Abstract
Study Objectives
The steep decline in slow-wave (delta) electroencephalogram (EEG) intensity across adolescence is a prominent feature of late brain maturation. As a first step in determining whether the adolescent delta decline is similar in both sexes, we compared cross-sectional sleep EEG data from 9- and 12-year-old boys and girls.
Design
All-night EEG recordings, 6 months apart, were conducted on each subject.
Setting
EEG was recorded in the subjects’ homes.
Participants
Thirty-two 9-year-olds and 38 12-year-olds are enrolled in a 4-year longitudinal study of adolescent sleep. There are equal numbers of each sex in both age cohorts.
Interventions
N/A.
Measurements
Using ambulatory recorders, EEG was recorded in the subjects’ homes on their normal sleep schedule. For each of the 2 semi-annual recording periods, data from the 10 subjects from each age-sex group with the cleanest (fewest artifacts) signals were selected for cross-sectional comparisons of visual scoring and EEG variables. All artifact-free 20-second non-rapid eye movement epochs were analyzed with power spectral and period-amplitude analysis.
Results
In the 12-year-old cohort, delta power per minute was 37% higher in boys than girls. The 9-year-old cohort showed no sex difference. A second recording 6 months later produced similar results.
Conclusion
These cross-sectional data indicate that girls begin the steep adolescent decline in slow-wave EEG earlier than boys. We hypothesize that this reflects an earlier onset of adolescent synaptic pruning in females.
Keywords: EEG, adolescence, maturation, gender, delta
INTRODUCTION
The slow-wave electroencephalogram (eeg) of non-rapid eye movement (nrem) sleep changes across the life span. cross-sectional studies with either visual analysis of slow-wave sleep or computer measurement of EEG show that slow-wave EEG intensity peaks in early childhood and then decreases until about the fifth decade of life.1,2 The decline is steepest across adolescence. Between ages 10 and 20 years, slow-wave intensity declines by about 50%.3,4 We have hypothesized that the slow-wave EEG decrease across adolescence is one component of a widespread brain maturation process that involves roughly parallel decreases in synaptic density and cerebral metabolic rate.5,6 The dramatic decrease in slow-wave EEG is also accompanied by changes in sleep duration. As children progress through adolescence, their weekday sleep duration decreases, but they frequently extend their sleep duration on weekends.7
Age-related changes in the slow-wave EEG of NREM sleep are of particular interest because current theory holds that slow-wave EEG intensity reflects the recuperative processes of sleep.8,9 Therefore, studying this period of rapid change in slow-wave EEG could provide a deeper understanding of the biology of sleep homeostasis as well as of late brain development.
In addition to the well-established age-related differences in sleep EEG, the sleep EEG differs by sex. Adult women have greater slow-wave EEG intensity in NREM sleep than do adult men.10 It is not known whether a similar sex difference is present in adolescence. Here, we examined this possibility using cross-sectional data from 9- and 12-year-old boys and girls participating in a longitudinal study of sleep across adolescence. In the absence of empirical evidence or compelling theory, our a priori hypothesis was that children in these age groups would not show sex differences in the slow-wave EEG.
METHODS
Subjects
The data presented here were drawn from a semilongitudinal study of sleep EEG that follows 9- and 12-year-old cohorts with semiannual recordings for 4 years. Thirty-two 9-year-olds and thirty-eight 12-year-olds were enrolled in the study. There were an equal number of boys and girls in each cohort. The University of California Davis Human Research Protection Committee approved all procedures. Parents gave informed consent for their children to participate in the study, and the children gave assent. Prior to enrollment, subjects were screened via parental interviews and questionnaires for psychiatric disorders, a history of head injury, and neurologic disorders such as epilepsy. Data are presented here for the first 2 semiannual recordings. Mean subject ages for each age-sex group at each recording are presented in Table 1.
Table 1.
Subject Age and Tanner Stage for the 2 Recording Sessions*
| Cohort | ||||||
|---|---|---|---|---|---|---|
| 9-Year-Old | 12-Year-Old | 9-Year-Old Boys | 9-Year-Old Girls | 12-Year-Old Boys | 12-Year-Old Girls | |
| Age | ||||||
| First Recording | 9.31† ± 0.04 | 12:33† ± 04 | 9.31A ± 0.06 | 9.30A ± 0.06 | 12.26B ± 0.05 | 12.41C ± 0.05 |
| Second Recording | 9.83† ± 0.05 | 12.80† ± 0.04 | 9.80A ± 0.07 | 9.85A ± 0.07 | 12.71B ± 0.07 | 12.89C ± 0.04 |
| Tanner Score | ||||||
| First Recording | 1.32† ± 0.10 | 2.66† ± 0.22 | 1.20A ± 0.13 | 1.44A ± 0.18 | 2.20B ± 0.25 | 3.17C ± 0.29 |
| Second Recording | 1.31† ± 0.11 | 2.89† ± 0.22 | 1.20A ± 0.13 | 1.43A ± 0.18 | 2.61B ± 0.35 | 3.17B ± 0.25 |
Data are presented as mean ± SEM for the 2 age cohorts and for each age-sex group.
