Abstract
Acquired immunity is known to be a key modulator of the dynamics of many helminth parasites in domestic and human host populations, but its relative importance in natural populations is more controversial. A detailed long-term dataset on the gastrointestinal nematode Trichostrongylus retortaeformis in a wild population of European rabbits (Oryctolagus cuniculus) shows clear evidence of seasonal acquired immunity in the age-structured infection profiles. By fitting a hierarchy of demographic infection–immunity models to the observed age-structured infection patterns, we are able to quantify the importance of different components (seasonality, immunity and host age structure) of the parasite dynamics. We find strong evidence that the hosts' immunocompetence waxes and wanes with the seasons, but also contains a lifelong cohort factor, possibly acting through a maternal effect dependent on the host's month of birth. These observations have important and broad implications for the ecology of parasite infection in seasonal natural herbivore systems.
Keywords: parasite, seasonality, acquired immunity, mathematical modelling, Trichostrongylus retortaeformis, Oryctolagus cuniculus
1. Introduction
The rate at which a helminth parasite infects a host reflects the interaction between the host's exposure to infective stages and its susceptibility to the infective stages it encounters. The host exposure is primarily influenced by the local abundance of infectious stages, which varies seasonally with the rates of production and the extrinsic development and survival of free-living stages (Anderson 2000). Host susceptibility, on the other hand, is affected by intrinsic physiological and immunological components that depend on host characteristics like sex, health condition and reproductive status. Vertebrates often mount a significant immune response to helminth parasites, and this is found to be an important determinant of the epidemiology in human and animal hosts (Anderson & May 1991; Grenfell et al. 1995a; Lloyd 1995). Moreover, immune-mediated resistance is thought to have shaped the coevolutionary history of host–parasite relationships in natural populations (Dwyer et al. 1990; Boots & Bowers 2004). However, very little is known about the importance of immunity as a modulator of infection during the host's lifetime in natural populations. Indeed, standard models of parasite infection in wild populations typically neglect the immune response (Anderson & May 1978; May & Anderson 1978; Rosà et al. 2003) and focus on the density-dependent parasite effects on the host's mortality and fecundity, rather than the host's acquisition of immunity (Roberts 1995).
Different immunological responses are expected to generate distinct patterns in the relationship between host age and parasite intensity (Woolhouse 1992). In particular, the acquired immunity that results from repeated exposure to the parasite-infective stages should lead to a humpbacked age–intensity curve, where parasite intensity initially increases in young individuals, peaks at a certain host age and then decreases as the hosts get older and resistant to further infection (Anderson & May 1985; Woolhouse 1992; Hudson & Dobson 1995). An increased force of infection (i.e. abundance of infectious larvae) will cause both a more rapid accumulation of parasites and an accelerated acquisition of immunity, resulting in a higher peak of infection shifted towards the youngest hosts (Woolhouse 1998). This produces a negative relationship between host age and parasite intensity at the peak of infection, a phenomenon known as the ‘peak shift’ effect (Woolhouse 1998). A shift in the age at the peak of infection with a change in rate of exposure distinguishes an acquired immune response from one that simply strengthens with the host's age, or from the possibility that the humpbacked age–intensity profile is produced by parasite-induced host mortality (Cattadori et al. 2005).
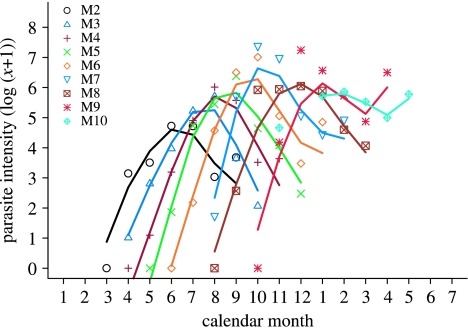
We have recently analysed a particularly rich, long-term dataset on a nematode parasite (Trichostrongylus retortaeformis) and its common host, the European rabbit (Oryctolagus cuniculus), from a free-living age-structured population in Scotland (Boag et al. 2001). We found there to be strong seasonal variability in the abundance of both host and parasite (Cattadori et al. 2005). Cohorts of rabbits born in different months show the characteristic humpbacked relationship between parasite intensity and host age (figure 1). In our rabbit–nematode system, it is apparent that the force of infection varies throughout the year, such that rabbits born in different months experience different infection rates, and while the age–intensity profiles have similar shape they differ in the peak intensity (Cattadori et al. 2005). When comparing the age–intensity relationship of different cohorts we find a significant peak shift effect, giving good grounds to suppose that wild rabbits mount an acquired immune response to T. retortaeformis infection and that the strength of the response depends on the individual's history of exposure (Cattadori et al. 2005).
Figure 1.
The raw age–intensity data (mean of log (number of parasites+1)) from each cohort where the key identifies the month of birth of each cohort (M2=born in February, etc.). Adapted from Cattadori et al. (2005).
