Abstract
Background
Atrial fibrillation (AF) has been linked to an inflammatory process detected through various biomarkers, including C-Reactive Protein (CRP). Early recurrence of AF within the first three months after curative AF ablation is not felt to reflect success or failure of the procedure. We hypothesized that this early recurrence is due to an inflammatory response to the ablation itself. We therefore sought to evaluate levels of CRP after AF ablation.
Methods
We prospectively enrolled subjects undergoing AF ablation. A control group of patients undergoing ablation for supraventricular tachycardia (SVT) was also enrolled. Each patient had CRP drawn on the day of the procedure (prior to ablation) and during their first follow-up (median 49 days, interquartile range [IQR] 37–93) and second follow-up (median 147 days, IQR 141–257) clinic visits. Patient interviews were performed and medical histories reviewed for evidence of recurrent AF prior to the first follow-up.
Results
CRP levels significantly increased from baseline to first follow-up in the AF ablation group (p=0.0017). CRP did not significantly change after SVT ablation (p=0.92). Seventeen (45%) of the AF subjects exhibited recurrence of AF prior to first follow-up. After adjusting for multiple potential confounders, AF ablation patients with recurrent AF prior to their first follow-up had a statistically significant greater odds of having an increase in CRP (OR 21, 95% CI 1.1–417, p=0.045).
Conclusions
AF ablation generates an inflammatory response that persists for several weeks. This inflammation may explain early recurrence of AF after curative ablation.
Introduction
Catheter based radio-frequency (RF) ablation is an effective treatment for atrial fibrillation (AF), with success rates generally varying from approximately 40–80%.1 Recurrent AF is common within 3 month after AF ablation, occurring in nearly half of these patients even despite antiarrhythmic therapy 2, 3 and with an increase in AF frequency reported in a substantial proportion.2
Interestingly, studies have shown that this early recurrence of AF likely has little to no prognostic value regarding long-term treatment failure or success.2–7 Nevertheless, recurrent AF in this timeframe is understandably disconcerting and can be a serious concern for these patients (who have just undergone a significant invasive procedure) and the practitioners that care for them. Currently, the mechanisms underlying early recurrence of AF in the ablation setting are unknown, and, with this knowledge gap, there remains much debate regarding the use of early repeat RF ablation and/or long-term use of antiarrhythmic medications in this setting.8
It is well known that inflammation is sufficient to facilitate the initiation of atrial arrhythmias,9 and recent evidence has demonstrated that an elevated C-Reactive Protein (CRP) may predict AF in some patients.10 Given the extensive tissue destruction involved in left atrial ablation procedures for AF, an acute inflammatory response is likely,11,12 but whether or not such a response might persist long enough to explain the recurrent AF occurring on a scale of weeks to months after the procedure has not been explored. With the hypothesis that early recurrence of AF after curative AF ablation occurs due to an increase in inflammation, we sought to assess CRP levels before and several weeks after AF ablation procedures.
Methods
Enrollment
We performed a prospective observational study. Consecutive adult patients undergoing SVT ablation or curative left atrial ablation for paroxysmal AF over a 1 year period were invited to return for study “first follow-up” between 1 and 3 months after their procedure. Only those who followed-up in that time period were included in the study; these patients were then asked to return for a “second follow-up” at approximately 6 months after the procedure. To evaluate whether or not those returning for follow-up in the allotted time generally represented the general population undergoing AF and SVT ablation procedures, baseline demographics and cardiovascular risk factors were compared between those returning for follow-up and a consecutive sample of all AF and SVT ablation patients over the study period. Patients were excluded if they had known acute or chronic inflammatory conditions (including infectious, autoimmune, or cancerous diseases), renal disease, a history of myocardial infarction, or heart failure.
Femoral venous blood was collected from all patients prior to any ablations on the day of the ablation procedure (baseline), and peripheral venous blood was collected at each subsequent follow up visit. High-sensitivity CRP was determined by ELISA (Alpha Diagnostic International, San Antonio, TX), with a lower rate of detection of 0.00035 mg/L.
