Abstract
Ehrlichia risticii has a close antigenic relationship to E. sennetsu. Sera of ponies experimentally infected with E. risticii, the etiologic agent of Potomac horse fever, consistently reacted with E. sennetsu, a human pathogen, in indirect fluorescent-antibody (IFA) testing, while human E. sennetsu convalescent serum reacted with E. risticii by IFA testing and immunoferritin labeling of cells infected in vitro. Two ponies injected intravenously with live E. sennetsu did no develop clinical illness. Subsequent injection with live E. sennetsu did not develop clinical illness. Subsequent injection with live E. risticii also did not induce any disease, in contrast to two control ponies given E. risticii without prior exposure to E. sennetsu. Both controls developed fever, anorexia, depression, dehydration, and diarrhea, which are typical clinical signs of Potomac horse fever, and had characteristic lesions of enteritis and lymph node histiocytosis at postmortem examination. E. sennetsu-exposed ponies had normal gastrointestinal morphologies and lymph node hyperplasia. Ponies primed with E. sennetsu before E. risticii challenge developed high titers of immunoglobulin G antibody which reacted against both E. sennetsu and E. risticii antigens by IFA testing. The most prominent antigenic polypeptide in Western (immuno-) blot analysis of sera collected from ponies primed with E. sennetsu before subsequent challenge with E. risticii was present in lysates of both Ehrlichia species and had an apparent molecular mass of 44 kilodaltons. This band was not prominent in Western blots performed with sera of ponies injected with E. risticii alone. Thus, injection of E. sennetsu protects ponies from clinical and pathological manifestations of the disease induced by injection with E. risticii. Immunologic cross-reactivity of the two organisms with IFA testing and strong immunologic recognition by ponies of the 44-kilodalton antigen common to the two organisms may be related to the development of protective immunity against E. risticii.
