Abstract
Sibling competition has been shown to affect overall growth rates in birds. However, growth consists on the coordinated development of a multitude of structures, and there is ample scope for developmental plasticity and trade-offs among these structures. We would expect that the growth of structures that are used in sibling competition, such as the gape of altricial nestlings, should be prioritized under intense competition. We conducted an experiment in the spotless starling (Sturnus unicolor), cross-fostering nestlings to nests with different levels of sibling competition. We predicted that nestlings subjected to higher levels of sibling competition should develop larger gapes than control birds. We found that, halfway through the nestling period, overall size (a composite index of mass, wing, tarsus and bill) was reduced in nests with intense sibling competition, whereas gape width remained unaffected. At the end of the nestling period, experimental nestlings had wider gapes than controls. Additionally, a correlative study showed that nestling gape width increased when feeding conditions worsened and overall size decreased. These patterns could either be due to increased growth of gape flanges or to delayed reabsorption of this structure. Our results show that birds can invest differentially in the development of organs during growth, and that the growth of organs used in sibling competition is prioritized over structural growth.
Keywords: differential growth, sibling competition, spotless starling, nutritional stress, developmental plasticity, gape width
1. Introduction
Sibling competition has long been proposed to be an important factor in shaping growth rates in birds (Werschkul & Jackson 1979; Ricklefs 1982, 1993). A recent comparative study has given support for this hypothesis, showing that overall growth rate is higher in species where extra-pair paternity is common (Royle et al. 1999). However, during development, most of the bird's anatomical structures develop simultaneously, and may thus compete for resources (O'Connor 1984; Schew & Ricklefs 1998). When food becomes limited, organisms may either reduce their overall growth or balance their investment among different sets of organs and structures (Schew & Ricklefs 1998). Selection pressures may greatly vary between different traits, therefore it is expected that selection will favour allocation shifts among these different structures (Schew & Ricklefs 1998), and favour those with the highest survival value. Under intense sibling competition levels, we would expect birds to prioritize the development of structures that would maximize their competitive potential.
Some observational evidence for this prediction comes from interspecific comparisons that suggest that the early development of the digestive apparatus in altricial species is an adaptation for their high food intake (Ricklefs 1967; Dunn 1975). Similarly, the differential relative growth of characters through developmental time has been proposed to arise as a consequence of sibling competition (O'Connor 1977, 1978). An experiment carried out in the asynchronously hatching marsh tit (Parus palustris) has shown that runts give priority to the development of their wing feathers as a response to strong selection for synchronized fledging (Nilsson & Svensson 1996). In the spotted owl (Strix occidentalis), a species characterized by an early nest leave, it has been suggested that nestlings develop tarsi and bill early in development as an adaptation to locomotion in nearby branches (Kristan et al. 1996).
Patterns such as these may be especially relevant for structures that only have a temporary function (e.g. sibling competition). A unique example of such a structure is the gape of altricial birds. In contrast to precocial species, gape width is exaggerated in altricial nestlings by fleshy flanges, often conspicuously coloured, and that are used to guide parental feedings (O'Connor 1978; Kilner 1997; Saino et al. 2000; Jourdie et al. 2004). The particular development of flanges, which typically reaches a maximum width around the middle of the nestling period and regresses thereafter, suggests that it may have been shaped by patterns of sibling competition (O'Connor 1978). The gape thus works as a passive attractant when nestlings are small. Later, once sight and locomotion are better developed, nestlings can use more active ways to obtain food from parents.
Dwelling on this idea, Ortega & Cruz (1992) have proposed that increased development of nestling gapes may be an adaptation of some brood parasites to exploit host parental feedings, in the same way that begging signals of brood parasites often constitute super-stimulus for hosts (Davies et al. 1998; Tanaka & Ueda 2005). Thus, they have shown that nestlings of the brood-parasitic brown-headed cowbird (Molothrus ater) have wider gapes for their weight than yellow-headed blackbirds (Xanthocephalus xanthocephalus; but see Clark 1995).
In this study, we tested the hypothesis that gape width in passerines develops out of proportion compared with other body structures as a response to increased sibling competition (O'Connor 1978). We chose as a model the spotless starling (Sturnus unicolor), a species that shows a conspicuous yellow gape at the nestling stage. We first report data from an experiment in which nestlings were subjected to high levels of sibling competition by cross-fostering them to adoption nests with larger nestlings. In addition, we report observational data analysing how body size and gape width change with differences in natural food availability. The objective of our study is to investigate whether birds are able to invest differentially in the development of structures and whether organs of immediate use, such as nestling gapes, are prioritized over structural growth.