Significant (α = .05) age effect in analysis of variance. Post hoc multiple comparisons were conducted between sexes within the 2 age cohorts and between ages within the 2 sexes.
Age-sex groups that do not differ share a common superscript.
Study Design
At 6-month intervals, sleep EEG was recorded in the subjects’ homes for 4 consecutive nights. Subjects maintained their habitual weekday sleep schedules and refrained from daytime napping for 5 days prior to the recording. Actigraphy recordings monitored the subjects’ compliance. On the first 2 nights, baseline EEG was recorded from the subjects’ habitual weekday bedtimes until their usual weekday wake times. Night 3 and 4 recordings began at the habitual weekday bedtime, but subjects were instructed to sleep as long as possible on the following morning. Data presented here are from the second baseline night of the first 2 semiannual recordings.
In addition to sleep recordings, other pertinent developmental data were collected. These included Tanner staging of sexual maturation, conducted by a physician within 1 month of the sleep recording.
EEG Recording and Analysis
The EEG and electrooculogram were recorded on Grass H2O ambulatory EEG recorders. The low-frequency hardware filter on this recorder is a 0.3-Hz half-amplitude filter. EEG was recorded from C3, C4, O1, and O2 versus a reference and referred to the contralateral mastoid via subtraction. Electrooculograms from left and right outer canthus leads were recorded versus reference and referred to the forehead via subtraction. All signals were digitized at 200 Hz by the H2O recorder and stored on its removable hard disk.
The digitized data were exported to laboratory computers where they were analyzed by PASSPLUS EEG analysis software (Delta Software, St. Louis, Mo.). For visual scoring, each 20-second epoch was displayed on a computer monitor and scored as stage 1, NREM, REM, wake, or movement according to modified Rechtschaffen and Kales criteria.11 Independent of stage, each epoch was marked for the presence of artifacts. A second scorer checked all records, and a third person reconciled discrepancies. Epochs with artifacts were included in the stage totals but not in the EEG analysis. For each of the 2 semiannual recording periods, data from the 10 subjects from each age-sex group with the cleanest (fewest artifacts) signals were selected for cross-sectional comparisons of visual scoring and EEG variables. The 40 subjects chosen for comparison were selected independently for each recording period. Data from 21 subjects were included in both recording periods.
Power spectral and period-amplitude analyses were performed on signals from either C3 or C4 versus the contralateral mastoid for all artifact-free epochs of NREM sleep. Power spectral analysis was fast Fourier transform with 5.12-second windows with 2.62-second overlap, yielding 8 windows per 20-second epoch. The delta-frequency band was specified a priori as 0.3 to 3 Hz. We also analyzed the following frequencies in the delta range (within and slightly above delta): 0.3 to 1, 1 to 2, 2 to 3, and 3 to 4 Hz. Spectral power in 4 to 6, 6 to 8, 8 to 12, 12 to 15, 15 to 23, and 23 to 30 Hz was also analyzed. We calculated all-night power density (power per minute of NREM sleep) for each frequency band. In addition to power spectral analysis, we analyzed the 0.3 to 3 Hz band with period-amplitude analysis. Using half-wave detection by zero crossings, we measured amplitude and incidence of waves in the delta range. Average sample amplitude (μV) was the measure of wave amplitude, and time in band (seconds per minute) indexed wave incidence.12
Statistical Analysis
Age and sex effects on all sleep and EEG variables were evaluated with an analysis of variance with age and sex factors. Post hoc t tests compared the 4 age-sex groups. We also computed correlation coefficients between delta power density and Tanner stage.
RESULTS
Sleep Schedule and Vigilance States
For the first recording session, the sleep schedule differed significantly between the 9- and 12-year-old cohorts. Analyses of variance showed a significant age effect on bedtime (F(1,36) =18.61, P = 4.32, P = .045), and time in bed (F(1,36) = .0001), wake time (F(1,36) = 20.65, P = .0001). The bedtime of the 9-year-olds was approximately 40 minutes earlier (Table 2) and the wake time was about 20 minutes later than that of the 12-year-olds. Within the 9-year-old cohort, girls had a significantly longer (37 minutes) time in bed than boys (t18 = 2.81, P = .011). Within the 12-year-old cohort, girls had an earlier (26 minutes) wake time than did boys (t18 = 2.67, P = .016), but their 20-minute earlier bedtime was not significantly different from that of the boys. Time in bed was similar in 12-year-old boys and girls.
Table 2.