Patient history was determined by chart review, and patient interview and details regarding the ablation procedure were recorded. In the AF ablation subjects, AF recurrence at first follow-up was defined as either evidence of AF by electrocardiogram at anytime between completion of the ablation procedure and time of first follow-up or a subjective report by the patient of either no improvement or worsening in symptoms previously attributed to AF. Because not all subjects returned for second follow-up, success of the procedure at second follow-up was determined by either patient visit or phone call and relied on report of symptoms only.
Statistical analysis
Normally distributed continuous variables are expressed as means ±SD. Variables not normally distributed are expressed as medians and interquartile ranges (IQR). Normally distributed continuous variables were compared using t-tests and not normally distributed variables were compared using the Wilcoxon rank sum and sign rank tests as appropriate. Categorical variables were compared using the χ2 test. Multivariable analysis was performed using logistic regression analysis. Covariates included in the regression model were based on variables previously established as potential confounders or important demographics (e.g. age, gender and race) and covariates significantly associated with both the predictor and outcome with p values < 0.10. Consistent with previous studies, CRP was found to be right skewed,1 and therefore, the primary outcome used in the logistic regression was a dichotomous positive versus negative change in CRP between baseline and first follow-up. Two tailed p values < 0.05 were considered statistically significant.
Results
A total of 38 AF ablation patients and 38 SVT ablation patients were included in the analysis (all with first follow-up). While the AF subjects were older and more often male, none of the other baseline demographics and cardiovascular risk factors differed between the 2 groups (Table 1). None of the demographic or cardiovascular risk factor differences listed in Table 1 were statistically significant different between the sample study population and consecutive subjects presenting for AF or SVT ablation over the same time period.
Table 1.
Baseline characteristics of subjects with atrial fibrillation (AF) and supraventricular tachycardia (SVT).
| AF (n=38) | SVT (n=38) | p value | |
|---|---|---|---|
| Age (years) | 53 ± 8 | 47 ± 17 | 0.032 |
| Male | 29 (76%) | 16 (42%) | 0.002 |
| Race | |||
| White | 31 (82%) | 25 (66%) | |
| Black | 0 | 2 (5%) | |
| Asian | 3 (8%) | 7 (18%) | |
| Latino | 3 (8%) | 3 (8%) | 0.37 |
| Hypertension | 12 (32%) | 11 (29%) | 0.80 |
| Type II diabetes mellitus | 2 (5%) | 3 (8%) | 0.64 |
| Left ventricular ejectionfraction (%) | 63 ± 12 | 61 ± 6 | 0.50 |
| Body mass index (kg/m2) | 28 ± 5 | 27 ± 7 | 0.40 |
| ACEI/ ARB* | 4 (11%) | 5 (13%) | 0.72 |
| Statin | 6 (16%) | 8 (22%) | 0.55 |
ACEI/ARB denotes ace inhibitor or angiotensin receptor blocker.
Median time to first follow-up amongst AF and SVT patients was 49 days (IQR 37–93) and 48 days (IQR 33–73), respectively (p = 0.36). Twenty-three (61%) of the AF patients and 9 (24%) of the SVT patients returned for second follow-up a median of 179 days (IQR 141–257) and 206 days (IQR 103–339) after enrollment, respectively (p = 0.89 for the comparison in follow-up times).
There was no statistically significant difference in baseline CRP levels between the AF (median 1.8 mg/L, IQR 0.8–5.5) and SVT patients (median 2.5 mg/L, IQR 0.9–5.2), p = 0.65. All AF ablations were performed using radiofrequency energy delivered with an 8 mm tipped catheter. In 31 (82%) of the AF procedures, ablation lines were drawn in 2 circles, each 1–2 cm outside and encompassing the left or right pulmonary veins, and the remaining 7 underwent ablations 1–2 cm outside and encompassing each of the individual veins. In addition, fractionated electrocardiograms were targeted in 22 (58%), and 6 (16%) underwent ablation from the left lower pulmonary vein to the mitral valve isthmus. Of the 38 SVT patients, 18 had typical AV nodal reentrant tachycardia (AVNRT), 9 had atrioventricular reciprocating tachycardia, 6 had focal atrial tachycardia, 1 had atypical AVNRT, 1 had junctional tachycardia, and 3 failed to exhibit an inducible tachycardia. Of these, radiofrequency energy was used in 29 (28 with a 4 mm tipped catheter and 1 with an 8 mm catheter), cryoablation was used in 4, and ablation was not performed in 2 parahisian atrial tachycardias as well as the 3 cases wherein the clinical tachycardia could not be induced. Compared to the SVT ablations, AF ablations involved significantly more radiofrequency applications (69 ± 33 versus 13 ± 13, p<0.001), longer total ablation time (median 1,911 seconds, IQR 1,420-2,735 versus median 416 seconds, IQR 180–669, p<0.001) and higher mean power (41 ± 9 Watts versus 31 ± 15 Watts, p=0.0051).