2. Material and methods
The spotless starling is a close relative of the more frequently studied European starling (Sturnus vulgaris). It is a medium-sized, facultative polygamous passerine that nests opportunistically in tree holes and buildings. The species can be highly colonial, although solitary nesters also exist. Most females produce two broods per season, and males vary considerably in the amount of parental care they provide (Moreno et al. 1999; Veiga et al. 2001). In our study area, food availability decreases as the season advances (i.e. from first to second broods), and nestling mass decreases with date, with frequent starvation, nest abandonment and brood reduction events in the last weeks of the season (D. Gil et al. 2006, unpublished data). Thus, we can use date as a surrogate of nutritional stress in this population (see §3 for a test of this assumption).
We conducted this study in an open woodland (dehesa) of oak (Quercus pyrenaica) and ash (Fraxinus angustifolius) with scattered areas of pasture land, in Soto del Real (Madrid, Spain) in 2004 and 2005. This woodland is used for cattle grazing, and farmers keep the soil well watered in spring by diverting the course of several brooks into the woodland. Since 2001 we have studied a breeding population of starlings that nests in a total of 250 nest-boxes (L×W×H: 18×18×40 cm), which are set up at varying densities throughout the wood.
(a) Cross-fostering experiment
The cross-fostering experiment was conducted in 2005 in 84 nest-boxes different from those which provided the observational data (see below). The protocol was as follows: when nestlings were 2 days old, we chose two similar-sized nestlings from each of 28 different nests (source nests) to be cross fostered to nests of adoption. Nests of adoption consisted of two types: (i) control nests, in which nestlings were of similar age, mass and size to cross-fostered nestlings and (ii) experimental nests, in which nestlings were 1–2 days older, heavier and larger than the cross-fostered nestlings (mass of experimental nests (s.e.)=21.32 (0.81) versus control nests=8.66 (0.58); t28=16.57, p<0.001). The difference in mass between cross-fostered nestlings and nestlings in the adoption nest in experimental conditions (12.6 g) corresponds to 2 s.d. over the mean of within-nest mass differences found in natural nests, caused by hatching asynchrony (in a sample of 56 non-manipulated nests, the average mass difference between the lighter and the heavier nestling at 2 days of age was 4.2 g (s.d.=3.8, range: 0.15–21.5 g)). Each dyad of nestlings was thus split by taking one nestling to a nest with similar-sized chicks (control), and the other to a nest with bigger chicks (experimental). There were no differences in brood size between control and experimental groups (t52=0.68, p=0.49; mean (s.d.): control: 3.59 (0.97), experimental: 3.40 (1.03)). We predicted that focal chicks would suffer higher levels of sibling competition in the experimental nest than in the control (Skagen 1987).
All nestlings were weighed and measured at 6 and 14 days of age. We weighed nestlings to the nearest 0.1 g with a digital balance (Ohaus Scout SC2020, NJ, USA), and we measured wing length, tarsus length, bill length and gape width with digital callipers (Mitutoyo, Japan) to the nearest 0.1 mm. Wing length was recorded as the distance between the humerus–femural joint and the tip of the top phalange. Tarsus length was measured by holding the nestling's fingers folded against the tarsus, and taking the full distance between the outermost bent of the fingers and the tibia–tarsus joint (held at 90° with respect to the tibia). Bill length was defined as the distance between the tip of the bill and the proximal end of the narines, and was measured by gently inserting the calliper's pointed probe in the narines and sliding the callipers until the bill's tip was reached. Gape width was measured by gently holding the head of the bird horizontally and sliding the callipers to encompass the full gape width, that is, the fleshy and conspicuously yellow flange at both sides of the bill (see Clark (1995) for an illustration).
(b) Correlative study
For the observational data, we followed egg laying and incubation in a total of 77 broods in 2006. Nests were visited daily near the predicted hatching date, which we recorded with a precision of 1 day. Measurements were taken at day 14 only. In this dataset we measured both gape and head widths, and subtracted both values so as to obtain a measurement of gape width that was not affected by head size, called hereafter flange width. Considering that brood hatching date refers to that of the first nestling to hatch in a given nest, and that hatching asynchrony is common in this species, we avoided the use of nest averages, since this would be affected by the variance in age in the brood. Instead, we used data from the heaviest nestling in each nest, which is typically the first to be hatched (Skagen 1987), and was thus the only nestling for which age was certain.
