Abstract
The mechanisms and temporal aspects of mate choice according to genetic constitution are still puzzling. Recent studies indicate that fitness is positively related to diversity in immune genes (MHC). Both sexes should therefore choose mates of high genetic quality and/or compatibility. However, studies addressing the role of MHC diversity in pre- and post-copulatory mate choice decisions in wild-living animals are few. We investigated the impact of MHC constitution and of neutral microsatellite variability on pre- and post-copulatory mate choice in both sexes in a wild population of a promiscuous primate, the grey mouse lemur (Microcebus murinus). There was no support for pre-copulatory male or female mate choice, but our data indicate post-copulatory mate choice that is associated with genetic constitution. Fathers had a higher number of MHC supertypes different from those of the mother than randomly assigned males. Fathers also had a higher amino acid distance to the females' MHC as well as a higher total number of MHC supertypes and a higher degree of microsatellite heterozygosity than randomly assigned males. Female cryptic choice may be the underlying mechanism that operates towards an optimization of the genetic constitution of offspring. This is the first study that provides support for the importance of the MHC constitution in post-copulatory mate choice in non-human primates.
Keywords: cryptic mate choice, MHC class II, good genes, promiscuity, Microcebus murinus, Madagascar
1. Introduction
Immunocompetence is an important determinant of fitness (Altizer et al. 2003; Bernatchez & Landry 2003; Ziegler et al. 2005). Both sexes are therefore expected to tune their mating strategies towards obtaining genetic constitutions for their offspring which provide them with superior immunocompetence. Recent studies have indicated that immunocompetence is directly related to immune gene (MHC) diversity (Sommer 2005; Piertney & Oliver 2006; Eyto et al. 2007). In order to increase immune gene diversity in offspring, individuals can mate promiscuously and/or perform mate choice (Nunn et al. 2000; Neff & Pitcher 2005). Females of many species are highly selective when it comes to mating (Darwin 1871; Bateson 1983; Andersson 1994; Kokko et al. 2003). Their fitness is mainly determined by their offspring's survival, selecting for female choosiness (Eberhard 1998; Jennions & Petrie 2000; Gavrilets et al. 2001; Fedorka & Mousseau 2002; Kokko et al. 2003). Females can exercise choice before, during and even after mating via cryptic choice. On a proximate level, they can exercise choice either directly, through preference or resistance, or indirectly, e.g. through oestrus advertisement that biases the operational sex ratio towards males and increases male–male competition. On an ultimate level, females can choose for direct benefit like paternal care, or indirect benefits such as genetic compatibility or ‘good genes’ (Birkhead & Møller 1993; Birkhead 2000; Jennions & Petrie 2000; Tregenza & Wedell 2000; Fedorka & Mousseau 2002; Paul 2002; Kokko et al. 2003; Reeder 2003; Birkhead & Kappeler 2004). Male reproductive success, in turn, is primarily limited by the number of females they can fertilize, selecting them to tune their mating strategies primarily towards monopolization of as many receptive females as possible (Clutton-Brock & Parker 1992). However, mate choice in terms of maximization of the quality of the genetic constitution of offspring may also be a rewarding strategy for males, especially in species with low male monopolization potential.
MHC genes are of crucial importance to the immune system since they code for cell surface glycoproteins that recognize, bind and present small peptide antigens to T-cells, which initiate an appropriate immune reaction (Klein 1986; Janeway & Travers 2002). A growing body of recent human medical and non-human primate studies indicate that certain MHC alleles functionally overlap due to similar antigen-binding motifs and emphasize the biological relevance of so-called MHC supertypes in disease resistance (Southwood et al. 1998; Sette & Sidney 1999; Trachtenberg et al. 2003; Lund et al. 2004; Schwensow et al. 2007b). In addition, several studies have revealed that the MHC constitution is an important factor that participates in mate choice decisions (e.g. reviewed by Jordan & Bruford 1998; Hedrick 1999; Penn & Potts 1999; Penn 2002; Ziegler et al. 2005). There is increasing support that the MHC constitution is olfactorily perceptible, although the exact mechanism is still subject to debates (Penn 2002; Potts 2002; Leinders-Zufall et al. 2004; Milinski et al. 2005). A direct role for MHC genes or MHC-linked loci in reproduction is also suggested by the demonstration that mouse oocytes can select sperm bearing a foreign MHC haplotype (Wedekind et al. 1996; Rülicke et al. 1998). However, studies addressing the role of MHC diversity in pre- and post-copulatory mate choice decisions in wild-living animals are few.
The most important hypotheses explaining mate choice mechanisms are the disassortative mating hypothesis and the good-genes-as-heterozygosity hypothesis (Brown 1997, 1999; Penn & Potts 1999; Tregenza & Wedell 2000; Penn 2002; Ziegler et al. 2005). According to the disassortative mating hypothesis, mate choice may function to avoid incompatibility between maternal and paternal genes or inbreeding and would lead to offspring being genetically different from each parent. The good-genes-as-heterozygosity hypothesis refers to a preference of mates having good genes which could mean either a specific good genetic constitution or heterozygosity. Both hypotheses are not mutually exclusive but can act simultaneously and both potentially increase the MHC variability of the offspring. Here we refer to ‘incompatibility’ in the sense of a too high degree of similarity of parental MHC genes, whereas we define ‘compatibility’ as dissimilarity between MHC constitutions. Thus, compatibility might be measured by the number of different MHC alleles between pairs, by genetic distances between the partners or by relatedness (Queller & Goodnight 1989). Heterozygotes might be selectively favoured due to the ability to recognize a broader array of pathogens (Doherty & Zinkernagel 1975). In case of the preference of heterozygous partners (which potentially leads to diverse offspring), individuals carrying divergent alleles might be favoured over those carrying similar alleles. Several studies indicate that specific MHC alleles are advantageous, and that frequency-dependent selection favours rare MHC alleles (for reviews see Penn & Potts 1999; Bernatchez & Landry 2003; Sommer 2005; Piertney & Oliver 2006). In contrast to MHC loci that are directly related to individual fitness (Paterson et al. 1998; Langefors et al. 2001; Lohm et al. 2002), microsatellites are assumed to behave neutrally and variation in microsatellites is thought to be primarily driven by non-selective evolutionary factors such as mutations, gene flow or genetic drift (Nei 1987). Combining analyses of neutral and adaptive genetic markers provides an efficient tool for evaluating the importance of various mechanisms on the evolution of immunogenetic constitutions such as selection driven by mate choice.