Sleep Schedule and Vigilance-State Durations for the First Recording Session*
| 9-Year-Old | 12-Year-Old | 9-Year-Old Boys | 9-Year-Old Girls | 12-Year-Old Boys | 12-Year-Old Girls | |
|---|---|---|---|---|---|---|
| Bedtime | 21:15† ± :06 | 21:54† ± :07 | 21:29A ± :10 | 21:01B ± :04 | 22:04C ±:08 | 21:44C ±:11 |
| Wake Time | 06:50† ± :05 | 06:34† ± :05 | 06:46A ± :06 | 06:55A ± :09 | 06:47A ± :07 | 06:21B ± :06 |
| Time in Bed, min | 575† ± 8 | 520† ± 10 | 557 A ± 10 | 594B ± 8 | 517C ± 16 | 523C ± 13 |
| NREM, min | 400† ± 8 | 361† ± 8 | 386AB ± 13 | 415A ± 8 | 367BC ± 9 | 356C ± 13 |
| REM, min | 121 ± 4 | 113 ± 5 | 117 ± 5 | 126 ± 7 | 108 ± 6 | 118 ± 7 |
Data are presented as mean ± SEM for the 2 age cohorts and for each age-sex group.
Significant (α= .05) age effect in analysis of variance. Post hoc multiple comparisons were conducted between sexes within the 2 age cohorts and between ages within the 2 sexes. NREM refers to non-rapid eye movement sleep; REM, rapid eye movement sleep.
Age-sex groups that do not differ share a common superscript.
As shown in Table 2, for the first recording, NREM duration in the 9-year-old cohort exceeded that in the 12-year-old cohort by 39 minutes (F(1,36)= 13.27, P = .0008). NREM duration did not differ by sex (F(1,36) = 0.73, P = .4). REM sleep duration did not differ by age (F(1,36) = 1.71, P = .2) or sex (F(1,36) = 2.19, P = .15) groups.
Table 3 shows that, in the second recording 6 months later, the differences in sleep schedule and vigilance state resembled those in the first recording.
Table 3.
Sleep Schedule and Vigilance-State Durations for the Second Recording Session*
| 9-Year-Old | 12-Year-Old | 9-Year-Old Boys | 9-Year-Old Girls | 12-Year-Old Boys | 12-Year-Old Girls | |
|---|---|---|---|---|---|---|
| Bedtime | 21:40† ± :12 | 22:13† ± :08 | 21:55AB ± :18 | 21:25A ± :16 | 22:06BC ±:09 | 22:17C ±:13 |
| Wake Time | 07:13 ± :11 | 06:47 ± :10 | 07:13 ± :17 | 07:14 ± :16 | 06:50 ± :20 | 06:44 ± :06 |
| Time in Bed, min | 573† ± 8 | 514† ± 10 | 559 ± 10 | 587 ± 10 | 511 ± 20 | 518 ± 6 |
| NREM, min | 422† ± 7 | 363† ± 7 | 403A ± 7 | 440B ± 7 | 367C ± 7 | 358C ± 13 |
| REM, min | 102 ± 3 | 106 ± 5 | 102 ± 5 | 102 ± 3 | 105 ± 5 | 108 ± 7 |
Data are presented as mean ± SEM for the 2 age cohorts and for each age-sex group.
Significant (α = .05) age effect in analysis of variance. Post hoc multiple comparisons were conducted between sexes within the 2 age cohorts and between ages within the 2 sexes. NREM refers to non-rapid eye movement sleep; REM, rapid eye movement sleep.
Age-sex groups that do not differ share a common superscript.
Age and Sex Differences in Delta EEG Power Density
The NREM 0.3 to 3 Hz power density in the 9-year-old cohort was 15% higher than that in the 12-year-old cohort, but this difference did not reach significance (F1,36 = 1.92, P = .17). There was also no significant sex effect (F1,36 = 1.20, P = .28) on delta power density. The interaction between the age and sex factors approached significance (F1,36 = 3.39, P = .074) for the first recording and was significant for the second recording (F1,36 = 4.23, P = .047). Therefore, we separately analyzed sex effects within each age group and age effects within each sex. Within the 12-year-old cohort (Figure 1A), delta power density was 37% higher in boys than in girls (t18 = 2.29, P = .034). In the 9-year-old cohort, delta power density did not differ between sexes (Figure 1A). Girls in the 9-year-old cohort had 41% higher delta power density than girls in the 12-year-old cohort (t18 = 2.28, P = .035). Among boys, delta power density did not differ between the 2 age groups. As shown in Figure 1B, the second recording 6 months later yielded similar results. Again, girls in the 12-year-old cohort had significantly lower delta power density than did boys in the 12-year-old cohort (t18 = 2.18, P = .043) and girls in the 9-year-old cohort (t18 = 2.77, P = .012).
Figure 1.