While the existence of an acquired immune response can be inferred from the qualitative properties of the age–intensity profiles, in the absence of immunological data we need explicit mechanistic modelling to understand how the immune response is influenced by ecological and physiological factors. Since the intensity of an individual's infection is the result of nonlinear interactions between confounding processes, such as history of infection, immunity and parasite mortality, we need to tease apart the role of each component in order to identify the major forces driving the host–parasite interaction. There is a distinguished body of mathematical models for different vertebrate–nematode systems with varying levels of complexity (for a review, see Cornell 2005). These models expose the many nuances of how parasite intensity depends on exposure and immunity. Much effort has been spent in estimating parameters for these models in domestic hosts (Kao et al. 2000), but their validity in wild hosts has received little attention (Grenfell et al. (1995b) being a notable exception). This is partly due to the lack, until now, of a suitable long-term ecological dataset.
In this study, we use the T. retortaeformis–rabbit system to parametrize and test models of how acquired immunity develops to a gastrointestinal infection in a seasonal environment. We first develop a hierarchy of explicit demographic models that capture the various seasonal components of the host–parasite interaction. These include seasonality in the force of infection and in the development of immunity, and we examine how this can vary with host cohort. Second, we use a maximum-likelihood framework to fit these models to the individual-based data on parasite intensities in wild rabbits of different ages and cohorts. This allows us to use information theoretic criteria (Burnham & Anderson 1998) to compare the merit of the various models, and hence identify the most probable factors affecting the host–parasite interaction. The modelling gives strong evidence for variations of the immune response between individuals and over time, which have direct interpretations in terms of the ecology of the rabbit–nematode interaction.
2. Material and methods
(a) The system and data
This study is based on the long-term data of a wild European rabbit population and its common gastrointestinal parasite T. retortaeformis, a nematode with a direct life cycle. Free-living infective larval stages are ingested through feeding and in a few hours settle in the small intestine where they develop into mature adult worms that reproduce sexually; eggs are expelled through the host's faeces and shed into the environment approximately two weeks after the initial infection (Audebert et al. 2002). The survival and development of the free-living stages is strongly affected by temperature and humidity (Soulsby 1982; Anderson 2000). In general, when temperature is below 10°C development is arrested or very slow, and migration of infective larvae onto vegetation cease below 2.7°C and at low humidity (Crofton 1948). Owing to this, seasonal climatic fluctuations greatly influence the abundance of larval infective stages on the pasture (Cattadori et al. 2005, 2007a).
Rabbits were shot monthly with a .22 rifle, while walking transects across a multi-warren population in a 400 ha area in eastern Scotland from 1977 to 2002 (in Great Britain this is an approved humane way to control this pest species). For each individual, the infection intensity, i.e. the total number of adult T. retortaeformis, was recorded, as well as a number of host characteristics, such as sex, body mass, breeding status and co-infections (Boag et al. 2001). Our recent studies on the interactions between the most common parasites and pathogens in this rabbit population suggested that T. retortaeformis intensity is strongly affected by the immunosuppressive myxoma virus and probably weakly affected by the stomach nematode Graphidium strigosum (Cattadori et al. 2007a,b). To simplify the present study, we removed rabbits co-infected with myxoma or Graphidium from the dataset. Previous studies on the monthly changes in T. retortaeformis intensity with rabbit age found strong seasonal patterns in both host reproduction and parasite force of infection (Cattadori et al. 2005). In this rabbit population, the main reproductive season occurs between April and July. The newborns spend most of their first month of life in the burrows feeding from their mothers and by the second month they emerge from the burrows and are then exposed to the infective stages through feeding on the parasite-contaminated pasture. Host body mass was used to estimate the date of birth of each individual according to Cattadori et al. (2005), and thus reconstruct the age structure of the rabbit population. For the purpose of presentation, individuals are grouped into nine cohorts by month of birth (February to October).
(b) Immuno-epidemiological models
The humpbacked age–intensity relationships (figure 1), together with the inverse correlation between peak parasite intensity and age of peak infection (Cattadori et al. 2005), are qualitatively consistent with the standard autocatalytic model that assumes that the hosts' acquired immunity increases in proportion to the cumulative exposure to the parasite (Woolhouse 1992, 1998). Whenever this immune response acts to reduce the establishment of ingested infective stages, the infection rate will decay with exposure so that, with age, the average parasite burden eventually decreases as mortality and/or expulsion outweighs establishment.
Here we test whether an infection–immunity model of this type is quantitatively consistent with our data. In particular, we investigate whether and what additional biological realism needs to be incorporated in the basic model in a number of ways. Most importantly, we ask what kind of drivers might generate the observed seasonal patterns. We begin by investigating whether the data can be explained by seasonal variation in the exposure to infection alone. We then explore whether it is necessary to introduce additional seasonal modulations in the immune response. We also investigate whether the model fit is improved by adding realism to exposure rates (for example, if feeding rate depends on age).
In all of our models, we assume that the host's total immune response at any time is a function of the total number of infective larvae it has encountered multiplied by a quantity that we call ‘immunocompetence’. This immune response acts to inhibit infection. In §2c,d we give a verbal description of the hierarchy of models; appendix A in the electronic supplementary material contains a full mathematical definition of these models together with a detailed exposé of our statistical methods for estimating parameters and comparing goodness-of-fit of the different models. For brevity, we shall sometimes denote each model component by a letter, as defined in the legend to table 1. Our seasonal age–intensity models are expressed as differential equations, and use continuous variables for time (in months), age and date of birth of the rabbits. However, the models need to be solved by numerical integration, for which we used a fixed time step of 0.001. When presenting our results and data we aggregate time, age and cohort by discrete months.