Primate species are promising models to investigate MHC-associated mate choice because this relatively small mammalian order exhibits a stunning diversity of social systems (Crook & Gartlan 1966; Eisenberg et al. 1972; Clutton-Brock 1974). Their social organization ranges from living solitarily, in pairs or in groups with up to several hundred individuals, and most mammalian mating systems can also be found in primates (reviewed by Kappeler & van Schaik 2002). This diversity potentially allows for detection of universal mechanisms of adaptive evolution. In this study, we investigate the impact of both the MHC constitution and the neutral microsatellite variability on pre- and post-copulatory mate choice in both sexes in a wild population of a promiscuous primate from western Madagascar, the grey mouse lemur (Microcebus murinus). Grey mouse lemurs are small (60 g), nocturnal solitary foragers. The mating system in the populations of Kirindy Forest in western Madagascar is characterized by highly seasonal reproduction, with matings being annually limited to a 4-week period in the austral spring, and by a high degree of home range overlap within and between sexes. Male grey mouse lemurs have relatively large testes (Fietz & Ganzhorn 1999; Aslam et al. 2002) and roam and search for receptive females. They aggressively defend access to females, but they cannot monopolize them or enforce copulations. Females become oestrous asynchronously and each female becomes receptive and mates during only a single night of the annual mating season. During their receptive night, the females are approached by up to 14 different males and most females mate promiscuously with up to seven males (Eberle & Kappeler 2004b). Male paternal care does not exist (Eberle & Kappeler 2004b). Studying this species may be especially rewarding for attempts at understanding the relationship between genetic constitution and mate choice in primates owing to the highly male-biased operational sex ratio and the high degree of promiscuity. Moreover, the study population is probably one of the best characterized wild non-human primate populations. It has been studied continuously since 1994 under various socio-biological and behavioural aspects (Eberle & Kappeler 2002, 2004a,b, 2006). This comprehensive information provides the rare opportunity to study MHC-associated mate choice in a wild population of a non-human primate.
The specific aims of our study were to investigate (1) the effects of MHC constitution in relation to overall genetic diversity (measured by microsatellites) on mate choice decisions, (2) whether pre- and/or post-copulatory MHC-associated mate preferences exist, and if they do exist, (3) the mechanisms that underlie these mate choice decisions. According to the hypotheses outlined above, mating partners, or parents, respectively, should have MHC constitutions as compatible as possible. We therefore predict that of all males that share (part of) their home range with that of a particular female, those males that approach this female during her receptive night are more MHC-dissimilar than those males that do not show up. Second, if pre-copulatory mate choice is important in grey mouse lemurs, we predict that of all males that locate a female during her receptive night, those that mate with her are more MHC-dissimilar, more variable or have specific, preferred MHC alleles compared with males that do not mate with her. Since field studies in this population did not provide support for direct pre-copulatory female choice (Eberle & Kappeler 2004a), females may opt to exercise post-copulatory cryptic choice. If they are able to do so, we expect fathers to be less MHC-similar to the mother or have a higher degree of variability than males that were available but did not sire the offspring of a female. This is the first study that investigates the importance of the MHC constitution in pre- and post-copulatory mate choice in non-human primates.
2. Material and methods
(a) Study site and tissue sampling
Field work for this study was conducted in the dry deciduous Kirindy Forest/CFPF located at approximately 60 km northeast of Morondava in western Madagascar. A detailed description of the area is provided by Ganzhorn & Sorg (1996) and Sorg et al. (2003). In the study population, locally known as CS7, trappings have been conducted three to five times per year since 1994, and on a monthly basis since 1999. In order to trap grey mouse lemurs, Sherman life traps (7.7×7.7×30.5) were baited with banana and set in the late afternoon. Captured individuals were collected early in the next morning. New animals were briefly anaesthetized for tissue collection and individually marked with subdermal transponders. All trapped animals were set to standard morphological measurements and released at their capture sites in the late afternoon of the same day (for detailed information see Eberle & Kappeler (2002, 2004a,b, 2006)).
(b) Behavioural observations
Detailed behavioural data on pre-copulatory mate choice activities were available from a previous study where 21 females were observed during their complete night of oestrus and the encountered males were recorded (Eberle & Kappeler 2004a,b). Each of these 21 females was encountered by 2–14 different males and mated with between one and six males. Thus, not every encountered male was mated; the females did not mate with 1–12 of the males in vicinity. Each observed female shared (part of) her home range with 21–38 (mean 31±4.7) males per year, and all their genetic data are available.
(c) Genotyping
Genome-wide heterozygosity was assessed in 313 individuals by a detailed microsatellite analysis of 17 loci with an average of 17 alleles using the same methods and dataset as described by Wimmer (2000), Hapke et al. (2003) and Eberle & Kappeler (2006). We have detailed knowledge about the pedigrees of 79 young born in the study area between 1994 and 2001. It can be expected that virtually all individuals of the study population are known individually because during the monthly captures since 1999, nearly all newly trapped animals were juveniles and almost all trapped adults were already individually marked (based on 682 individuals trapped 6.709 times since 1999 in the study population, see also Eberle & Kappeler (2002, 2004a,b, 2006)). This comprehensive information provides the rare opportunity to study MHC-associated mate choice in a wild-living non-human primate.
We genotyped a total of 180 individuals (108 males and 72 females) at the highly polymorphic MHC DRB exon 2, including the functionally important antigen-binding sites (ABS). A 171 bp-fragment was PCR-amplified using the primers JS1 und JS2 as described in Schad et al. (2004). For genotyping we carried out SSCP (single-stranded conformation polymorphism) electrophoresis (Orita et al. 1989a,b). We loaded PCR products on 15% polyacrylamide gels following the manufacturer's instructions (ETC, Elektrophoresetechnik, Kirchentellinsfurt, Germany) and carried out runs using a horizontal cooling electrophoresis system (Amersham Pharmacia Biotech, Freiburg, Germany). After electrophoresis, we fixed the gels and silver-stained them to visualize the resulting bands which we sequenced bidirectionally. We cut at least two different samples of each band out of the gel and dissolved them in 1×TBE-buffer. We carried out subsequent reamplification under the same PCR conditions as before but with a reduced number of cycles. We purified reamplificants by ethanol precipitation, performed cycle sequencing using a dye terminator sequencing kit (Applied Biosystems, Foster City, CA) and then analysed them with an Applied Biosystems sequencer Model 3100 following the manufacturer's protocol. Details on the molecular techniques are provided elsewhere (Sommer et al. 2002; Sommer 2003; Schad et al. 2004). The nucleotide sequences are deposited in GenBank (Accession numbers EU137061–EU137103).
(d) Analysis and statistical treatment
We calculated individual genetic diversity, i.e. the number of heterozygous microsatellite loci per total number of typed loci (Hobs). To include information on the genetic distance between microsatellite alleles, we additionally calculated d2 for each individual as: , where ai and aj are the length in repeat numbers of each allele at a locus, averaged over n typed loci. In order to investigate inbreeding avoidance between the potential partners, we calculated Queller & Goodnight's relatedness R using the program Relatedness v. 5.08 (Queller & Goodnight 1989). A detailed parentage analysis was performed by a combined mismatch and likelihood analysis using Cervus v. 2.0 (see Marshall et al. 1998; Eberle & Kappeler 2004a). We used Mega v. 3 (Kumar et al. 2004) to edit and align MHC DRB sequences and to calculate the mean number of amino acid differences between all MHC DRB sequences of an individual as a measure of individual MHC diversity. There is support for positive selection on MHC DRB in M. murinus since higher rates of non-synonymous substitutions in the ABS have been found (N. Schwensow, M. Eberle & S. Sommer 2007, unpublished data). Second, a maximum likelihood calculation using the computer program CodeML implemented in the PAML v. 3.15 software package (Yang 1997) identified nine positively selected sites (PSS; 15.8% of all investigated sites) after Bayes empirical analysis and two further sites that just lacked significance. In the next step, we defined MHC supertypes by applying amino acid sequence-based cluster analysis including all identified PSS as proposed by Doytchinova & Flower (2005). We also included the two sites that lacked significance since they are ABS in humans and in the closely related lemur species Cheirogaleus medius (Schwensow et al. 2007a,b). Details on the method are given in Schwensow et al. (2007b). The biological relevance of such MHC supertypes is supported by recent human and non-human primate studies (Southwood et al. 1998; Sette & Sidney 1999; Trachtenberg et al. 2003; Lund et al. 2004; Schwensow et al. 2007b) which indicate that certain MHC alleles functionally overlap due to similar antigen-binding motifs.