Full text
PDF

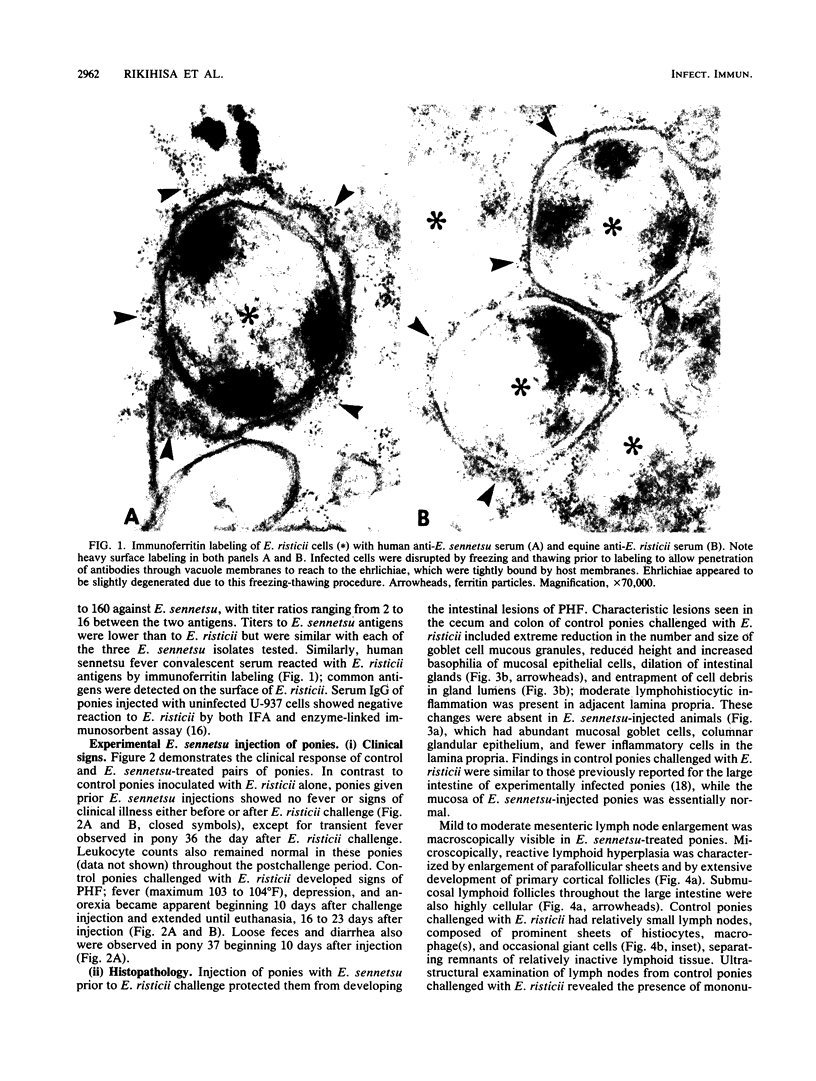
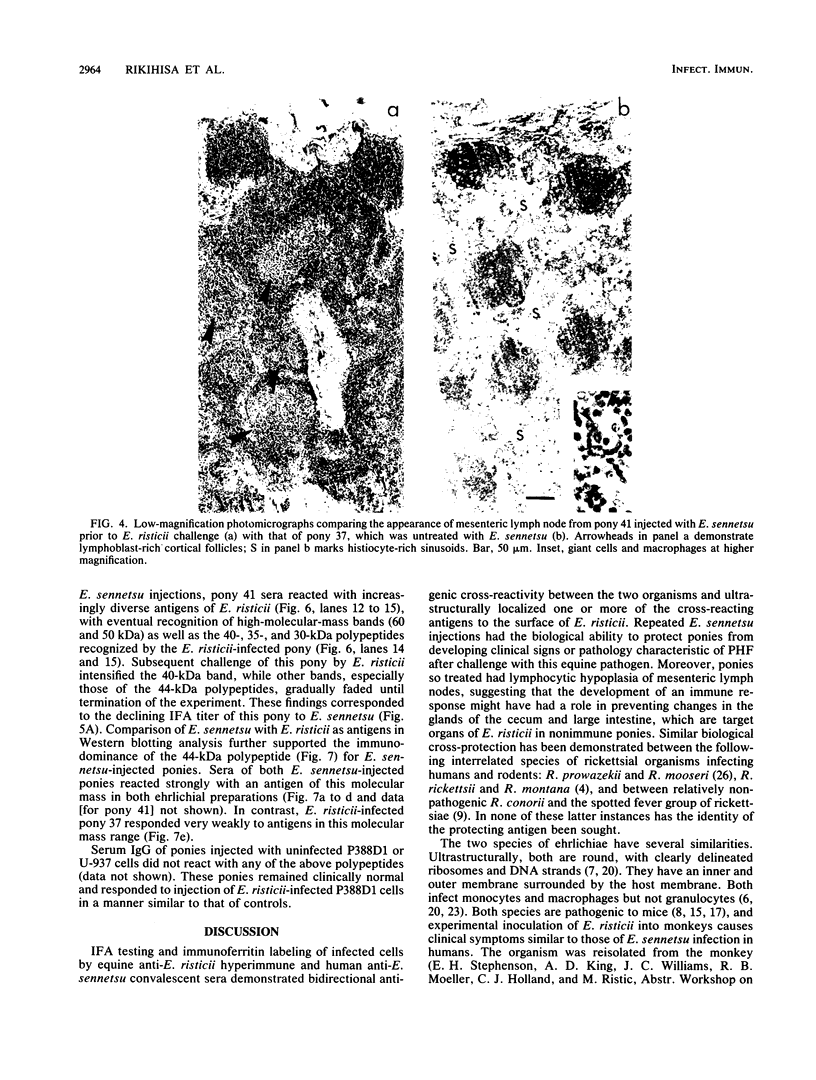
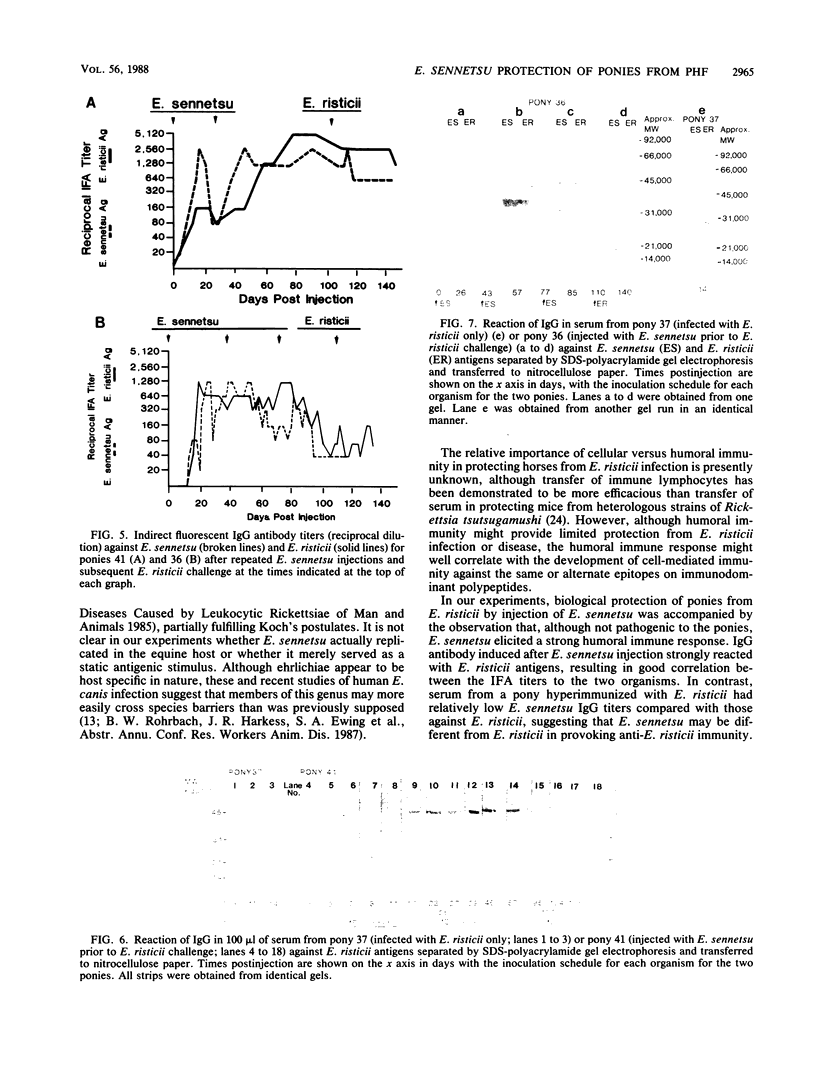

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cordes D. O., Perry B. D., Rikihisa Y., Chickering W. R. Enterocolitis caused by Ehrlichia sp. in the horse (Potomac horse fever). Vet Pathol. 1986 Jul;23(4):471–477. doi: 10.1177/030098588602300418. [DOI] [PubMed] [Google Scholar]
- Dutta S. K., Myrup A. C., Rice R. M., Robl M. G., Hammond R. C. Experimental reproduction of Potomac horse fever in horses with a newly isolated Ehrlichia organism. J Clin Microbiol. 1985 Aug;22(2):265–269. doi: 10.1128/jcm.22.2.265-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W. C., Waner J. L. Serological cross-reaction and cross-protection in guinea pigs infected with Rickettsia rickettsii and Rickettsia montana. Infect Immun. 1980 May;28(2):627–629. doi: 10.1128/iai.28.2.627-629.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. J., Ristic M., Cole A. I., Johnson P., Baker G., Goetz T. Isolation, experimental transmission, and characterization of causative agent of Potomac horse fever. Science. 1985 Feb 1;227(4686):522–524. doi: 10.1126/science.3880925. [DOI] [PubMed] [Google Scholar]
- Holland C. J., Ristic M., Huxsoll D. L., Cole A. I., Rapmund G. Adaptation of Ehrlichia sennetsu to canine blood monocytes: preliminary structural and serological studies with cell culture-derived Ehrlichia sennetsu. Infect Immun. 1985 May;48(2):366–371. doi: 10.1128/iai.48.2.366-371.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins S. J., Jones N. K., Jenny A. L. Potomac horse fever agent in mice. Vet Rec. 1985 Nov 23;117(21):556–557. doi: 10.1136/vr.117.21.556. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Jarboe D. L., Eisemann C. S. Cross-reactive lymphocyte responses and protective immunity against other spotted fever group rickettsiae in mice immunized with Rickettsia conorii. Infect Immun. 