Table 1.
Maximum log likelihood and Akaike information criterion scores relative to the best-fitting model (ΔAIC) for different models. (The three models with strong support (ΔAIC<2) are marked in bold face with a double asterisk in the final column; the six models with medium support (2<ΔAIC<3) are marked in italics with a single asterisk in the final column. Letters in the first eight columns denote whether that component is present. The model components are: G, feeding rate depends on age; F, seasonal force of infection; H, second harmonic (six-monthly) component in force of infection; P, phase modulated force of infection; I, age-independent component to acquired immunity; A, age-dependent component to acquired immunity; S, seasonal factor in acquired immunity; C, acquired immunity depends on month of birth. Not all of the 72 possible model combinations are shown; no unlisted model has a ΔAIC of less than 12.66.)
| G | F | H | P | I | A | S | C | log likelihood | no. params | ΔAIC |
|---|---|---|---|---|---|---|---|---|---|---|
| G | I | −18108.88 | 5 | 146.7 | ||||||
| G | F | I | −18064.30 | 7 | 61.54 | |||||
| G | F | H | I | −18060.32 | 9 | 57.58 | ||||
| G | F | P | I | −18058.63 | 9 | 54.20 | ||||
| F | P | I | −18058.63 | 8 | 52.20 | |||||
| G | F | I | A | −18053.66 | 8 | 42.26 | ||||
| G | F | I | A | C | −18045.00 | 10 | 28.94 | |||
| G | F | I | A | S | −18042.83 | 10 | 24.60 | |||
| G | F | I | A | S | C | −18029.53 | 12 | *2.00 | ||
| G | F | A | S | C | −18030.71 | 11 | *2.36 | |||
| G | F | I | S | C | −18029.53 | 11 | **0.00 | |||
| G | F | H | I | A | −18048.18 | 10 | 35.90 | |||
| G | F | H | I | A | C | −18038.71 | 12 | 20.36 | ||
| G | F | H | I | A | S | −18036.34 | 12 | 15.62 | ||
| F | H | I | A | S | C | −18038.86 | 13 | 22.66 | ||
| G | F | H | I | A | S | C | −18027.93 | 14 | *2.80 | |
| G | F | H | A | S | C | −18028.95 | 13 | *2.84 | ||
| G | F | H | I | S | C | −18027.93 | 13 | **0.80 | ||
| G | F | P | I | A | −18044.72 | 10 | 28.38 | |||
| G | F | P | I | A | C | −18036.30 | 12 | 15.54 | ||
| G | F | P | I | A | S | −18036.25 | 12 | 15.44 | ||
| F | P | I | A | S | C | −18038.92 | 13 | 22.77 | ||
| G | F | P | I | A | S | C | −18027.57 | 14 | *2.08 | |
| G | F | P | A | S | C | −18028.56 | 13 | *2.06 | ||
| G | F | P | I | S | C | −18027.57 | 13 | **0.08 |
(c) Heterogeneities in exposure
(i) Seasonal force of infection
Different cohorts of rabbits display different peak infection intensities, which reflect a different history of exposure for hosts born in different months. We therefore need to allow the force of infection to vary with time. While we do not have independent data on the abundance of the free-living infective stage on the pasture, previous analyses on the relationship between T. retortaeformis intensity and monthly climatic variables highlighted a seasonal effect of the minimum grass temperature on nematode intensity (Cattadori et al. 2005). In our simplest model, we assume that the force of infection is a sinusoidal function with period 1 year, with an amplitude and phase that are determined by fitting the predicted infection intensity to the observed data. We also investigate the possibility that the force of infection is non-sinusoidal.
(ii) Age-dependent exposure
The rate at which a host encounters infectious larvae depends not only on the abundance of larvae in the environment but also on the rate at which the host ingests herbage. Feeding rate changes with age, being lower in juveniles than in adults (Harkness & Wagner 1995), therefore we assume that the rabbits' rate of exposure to infectious larvae is an increasing function of age.
(d) Heterogeneities in immunocompetence
In addition to examining components that affect the force of infection, we also consider a suite of model elaborations which may modulate the hosts' immunocompetence. In the absence of these model components, immunocompetence is assumed to be the same for all hosts at all times.
(i) Age-dependent immunity
We allow for the possibility that older animals have a stronger immune system by assuming that the immunocompetence may depend on age.
(ii) Season-dependent immunity
Several factors, such as variations in food quality and/or availability, could cause the hosts' condition—and hence the immune response—to fluctuate seasonally (Rogers et al. 1994; Veloso & Bozinovic 2000). Here, we assume that this may lead to a factor in each individual's immunocompetence that varies in a sinusoidal way through the year.