The pre-copulatory mate choice was tested by comparing males that approached or not a receptive female that shared (a part of) the males' home range in the respective year and by comparing all males that mated or not while in close proximity of the female (less than 10 m) during her receptive night. We compared the mean values of each tested parameter (see below) of the groups using a pairwise test. Whenever the requirements were fulfilled, we used a parametric test.
We also investigated mate choice by comparing the genetic fathers of 79 young ones with males that did not achieve paternity of the respective young ones. We generated a random model where we let each female choose randomly 15 000 times between all available males of the same year (N=7–40, mean 22.3±12.2, depending on year) to generate a null distribution. Subsequently, we checked if the observed mean value (i.e. the mean value of the males chosen as genetic fathers) falls within or outside of 95% of the simulated values. As test parameters for disassortative choice, we used the males' number of MHC DRB sequences and MHC supertypes different from the female, the amino acid distance between the most similar DRB sequences of female and males and the microsatellite relatedness. As the DRB-gene is assumedly duplicated in this species, we were unable to assess heterozygosity. Instead, we used the number of MHC DRB sequences and MHC supertypes present in males and the individual internal amino acid distance between the MHC DRB sequences of a male. To assess microsatellite heterozygosity, we used Hobs and the d2-value. All statistical calculations were carried out using SPSS v. 15.0 (SPSS, Inc., Chicago, IL, USA) and ResamplingStats v. 5.0.2 (ResamplingStats, Inc., Arlington, VA, USA), respectively.
3. Results
In the 180 genotyped individuals, we found 43 new MHC DRB sequences. Each individual carried between one and four different MHC DRB sequences indicating a duplication of the locus. All nucleotide sequences code for a unique amino acid sequence. There was no indication that either locus was a pseudogene. All loci seem to be under positive selection (N. Schwensow, M. Eberle & S. Sommer 2007, unpublished data) and were thus assumed to be functional. The MHC DRB sequences were grouped into five MHC supertypes differing in their antigen-binding motifs. The individual number of MHC DRB sequences or MHC supertypes did not correlate with the degree of neutral heterozygosity measured by 17 microsatellites (number of MHC DRB sequences −Hobs: Spearman's rho=−0.003, p=0.967; number of MHC supertypes −Hobs: Spearman's rho=0.047, p=0.536).
(a) Disassortative mate choice
(i) Female disassortative choice: mated versus not-mated males
In order to test for pre-copulatory disassortative female choice, we used all males that appeared in the immediate vicinity (less than 10 m) of an observed receptive female and compared males that mated with those that did not. Mated and not-mated males did neither differ in the number of MHC DRB sequences different from the female nor in the number of MHC supertypes different from the female (paired t-tests: number of MHC-alleles different from female: t=0.517, p=0.611; number of MHC-supertypes different from female: t=0.139, p=0.891; figure 1a,b in the electronic supplementary material). The amino acid distance between the most similar MHC DRB sequences of the female and males did not differ between mated and not-mated males (paired t-test, t=0.061, p=0.952, figure 1c in the electronic supplementary material) and both groups of males also did not differ in the degree of relatedness to the female (paired t-test, t=−0.039, p=0.969, figure 1d in the electronic supplementary material).
(ii) Female disassortative choice: fathers versus randomly assigned males
In another test for MHC-associated disassortative female choice, we compared fathers with randomly assigned males, i.e. males that did not achieve paternity but could have reached the respective mother. Randomly assigned males had a higher number of MHC DRB sequences different from those of the female than fathers (fathers: 1.785 and random males: 1.821, p<0.001, table 1, figure 1a), but fathers had a higher number of MHC supertypes different from those of the female than randomly assigned males (fathers: 1.063 and random males: 1.027, p=0.001, table 1, figure 1b). We also tested for differences in the amino acid distance between the two most similar MHC DRB sequences of the female and the males. Fathers had a higher amino acid distance to the most similar female MHC DRB sequence than randomly assigned males (fathers: 6.582 and random males: 6.439, p=0.015, table 1, figure 1c). We found no difference between genetic fathers and randomly assigned males with respect to the degree of genetic relatedness to the mother (fathers: −0.010 and random males: −0.010, p=0.921, table 1).
Table 1.
Tests for differences in genetic parameters in fathers versus (by simulation of female choice) randomly assigned males.
| hypotheses | genetic parameters | fathers | random males | p |
|---|---|---|---|---|
| disassortative mate choice | number of MHC DRB sequences different from female | 1.785 | 1.821 | <0.001 |
| number of MHC supertypes different from female | 1.063 | 1.027 | 0.001 | |
| amino acid distance between most similar MHC DRB sequences of females and males | 6.582 | 6.439 | 0.015 | |
| relatedness (microsatellites) | −0.010 | −0.010 | 0.921 | |
| choice for good genes | number of MHC DRB sequences present in males | 1.949 | 2.013 | <0.001 |
| number of MHC supertypes present in males | 1.861 | 1.829 | <0.001 | |
| male individual internal MHC DRB sequence distance | 9.266 | 10.092 | <0.001 | |
| Hobs (microsatellites) | 0.740 | 0.724 | <0.001 | |
| d2 (microsatellites) | 273.244 | 482.080 | <0.001 |
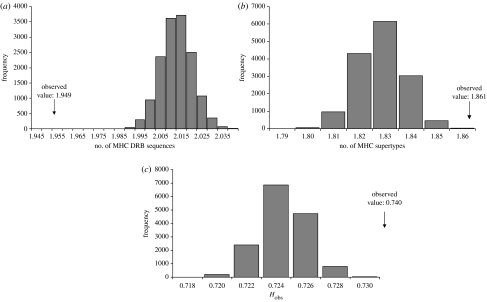
Figure 1.
Support for disassortative choice of fathers. Shown are the frequencies of individual genetic parameters of randomly assigned males. (a) Number of MHC DRB sequences different from that of female, (b) number of MHC supertypes different from that of female and (c) amino acid distance between most similar MHC DRB sequences of females and males. The observed values for fathers are indicated by arrows and lie outside of 95% of the frequency values for randomly assigned males. Details are given in the text.