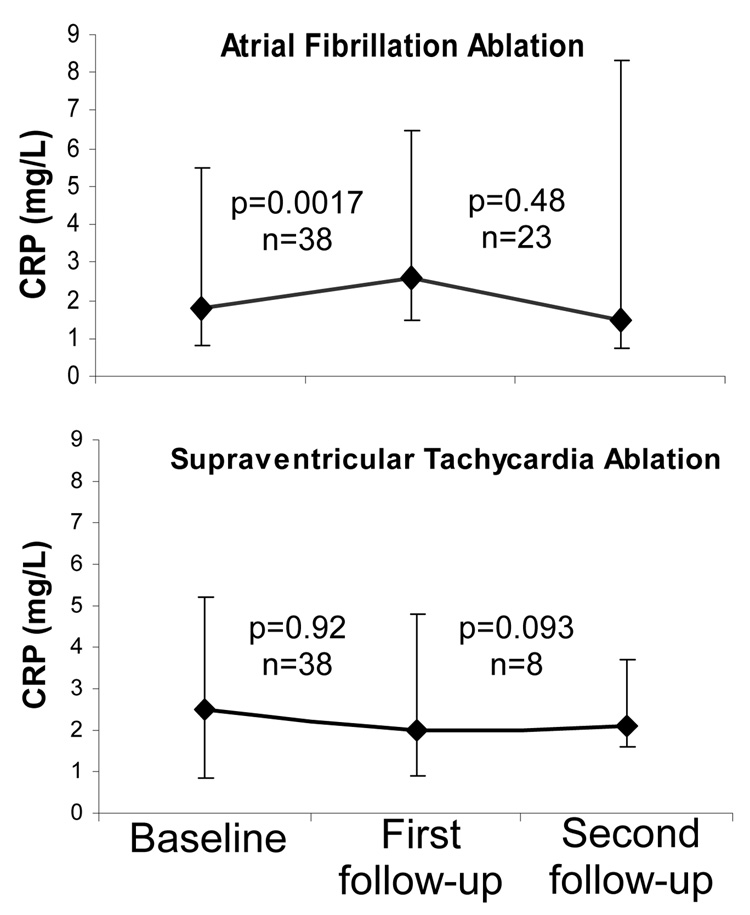
CRP levels significantly increased from baseline to first follow-up in the AF ablation group (Figure 1). CRP levels did not significantly change in the SVT group. Those with a rise in CRP after ablation were older than those without, but none of the other baseline demographics or cardiovascular risk factors were significantly associated with a rise or fall in CRP (Table 2). Among the AF patients, the type of AF ablation did not significantly influence the change in CRP, and, among the SVT patients, the type of SVT ablation did not meaningfully influence the change in CRP. A sensitivity analysis was performed excluding the SVT subjects that did not undergo RF ablation, and the findings did not meaningfully change the results (no significant change in CRP was observed after excluding these patients). In the 4 SVT patients treated with cryoablation, 3 exhibited an increase in CRP and 1 exhibited a decrease.
Figure 1.
Median levels of CRP after atrial fibrillation and supraventricular tachycardia ablation. See text for median time to first and second follow-up visits. Y error bars denote interquartile ranges of CRP.
Table 2.
