
- 27.Wiedemann A, Patel JC, Lim J, Tsun A, van Kooyk Y, Caron E. Two distinct cytoplasmic regions of the beta2 integrin chain regulate RhoA function during phagocytosis. J Cell Biol. 2006;172:1069–1079. doi: 10.1083/jcb.200508075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J, Kim JS, Jung KC, Lee HJ, Kim JI, Kim J, Lee JY, et al. Exoenzyme Tat-C3 inhibits association of zymosan particles, phagocytosis, adhesion, and complement binding in macrophage cells. Mol Cells. 2003;16:216–223. [PubMed] [Google Scholar]
- 29.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, et al. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popov Y, Patsenker E, Stickel F, Zaks J, Bhaskar KR, Niedobitek G, Kolb A, et al. Integrin alphavbeta6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J Hepatol. 2008;48:453–464. doi: 10.1016/j.jhep.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Kim JS, Diebold BA, Kim JI, Kim J, Lee JY, Park JB. Rho is involved in superoxide formation during phagocytosis of opsonized zymosans. J Biol Chem. 2004;279:21589–21597. doi: 10.1074/jbc.M308386200. [DOI] [PubMed] [Google Scholar]