(iii) Male disassortative choice
Each observed female shared (part of) her home range with 21–38 (mean 31±4.7) males of which genetic data are available. The comparison of males that approached a receptive female versus those that did not (despite sharing part of their home ranges) revealed no support for male MHC-associated mate choice, since both classes of males did not differ either in the mean number of MHC DRB sequences different from that of the female (Wilcoxon test, Z=−0.226, p=0.821) or MHC supertypes different from that of the female (Z=−1.269, p=0.204; figures not shown).
(b) Choice for good genes, i.e. number of MHC DRB sequences or specific MHC DRB sequences
(i) Female choice for good genes: mated versus not-mated males
For the following tests, we again used all males that appeared in the immediate vicinity (less than 10 m) of an observed receptive female. We compared males that mated with those that did not. Mated and not-mated males differed neither in their individual number of MHC DRB sequences nor in their individual number of MHC supertypes (Wilcoxon tests: number of MHC DRB sequences: Z=−0.804, p=0.421; number of MHC supertypes: Z=−0.501, p=0.616; figure 2a,b in the electronic supplementary material). We found no indication for an effect of the individual internal amino acid distance between the MHC DRB sequences of a male (paired t-test, t=−0.593, p=0.560, figure 2c in the electronic supplementary material). There was no difference in the observed microsatellite heterozygosity (Hobs, paired t-test, t=0.365, p=0.719, figure 2d in the electronic supplementary material) or in the degree of dissimilarity in microsatellites (d2-value, paired t-test, t=1.923, p=0.069, figure 2e in the electronic supplementary material) between mated and not-mated males. The high degree of MHC variability implies low frequencies of several MHC DRB sequences and limited MHC DRB sequence-specific analyses. Since there was no difference in the MHC supertype frequencies between mated and not-mated males, no preference for the certain (=advantageous) MHC supertypes was indicated (Χ2=0.822, FG=4, p=0.936; figure not shown).
(ii) Female choice for good genes: fathers versus randomly assigned males
Further, we investigated MHC-associated female choice for good genes by comparing fathers with randomly assigned males, i.e. males that did not achieve paternity but that could have reached the respective mother. There is no indication that the number of MHC DRB sequences had an influence on the paternity since randomly assigned males had a higher number of MHC DRB sequences than fathers (fathers: 1.949 and random males: 2.013, p<0.001, table 1, figure 2a). However, genetic fathers of offspring had a higher number of MHC supertypes than randomly assigned males (fathers: 1.861 and random males: 1.829, p<0.001, table 1, figure 2b). The individual internal amino acid distance of MHC DRB sequences of a male also did not influence mate choice since it was higher in randomly assigned males (fathers: 9.266 and random males: 10.092, p<0.001, table 1). The degree of microsatellite heterozygosity (Hobs) was higher in fathers than in randomly assigned males (0.740 versus 0.724, p<0.001, table 1, figure 2c). Accordingly, the d2-value that reflects the degree of similarity between the microsatellite alleles was higher in random males (fathers: 273.24 and random males: 482.08, p<0.001, table 1). As above, low frequencies of several MHC DRB sequences did not allow statistical comparisons, but no preferences for specific MHC supertypes were detected since MHC supertype frequencies did not differ between the genetic fathers and non-fathers (Χ2=3.079, FG=4, p=0.545; figure not shown).
Figure 2.
Support for choice of fathers with good genes. Shown are the frequencies of individual genetic parameters of randomly assigned males as (a) the total number of MHC DRB sequences present in a male, (b) the total number of MHC supertypes present in a male and (c) the degree of heterozygosity (based on microsatellites). The observed values of fathers are indicated by arrows and lie outside of 95% of the frequency values of randomly assigned males.
4. Discussion
By theory, both promiscuity and pre- or post-copulatory mate choice can improve fitness, and this holds true for both sexes. Female grey mouse lemurs are receptive for only one night per year during which they are approached by different males. Behavioural observations indicated that females mate indiscriminately with multiple males and usually do not reject males between the first and last one they mate with (Eberle & Kappeler 2004a; Eberle et al. 2007), although females dominate males when not in oestrus (Schmid & Kappeler 1998; Andrès et al. 2003). Male mate guarding is prevalent in grey mouse lemurs, but females might be able to choose males indirectly by terminating matings or by escaping guarding males (Eberle & Kappeler 2004b). In M. murinus, neither sex performs pre-copulatory mate choice associated with genetic constitution, but our results provided evidence of post-copulatory female choice. Below we discuss potential causes of these findings and possible mechanisms of the post-copulatory mate choice.
Our study does not provide any indication for the occurrence of pre-copulatory MHC-associated female mate choice in M. murinus, which is in concordance with previous behavioural observations in this species (Eberle & Kappeler 2004a, 2006; Eberle et al. 2007). No differences between mated and not-mated males in vicinity of a receptive female with respect to MHC similarity or microsatellite relatedness to the female have been observed. We also did not find that the number of MHC DRB sequences/MHC supertypes or the internal distance between the MHC DRB sequences of a male is an important factor in pre-copulatory female mate choice. There was also no indication for the preference or avoidance of certain or rare MHC supertypes. So far, female preference of certain MHC constitutions has only been described in the great snipe (Gallinago media) with some MHC allelic lineages (grouped by similarity and not functionality as in this present study) being more frequent in males with mating success (Ekblom et al. 2004). It was suggested that in mammals, females' direct rejection of males might be too costly due to a highly male biased operational sex ratio or the risk of harassment (Clutton-Brock & Parker 1995; Eberle & Kappeler 2004b).
Despite lacking evidence for pre-copulatory female mate choice in M. murinus, male pre-copulatory mate choice could still operate, especially in species like grey mouse lemurs where males cannot monopolize females and where female receptivity is restricted to a very short period of time. There is good evidence that not only human men but also non-human primate males are choosy (reviewed by Paul 2002). In grey mouse lemurs, males that mate early with the receptive female have a higher probability of siring offspring (Eberle & Kappeler 2004b; Eberle et al. 2007). We are aware that the most reliable way to test male pre-copulatory mate choice would be male focal observation; however, this was not feasible in this study. We therefore tested whether males that match the female in terms of MHC compatibility approach a receptive female sharing (part of) their home range whereas less compatible males do not seek to encounter the respective female. This was not supported by our data since males that approached a receptive female in their home range did not have more MHC DRB sequences different from the female than those that did not approach. This suggests that the MHC constitution might not be important in the context of pre-copulatory choice in M. murinus. Because oestrus synchrony is low (or incidental) in neighbouring females in this species (Eberle & Kappeler 2004a), most males may have no more than one option to mate during a single night. Moreover, due to the highly male-biased operational sex ratio and the fact that mouse lemurs are faced with a very high risk of mortality (Goodman et al. 1993; Rasoloarison et al. 1995; M. Eberle 1995–2005, unpublished data), males may have only a few options to mate at all during their life. They may therefore rarely have the option to choose and seize any opportunity.