(c) Repeatability of measurements
Repeatability estimates were very high, as shown by intra-class correlation coefficients (ri) calculated from two different measurements of the same bird taken with an interval of several minutes between them (p-values correspond to ANOVA's F (d.f.=8, 11): body mass: ri=0.784, p<0.01; wing length: ri=0.913, p<0.001; tarsus length: ri=0.834, p<0.001; bill length: ri=0.979, p<0.001; flange width: ri=0.862, p<0.001 and head width: ri=0.773, p<0.01).
(d) Statistics
Most of the structures that we measured were highly correlated with each other, and this collinearity prevented their joint inclusion in MANOVA (Quinn & Keough 2002). The correlation matrices for all traits in the experimental and observational datasets showed that all traits except gape width were on the whole positively correlated with each other (tables 1 and 2). For 6-day-old nestlings, the correlation of gape with the other traits was much smaller than that of the rest of traits. At the age of 14 years, gape size was actually negatively related to several of the other traits. Thus, we performed principal component analyses (PCA) on the entire dataset using normalized values of body mass, wing length, tarsus length and bill length in order to obtain a composite index of body size. The reason we dropped gape width from the PCA is that its contribution to the PC1 was negative, thus making it impossible to compare the response of body size versus gape width to the treatment. The first principal component of the analysis conducted for the experimental data explained 87.8% of the total variance of and was positively loaded by all variables (all loadings greater than 0.96). In the case of the correlative study, PC1 accounted for 69.7% of the variance and was equally loaded by all variables (all loadings greater than 0.85). These first principal components will be referred to as ‘overall size’ in the rest of the text.
Table 1.
Pearson's correlations among measured nestling traits at 6 and 14 days of age in the cross-fostering experiment. (Probability values are *p<0.05; **p<0.01; n.s., non-significant (N=56 (age 6), N=54 (age 14)).)
| age 6 | body mass | tarsus l | wing l | bill l |
|---|---|---|---|---|
| tarsus length | 0.877** | |||
| wing length | 0.827** | 0.918** | ||
| bill length | 0.539** | 0.671** | 0.684** | |
| gape width | 0.335* | 0.348* | 0.167 | 0.215 |
| age 14 | body mass | tarsus l | wing l | bill l |
|---|---|---|---|---|
| tarsus length | 0.240n.s. | |||
| wing length | 0.603** | 0.352** | ||
| bill length | 0.466** | 0.179n.s. | 0.576** | |
| gape width | −0.127n.s. | −0.198n.s. | −0.486** | −0.279* |
Table 2.
Pearson's correlations among measured nestling traits in the correlative study. (Probability values are *p<0.05; **p<0.01; n.s., non-significant (N=77).)
| body mass | tarsus l | wing l | bill l | |
|---|---|---|---|---|
| tarsus length | 0.581** | |||
| wing length | 0.502** | 0.583** | ||
| bill length | 0.607** | 0.590** | 0.711** | |
| gape width | −0.221n.s. | −0.075n.s. | −0.218n.s. | −0.270* |
Since there was variance in mass and size on the day of manipulation (day 2) for the experimental data, and these values significantly affected all measures at later ages, we included in the analysis overall size at manipulation to account for these initial differences.
All data were checked for normality and transformed (log) when necessary. A further test of normality was performed on the residuals of the model, which in all cases were found not to deviate from the normality. Body mass was cube root transformed to allow a direct comparison with linear measurements (Clark 1995). We used parametric statistics throughout and the α value was set at 0.05. Analyses were performed on SPSS v. 11.5 and SAS v. 8.