(iii) Cohort-dependent immunity
The quality of each rabbit cohort may depend on their time of birth. This might be caused by a seasonal variation in the maternal investment into foetus quality or provisioning for kittens, perhaps as a function of the number of litters produced in the lifetime or as a function of the mother's own (seasonally fluctuating) condition (Veloso & Bozinovic 2000; Côté & Festa-Bianchet 2001). Also, rabbits born at the end of the breeding season (late summer) have less time to gain weight before the winter, and may therefore be less able to compete for resources than their heavier peers born earlier in the season (Gibb et al. 1985; Côté & Festa-Bianchet 2001). We model this cohort effect by assuming that each rabbit may have a lifelong factor in its immunocompetence that varies sinusoidally with its date of birth.
3. Results
Table 1 lists the Akaike information criterion (AIC) scores obtained by a maximum-likelihood fit of a range of models. For brevity, we have not listed all of the 72 possible combinations of model components, but the hierarchical structure of our models is such that the maximum likelihood of any given model cannot be greater than that for a more general model (i.e. one whose components are a strict superset of the first). None of the unlisted models have an AIC within 12.66 of the model with the best AIC score, and they are therefore generally not supported by the data.
(a) Heterogeneities in exposure
We first consider the set of model combinations obtained by including, or omitting, age-dependent exposure and seasonal force of infection (first five lines in table 1). A seasonal force of infection (component F) significantly improves the quality of fit relative to models with a constant force of infection, and the fit further improves if the force of infection is non-sinusoidal (model components H and P). Parameters for the best-fitting model in this class are given in table 2 under column ‘GFPI’; here, age-dependent exposure (component G) does not improve the quality of fit when the force of infection is seasonal, as seen by the fitted value of γ being close to 0.
Table 2.
Maximum-likelihood values of the parameters for four models. (The first model (GFPI) is the best-fitting model where immunocompetence is constant; the other three are the models with ‘strong’ support from the data. ‘FOI’ is an abbreviation of ‘force of infection’. Letters denoting model components are the same as given in the table caption 1.)
| parameter | symbol | GFPI | GFISC | GFHISC | GFPISC |
|---|---|---|---|---|---|
| weight-feeding rate exponent | γ | 1.45×10−6 | 1.59 | 1.56 | 1.55 |
| feeding rate proportionality constant | ϕ0 | 8.6×103 | 1.0×104 | 9.9×103 | 1.4×104 |
| amplitude of sinusoidal yearly variation in FOI | f1 | 0.99 | 0.77 | 0.92 | 0.78 |
| phase of sinusoidal yearly variation in FOI | θ1 | 10.36 | 9.21 | 8.96 | 8.92 |
| amplitude of sinusoidal six-monthly variation in FOI | f2 | — | — | 0.24 | — |
| phase of sinusoidal six-monthly variation in FOI | θ2 | — | — | 4.56 | — |
| amplitude of phase modulation in FOI | fP | 0.97 | — | — | 0.72 |
| phase of phase modulation in FOI | θp | 1.84 | — | — | 0.34 |
| component of immunity independent of age | β0 | 9.32 | 9.1×10−4 | 8.1×10−4 | 7.8×10−4 |
| component of immunity proportional to age | β1 | — | — | — | — |
| amplitude of seasonally varying immunity | β1T | — | 0.76 | 0.73 | 0.72 |
| phase of seasonally varying immunity | ψT | — | 2.66 | 2.78 | 2.80 |
| amplitude of cohort-dependent immunity | β1C | — | 0.87 | 0.88 | 0.88 |
| phase of cohort-dependent immunity | ψC | — | 3.54 | 3.46 | 3.43 |
| mortality of adult worms within the host | μ | 0.10 | 0.43 | 0.48 | 0.49 |
| negative binomial shape parameter | k | 0.33 | 0.33 | 0.33 | 0.33 |
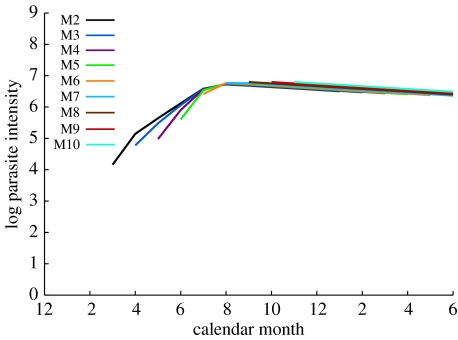
However, the age–intensity profiles for this model, shown in figure 2, correspond very poorly to the patterns in the real data (figure 1). Specifically, the height of the peak infection intensity is much less variable in the model than in the data, and the rate of decay after the peak is much slower. The reason for the low variability in model peak infection intensity is that hosts are assumed to acquire immunity at the same rate that they ingest larvae, so an increase in the force of infection will result in the host becoming protected at an earlier age. This weakens the relationship between the peak infection intensity and the force of infection (for example, in the mathematical model of Woolhouse 1998 the peak varies only logarithmically with the force of infection when the latter is large), making it very difficult to produce a highly variable peak infection intensity by modulating the force of infection alone. This is compounded by the fact that host cohorts overlap in time, so the force of infection cannot be varied independently for each cohort.
Figure 2.
Fit of best model where immunocompetence is constant (model GFPI).