Taking all this into account, we assume that direct pre-copulatory mate choice can be ruled out for both sexes in grey mouse lemurs. So why do females mate promiscuously? Females might have no choice other than mating with several males although frequent sexual encounters inevitably carry costs–-they take time and energy and involve the risk of catching sexually transmitted diseases (Arnqvist & Rowe 2005). Several possible benefits of female promiscuity have been discussed to explain this behaviour. Promiscuity provides the option of less expensive post-copulatory cryptic mate choice, such as differential fertilization or differential investment in embryos (Jennions & Petrie 2000; Birkhead & Pizzari 2002; Simmons 2005). Benefits of female promiscuity may also include fertilization insurance, fertilization with the most competitive sperm, protection against infanticide and increased genetic diversity among offspring (Nunn et al. 2000; Zeh & Zeh 2001; Stockley 2003; Wolff & Macdonald 2004; Neff & Pitcher 2005; Simmons 2005). Infanticide is an unlikely threat in the species studied by us. In Kirindy forest, the annual period of time is too short to reproduce twice per year (M. Eberle 1995–2005, unpublished data). Infanticide would therefore not shorten the period of time to the following oestrus. However, fertilization insurance, fertilization with the most competitive sperm or avoidance of harassment and/or genetic incompatibility are probable benefits of female promiscuity in grey mouse lemurs (see also Zeh & Zeh 1997; Jennions & Petrie 2000).
In M. murinus the detected pattern of MHC-associated disassortative choice might be best explained by post-copulatory mechanisms like cryptic female choice or sperm competition. Discrimination of post-copulatory female sperm selection and sperm competition is difficult, but it has been argued that sperm competition sensu stricto almost never occurs without influences from the female's reproductive tract which affect the outcome and thus cryptic females should always be a factor (summarized by Jennions & Petrie 2000). Field studies suggest that post-mating sexual selection biases fertilization towards genetically more compatible males (Olsson et al. 1996; Thuman & Griffiths 2005). In M. murinus, fathers had a higher number of MHC supertypes different from the mother than (potentially available) randomly assigned males that did not sire offspring. Interestingly, we found that randomly assigned males had a higher number of MHC DRB sequences different from the female than genetic fathers. This strongly indicates the importance of functional differences in the ABS of MHC molecules since MHC DRB sequences were grouped into MHC supertypes based on their functional similarities although our supertypes might not be exactly comparable to the supertypes in humans, as the lemur data are wholly derived from genomic DNA and cluster analysis. In line with this is the fact that the amino acid distance between the most similar MHC DRB sequences between the female and the males is higher in fathers than in randomly assigned males. This is highly interesting since we found that a high individual amino acid distance between MHC DRB sequences (i.e. a high individual MHC divergence) is beneficial with respect to intestinal parasite resistance in this species (N. Schwensow, M. Eberle & S. Sommer 2007, unpublished data). Fertilization with sperm from partners having a dissimilar MHC constitution could be beneficial for several reasons. It can function as a mechanism to avoid the deleterious effects of inbreeding. Second, it leads to offspring being different to each parent. This might increase the adaptability of the offspring to future environmental changes which might be important in the coevolutionary ‘arms race’ between hosts and parasites (Clarke & Kirby 1966; Takahata & Nei 1990). Parasite-driven selection for MHC diversity has been indicated by several studies (for reviews see Hedrick 1999; Sommer 2005; Piertney & Oliver 2006) and has also been found to be important in grey mouse lemurs (Schad et al. 2005; N. Schwensow, M. Eberle & S. Sommer 2007, unpublished data) and in the closely related fat-tailed dwarf lemur (Cheirogaleus medius; Schwensow et al. 2007b). Third, if individuals with a higher number of MHC DRB sequences have a better immunocompetence, fertilization among gametes with dissimilar MHC constitution may increase the potential of offspring to recognize a broader array of different antigens because it potentially leads to MHC heterozygosity.
Alternatively, sperm competition could select for males that produce ejaculates containing more sperm and/or for males that produce sperm that are more competitive in terms of fertilization (reviewed by Birkhead & Pizzari 2002). Sperm competition has earlier been suggested as an important factor in M. murinus because it is physically indicated by large testis (Fietz 1999). Heterozygosity might influence the condition or immunocompetence of a male and thus affect the quality or amount of ejaculated sperm (Gage et al. 2006). Heterozygosity might be fitness-relevant for male mouse lemurs since fathers had a higher observed microsatellite heterozygosity and a higher number of MHC supertypes than randomly assigned males. Interestingly, the number of MHC DRB sequences had no influence on paternity. Randomly assigned males had a higher number of MHC DRB sequences than fathers, which again might indicate that functional MHC diversity in terms of MHC supertypes is of greater importance than the quantity of MHC DRB sequences. Since male heterozygosity or MHC constitution cannot be influenced by females, we cannot rule out possible effects of sperm competition in M. murinus; but so far it is not clear whether a male's MHC constitution has an impact on the sperm's ability to outcompete other sperm. An alternative explanation might be that a higher number of MHC supertypes increases the probability that at least part of the sperm carry compatible alleles and might have a higher chance of fertilization. Since MHC DRB sequence sharing between non-relatives was low in our study population and the allelic status of the MHC DRB sequences is unknown, our data do not allow us to discriminate between heterozygote advantage and disassortative mate choice; however since both mechanisms are not mutually exclusive, they possibly could work simultaneously. Future studies addressing this issue are needed.
5. Conclusion
Our data provide the first support for hypotheses of MHC-associated post-copulatory mate choice from a wild living non-human primate, with sperm competition and female cryptic choice possibly acting simultaneously. This finding also provides a strong additional explanation for female promiscuity in grey mouse lemurs. Cryptic mate choice might be a good alternative mating strategy when the operational sex ratio is highly male biased. Promiscuity in combination with post-copulatory mate choice avoids the high costs of direct female choice but still provides the females with the opportunity to maximize offspring genetic constitution.
Acknowledgments
We are grateful to the late B. Rakotosamimanana, to P. M. Kappeler, R. Rasoloarison and L. Razafimanantsoa, and the following institutions for their authorization and support of our work in Madagascar: Commission Tripartite of the Malagasy Government, Laboratoire de Primatologie et des Vertébrés de l'Université d'Antananarivo, Parc Botanique et Zoologique de Tsimbazaza, Ministère pour la Production Animale, Département des Eaux et Forêts, Centre de Formation Professionnelle Forestière (CFPF) de Morondava and the German Primate Center (DPZ). We thank J. Streich for statistical advice, I. Tomaschweski for technical assistance in the laboratory and J. Ganzhorn for unflagging support. Two anonymous reviewers provided helpful comments on a former version of this manuscript. This study was made possible by the German Science Foundation (DFG; So 428/4-1, So 428/4-2).
Supplementary Material
No support for pre-copulatory disassortative mate choice from the comparison of genetic parameters (means±s.d.) of males that mated and males that did not mate but were in the vicinity (less than 10 m) of receptive females: (a) number of MHC DRB sequences different from the female, (b) number of MHC supertypes different from the female, (c) amino acid distance between the most similar MHC DRB sequences of female and males and (d) relatedness (based on microsatellites). Details are given in the text.
No support for pre-copulatory choice for ‘good genes’ from the comparison of genetic parameters (means±standard deviation) of males that mated and males that did not mate but were in the vicinity (<10 m) of receptive females: (a) number of MHC DRB sequences, (b) number of MHC supertypes, (c) individual internal amino acid distance between the MHC DRB sequences of a male, (d) degree of heterozygosity measured by microsatellites (Hobs) and (e) d2-value (microsatellites). Details are given in the text.