3. Results
(a) Cross-fostering experiment
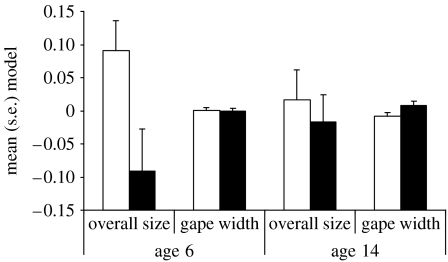
To test the effect of the experimental treatment on development, we ran a repeated measures MANOVA. This model examined the effect of the treatment in two traits (overall size and gape width) at the two ages at which they were measured. The model included overall size at manipulation as a covariate and the adoption nest as a random effect. The results show that the experimental treatment did not affect overall development uniformly (response×treatment interaction: Wilks' λ=0.87, F2.22=1.61, p=0.22). However, the interaction of response×age×treatment was significant (Wilks' λ=0.74, F2,22=3.68, p<0.05), showing that gape width and overall size responded to the treatment in different ways at the two ages when these were measured. The nature of the interaction can be appreciated in figure 1: 4 days after manipulation (at day 6), nestlings in experimental broods had a smaller overall size than nestlings in control broods, confirming the success of the manipulation. In striking contrast, gape size was not affected by the experimental treatment and was similar in the two groups. However, at day 14, experimental birds had compensated for the initial handicap, and overall size was no longer different between treatments. In marked contrast to overall size, gape width was larger in experimental than in control birds at this age.
Figure 1.
Means (s.e.) for overall size and gape width for control (open bars) and experimental (filled bars) nestlings at 6 and 14 days of age.
(b) Correlative study
In the unmanipulated sample size, we used hatching date as a surrogate of feeding conditions. Owing to diminishing food availability, average nestling mass decreased with date, while within-nest variation in nestling mass increased (data for broods of brood size greater than 1; mean brood mass: F1,75=239.07, p<0.001, slope=−0.11 (0.001), r2=0.76; within brood mass CV: F1,75=15.63, p<0.001, slope=0.03 (0.007), r2=0.16). This effect was not exclusively due to the differences in parental quality, since most pairs produce two full clutches, and a pairwise comparison of first versus second broods produced by the same parents revealed a dramatic decrease in nestling mass at 15 days of age (paired t-test: t=8.81, d.f.=15, p<0.001; means (s.d.): first broods: 81.1 (3.1) and second broods: 59.6 (8.1)). We thus use date as a surrogate of the strength of sibling competition in this species.
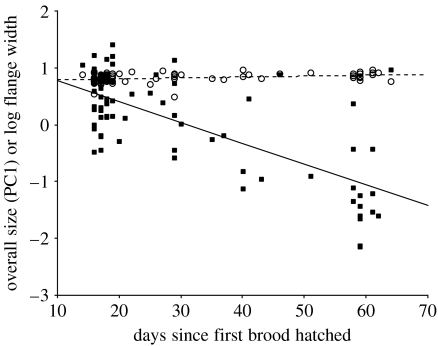
We included overall size and flange width in a multiple analysis of variance, with hatching date as a predictor and nest as a random factor. The analysis revealed that hatching date had a strong effect on these traits (Wilks' λ=0.29, F2.26=31.3, p<0.001). However, the slopes of the two traits with respect to date differed greatly: while overall size decreased with date (B=−0.033 (s.e.=0.004)), the flange width increased (B=0.012 (s.e.=0.003). The two slopes were very different to one another (t=−9.02, d.f.=73; p<0.001; figure 2).
Figure 2.
Relationship between overall body size (PC1, filled squares) and flange width (open circles) with date. Lines represent linear regressions (PC1, solid line and flange width, dotted line). See §3 for statistics.
4. Discussion
Sibling competition is a key factor in the evolution of life history, behaviour and physiology in a wide variety of organisms in which siblings compete for resources (Trivers 1974; Mock & Parker 1997). Growth rates are higher in species in which extra-pair paternity is common (Royle et al. 1999), suggesting that sibling competition has promoted the evolution of high growth rates (Werschkul & Jackson 1979). We tested whether intense sibling competition levels have an influence on the differential growth of traits that are used to obtain food from parents. Since altricial birds use their gapes to signal their need to parents, we predicted that this trait should increase under elevated levels of sibling competition. We found that, 4 days after manipulation, nestlings that were cross fostered to broods with larger nest mates maintained the growth of their gapes. This contrasted markedly with overall body size, which grew less due to increased sibling competition. In the second period of growth, until day 14, we found that nestlings in the experimental group had compensated their overall body size growth, and that gape width was larger than in the control group. These data suggest that birds that are subjected to high levels of sibling competition increase the size of their gapes in order to obtain a largest share of food than their nest mates (O'Connor 1978). Confirming these results, our correlative study, which used hatching date as a surrogate for food availability, showed that although body size was notably reduced in broods that hatched late in the season, flange width remained unaffected (it actually increased).