(b) Heterogeneities in immunocompetence
Since modulation of the force of infection cannot by itself account for the observed patterns of parasite intensity, we consider the effect of allowing immunocompetence to differ between individuals and over time. We find that adding any of the three components A, S and C—which correspond, respectively, to an immunocompetence that varies with host age, current month and month of birth—significantly improves the quality of fit. Adding combinations of these model components improves the fit still further, and in some instances the improvement is greater than adding the components individually. For instance, adding component S or C to model GFIA improves the AIC score by 17.66 and 13.32, respectively, but adding both improves the AIC by 40.26. We also find that component G (age-dependent feeding rate)—which did not improve the fit in combination solely with other heterogeneities in exposure—does significantly improve the quality of fit in combination with heterogeneities in immunocompetence, as seen for instance by the AIC score of FHIASC being 19.86 higher than GFHIASC.
When all possible model combinations are considered, we find that they fall into three distinct groups (see table 1): models that fit the data very well, with AIC scores within 0.8 of the best-fitting model (showing ‘strong’ support from the data); those with AIC scores of between 2 and 3 of the best model (showing ‘some support’ from the data); and those with AIC scores differing by 12 or more from the best model (showing ‘no support’). The AIC favours parsimony by penalizing models with many parameters in a particular way, but the differences in likelihood between these groups of model is so great that we are confident that our conclusions are not sensitive to the details of how this penalty is implemented (for example, we find that the model with the best AIC also has the lowest Bayes information criterion).
The models that show ‘strong’ support all have both season- and cohort-dependent immunity, as well as seasonal force of infection, age-dependent exposure and age-independent immunocompetence. The use of a non-sinusoidal force of infection improves the likelihood of the fit; however, this change is not enough to compensate for the additional parameters (i.e. the AIC score in table 1 is not significantly improved by components H and P). The models with ‘some’ support differ from the best models only in whether immunocompetence is age-dependent; since an age-dependent component to the immunocompetence does not improve the likelihood of the fit, we conclude that there is no statistical evidence for age-dependent immunocompetence. The majority of nematode–host models assume that immunocompetence does not depend on age (Roberts & Grenfell 1991; Woolhouse 1992), though to our knowledge ours is the first quantitative test of this hypothesis for a wild host population.
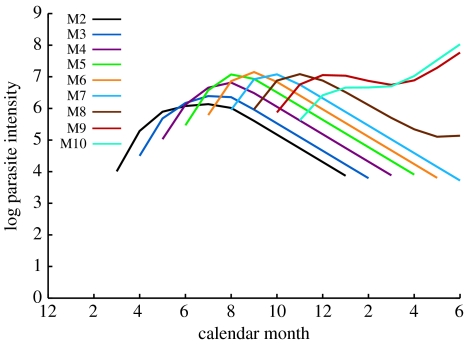
The seasonal age–intensity curves of the best-fitting model (best AIC score, model GFISC) are shown in figure 3. This model clearly mimics the data much better than the simple seasonal exposure model (figure 2). Specifically, the peaks are highly variable, and the rates of increase towards and decay away from the peak are also consistent with the data. Moreover, the model captures the second rise in intensity between April and June (months 4–6) for cohorts born in September and October (M9 and M10).
Figure 3.
Fit of the most parsimonious model where immunity depends on season and cohort (model GFISC).
There is very little variation in the estimated parameters (table 2) for the three best-fitting models (denoted by GFISC, GFHISC and GFPISC, respectively). Our estimates of the adult parasite mortality μ are in the range 0.43–0.49, suggesting that the parasites live for approximately two to three months on average. The maximum parasite lifespan is reported to be about six months (Audebert et al. 2002), so it is not unreasonable that the average lifespan would be a few months. The estimates of the parameter γ, which describes the allometric relationship between feeding rate and weight ((feeding rate)∝(weight)γ), are in the range 1.5–1.6, suggesting that rabbits' feeding rate increases rapidly with individual size. We would usually expect this exponent to be less than unity (2/3 for a von-Bertallanffy growth curve), and it may be that the high fitted value of this parameter acts as a proxy for further biological mechanism that reduces the susceptibility or infection rate of particularly young rabbits, such as maternal antibodies or the possibility that they are not yet fully weaned.
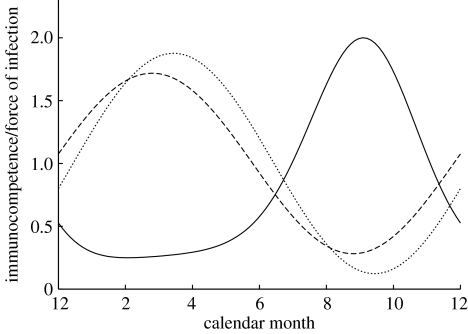
The fitted seasonal components for the model that best described the observed age–intensity curves (i.e. had the highest likelihood, GFPISC) are shown in figure 4 (the other two models with good support, GFISC and GFHISC, give very similar results). It is evident from the shape of the force of infection curve that deviations from sinusoidality are weak. The force of infection begins to rise in late spring, peaking in September before decaying to a low level during the winter. Both the temperature (which increases the larval development rate) and the breeding rate of the rabbit population peak a little earlier than this. However, we would expect the force of infection to lag behind these processes, since the density of infective larvae in herbage reflects the cumulative deposition of eggs (and therefore number of infected hosts) and their development into infectious larvae (Cattadori et al. 2005). The season-dependent component of immunity peaks in the spring and is weakest in the autumn, while the cohort-dependent component of immunity is strongest for rabbits born in the spring and weakest for those born late in the season.