References
- Altizer S, Harvell D, Friedle E. Rapid evolutionary dynamics and disease threats to biodiversity. Trends Ecol. Evol. 2003;18:589–596. doi:10.1016/j.tree.2003.08.013 [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Andrès M, Solignac M, Perret M. Mating system in mouse lemurs: theories and facts, using analysis of paternity. Folia Primatol. 2003;74:355–366. doi: 10.1159/000073319. doi:10.1159/000073319 [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Princeton University Press; Princeton, NJ: 2005. Sexual conflict. [Google Scholar]
- Aslam H, Schneiders A, Perret M, Weinbauer G.F, Hodges J.K. Quantitative assessment of testicular germ cell production and kinematic and morphometric parameters of ejaculated spermatozoa in the grey mouse lemur, Microcebus murinus. Reproduction. 2002;123:323–332. doi: 10.1530/rep.0.1230323. doi:10.1530/rep.0.1230323 [DOI] [PubMed] [Google Scholar]
- Bateson P.P.G. Cambridge University Press; Cambridge, UK: 1983. Mate choice. [Google Scholar]
- Bernatchez L, Landry C. MHC studies in nonmodel vertebrates: what have we learned about natural selection in 15 years? J. Evol. Biol. 2003;16:363–377. doi: 10.1046/j.1420-9101.2003.00531.x. doi:10.1046/j.1420-9101.2003.00531.x [DOI] [PubMed] [Google Scholar]
- Birkhead T.R. Hidden choices of females. Nat. Hist. 2000;10:66–71. [Google Scholar]
- Birkhead T.R, Kappeler P.M. Post-copulatory sexual selection in birds and primates. In: Kappeler P.M, van Schaik C.P, editors. Sexual selection in primates: new and comparative perspectives. Cambridge University Press; Cambridge, UK: 2004. pp. 151–171. [Google Scholar]
- Birkhead T.R, Møller A.P. Female control of paternity. Trends Ecol. Evol. 1993;8:100–104. doi: 10.1016/0169-5347(93)90060-3. doi:10.1016/0169-5347(93)90060-3 [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Pizzari T. Postcopulatory sexual selection. Nature. 2002;3:262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- Brown J.L. A theory of mate choice based on heterozygosity. Behav. Biol. 1997;8:60–65. [Google Scholar]
- Brown J.L. The new heterozygosity theory of mate choice and the MHC. Genetica. 1999;104:215–221. doi: 10.1023/a:1026409220292. doi:10.1023/A:1026409220292 [DOI] [PubMed] [Google Scholar]
- Clarke B, Kirby D.R. Maintenance of histocompatibility polymorphisms. Nature. 1966;211:999–1000. doi: 10.1038/211999a0. doi:10.1038/211999a0 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H. Primate social organization and ecology. Nature. 1974;250:539–542. doi:10.1038/250539a0 [Google Scholar]
- Clutton-Brock T.H, Parker G.A. Potential reproductive rates and the operation of sexual selection. Q. Rev. Biol. 1992;67:437–456. doi:10.1086/417793 [Google Scholar]
- Clutton-Brock T.H, Parker G.A. Sexual coercion in animal societies. Anim. Behav. 1995;49:1345–1365. doi:10.1006/anbe.1995.0166 [Google Scholar]
- Crook J.H, Gartlan J.C. Evolution of primate societies. Nature. 1966;210:1200–1203. doi: 10.1038/2101200a0. doi:10.1038/2101200a0 [DOI] [PubMed] [Google Scholar]
- Darwin C. John Murray; London, UK: 1871. The descent of man, and selection in relation to sex. [Google Scholar]
- Doherty P.C, Zinkernagel R.M. Enhanced immunological surveillance in mice heterozygous at the H-2 gene complex. Nature. 1975;256:50–52. doi: 10.1038/256050a0. doi:10.1038/256050a0 [DOI] [PubMed] [Google Scholar]
- Doytchinova I.A, Flower D.R. In silico identification of supertypes for class II MHCs. J. Immunol. 2005;174:7085–7095. doi: 10.4049/jimmunol.174.11.7085. [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Female roles in sperm competition. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London, UK: 1998. pp. 91–116. [Google Scholar]
- Eberle M, Kappeler P.M. Mouse lemurs in space and time: a test of the socioecological model. Behav. Ecol. Sociobiol. 2002;51:131–139. doi:10.1007/s002650100409 [Google Scholar]
- Eberle M, Kappeler P.M. Selected polyandry: female choice and inter-sexual conflict in a small nocturnal solitary primate (Microcebus murinus) Behav. Ecol. Sociobiol. 2004a;57:91–100. doi:10.1007/s00265-004-0823-4 [Google Scholar]
- Eberle M, Kappeler P.M. Sex in the dark: determinats and consequences of mixed male mating tactics in Microcebus murinus, a small solitary nocturnal primate. Behav. Ecol. Sociobiol. 2004b;57:77–90. doi:10.1007/s00265-004-0826-1 [Google Scholar]
- Eberle M, Kappeler P.M. Family insurance: kin selection and cooperative breeding in a solitary primate (Microcebus murinus) Behav. Ecol. Sociobiol. 2006;60:582–588. doi:10.1007/s00265-006-0203-3 [Google Scholar]
- Eberle, M., Perret, M. & Kappeler, P. M. 2007 Sperm competition and optimal timing of matings in Microcebus murinus Int. J. Primatol (doi:10.1007/s10764-007-9220-y)
- Eisenberg J.F, Muckenhirn N.A, Rudran R. The relation between ecology and social structure in primates. Science. 1972;176:863–874. doi: 10.1126/science.176.4037.863. doi:10.1126/science.176.4037.863 [DOI] [PubMed] [Google Scholar]
- Ekblom R, Saether S.A, Grahn M, Fiske P, Kalas J.A, Höglund J. Major histocompatibility complex variation and mate choice in a lekking bird, the great snipe (Gallinago media) Mol. Ecol. 2004;13:3821–3828. doi: 10.1111/j.1365-294X.2004.02361.x. doi:10.1111/j.1365-294X.2004.02361.x [DOI] [PubMed] [Google Scholar]
- Eyto E, et al. Natural selection acts on Atlantic salmon major histocompatibility (MH) variability in the wild. Proc. R. Soc. B. 2007;274:861–869. doi: 10.1098/rspb.2006.0053. doi:10.1098/rspb.2006.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorka K.M, Mousseau T.A. Material and genetic benefits of female multiple mating and polyandry. Anim. Behav. 2002;64:361–367. doi:10.1006/anbe.2002.3052 [Google Scholar]
- Fietz J. Mating system of Microcebus murinus. Am. J. Primatol. 1999;48:127–133. doi: 10.1002/(SICI)1098-2345(1999)48:2<127::AID-AJP4>3.0.CO;2-4. doi:10.1002/(SICI)1098-2345(1999)48:2<127::AID-AJP4>3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- Fietz J, Ganzhorn J.U. Feeding ecology of the hibernating primate Cheirogaleus medius: how does it get so fat? Oecologia. 1999;121:157–164. doi: 10.1007/s004420050917. doi:10.1007/s004420050917 [DOI] [PubMed] [Google Scholar]
- Gage M.J.G, Surridge A, Tomkins J.L, Green E, Wiskin L, Bell D.J, Hewitt G.M. Reduced heterozygosity depresses sperm quality in wild rabbits, Oryctolagus cuniculus. Curr. Biol. 2006;16:612–617. doi: 10.1016/j.cub.2006.02.059. doi:10.1016/j.cub.2006.02.059 [DOI] [PubMed] [Google Scholar]
- Ganzhorn, J. U. & Sorg, J. P. 1996 Ecology and economy of a tropical dry forest in Madagascar. Primate report no. 46, Goltz, Göttingen.
