Abstract
The maintenance of colour polymorphisms within populations has been a long-standing interest in evolutionary ecology. African cichlid fish contain some of the most striking known cases of this phenomenon. Intrasexual selection can be negative frequency dependent when males bias aggression towards phenotypically similar rivals, stabilizing male colour polymorphisms. We propose that where females are territorial and competitive, aggression biases in females may also promote coexistence of female morphs. We studied a polymorphic population of the cichlid fish Neochromis omnicaeruleus from Lake Victoria, in which three distinct female colour morphs coexist: one plain brown and two blotched morphs. Using simulated intruder choice tests in the laboratory, we show that wild-caught females of each morph bias aggression towards females of their own morph, suggesting that females of all three morphs may have an advantage when their morph is locally the least abundant. This mechanism may contribute to the establishment and stabilization of colour polymorphisms. Next, by crossing the morphs, we generated sisters belonging to different colour morphs. We find no sign of aggression bias in these sisters, making pleiotropy unlikely to explain the association between colour and aggression bias in wild fish, which is maintained in the face of gene flow. We conclude that female–female aggression may be one important force for stabilizing colour polymorphism in cichlid fish.
Keywords: intrasexual selection, speciation, female–female competition, Lake Victoria
1. Introduction
The maintenance of colour polymorphism in natural populations has been a major theme in evolutionary ecology for more than 50 years (Huxley 1955; Gray & McKinnon 2007). Recent work has focused on the question of when colour polymorphisms may be transient stages in sympatric speciation through disruptive and negative frequency-dependent selection (Gray & McKinnon 2007). The colourful haplochromine cichlid species flock of the Great African Lake Victoria is perhaps the most outstanding case both of explosive speciation and intraspecific colour polymorphism among vertebrates (Seehausen 2006). A great diversity of trophic adaptations have evolved rapidly, associated with strong habitat and trophic resource partitioning among the major lineages (Greenwood 1981). However, many sibling species coexist sympatrically with very little apparent ecological differentiation but with very marked differences in coloration (Seehausen 1996; Bouton et al. 1997; Seehausen et al. 1999a,b). A similar pattern is observed in the older radiation of haplochromines in Lake Malawi (Fryer & Iles 1972; Genner et al. 1999a; Danley & Kocher 2001). Female mate choice based on male nuptial coloration has been hypothesized to play an important role in generating and maintaining reproductive isolation between species (Seehausen & van Alphen 1998; Knight & Turner 2004). However, the stable coexistence of ecologically similar species is a long-standing problem in ecology (Hutchinson 1961; Scheffer & van Nes 2006; in cichlids: Bouton et al. 1997; Genner & Turner 2005). The coexistence problem converges on that of the maintenance of colour polymorphism when reproductive isolation is incomplete.
One hypothetical mechanism for the maintenance of both is negative frequency-dependent selection on male nuptial coloration arising from male–male aggression (Mikami et al. 2004; Seehausen & Schluter 2004; Dijkstra et al. 2007). Haplochromine males vigorously defend long-term mating territories to secure spawnings (Maan et al. 2004). Male competition over territories is intense and affects reproductive success and sexual selection (Genner et al. 1999b; Dijkstra et al. in press a). Negative frequency-dependent selection on colour could arise if territorial males direct more aggression towards phenotypically similar than dissimilar competitors. Such an aggression bias would generate a fitness advantage for rare phenotypes relative to more abundant phenotypes. Experimental evidence confirms that males of some haplochromine cichlids bias aggression towards males of their own species and that this bias is induced by colour signals (Dijkstra et al. 2006, 2007). Field data suggest that such interactions may structure haplochromine communities in nature (Seehausen & Schluter 2004).
The reproductive output of haplochromine populations is probably limited by survival and energy intake of females more than by those of males. Males provide no parental care and can spawn far more frequently than females (Genner et al. 1999b). Hence, in terms of recruitment and coexistence of species or morphs, it is important to consider forces that affect fitness of females. We suggest that female–female aggression could be one important force for stabilizing colour polymorphism. Haplochromines are female mouth brooders and many species guard their fry for extended periods. Mothers aggressively defend small feeding territories for their fry (Fryer & Iles 1972; Seehausen 1996; P. D. Dijkstra 2002–2003, personal observation). In algae-scraping species, such as Neochromis omnicaeruleus, females spend most of their time on the surface of rocks from which they scrape algae and where they can often be seen chasing one another (Seehausen 1996; Maan et al. submitted). Some algae-scraping species also occupy short-term feeding territories on rock substrate (Ribbink 1991; Seehausen 1996). Finally, territories can provide shelter against piscine and avian predators. Suitable territories and foraging sites are probably scarce, resulting in interference competition among females for territories and dominance (Chase et al. 2002, 2003). If females use colour as a cue in their social interactions, female–female competition may exert selection on female coloration.