Baseline demographics of AF and SVT subjects with either a rise or fall in CRP between baseline and first follow-up visits. No subject exhibited identical CRP levels on these 2 visits.
| Increase in CRP (n=45) |
Decrease in CRP (n=31) |
p value | |
|---|---|---|---|
| Age (years) | 53 ± 8 | 47 ± 16 | 0.040 |
| Male | 28 (61%) | 17 (57%) | 0.72 |
| Hypertension | 17 (37%) | 6 (20%) | 0.12 |
| Ejection fraction (%) | 63 ± 11 | 61 ± 6 | 0.50 |
| Body mass index (kg/m2) | 28 ± 5 | 27 ± 6 | 0.40 |
| Type II Diabetes | 3 (7%) | 2 (7%) | 0.98 |
None of the individual ablation parameters (total number of ablations, total ablation time, or mean power delivered) were significantly associated with the change in CRP from baseline to first follow-up in bivariate analysis. However, in logistic regression analysis adjusting for each of these variables, an 8 mm ablation catheter ablator size was associated with a greater odds of an increase in CRP (odds ratio [OR] 8.5, 95% confidence interval [CI] 1.07–68.0, p=0.043).
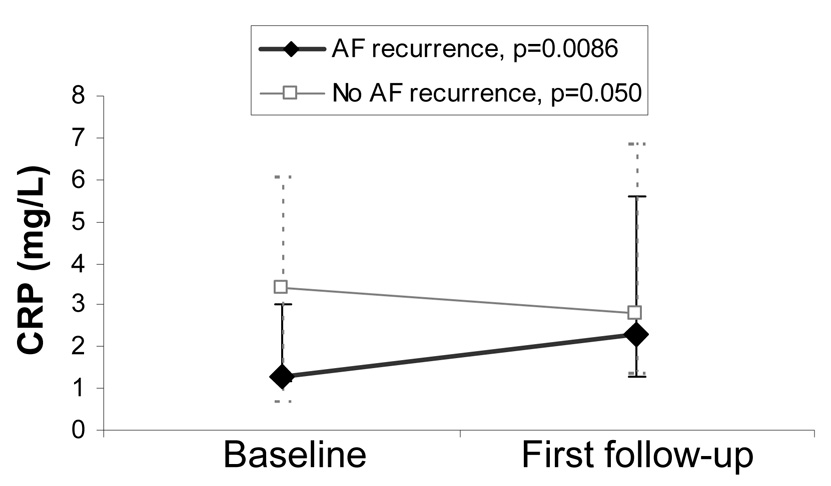
Of the 38 AF patients, 17 had evidence of recurrent AF prior to their first follow-up visit (10 with documented AF and 7 with symptoms consistent with AF that were the same or worse than prior to their procedure). Examining only those with early recurrent AF (AF detected by the time of first follow-up), median CRP significantly increased (Figure 2). In contrast, the median CRP decreased after AF ablation in those without evidence of AF recurrence at first follow-up; importantly, in those without early recurrence of AF, the overall distribution of CRP increased to a nearly statistically significant degree (as can be seen by the increase in the interquartile range in Figure 2, p=0.050).
Figure 2.
Median baseline and first follow-up CRP levels of atrial fibrillation ablation patients with and without evidence of recurrent atrial fibrillation by first follow-up. Solid and dashed Y error bars denote interquartile ranges for those with and without recurrent atrial fibrillation, respectively. Although the median CRP level declined in those without recurrent atrial fibrillation, the p value of 0.050 represents the overall increase in CRP (as demonstrated by the interquartile range).
Although a larger proportion of subjects with AF recurrence prior to first follow-up exhibited an increase in CRP (77%) compared to those without any known AF recurrence (67%), this difference was not statistically significant in the bivariate analysis (p=0.51). After adjusting for multiple potential confounders, including age, gender, body mass index (BMI), statin therapy, and antiarrhythmic agents, those with recurrence of AF prior to first follow-up had a statistically significant greater odds of having an increase in CRP (OR 21, 95% CI 1.1–417, p=0.045).
A total of 33 AF ablation patients were interviewed either during their second follow-up visit or by phone a median 194 days (IQR 146–271) after their procedure. Of these, 22 had an improvement or elimination of symptoms (approximately half of these with complete elimination). There was no correlation with a rise in CRP and change in symptoms.
Discussion
We demonstrated a significant rise in CRP a median 49 days after AF ablation. Although the subsequent decline in CRP observed at second follow-up (a median 179 days after ablation) was not statistically significant, the trend and in particular the lower median CRP demonstrate that this inflammatory response is likely declining by that time. A similar elevation in CRP was not seen in patients undergoing SVT ablation, suggesting that the rise in CRP is not due to the electrophysiology study and ablation per se, but rather something unique to curative AF ablation procedures (such as the extensive left atrial ablation involved).