- Gavrilets S, Arnqvist G, Friberg U. The evolution of female mate choice by sexual conflict. Proc. R. Soc. B. 2001;268:531–539. doi: 10.1098/rspb.2000.1382. doi:10.1098/rspb.2000.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S.M, Langrand O, Raxworthy C.J. The food habits of the barn owl Tyto alba at three sites on Madagascar. Ostrich. 1993;64:160–171. [Google Scholar]
- Hapke A, Eberle M, Zischler H. Isolation of new microsatellite markers and application in four species of mouse lemurs (Microcebus spec.) Mol. Ecol. Notes. 2003;3:205–208. doi:10.1046/j.1471-8286.2003.00398.x [Google Scholar]
- Hedrick P.W. Balancing selection and MHC. Genetica. 1999;104:207–214. doi: 10.1023/a:1026494212540. doi:10.1023/A:1026494212540 [DOI] [PubMed] [Google Scholar]
- Janeway C.A, Travers P. Spektrum Akademischer Verlag GmbH; Heidelberg, Germany; Oxford, UK: 2002. Immunology. [Google Scholar]
- Jennions M.D, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. doi:10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Jordan W.C, Bruford M.W. New perspectives on mate choie and the MHC. Heredity. 1998;81:127–133. doi: 10.1046/j.1365-2540.1998.00428.x. doi:10.1038/sj.hdy.6884281 [DOI] [PubMed] [Google Scholar]
- Kappeler P.M, van Schaik C.P. Evolution of primate social systems. Int. J. Primatol. 2002;23:707–740. doi:10.1023/A:1015520830318 [Google Scholar]
- Klein J. Wiley; New York, NY: 1986. Natural history of the major histocompatibility complex. [Google Scholar]
- Kokko H, Brooks R, Jennions M.D, Morley J. The evolution of mate choice and mating biases. Proc. R. Soc. B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. doi:10.1098/rspb.2002.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. Mega3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. doi:10.1093/bib/5.2.150 [DOI] [PubMed] [Google Scholar]
- Langefors A, Lohm J, Grahn M, Andersen O, von Schantz T. Association between major histocompatibility complex class IIB alleles and resistance to Aeromonas salmonicida in Atlantic salmon. Proc. R. Soc. B. 2001;268:479–485. doi: 10.1098/rspb.2000.1378. doi:10.1098/rspb.2000.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, et al. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. doi:10.1126/science.1102818 [DOI] [PubMed] [Google Scholar]
- Lohm J, Grahn M, Langefors A, Andersen O, Storset A, von Schantz T. Experimental evidence for major histocompatibility complex-allele-specific resistance to a bacterial infection. Proc. R. Soc. B. 2002;269:2029–2033. doi: 10.1098/rspb.2002.2114. doi:10.1098/rspb.2002.2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund O, et al. Definition of supertypes for HLA molecules using clustering of specifity matrices. Immunogenetics. 2004;55:797–810. doi: 10.1007/s00251-004-0647-4. doi:10.1007/s00251-004-0647-4 [DOI] [PubMed] [Google Scholar]
- Marshall T.C, Slate J, Kruuk L.E.B, Pemberton J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. doi:10.1046/j.1365-294x.1998.00374.x [DOI] [PubMed] [Google Scholar]
- Milinski M, Griffiths S, Wegner K.M, Reusch T.B.H, Haas-Assenbaum A, Boehm T. Mate choice decisions of stickleback females predictably modified by MHC peptide ligands. Proc. Natl Acad. Sci. USA. 2005;102:4414–4418. doi: 10.1073/pnas.0408264102. doi:10.1073/pnas.0408264102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. doi:10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Nei M. Columbia University Press; New York, NY: 1987. Molecular evolutionary genetics. [Google Scholar]
- Nunn C.L, Gittleman J.L, Antonovics J. Promiscuity and the primate immune system. Science. 2000;290:1168–1170. doi: 10.1126/science.290.5494.1168. doi:10.1126/science.290.5494.1168 [DOI] [PubMed] [Google Scholar]
- Olsson M, Shine R, Madsen T, Gullberg A, Tegelstrom H. Sperm selection by females. Nature. 1996;383:585. doi:10.1038/383585a0 [Google Scholar]
- Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T. Detection of polymorphism of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc. Natl Acad. Sci. USA. 1989a;86:2766–2770. doi: 10.1073/pnas.86.8.2766. doi:10.1073/pnas.86.8.2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M, Sekiya T, Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989b;5:874–879. doi: 10.1016/0888-7543(89)90129-8. doi:10.1016/0888-7543(89)90129-8 [DOI] [PubMed] [Google Scholar]
- Paterson S, Wilson A.C.C, Pemberton J.M. Major histocompatibility complex variation associated with juvenile survival and parasite resistance in a large unmanaged ungulate population (Ovis aries) Evolution. 1998;95:3714–3719. doi: 10.1073/pnas.95.7.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A. Sexual selection and mate choice. Int. J. Primatol. 2002;23:877–904. doi:10.1023/A:1015533100275 [Google Scholar]
- Penn D.J. The scent of genetic compatibility: sexual selection and the major histocompatibiliy complex. Ethology. 2002;108:1–21. doi:10.1046/j.1439-0310.2002.00768.x [Google Scholar]
- Penn D.J, Potts W.K. The evolution of mating preferences and major histocompatibility complex genes. Am. Nat. 1999;153:145–164. doi: 10.1086/303166. doi:10.1086/303166 [DOI] [PubMed] [Google Scholar]
- Piertney S.B, Oliver M.K. The evolutionary ecology of the major histocompatibility complex. Heredity. 2006;96:7–21. doi: 10.1038/sj.hdy.6800724. [DOI] [PubMed] [Google Scholar]
- Potts W.K. Wisdom though immunogenetics. Nat. Genet. 2002;30:130–131. doi: 10.1038/ng0202-130. doi:10.1038/ng0202-130 [DOI] [PubMed] [Google Scholar]
- Queller D.C, Goodnight K.F. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. doi:10.2307/2409206 [DOI] [PubMed] [Google Scholar]
- Rasoloarison R.M, Rasolonandrasana B.P.N, Ganzhorn J.U, Goodman S.M. Predation on vertebrates in the Kirindy forest, Western Madagascar. Ecotropica. 1995;1:59–65. [Google Scholar]
- Reeder D.M. The potential for cryptic female choice in primates: behavioral, physiological, and anatomical considerations. In: Jones C.B, editor. Sexual selection and reproductive competition in primates: new perspectives and directions. American Society of Primatologists; Norman, OK: 2003. pp. 255–303. [Google Scholar]