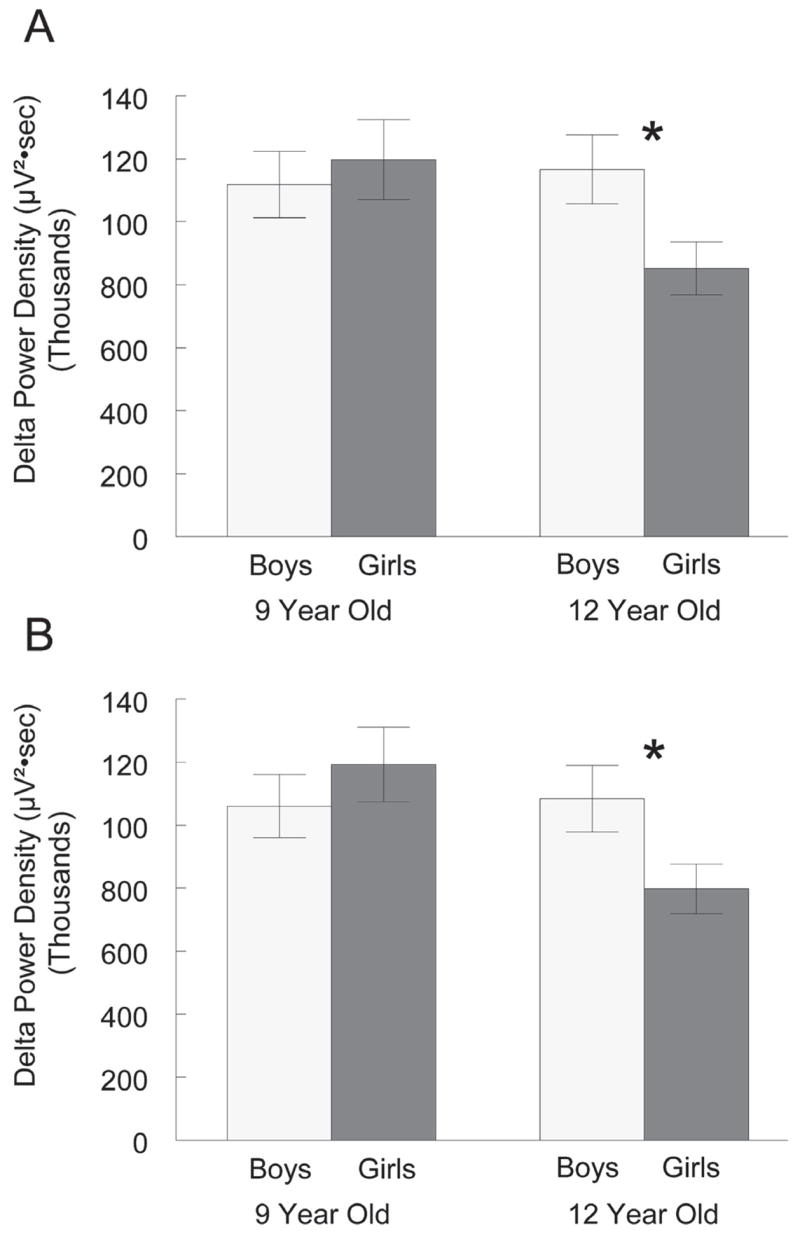
For (A) the first recording period and (B) the second recording 6 months later, mean delta (0.3 – 3 Hz) power density (± SEM) is compared between boys and girls in the 9- and 12-year-old cohorts. In both recording periods, girls in the 12-year-old cohort had significantly (*) lower delta power density than boys in the 12-year-old cohort or girls in the 9-year-old cohort. Within the 9-year-old cohort, delta power density did not differ between boys and girls in either recording period.
Sex Difference in Delta Power Is Due to Amplitude Rather Than Incidence Difference
Current methods of spectral analysis cannot distinguish changes in power caused by changes in the amplitude or incidence of waves. We, therefore, examined the period-amplitude data to determine whether the differences in delta power density were due to differences in the delta-wave amplitude, incidence, or both. Figure 2B shows that the sex difference in delta power density in the 12-year-old cohort resulted from a 17% higher delta-wave amplitude in boys than in girls (t18 = 2.67, P = .016). Delta-wave incidence did not differ between boys and girls in the 12-year-old cohort (t18 = 0.31, P = .76). In the 9-year-old cohort, neither delta-wave amplitude nor incidence differed between the sexes (Figure 2A).
Figure 2.