Alternatively, since the brain is one of the organs of highest buffering against nutritional stress, it has been suggested by some authors (Caccamise 1980; Gille & Salomon 1999) that the large development of gape at hatching could respond to developmental constraints for a large skull rather than to the need for a large feeding apparatus (Ricklefs 1967; Dunn 1975; O'Connor 1978). Since gape width was measured in our experiment as the total width between the extremes of the rectal flanges, thus encompassing skull width, it could be argued that our findings could be explained by the skull's developmental buffering rather than by a special development of the gape width. However, although this constraint may explain the low lability of gape width under high levels of sibling competition at day 6, it is unlikely to explain the presence of larger gapes found at day 14. Furthermore, in our observational study we measured flange width independently of skull width, and the results support the experimental data, showing increased flange growth in response to increased competition for food.
Although the results of our study suggest that birds under high levels of sibling competition develop larger gapes than controls birds, an alternative explanation would also fit the data. This is because gape width grows to a maximum around the middle of the nestling period, and then decreases until it is completely reabsorbed some days after fledging (O'Connor 1978). Since we did not take daily measurements of the gapes of experimental nestlings, the larger gapes that we found at day 14 could actually be unregressed gapes that had maintained their maximum size for longer. This would imply delayed maturation of this character rather than increased growth. However, regardless of the mechanism, our data show that this trait shows adaptive developmental plasticity in situations of increased sibling competition.
The observed patterns are most likely to be explained by strong selection against nestlings showing smaller (i.e. less conspicuous) signals of need in situations of intense sibling competition. Although to our knowledge no study so far has manipulated gape width, many studies have shown that parents preferentially feed nestlings that display open gapes to them (Ryden & Bengtsson 1980) and that gape colour modifications can alter the number of feedings received (Kilner 1997; Jourdie et al. 2004). Therefore, any nestling with a small gape would be perceived by parents to be less motivated than its fellow nestlings and, as a result, receive less food. All other factors being equal, we would expect a positive correlation between gape size and the amount of food received by a nestling when sibling competition is intense. Additional evidence for a role of gapes in sibling competition comes from a recent study in which nestlings hatching from androgen-injected eggs were found to show wider gapes than controls (Mu¨ller et al. 2007), a similar mechanism to the increased neck muscle development that yolk androgens has been shown to promote (Lipar et al. 2000).
If wide gapes are beneficial to nestlings, why are they not maintained for the whole nestling period? A first possibility is that there might be some type of developmental incompatibility between growing the keratinized adult beak and maintaining the fleshy flange at the same time. Secondly, since older nestlings have more active ways of getting food from their parents, it is to be expected that the role of passive signals may decrease as nestlings get older.
Nutritional conditions during growth have a major role in determining the fitness of individuals, and research has shown that they may affect morphology, behaviour, life-history strategies and physiological processes later on in life (Lindström 1999; Metcalfe & Monaghan 2001). Intense sibling competition levels when resources are scarce may result in unequal sharing of resources, leading to some offspring suffering considerable levels of nutritional stress (Skagen 1987; Nilsson & Gårdmark 2001). Our study supports previous evidence showing that birds can use adaptive developmental plasticity responses as a function of environmental conditions (Zach 1982; Kristan et al. 1996; Nilsson & Svensson 1996; Brzek & Konarzewski 2001; Nilsson & Gårdmark 2001).
Although nestlings may use behavioural and physiological adaptations to compensate inequalities caused by a limited food supply (Emlen et al. 1991; Nilsson & Svensson 1996; Brzek & Konarzewski 2001; Naguib et al. 2004; this study), evidence shows that growth deficiencies in response to malnutrition may bring about a multitude of costs in adult life (Richner 1989). In the particular case of gape width, a structure that is determined by the growth of fleshy flanges on the rectal regions of the bill, we can speculate that the growth of larger gapes or a retardation of flange reabsorption might induce changes in the overall structure of the bill shape in the adult bird. This could have important implications for feeding adaptations (Smith et al. 1978), and we would expect that deviations from optimal bill shapes caused by nutritional stress could lead to changes in food choice in adult birds. Although this might have little influence on fitness in the omnivorous spotless starling (Peris 1980), specialized seed eaters such as finches could encounter higher selection pressures that might limit the effectiveness of such an adaptation.
Acknowledgments
The study complied with the legal requirements of Spanish law for experimentation with animals (Ministerio de Educacio´n y Ciencia) and for working with wild bird species (Consejería de Medio Ambiente, CAM).