1986 Mar;51(3):832–837. doi: 10.1128/iai.51.3.832-837.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maeda K., Markowitz N., Hawley R. C., Ristic M., Cox D., McDade J. E. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987 Apr 2;316(14):853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- Minamishima Y. Persistent infection of Rickettsia sennetsu in cell culture system. Jpn J Microbiol. 1965 Jun;9(2):75–86. doi: 10.1111/j.1348-0421.1965.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Pretzman C. I., Rikihisa Y., Ralph D., Gordon J. C., Bech-Nielsen S. Enzyme-linked immunosorbent assay for Potomac horse fever disease. J Clin Microbiol. 1987 Jan;25(1):31–36. doi: 10.1128/jcm.25.1.31-36.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Johnson G. C., Burger C. J. Reduced immune responsiveness and lymphoid depletion in mice infected with Ehrlichia risticii. Infect Immun. 1987 Sep;55(9):2215–2222. doi: 10.1128/iai.55.9.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D. Causative ehrlichial organisms in Potomac horse fever. Infect Immun. 1985 Sep;49(3):513–517. doi: 10.1128/iai.49.3.513-517.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D., Cordes D. O. Ultrastructural study of ehrlichial organisms in the large colons of ponies infected with Potomac horse fever. Infect Immun. 1985 Sep;49(3):505–512. doi: 10.1128/iai.49.3.505-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Rota T., Lee T. H., MacDonald A. B., Ito S. Changes in immunoferritin labeling of Rickettsia tsutsugamushi after serial cultivation in 60Co-irradiated BHK cells. Infect Immun. 1979 Nov;26(2):638–650. doi: 10.1128/iai.26.2.638-650.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic M., Holland C. J., Dawson J. E., Sessions J., Palmer J. Diagnosis of equine monocytic ehrlichiosis (Potomac horse fever) by indirect immunofluorescence. J Am Vet Med Assoc. 1986 Jul 1;189(1):39–46. [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]