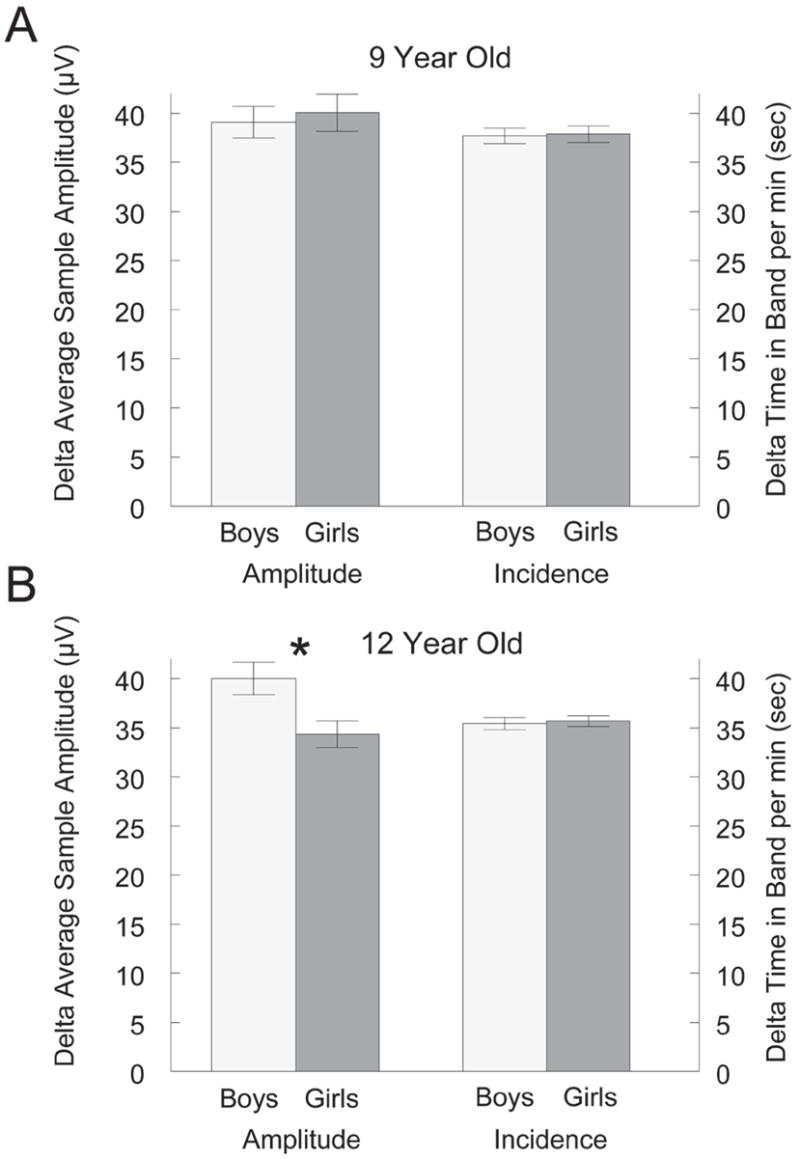
Sex effects on mean delta wave amplitude and incidence in the (A) 9-year-old cohort and (B) the 12-year-old cohort. Within the 9-year-old cohort, neither delta-wave amplitude nor incidence differed between boys and girls. Within the 12-year-old cohort, boys had significantly (*) higher delta-wave amplitude than girls, but incidence was the same for both sexes. Data shown are for the first recording period.
Sex Difference in Frequencies Within the Delta Range
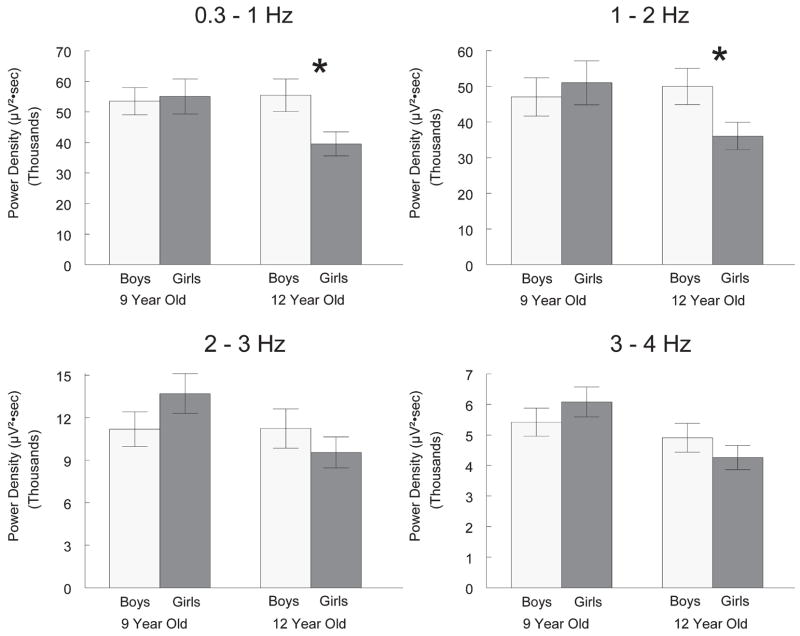
Frequencies within the delta frequency range exhibit different across-night trends.13 Therefore, we examined sex differences in 0.3 to 1, 1 to 2, 2 to 3 and 3 to 4 Hz power density. Figure 3 shows that the sex difference was stronger at the lower frequencies. Within the 12-year-old cohort, boys had 40% higher 0.3 to 1 Hz power density (t18 = 2.41, P = .027) and 39% higher 1 to 2 Hz power density (t18 = 2.18, P = .043) than girls. The 18% difference in 2 to 3 Hz power density and the 15% difference in 3 to 4 Hz power density were not significant (t18 = .35 and t18 = 0.96, P = 1.06, P = .30, respectively). In contrast to the 12-year-old cohort, the 9-year-old cohort showed no sex differences in power density for any of the 4 delta range frequencies.