Figure 4.
Force of infection, seasonal immunocompetence and cohort immunocompetence for the model with the highest likelihood fit (GFPISC). Solid line, force of infection; dashed line, seasonal immunocompetence; dotted line, cohort immunocompetence.
In summary, the statistical analysis reveals that the most parsimonious model should contain the following components
force of infection is highly seasonal and approximately sinusoidal;
strength of immune response varies both seasonally (depends on the current time of year) and by cohort (depends on the month of birth of the host);
host ingestion rate of infectious larvae increases with age; and
immunocompetence does not increase notably with age.
4. Discussion
We provide evidence to support the hypothesis, using statistical and mathematical modelling, that the immune response of wild rabbits to T. retortaeformis develops with cumulative exposure to the infection challenge but this alone is insufficient to account for the patterns observed. In addition, we found evidence consistent with immunocompetence depending on both the current season and the month in which the host was born. By immunocompetence, we specifically mean the relative ability of a host to resist or clear infection conditionally on its history of exposure. The biological validity of the best-fit model is confirmed not only by the good agreement between the data and fitted host age-parasite intensity curves but also by the concordance between our independent biological expectations, based on the published literature, and the fitted values of parameters, such as the parasite mortality rate and seasonal variation in the force of infection. Intriguingly, cohort-specific variables are necessary to generate the variability in the peak parasite intensity observed between cohorts. Our findings further stress the need to consider seasonal variations not only in the force of infection but also in the immunocompetence of cohorts. These last two components give rise to qualitatively similar effects on the infection intensity, and it is only by explicit modelling of the corresponding demographic processes that we can distinguish their relative contribution.
The model predictions for the strength and phase of the seasonal and cohort components of immunocompetence (figure 4) can be interpreted in the context of the rabbit's ecology. Immunocompetence appears the strongest in early spring and the weakest in the autumn. This may perhaps reflect that rabbits are in good condition early in the season and hence can allocate more resources to their immune response. A similar pattern is seen in the cohort-dependence in immunocompetence: individuals born in early spring appear to have a better ability to fight the infection than individuals born late in the season. This is suggestive of the importance of condition early in life or perhaps a maternal effect of the mother's condition during pregnancy and/or weaning. Perhaps the rabbits' condition declines due to competition for resources and space, as the population size increases during the summer. It is also conceivable that optimal patterns of maternal investment may change during the season such that mothers may invest more in offspring early in the season. Finally, it may be that secondary infections reduce condition later in the season; indeed, analyses of our data reported elsewhere (Cattadori et al. 2007a,b) show an increase in the prevalence of myxoma co-infections at the end of the summer.
Since we have not made direct immunological measurements in the field, it is conceivable that the patterns of parasite intensity are in fact generated by some other mechanism than acquired immunity. As discussed in Cattadori et al. (2005), processes that depend only on the age or current level of infection would not produce the observed peak shift, so a non-immunological explanation for the data would need to have the same mathematical behaviour as the immunological mechanisms our model. Specifically, the infection rate must be inhibited by cumulative exposure to infection risk, and the degree of inhibition modulated with season and cohort in the same way as in figure 4. For instance, experience might teach rabbits to avoid infective stages, in which case ‘immunocompetence’ would correspond to ability to learn. The seasonal component in figure 4 is approximately a half cycle out of phase with the force of infection and the host density, which is reasonable since rabbits might find it more difficult to find uninfested herbage when infestation and/or competition for food is high. However, the cohort effect is difficult to interpret as a behavioural effect, as this would imply that rabbits born in a particular month (early in the season) would have a lifelong propensity to be better at learning to avoid infection. We believe that acquired immunity is the most reasonable and parsimonious explanation, not only owing to strongly suggestive patterns in the data such as the observed periparturient rise of parasite intensity in breeding females (Cattadori et al. 2005, 2007b), and apparent immunosuppressive effects of myxoma on the nematode infection (Cattadori et al. 2007a,b), but also owing to independent empirical evidence for acquired immunity in this system. Laboratory experiments have shown that repeated infective doses increase the rate of arrested larval development and clearance of the infection (Michel 1952a,b). More recently, Audebert et al. (2003) recorded an inflammatory response to T. retortaeformis in the rabbits' stromal capillaries. That study was based on tissue sections collected over 15 days after a single infection dose and, therefore, does not speak to the gradually acquired immunity in our model. However, the tendency of this nematode to migrate into the intestinal mucosa during the initial establishment phase may stimulate the acquired immune response in repeatedly exposed hosts.