The inflammatory response to AF ablation suggests a mechanism that might explain early recurrence of AF, a process that commonly occurs within 3 months of the procedure. While those with recurrence of AF prior to first follow-up exhibited a significant rise in CRP, those without early recurrent AF exhibited only a trend towards such an increase. After adjusting for multiple potential confounders, early recurrence of AF was significantly associated with a rise in CRP.
Ablation has proven to be a successful therapy for symptomatic AF, comparing favorably to alternative strategies of normal sinus rhythm maintenance with a reported success rate as high as 80%.2, 7, 13–15 However, even in those cases that are ultimately successful, an early recurrence of AF will often occur.2–6, 16 In fact, this problem is so prevalent that current guidelines suggest that monitoring for AF ablation success or practice, recurrent AF occurring after such a major procedure can be concerning to patients, particularly as symptoms of this early recurrence may be worse than AF symptoms prior to the procedure,2 and the most appropriate subsequent management (including pharmacologic and/or repeat ablation therapies) is often difficult to discern. In addition, the possibility that the favorable prognosis of early recurrence of AF represents a follow-up bias in some studies can not be excluded: for example, it is not inconceivable that AF is more often detected shortly after procedures because that is when clinical follow-up is most complete. Therefore, this process is important enough that an investigation of possible underlying mechanisms are worthwhile, both to inform future studies with prevention and/or risk stratification strategies and to provide some biological plausibility that will reinforce epidemiologic data gleaned from clinical studies.
The initiation of inflammation in the sterile pericarditis model is known to render an animal more prone to atrial arrhythmias.9 Post-operative AF that commonly occurs after open heart surgery has been attributed to a pro-inflammatory process, with some evidence that serologic markers of inflammation may portend a greater risk.17 In addition, an important marker of systemic inflammation, CRP, has been shown to predict non post-operative AF in one large cohort study.10 AF ablation involves prolonged ablation times and burns throughout the left atrium, resulting in significant histopathologic tissue damage in animal models and human hearts alike,11,12 which supports a plausible mechanism for inflammation in the post AF ablation period. These extensive ablations may also result in pericarditis, which itself may be pro-arrhythmic, and future studies examining echocardiograms for pericardial effusions after AF ablations may help to elucidate this further. However, evidence of persistent inflammation for weeks after the procedure has not previously been studied. Our data suggest that this inflammation does indeed persist, potentially providing a ready substrate for recurrent AF.
As expected, our AF ablation patients had a significantly greater number of ablations, with a longer ablation time and greater power delivered than those undergoing SVT ablation. Although none of these factors were by themselves significantly associated with an increase in CRP in bivariate analysis, multivariate analysis demonstrated that the use of an 8mm catheter tip was associated with a significantly greater odds of an increased CRP. Importantly, all AF ablation procedures utilized 8 mm ablation catheters as compared to only one SVT procedure (during which 4mm catheters were predominantly used). Therefore, we cannot exclude the possibility that an 8mm catheter tip may simply be a surrogate for AF ablation itself. Since larger catheter tip diameters provide a greater surface area for convection of heat to the blood pool, enabling them to deliver more power to heat the underlying tissue,18 the 8 mm catheter likely contributed to greater tissue destruction with each radiofrequency application.
Of note, we recently published a study wherein serologic markers of inflammation significantly declined after successful ablation of atrial flutter, demonstrating that an atrial tachyarrhythmia may contribute to inflammation.24 This may appear contradictory to the supposition that inflammation precedes or causes AF, but in fact these findings are not mutually exclusive. It may be that a vicious cycle ensues, with “AF begetting AF” due to an aggravation of a pro-inflammatory abnormal atrial substrate that itself leads to propagation of more AF. However, given the possibility that the inflammation seen in atrial arrhythmias is in fact entirely a cause of the arrhythmia, we can not exclude the possibility that the rise in CRP observed in this study was an effect (rather than a cause) of more post-ablation AF, leaving the cause of increased early AF after ablation unknown. Future studies may measure CRP levels both at a designated time after AF ablation and at the time of AF recurrence to help determine the cause and effect nature of the relationship.