Figure 3.
Sex effects on power density in electroencephalogram subfrequencies within the delta range. Within the 12-year-old cohort, girls had significantly (*) lower power density than boys in the 0.3 to 1 Hz and 1 to 2 Hz bands but not in the 2 to 3 Hz and 3 to 4 Hz bands. Within the 9-year-old cohort, no significant sex differences were seen in any frequency band.
Sex Difference in Other EEG Frequencies
Table 4 shows the analysis of sex differences in power density across a wide range of EEG frequencies for the 12-year-old cohort. In the first recording period, power density in the alpha and beta range was higher in boys than in girls. In the second recording period, theta, alpha, and sigma power density but not beta power density were higher in boys than girls. Although the frequencies showing a sex effect differed between the 2 recording sessions, these data raise the possibility that the adolescent sex differences in EEG are not limited to low-frequency EEG.
Table 4.
Sex Differences in Electroencephalogram Power Density of Frequencies from 0.3 to 50 Hz for the 12-Year-Old Cohort Only
| Percentage difference | ||
|---|---|---|
| Frequency Band, Hz | First Recording | Second Recording |
| 0.3–3 | 37* | 36* |
| 3–4 | 15 | 29 |
| 4–6 | 18 | 36* |
| 6–8 | 31 | 70* |
| 8–12 | 30* | 82* |
| 12–15 | 42 | 69* |
| 15–23 | 27* | 14 |
| 23–30 | 32* | 1 |
| 30–50 | 26 | −17 |
t tests indicates significant difference between sexes at α = .05. The sex difference in power density extended across a wide spectrum of frequencies, but the difference was significant for both recordings in only delta and alpha. Percentage difference refers to the percentage by which the mean power density for the boys exceeded that for the girls.
Delta Power Density and Tanner Stage
To test for a possible relationship between sexual maturation and the decrease in delta power density, we computed correlation coefficients between delta power density and Tanner stage. We analyzed each age cohort separately to remove the strong age effects. For girls, both the 9- and 12-year-old cohorts showed weak negative correlations (r = −0.38 and r = −0.46, respectively) between Tanner stage and delta power density at the first recording period. However, correlation coefficients at the second recording period (r = −0.11 and r = 0.06) did not approach significance. In boys, correlations between Tanner stage and delta power density were not significant at either recording period.
DISCUSSION
This cross-sectional analysis of sleep EEG in 9- and 12-year-old cohorts revealed that NREM delta power density in 12-year-old girls is lower than in 12-year-old boys or in 9-year-old girls. NREM delta power density does not differ between 9-year-old girls and boys or between 12-year-old boys and 9-year-old boys. This finding contradicts our hypothesis that the decline in NREM slow-wave EEG across adolescence would not show a sex difference. Obtaining the same result in 2 different recordings with different (albeit overlapping) groups of subjects adds confidence to this finding. Below, we discuss the possibility that the sex difference in slow-wave EEG at age 12 years indicates that girls begin adolescent brain maturation earlier than boys.
The NREM delta EEG is thought to reflect sleep-dependent recuperation from the effects of waking brain activity. We have hypothesized that the intensity of recuperation depends not only upon the duration of prior waking, but also upon the intensity of brain activity during waking.9 Consistent with this hypothesis are findings that the ontogenic curve for delta EEG amplitude parallels the curves for cortical metabolic rate and synaptic density,6 as well as for cortical gray matter.14 The curves for all 4 variables increase steeply in infancy, peak around age 5 to 8 years, and decrease steeply across adolescence.