- Rülicke T, Chapuisat M, Homberger F.R, Macas E, Wedekind C. MHC-genotype of progeny influenced by parental infection. Proc. R. Soc. B. 1998;265:711–716. doi: 10.1098/rspb.1998.0351. doi:10.1098/rspb.1998.0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad J, Sommer S, Ganzhorn J.U. MHC variability of a small lemur in the littoral forest fragments of southeastern Madagascar. Conserv. Genet. 2004;5:299–309. doi:10.1023/B:COGE.0000031137.50239.d3 [Google Scholar]
- Schad J, Ganzhorn J.U, Sommer S. Parasite burden and constitution of major histocopatibility complex in the Malagasy mouse lemur, Microcebus murinus. Evolution. 2005;59:439–450. [PubMed] [Google Scholar]
- Schmid J, Kappeler P.M. Fluctuating sexual dimorphism and differential hibernation by sex in a primate, the grey mouse lemur (Microcebus murinus) Behav. Ecol. Sociobiol. 1998;43:125–132. doi:10.1007/s002650050474 [Google Scholar]
- Schwensow, N., Fietz, J., Dausmann, K. & Sommer, S. 2007a MHC-associated mating strategies and the importance of overall genetic diversity in an obligate pair-living primate. Evol. Ecol. (doi:10.1007/s10682-007-9186-4)
- Schwensow N, Fietz J, Dausmann K, Sommer S. Neutral versus adaptive variation in parasite resistance: importance of MHC-supertypes in a free-ranging primate. Heredity. 2007b;99:265–277. doi: 10.1038/sj.hdy.6800993. doi:10.1038/sj.hdy.6800993 [DOI] [PubMed] [Google Scholar]
- Sette A, Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics. 1999;50:201–212. doi: 10.1007/s002510050594. doi:10.1007/s002510050594 [DOI] [PubMed] [Google Scholar]
- Simmons L.W. The evolution of polyandry: sperm competition, sperm selection and offspring viability. Annu. Rev. Ecol. Evol. Syst. 2005;35:125–146. doi:10.1146/annurev.ecolsys.36.102403.112501 [Google Scholar]
- Sommer S. Effects of habitat fragmentation and changes of dispersal behaviour after a recent population decline on the genetic variability of noncoding and coding DNA of monogamous Madagasy rodent. Mol. Ecol. 2003;12:2845–2851. doi: 10.1046/j.1365-294x.2003.01906.x. doi:10.1046/j.1365-294X.2003.01906.x [DOI] [PubMed] [Google Scholar]
- Sommer S. The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front. Zool. 2005;2:16. doi: 10.1186/1742-9994-2-16. doi:10.1186/1742-9994-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S, Schwab D, Ganzhorn J.U. MHC diversity of endemic Malagasy rodents in relation to range contraction and social system. Behav. Ecol. Sociobiol. 2002;51:214–221. doi:10.1007/s00265-001-0432-4 [Google Scholar]
- Sorg J.-P, Ganzhorn J.U, Kappeler P.M. Forestry and research in the Kirindy Forest/Centre de Formation Professionelle Forestière. In: Goodman S.M, Benstead J.P, editors. The natural history of Madagascar. The University of Chicago Press; Chicago, IL: 2003. pp. 1512–1519. [Google Scholar]
- Southwood S, et al. Several common HLA-DR types share leagely overlapping peptide binding repertoires. J. Immunol. 1998;160:3363–3373. [PubMed] [Google Scholar]
- Stockley P. Female multiple mating behaviour, early reproductive failure and litter size variation in mammals. Proc. R. Soc. B. 2003;270:271–278. doi: 10.1098/rspb.2002.2228. doi:10.1098/rspb.2002.2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata N, Nei M. Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics. 1990;124:967–978. doi: 10.1093/genetics/124.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuman K.A, Griffiths S.C. Genetic similarity and the nonrandom distribution of paternity in a genetically highly polyandrous shorebird. Anim. Behav. 2005;69:765–770. doi:10.1016/j.anbehav.2004.10.003 [Google Scholar]
- Trachtenberg E, et al. Advantage of rare HLA supertype in HIV disease progression. Nat. Med. 2003;9:928–935. doi: 10.1038/nm893. doi:10.1038/nm893 [DOI] [PubMed] [Google Scholar]
- Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. doi:10.1046/j.1365-294x.2000.00964.x [DOI] [PubMed] [Google Scholar]
- Wedekind C, Chapuisat M, Macas E, Rülicke T. Non-random fertilization in mice correlates with the MHC and something else. Heredity. 1996;400:409. doi: 10.1038/hdy.1996.160. [DOI] [PubMed] [Google Scholar]
- Wimmer, B. 2000 Untersuchung der Paarungssysteme und Populationsstruktur von Lemuren an Coquerel's Zwergmaki (Mirza coquereli), dem grauen Mauslemur (Microcebus murinus), dem Rotstirnmaki (Eulemur fulvus fulvus) und dem Larvensifaka (Propithecus verreauxi verreauxi). PhD thesis, Universität München.
- Wolff J.O, Macdonald D.W. Promiscuous females protect their offspring. Trends Ecol. Evol. 2004;19:127–134. doi: 10.1016/j.tree.2003.12.009. doi:10.1016/j.tree.2003.12.009 [DOI] [PubMed] [Google Scholar]
- Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Cabios. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- Zeh J.A, Zeh D.W. The evolution of polyandry II: post-copulatory defences against gentic incompatibility. Proc. R. Soc. B. 1997;264:69–75. doi:10.1098/rspb.1997.0010 [Google Scholar]
- Zeh J.A, Zeh D.W. Reproductive mode and the genetic benefits of polyandry. Anim. Behav. 2001;61:1051–1063. doi:10.1006/anbe.2000.1705 [Google Scholar]
- Ziegler A, Kentenich H, Uchanska-Ziegler B. Female choice and the MHC. Trends Immunol. 2005;26:496–502. doi: 10.1016/j.it.2005.07.003. doi:10.1016/j.it.2005.07.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
No support for pre-copulatory disassortative mate choice from the comparison of genetic parameters (means±s.d.) of males that mated and males that did not mate but were in the vicinity (less than 10 m) of receptive females: (a) number of MHC DRB sequences different from the female, (b) number of MHC supertypes different from the female, (c) amino acid distance between the most similar MHC DRB sequences of female and males and (d) relatedness (based on microsatellites). Details are given in the text.
No support for pre-copulatory choice for ‘good genes’ from the comparison of genetic parameters (means±standard deviation) of males that mated and males that did not mate but were in the vicinity (<10 m) of receptive females: (a) number of MHC DRB sequences, (b) number of MHC supertypes, (c) individual internal amino acid distance between the MHC DRB sequences of a male, (d) degree of heterozygosity measured by microsatellites (Hobs) and (e) d2-value (microsatellites). Details are given in the text.