Study Limitations
Our study has several limitations. First, our data was not derived from consecutive patients, but instead represented a sample of patients who returned for a first follow-up visit. However, the baseline demographic and clinical characteristics of the patients sampled was compared to consecutive subjects presenting for AF and SVT ablation procedures over the study period and was found to be similar in every way measured. Of note, all subjects were invited for follow-up in the same manner. Among the patients evaluated for SVT, not all underwent RF ablation. Importantly, a sensitivity analysis excluding these subjects did not change any of our findings. Finally, to ascertain AF recurrence, we were dependent on patient symptoms consistent with previous episodes of AF and/or 12 lead documentation of the arrhythmia, and, given that multiple studies have demonstrated that asymptomatic AF commonly occurs within the first 3 months after AF ablation (even in patients with previously symptomatic AF), we may have missed some episodes.8, 13, 16, 19–23 Therefore, while the increase in CRP after AF ablation appears to be a robust finding, the association between the rise in CRP and early recurrent AF should be validated in a separate study with more rigorous monitoring for AF.
Conclusion
This study demonstrated that AF ablation was associated with a significant increase in CRP measured a median 49 days after the procedure. This period of time is consistent with published timeframes for early recurrence of AF, a common and potentially concerning finding after AF ablation, and it appears that those with a rise in CRP were more likely to have recurrent AF. These data lend support to the role of inflammation in the genesis and maintenance of AF and suggest that the extent of left atrial tissue damage inherent to curative AF ablation generates a protracted inflammatory state with proarrhythmic effects.
Acknowledgments
Funding Sources
This work was made possible by grant number KL2 RR024130 (G.M.M.) from the National Center for Research Resources (NCRR), a component of the NIH, and the American Heart Association Western States Affiliate Beginning Grant-in-Aid Award (G. M. M.).
Footnotes
Author disclosures/ potential conflicts of interest: None
References
- 1.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Europace. 2007;9(6):335–379. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 2.Oral H, Knight BP, Ozaydin M, Tada H, Chugh A, Hassan S, Scharf C, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol. 2002;40(1):100–104. doi: 10.1016/s0735-1097(02)01939-3. [DOI] [PubMed] [Google Scholar]
- 3.Bertaglia E, Stabile G, Senatore G, Zoppo F, Turco P, Amellone C, De Simone A, et al. Predictive value of early atrial tachyarrhythmias recurrence after circumferential anatomical pulmonary vein ablation. PACE. 2005;28(5):366–371. doi: 10.1111/j.1540-8159.2005.09516.x. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Lu Z, Lei H, Zhao D, Yang B, Huang C. Predictors of early recurrence and delayed cure after segmental pulmonary vein isolation for paroxysmal atrial fibrillation without structural heart disease. J Interventional Cardiac Electrophysiol. 2006;15(3):157–163. doi: 10.1007/s10840-006-9003-y. [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, Yu WC, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interventional Cardiac Electrophysiol. 2004;10(3):221–226. doi: 10.1023/B:JICE.0000026915.02503.92. [DOI] [PubMed] [Google Scholar]
- 6.O'Donnell D, Furniss SS, Dunuwille A, Bourke JP. Delayed cure despite early recurrence after pulmonary vein isolation for atrial fibrillation. Am J Cardiol. 2003;91(1):83–85. doi: 10.1016/s0002-9149(02)03005-9. [DOI] [PubMed] [Google Scholar]
- 7.Vasamreddy CR, Lickfett L, Jayam VK, Nasir K, Bradley DJ, Eldadah Z, Dickfeld T, et al. Predictors of recurrence following catheter ablation of atrial fibrillation using an irrigated-tip ablation catheter. J Cardiovasc Electrophysiol. 2004;15(6):692–697. doi: 10.1046/j.1540-8167.2004.03538.x. [DOI] [PubMed] [Google Scholar]