The above-mentioned decrease in synaptic density could produce the decline in delta EEG amplitude across adolescence. The amplitude of EEG waves depends on the number of neurons whose membrane potentials oscillate in unison. As synaptic connectivity between neurons decreases, the size of these oscillating neuronal pools should decrease, thereby reducing delta-wave amplitude. A reduction in waking synaptic activity could also reduce the need for delta homeostasis. A recent hypothesis proposes that synaptic potentiation is the homeostatic function of slow wave sleep.15
The sex difference in delta power density found in our cross-sectional analysis suggests that adolescent synaptic pruning starts earlier in girls. Structural magnetic resonance imaging estimates of cortical gray matter volume show that frontal and parietal cortical gray matter volumes decrease across adolescence.14,16,17 Cortical gray volume largely depends on synaptic density18 but also is influenced by cell growth, cell atrophy, and cell death. The gray matter volume begins its ontogenic decline earlier in girls. Frontal-lobe gray matter volume decreases from a peak that occurs at 11 years of age in girls and 12.1 years in boys.17 Cross-sectional structural magnetic resonance imaging data indicate that adolescent boys show a steeper age-related decrease in gray matter and increase in white matter.19 We cannot make firm conclusions about the rate of slow-wave EEG decline across adolescence in girls and boys until we collect and analyze all the longitudinal data. Nevertheless, available evidence from cross-sectional data suggests that the slow-wave EEG decline would be steeper in boys. If males begin the delta decline later than females but, in adulthood, have lower delta levels than females, the rate of delta decline in boys must at some point exceed that in girls. More-rapid synaptic pruning during late adolescence in males could provide a greater opportunity for errors in circuitry, leading to earlier onset of schizophrenia in boys.19
Although the sex difference in delta EEG might be explained by synaptic pruning in the cortex, it remains possible that factors independent of brain maturation could produce the lower delta power density in the 12-year-old girls. We previously concluded that the difference in delta EEG intensity in young adults versus elderly indicates a change in the brain’s generation of delta waves for 2 reasons: (1) delta-wave incidence and amplitude are reduced in the elderly and (2) the amplitude difference is limited to low frequencies.20 However, here we show that lower delta power density in 12-year-old girls than in 12-year-old boys is entirely due to lower delta-wave amplitude. Delta-wave incidence showed no sex difference. Moreover, the range of frequencies showing a sex difference is uncertain in our data. Low-frequency (delta) power density is clearly reduced in 12-year-old girls, but the high-frequency power density may also be lower. Different frequency bands did not show consistent sex differences in the 2 recording periods. Thus, it remains possible that the sex difference in delta power density is caused by extracerebal factors, ie, decreased transmission of delta waves from the cortex to the recording sites on the scalp. For example, greater skull thickness in adolescent girls could produce decreased EEG signal transmission. This possibility is hypothetical; we know of no evidence indicating a sex difference in skull thickness at this age. While lower delta power density in men has been attributed to skull thickness by one group,21 this interpretation has been rejected by others.22
The change in decrease in delta EEG in the 12-year-old girls was opposite of what would be expected from a homeostatic response to a change in daytime waking. Both boys and girls within the 12-year-old cohort go to sleep later and arise earlier than do those in the 9-year-old cohort. The bedtime schedules we recorded with in-home EEG recordings agree nicely with previous data obtained with EEG measurements in the sleep laboratory and with home actigraphy (reviewed in 23). The recent meta-analysis by Ohayon et al24 reports that total sleep time decreases across adolescence on school days. We found decreased NREM sleep but not decreased REM sleep.
When not constrained by school-day schedules, older children typically have later wake times as well as later bedtimes.7 This change in sleep schedule has been attributed to a shift in circadian rhythms. Although we did not measure the circadian rhythms of the subjects in our study, it is unlikely that sex differences in circadian rhythms produce the sex difference in slow-wave EEG. The sex difference in delta power density that we observed in the 12-year-old cohort occurred in the absence of any sleep-schedule differences. Moreover, boys in the 12- and 9-year-old cohorts had similar delta power density although they had different sleep schedules.
The sex difference in NREM delta EEG power density likely reflects a sex difference in the need for the recuperative processes of sleep. Both recording periods show that this sex difference is greatest at the lower delta frequencies that, in the prefrontal cortex, are thought to have the strongest relationship to recuperation.25 However, sleep regulation involves more than just the recuperative portion related to slow-wave EEG. Boys and girls in the 12-year-old cohort were similar in other aspects of sleep, such as NREM duration, REM duration, and total sleep duration. It is interesting to note that, while the 9-year-old cohort averaged about 45 minutes more NREM sleep per night, REM sleep duration did not differ in the 2 age groups. This suggests that despite spending an hour longer in bed, the 9-year-olds were not extending their recuperative sleep beyond what is needed. If they were producing excess sleep, we would expect the higher levels of REM seen in the extended sleep of young adults.26,27 Our findings indicate that 9-year-olds have a greater total need for recuperation that requires longer NREM duration as well as more-intense delta.
The sex difference in delta power density in early adolescence raises the question of whether the delta decline is related to sexual maturation. We do not have sufficient data at this time to address this question. We found no significant correlation between Tanner stage and delta power, but the high intersubject variability in these measures precludes firm conclusions. This issue should be clarified by the longitudinal data we are now collecting.