To our knowledge, this is the first study that has quantified the immune response of a vertebrate host to a helminth parasite in a wild population. The high seasonal variability of the immune response points to the significant costs of mounting it, and has clear implications for the evolutionary ecology of host–parasite interactions. Other parasites will be affected by, and contribute to, the hosts' immune status in varying ways (Lello et al. 2004; Cattadori et al. 2007a,b). This study provides some new insights into parasite–host dynamics and for our studies sets the scene for future experimental investigations of the interaction of T. retortaeformis with the community of parasites in rabbits. We are now in the process of directly determining the immunological response of the host to T. retortaeformis infection through laboratory infections under controlled conditions, as well as measuring seasonal changes in the immune response from animals in the field.
Acknowledgments
I.M.C. and B.B. were funded by the Leverhulme Trust. This study was funded by the Leverhulme Trust and the Human Science Frontier Program (HSFP).
Supplementary Material
Details of the models and statistical techniques used in the paper
References
- Anderson R.C. Oxford University Press; Oxford, UK: 2000. Nematode parasites of vertebrates: their development and transmission. [Google Scholar]
- Anderson R.M, May R.M. Regulation and stability of host–parasite population interactions: I. Regulatory processes. J. Anim. Ecol. 1978;47:219–247. doi:10.2307/3933 [Google Scholar]
- Anderson R.M, May R.M. Herd immunity to helminth infection and implications for parasite control. Nature. 1985;315:493–496. doi: 10.1038/315493a0. doi:10.1038/315493a0 [DOI] [PubMed] [Google Scholar]
- Anderson R.M, May R.M. Oxford University Press; Oxford, UK: 1991. Infectious diseases of humans. [Google Scholar]
- Audebert F, Hoste H, Durette-Desset M.C. Life cycle of Trichostrongylus retortaeformis in its natural host, the rabbit (Oryctolagus cuniculus) J. Helminthol. 2002;76:189–192. doi: 10.1079/JOH2002126. doi:10.1079/JOH2002126 [DOI] [PubMed] [Google Scholar]
- Audebert F, Vuong P.N, Durette-Desset M.C. Intestinal migrations of Trichostrongylus retortaeformis (Trichostrongylina, Trichostrongylidea) in the rabbit. Vet. Parasitol. 2003;112:131–146. doi: 10.1016/s0304-4017(02)00386-2. doi:10.1016/S0304-4017(02)00386-2 [DOI] [PubMed] [Google Scholar]
- Boag B, Lello J, Fenton A, Tompkins D.M, Hudson P.J. Patterns of parasite aggregation in the wild European rabbit (Oryctolagus cuniculus) Int. J. Parasitol. 2001;31:1421–1428. doi: 10.1016/s0020-7519(01)00270-3. doi:10.1016/S0020-7519(01)00270-3 [DOI] [PubMed] [Google Scholar]
- Boots M, Bowers R.M. The evolution of resistance through costly acquired immunity. Proc. R. Soc. B. 2004;271:715–723. doi: 10.1098/rspb.2003.2655. doi:10.1098/rspb.2003.2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham K.P, Anderson D.A. Springer; New York, NY: 1998. Model selection and inference: a practical information theoretic approach. [Google Scholar]
- Cattadori I.M, Boag B, Bjørnstad O.N, Cornell S.J, Hudson P.J. Immuno-epidemiology and peak shift in a seasonal host–nematode system. Proc. R. Soc. B. 2005;272:1167–1169. doi: 10.1098/rspb.2004.3050. doi:10.1098/rspb.2004.3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattadori I.M, Albert R, Boag B. Variation in host susceptibility and infectiousness generated by co-infection: the myxoma—Trichostrongylus retortaeformis case in wild rabbits. J. R. Soc. Interface. 2007a;4:831–840. doi: 10.1098/rsif.2007.1075. doi:10.1098/rsif.2007.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattadori, I. M., Boag, B. & Hudson, P. J. 2007b Parasite co-infection and interaction as drivers of host heterogeneity. Int. J. Parasitol (doi:10.1016/j.ijpara.2007.08.004) [DOI] [PubMed]
- Cornell S.J. Modelling nematode populations: 20 years of progress. Trends Parasitol. 2005;21:542–546. doi: 10.1016/j.pt.2005.08.019. doi:10.1016/j.pt.2005.08.019 [DOI] [PubMed] [Google Scholar]
- Côté S.D, Festa-Bianchet M. Birthdate, mass and survival in mountain goat kids, effect of maternal characteristics and forage quality. Oecologia. 2001;127:230–238. doi: 10.1007/s004420000584. doi:10.1007/s004420000584 [DOI] [PubMed] [Google Scholar]
- Crofton H.D. The ecology of immature phases of Trichostrongyle nematodes: II. The effect of climatic factors on the availability of the infective larvae of Trichostrongylus retortaeformis to the host. Parasitology. 1948;39:26–38. doi: 10.1017/s0031182000083529. [DOI] [PubMed] [Google Scholar]