This study was financed by a research grant (BOS-2002-00105) from the Spanish Ministry of Science and Education (MEC). D.G. was supported by a Ramón y Cajal Fellowship and E.B. by a PhD grant, both from MEC. P.C. and I.L.-R. are recipients of PhD grants from CONACYT (Mexico). We are grateful to the Council of Soto del Real and to the Environment Department of the Autonomous Community of Madrid for allowing access to the study site. Three anonymous referees provided excellent advice. We would also like to thank our statistical advisor, Pepe Benavent, for his time and patience.
Supplementary Material
Alternative analysis of the experimental data
References
- Brzek P, Konarzewski M. Effect of food shortage on the physiology and competitive abilities of sand martin (Riparia riparia) nestlings. J. Exp. Biol. 2001;204:3065–3074. doi: 10.1242/jeb.204.17.3065. [DOI] [PubMed] [Google Scholar]
- Caccamise D.F. Growth and development of major body components in the Monk Parakeet. Wils. Bull. 1980;92:376–381. [Google Scholar]
- Clark A.B. Gapes of sexually dimorphic blackbird nestlings do not show sexually dimorphic growth. Auk. 1995;112:364–374. [Google Scholar]
- Davies N.B, Kilner R.M, Noble D.G. Nestling cuckoos Cuculus canorus, exploit hosts with begging signals that mimic a brood. Proc. R. Soc. B. 1998;265:673–678. doi:10.1098/rspb.1998.0346 [Google Scholar]
- Dunn E.H. Growth, body components and energy of nestling double-crested cormorants. Condor. 1975;77:431–438. doi:10.2307/1366090 [Google Scholar]
- Emlen S.T, Wrege P.H, Demong N.J, Hegner R.E. Flexible growth rates in nestling white fronted bee-eaters: a possible adaptation to short-term food shortage. Condor. 1991;93:591–597. doi:10.2307/1368191 [Google Scholar]
- Gille U, Salomon F.V. Growth of duck bills. Condor. 1999;101:710–713. doi:10.2307/1370207 [Google Scholar]
- Jourdie V, Moureau B, Bennett A.T.D, Heeb P. Ultraviolet reflectance by the skin of nestlings. Nature. 2004;431:262. doi: 10.1038/431262a. doi:10.1038/431262a [DOI] [PubMed] [Google Scholar]
- Kilner R. Mouth colour is a reliable signal of need in begging canary nestlings. Proc. R. Soc. B. 1997;264:963–968. doi:10.1098/rspb.1997.0133 [Google Scholar]
- Kristan D.M, Gutiérrez R.J, Franklin A.B. Adaptive significance of growth patterns in juvenile spotted owls. Can. J. Zool. 1996;74:1882–1886. [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. doi:10.1016/S0169-5347(99)01639-0 [DOI] [PubMed] [Google Scholar]
- Lipar J.L, Ketterson E.D. Maternally derived yolk testosterone enhances the development of the hatching muscle in the red-winged blackbird Agelaius phoeniceus. Proc. R. Soc. B. 2000;267:2005–2010. doi: 10.1098/rspb.2000.1242. doi:10.1098/rspb.2000.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe N.B, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. doi:10.1016/S0169-5347(01)02124-3 [DOI] [PubMed] [Google Scholar]
- Mock D.W, Parker G.A. Oxford University Press; Oxford, UK: 1997. The evolution of sibling rivalry. [Google Scholar]
- Moreno J, Veiga J.P, Cordero P.J, Minguez E. Effects of paternal care on reproductive success in the polygynous spotless starling Sturnus unicolor. Behav. Ecol. Sociobiol. 1999;47:47–53. doi:10.1007/s002650050648 [Google Scholar]
- Mu¨ller W, Deptuch K, Lo´pez-Rull I, Gil D. Elevated yolk androgen levels benefit offspring development in a between-clutch context. Behav. Ecol. 2007;18:929–936. doi:10.1093/beheco/arm060 [Google Scholar]
- Naguib M, Riebel K, Marzal A, Gil D. Nestling immunocompetence and testosterone covary with brood size in a songbird. Proc. R. Soc. B. 2004;271:833–838. doi: 10.1098/rspb.2003.2673. doi:10.1098/rspb.2003.2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J.-Å, Gårdmark A. Sibling competition affects individual growth strategies in marsh tit Parus palustris, nestlings. Anim. Behav. 2001;61:357–365. doi:10.1006/anbe.2000.1602 [Google Scholar]