Sleep EEG is the most easily measured and, perhaps, the most sensitive measure of the profound brain changes of adolescence. It may provide a direct index of the adolescent declines in synaptic density and cortical metabolism. It is increasingly apparent that the biology of adolescent maturation must be studied longitudinally.17,28 The longitudinal data we are collecting should enable us to describe the within-subject trajectory of sleep EEG changes in males and females and the relationship of these sleep changes to other developmental milestones.
Acknowledgments
NIH grant R01 MH62521 supported this work. Our undergraduate assistants provided excellent technical support in the data collection. We also thank the subjects who participated in this study.
Footnotes
Citation: Campbell IG; Darchia N; Khaw WY et al. Sleep EEG evidence of sex differences in adolescent brain maturation. SLEEP 2005;28(5):637–643.
Disclosure Statement
This was not an industry supported study. Drs. Campbell, Darchia, Khaw, Higgins, and Feinberg have indicated no financial conflicts of interest.
References
- 1.Feinberg I, Hibi S, Carlson VR. Changes in EEG amplitude during sleep with age. In: Nandy K, Sherwin I, editors. The Aging Brain and Senile Dementia. New York: Plenum Press; 1977. pp. 86–98. [Google Scholar]
- 2.Feinberg I, Carlson VR. Sleep variables as a function of age in man. Arch Gen Psychiatr. 1968;18:239–50. [Google Scholar]
- 3.Feinberg I, March JD, Flach K, Maloney T, Chern W-J, Travis F. Maturational changes in amplitude, incidence and cyclic pattern of the 0 to 3 Hz (delta) electroencephalogram of human sleep. Brain Dysfunction. 1990;3:183–92. [Google Scholar]
- 4.Coble PA, Reynolds CF, III, Kupfer DJ, Houck P. Electroencephalographic sleep of healthy children. Part II: Findings using automated delta and REM sleep measurement methods. Sleep. 1987;10:551–62. [PubMed] [Google Scholar]
- 5.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 198283;17:319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg I, Thode HC, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Bio. 1990;142:149–61. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 7.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–81. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- 8.Borbely A. A two-process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 9.Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 10.Ehlers CL, Kupfer DJ. Slow-wave sleep: do young adult men and women age differently? J Sleep Res. 1997;6:211–5. doi: 10.1046/j.1365-2869.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- 11.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Washington: Public Health Services, US Government Printing Office; 1968. [Google Scholar]
- 12.Feinberg I, March JD, Fein G, Floyd TC, Walker JM, Price L. Period and amplitude analysis of 0.5–3 c/sec activity in NREM sleep of young adults Electroencephalogr. Clin Neurophysiol. 1978;44:202–13. doi: 10.1016/0013-4694(78)90266-3. [DOI] [PubMed] [Google Scholar]
- 13.Achermann P, Borbely A. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;18:213–22. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- 14.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 15.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Jernigan TL, Trauner DA, Hesselink JR, Tallal P. Maturation of human cerebrum observed IN VIVO during adolescence. Brain. 1991;114:2037–49. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- 17.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 18.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 19.De Bellis MD, Keshavan MS, Beers SR, et al. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11:552–7. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 20.Feinberg I, March JD, Floyd TC, Fein G, aminoff MJ. Log amplitude is a linear function of log frequency in NREM sleep EEG of young and elderly normal subjects. Electroencephalogr Clin Neurophysiol. 1984;58:158–60. doi: 10.1016/0013-4694(84)90029-4. [DOI] [PubMed] [Google Scholar]
- 21.Dijk DJ, Beersma DGM, Bloem GM. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep. 1989;12:500–7. doi: 10.1093/sleep/12.6.500. [DOI] [PubMed] [Google Scholar]
- 22.Armitage R, Hoffmann RF. Sleep EEG, depression and gender. Sleep Med Rev. 2001;5:237–46. doi: 10.1053/smrv.2000.0144. [DOI] [PubMed] [Google Scholar]
- 23.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann NY Acad Sci. 2004;1021:276–91. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- 24.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 25.Anderson C, Horne J. Prefrontal cortex: Links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003;40:349–57. doi: 10.1111/1469-8986.00038. [DOI] [PubMed] [Google Scholar]
- 26.Feinberg I, Fein G, Floyd TC. EEG patterns during and following extended sleep in young adults. Electroencephalogr Clin Neurophysiol. 1980;50:467–76. doi: 10.1016/0013-4694(80)90013-9. [DOI] [PubMed] [Google Scholar]
- 27.Aserinsky E. The maximal capacity for sleep: rapid eye movement density as an index of sleep satiety. Biol Psychiatr. 1969;1:147–59. [PubMed] [Google Scholar]
- 28.Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab. 2000;85:1021–5. doi: 10.1210/jcem.85.3.6423. [DOI] [PubMed] [Google Scholar]