- Dwyer G, Levin S.A, Buttel L. A simulation model of the population dynamics and evolution of myxomatosis. Ecol. Monogr. 1990;60:423–447. doi:10.2307/1943014 [Google Scholar]
- Gibb J.A, White A.J, Ward C.P. Population ecology of rabbits in the Wairarapa, New Zealand. N Zeal.Z J. Ecol. 1985;8:55–82. [Google Scholar]
- Grenfell B.T, Dietz K, Roberts M.G. Modelling the immuno-epidemiology of macroparasites in wildlife host populations. In: Grenfell B.T, Dobson A.P, editors. Ecology of infectious diseases in natural populations. Cambridge University Press; Cambridge, UK: 1995a. [Google Scholar]
- Grenfell B.T, Wilson K, Isham V.S, Boyd H.E.G, Dietz K. Modelling patterns of parasite aggregation in natural populations: trichostrongylid nematode-ruminant interactions as a case study. Parasitology. 1995b;111:S135–S151. doi: 10.1017/s0031182000075867. [DOI] [PubMed] [Google Scholar]
- Harkness J.E, Wagner E. Williams & Wilkins; Baltimore, MD: 1995. Biology and medicine of rabbits and rodents. [Google Scholar]
- Hudson P.J, Dobson A.P. Macroparasites: observed patterns in naturally fluctuating animal populations. In: Grenfell B.T, Dobson A.P, editors. Ecology of infectious diseases in natural populations. Cambridge University Press; Cambridge, UK: 1995. pp. 144–176. [Google Scholar]
- Kao R.R, Leathwick D.M, Roberts M.G, Sutherland I.A. Nematode parasites of ruminants: a survey of epidemiological parameters and their application in a simple model. Parasitology. 2000;121:85–103. doi: 10.1017/s0031182099006095. doi:10.1017/S0031182099006095 [DOI] [PubMed] [Google Scholar]
- Lello J, Boag B, Fenton A, Stevenson I.R, Hudson P.J. Competition and mutualism among the gut helminths of a mammalian host. Nature. 2004;428:840–844. doi: 10.1038/nature02490. doi:10.1038/nature02490 [DOI] [PubMed] [Google Scholar]
- Lloyd S. Environmental influences on host immunity. In: Grenfell B.T, Dobson A.P, editors. Ecology of infectious diseases in natural populations. Cambridge University Press; Cambridge, UK: 1995. pp. 327–335. [Google Scholar]
- May R.M, Anderson R.M. Regulation and stability of host–parasite population interactions: II. Destabilizing processes. J. Anim. Ecol. 1978;47:249–267. doi:10.2307/3934 [Google Scholar]
- Michel J.F. Self-cure infection of Trichostrongylus retortaeformis and its causation. Nature. 1952a;169:881. doi: 10.1038/169881b0. doi:10.1038/169881b0 [DOI] [PubMed] [Google Scholar]
- Michel J.F. Inhibition of development of Trichostrongylus retortaeformis and its causation. Nature. 1952b;169:933. doi: 10.1038/169933a0. doi:10.1038/169933a0 [DOI] [PubMed] [Google Scholar]
- Roberts M.G. A pocket guide to host–parasite models. Parasitol. Today. 1995;11:172–177. doi: 10.1016/0169-4758(95)80150-2. doi:10.1016/0169-4758(95)80150-2 [DOI] [PubMed] [Google Scholar]
- Roberts M.G, Grenfell B.T. The population dynamics of nematode infections of ruminants: periodic perturbations as a model for management. IMA J. Math. Appl. Med. Biol. 1991;8:83–93. doi: 10.1093/imammb/8.2.83. doi:10.1093/imammb/8.2.83 [DOI] [PubMed] [Google Scholar]
- Rogers P.M, Arthur C.P, Soriguer R.C. The rabbit in continental Europe. In: Thompson H.V, King C.M, editors. The European rabbit: the history and biology of a successful colonizer. Oxford Scientific Publications; Oxford, UK: 1994. pp. 22–63. [Google Scholar]
- Rosà R, Pugliese A, Villani A, Rizzoli A. Individual-based vs. deterministic models for macroparasites: host cycles and extinction. Theor. Popul. Biol. 2003;63:295–307. doi: 10.1016/s0040-5809(03)00021-2. doi:10.1016/S0040-5809(03)00021-2 [DOI] [PubMed] [Google Scholar]
- Soulsby E.J.L. Lea and Febiger; Philadelphia, PA: 1982. Helminths, arthropods and protozoa of domesticated animals. [Google Scholar]
- Veloso C, Bozinovic F. Effect of food quality on the energetics of reproduction in a precocial rodent, Octodon degus. J. Mammal. 2000;81:971–978. doi:10.1644/1545-1542(2000)081<0971:EOFQOT>2.0.CO;2 [Google Scholar]
- Woolhouse M.E.J. A theoretical framework for the immunoepidemiology of helminth infection. Parasite Immunol. 1992;14:563–578. doi: 10.1111/j.1365-3024.1992.tb00029.x. doi:10.1111/j.1365-3024.1992.tb00029.x [DOI] [PubMed] [Google Scholar]
- Woolhouse M.E.J. Patterns in parasite epidemiology: the peak shift. Parasitol. Today. 1998;14:428–434. doi: 10.1016/s0169-4758(98)01318-0. doi:10.1016/S0169-4758(98)01318-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of the models and statistical techniques used in the paper