- Nilsson J.-Å, Svensson M. Sibling competition affects nestling growth strategies in marsh tits. J. Anim. Ecol. 1996;65:825–836. doi:10.2307/5680 [Google Scholar]
- O'Connor R.J. Growth strategies in nestling passerines. Living Bird. 1977;16:209–238. [Google Scholar]
- O'Connor R.J. Differential growth and body composition in altricial passerines. Ibis. 1978;119:147–166. [Google Scholar]
- O'Connor R.J. Wiley; Chichester, UK: 1984. The growth and development of birds. [Google Scholar]
- Ortega C.P, Cruz A. Differential growth patterns of nestling brown-headed cowbirds and yellow-headed blackbirds. Auk. 1992;109:368–376. [Google Scholar]
- Peris S.J. Biología del estornino negro (Sturnus unicolor Temm.): 1. Alimentación y variación de la dieta. Ardeola. 1980;25:207–240. [Google Scholar]
- Quinn G.P, Keough M.J. Cambridge University Press; Cambridge, UK: 2002. Experimental design and data analysis for biologists. [Google Scholar]
- Richner H. Habitat specific growth and fitness in carrion crows (Corvus corone corone) J. Anim. Ecol. 1989;58:427–440. doi:10.2307/4840 [Google Scholar]
- Ricklefs R.E. Relative growth, body constituents, and energy content of nestling barn swallows and red-winged blackbirds. Auk. 1967;84:560–570. [Google Scholar]
- Ricklefs R.E. Some considerations on sibling competition and avian growth rates. Auk. 1982;99:141–147. [Google Scholar]
- Ricklefs R.E. Sibling competition, hatching asynchrony, incubation period and lifespan in altricial birds. Curr. Ornithol. 1993;11:199–276. [Google Scholar]
- Royle N.J, Hartley I.R, Owens I.P.F, Parker G.A. Sibling competition and the evolution of growth rates in birds. Proc. R. Soc. B. 1999;266:923–932. doi:10.1098/rspb.1999.0725 [Google Scholar]
- Ryden O, Bengtsson H. Differential begging and locomotory behavior by early and late hatched nestlings affecting the distribution of food in asynchronously hatched broods of altricial birds. Z. Tierpsychol. 1980;53:209–224. [Google Scholar]
- Saino N, Ninni P, Calza S, Martinelli R, DeBernardi F, Møller A.P. Better red than dead: carotenoid-based mouth coloration reveals infection in barn swallow nestlings. Proc. R. Soc. B. 2000;267:57–61. doi: 10.1098/rspb.2000.0966. doi:10.1098/rspb.2000.0966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schew W.A, Ricklefs R.E. Developmental plasticity. In: Starck J.M, Ricklefs R.E, editors. Avian growth and development. Oxford University Press; New York, NY: 1998. pp. 288–304. [Google Scholar]
- Skagen S.K. Hatching asynchrony in American goldfinches—an experimental study. Ecology. 1987;68:1747–1759. doi: 10.2307/1939866. doi:10.2307/1939866 [DOI] [PubMed] [Google Scholar]
- Smith J.N.M, Grant P.R, Grant B.R, Abbott I, Abbott L.K. Seasonal variation in feeding habits of Darwin's ground finches. Ecology. 1978;59:1137–1150. doi:10.2307/1938228 [Google Scholar]
- Tanaka K.D, Ueda K. Horsfield's hawk-cuckoo nestlings simulate multiple gapes for begging. Science. 2005;308:653. doi: 10.1126/science.1109957. doi:10.1126/science.1109957 [DOI] [PubMed] [Google Scholar]
- Trivers R.L. Parent–offspring conflict. Am. Zool. 1974;14:249–264. [Google Scholar]
- Veiga J.P, Moreno J, Cordero P.J, Minguez E. Territory size and polygyny in the spotless starling: resource-holding potential or social inertia? Can. J. Zool. 2001;79:1951–1956. doi:10.1139/cjz-79-11-1951 [Google Scholar]
- Werschkul D.F, Jackson J.A. Sibling competition and avian growth rates. Ibis. 1979;121:97–102. [Google Scholar]
- Zach R. Hatching asynchrony, egg size, growth, and fledging in tree swallows. Auk. 1982;99:695–700. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alternative analysis of the experimental data