- 8.Kobza R, Hindricks G, Tanner H, Schirdewahn P, Dorszewski A, Piorkowski C, Gerds-Li JH, et al. Late recurrent arrhythmias after ablation of atrial fibrillation: incidence, mechanisms, and treatment. Heart Rhythm. 2004;1(6):676–683. doi: 10.1016/j.hrthm.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Ryu K, Li L, Khrestian CM, Matsumoto N, Sahadevan J, Ruehr ML, Van Wagoner DR, et al. Effects of sterile pericarditis on connexins 40 and 43 in the atria: correlation with abnormal conduction and atrial arrhythmias. Am J Physiol Heart Circ Pysiol. 2007;293(2):H1231–H1241. doi: 10.1152/ajpheart.00607.2006. [DOI] [PubMed] [Google Scholar]
- 10.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108(24):3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 11.Grubman E, Pavri BB, Lyle S, Reynolds C, Denofrio D, Kocovic DZ. Histopathologic effects of radiofrequency catheter ablation in previously infarcted human myocardium. J Cardiovasc Electrophysiol. 1999;10(3):336–342. doi: 10.1111/j.1540-8167.1999.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanno K, Kobayashi Y, Kurano K, Kikushima S, Yazawa T, Baba T, Inoue S, et al. Histopathology of canine hearts subjected to catheter ablation using radiofrequency energy. Jpn Circ J. 1994;58(2):123–135. doi: 10.1253/jcj.58.123. [DOI] [PubMed] [Google Scholar]
- 13.Kottkamp H, Tanner H, Kobza R, Schirdewahn P, Dorszewski A, Gerds-Li JH, Carbucicchio C, et al. Time courses and quantitative analysis of atrial fibrillation episode number and duration after circular plus linear left atrial lesions: trigger elimination or substrate modification: early or delayed cure? J Am Coll Cardiol. 2004;44(4):869–877. doi: 10.1016/j.jacc.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 14.Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, Bates ER, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354(9):934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 15.Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, Bash D, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293(21):2634–2640. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 16.Vasamreddy CR, Dalal D, Dong J, Cheng A, Spragg D, Lamiy SZ, Meininger G, et al. Symptomatic and asymptomatic atrial fibrillation in patients undergoing radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2006;17(2):134–139. doi: 10.1111/j.1540-8167.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 17.Ishida K, Kimura F, Imamaki M, Ishida A, Shimura H, Kohno H, Sakurai M, et al. Relation of inflammatory cytokines to atrial fibrillation after off-pump coronary artery bypass grafting. European J Cardio-thoracic Surg. 2006;29(4):501–505. doi: 10.1016/j.ejcts.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Haines D. The biophysics and pathophysiology of lesion formation during radiofrequency ablation. In: Zipes DP, et al., editors. Cardiac Electrophysiology: From Cell to Bedside. New York: Saunders Publishing; 2006. pp. 1018–1027. [Google Scholar]
- 19.Hindricks G, Piorkowski C, Tanner H, Kobza R, Gerds-Li JH, Carbucicchio C, Kottkamp H. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation. 2005;112(3):307–313. doi: 10.1161/CIRCULATIONAHA.104.518837. [DOI] [PubMed] [Google Scholar]
- 20.Karch MR, Zrenner B, Deisenhofer I, Schreieck J, Ndrepepa G, Dong J, Lamprecht K, et al. Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation. 2005;111(22):2875–2880. doi: 10.1161/CIRCULATIONAHA.104.491530. [DOI] [PubMed] [Google Scholar]
- 21.Klemm HU, Ventura R, Rostock T, Brandstrup B, Risius T, Meinertz T, Willems S. Correlation of symptoms to ECG diagnosis following atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2006;17(2):146–150. doi: 10.1111/j.1540-8167.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 22.Oral H, Veerareddy S, Good E, Hall B, Cheung P, Tamirisa K, Han J, et al. Prevalence of asymptomatic recurrences of atrial fibrillation after successful radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2004;15(8):920–924. doi: 10.1046/j.1540-8167.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 23.Senatore G, Stabile G, Bertaglia E, Donnici G, De Simone A, Zoppo F, Turco P, et al. Role of transtelephonic electrocardiographic monitoring in detecting short-term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation. J Am Col Cardiol. 2005;45(6):873–876. doi: 10.1016/j.jacc.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 24.Marcus GM, Smith LM, Glidden DV, Wilson E, McCabe J, Whiteman D, Tseng ZH, et al. Markers of Inflammation Before and After Curative Ablation of Atrial Flutter. Heart Rhythm. 2008;5(2):215–221. doi: 10.1016/j.hrthm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]