Females of many haplochromine cichlid species are cryptically coloured, consistent with their heavy investment in parental care (Fryer & Iles 1972; Seehausen 1996). Owing to this crypsis, females of closely related species often look phenotypically similar, providing limited scope for colour-based aggression biasing. However, conspicuous female colour phenotypes are common in several species (Lande et al. 2001). In many species in Lakes Victoria, Malawi and Kivu, particular types of conspicuous coloration occur much more frequently in females than in males (Lande et al. 2001). Such is the case in the highly polymorphic species N. omnicaeruleus from Lake Victoria (Seehausen et al. 1999b). In its Makobe island population, three distinct colour morphs occur in both sexes (Seehausen et al. 1999b): a plain presumably ancestral morph (P); and two conspicuously coloured blotched morphs, white blotched (WB; black blotches on white) and orange blotched (OB; black blotches on orange). The WB and OB morphs are predominantly found in females (Seehausen et al. 1999b). Intermediates between the WB and OB morphs exist but are rare. The species is abundant around Makobe Island and shows year-round reproductive activity (Seehausen et al. 1998). A long-term series of field data collected between 1991 and 2003 suggest that morph frequencies have fluctuated little over at least 12 years (Seehausen et al. 1999b; Maan et al. submitted). Females are often aggressive and territorial (Seehausen et al. 1999b; Maan et al. submitted). The microhabitat distribution of the morphs is completely overlapping (Seehausen & Bouton 1997; Maan et al. submitted). The intriguing question then is how this colour polymorphism is maintained (Seehausen et al. 1999b; Lande et al. 2001).
Seehausen et al. (1999b) suggest that morphs of N. omnicaeruleus are non-randomly mating incipient species with male and female mating preferences. Support for non-random mating was inferred from (i) morph frequencies in the wild (Seehausen et al. 1999b) and (ii) mate choice experiments in which morphs exhibited female and male mating preferences (Seehausen et al. 1999b; Pierotti & Seehausen 2007). Mutual mate choice would make females of the same morph stronger competitors for males than females of different morphs (see also Lande et al. 2001; van Doorn et al. 2004). We hypothesize that such intrasexual competition led to the evolution of own-morph aggression biases.
We first used a simulated intruder choice test in the laboratory to investigate whether wild-caught territory defending females of N. omnicaeruleus bias aggression towards competitors of their own colour morph. We show that females of each of the three colour morphs have significant own-morph aggression biases, which may generate negative frequency-dependent selection on female coloration. We then ask how the association between colour and aggression bias is maintained in the face of gene flow. To this end, we investigate aggression biases of full sib females of different colour generated by crossing plain and blotched morphs. We find that physical linkage or pleiotropy between colour and aggression bias is unlikely to maintain the own-morph bias, invoking instead disruptive selection to maintain the association between physically unlinked genes.
2. Material and methods
(a) Species
We studied a population of N. omnicaeruleus from Makobe Island in the western Speke Gulf (Tanzania) that consists of three fully sympatric morphs (Seehausen et al. 1999b). Plain (P) females are yellow to brown with 4–8 dark vertical bars, whereas most P males are blue and some are yellow. OB individuals have variably shaped brown blotches, superimposed on an orange or pink background. WB individuals have variably shaped black blotches on a white to brassy background. Even though half of the females in the population are blotched, less than 1.7% of the males are blotched.
(b) General housing
All aquarium walls, except the front, were covered with black plastic sheets. All aquaria were connected to a central biological filtration system and water was circulated continuously. Water temperature was kept at 25±2°C and a 12 L : 12 D cycle was maintained. Illumination was provided by 58 W fluorescent light tubes (cool white) in metal hoods without UV light. It should be born in mind that due to the relative turbidity of Lake Victoria, UV light hardly penetrates into the water (Seehausen et al. 1997). The bottom of the aquaria was covered with gravel. All fishes were fed with flake food (Tetra Min Tropical Fish Flakes) seven times per week and a mixture of ground shrimps and peas two times per week.
(c) Experiment I: aggression bias in wild-caught females
(i) Subjects
Females were collected in Lake Victoria around Makobe Island and transported to the laboratory in Haren, The Netherlands, in February 2003. We tested 15 OB, 16 P and 15 WB females, all wild-caught, between May and July 2003.
(ii) Pre-experimentation housing
For at least one week prior to the experiments, females were individually housed in separate compartments with a PVC tube as a refuge. Approximately 10 compartments were made in 100 or 170 l aquaria using transparent Perspex screen. Females had one or two direct neighbours and visual access to all other females in the tank. The arrangement avoided unwanted effects of social isolation, while preventing them engaging in physical interaction. All females reached territorial condition, as noted by boundary fighting with the neighbouring female(s). Females were visually exposed to females of all three morphs. Test females were never housed adjacent to the corresponding stimulus females.
(iii) Simulated intruder choice test
We used a simulated intruder choice test to estimate aggression biases (Dijkstra et al. 2006, 2007). Test females were allowed to become territorial and were then presented with stimulus females in paired combinations (WB–OB, P–OB and/or P–WB). Since we tested for own-type aggression biases, test females were confronted only with stimulus pair combinations that contained their own morph; each wild-caught female was thus presented with two stimulus pairs. We chose this two-choice context, rather than a three-choice context, because, although this was not our primary objective, in the former situation one can also examine whether the degree of aggression bias differs between the two alternative morphs. This is not possible in a three-choice context because any possible bias for own morph relative to morph A is affected by the presence of morph B and vice versa (Schuck-Paim et al. 2004). The two stimulus pairs were selected from standard length-matched stimulus trios (OB, P and WB). A test aquarium consisted of a large experimental compartment (55×35×39 cm, l×w×h) for the test fish, and a smaller compartment (5×35×39 cm) for a dither neighbour, needed to maintain territorial condition of the test fish. The dither neighbour was a small female of an unrelated Lake Victoria cichlid (Pundamilia nyererei Witte-Maas & Witte 1985). It was separated from the test fish by a transparent Perspex screen. Test females were transferred from the pre-experimentation housing aquarium to the experimental compartment one day before a trial to allow acclimatization to the test aquarium. A PVC tube was provided as a refuge.
At the start of a trial, two stimulus females, individually confined in transparent watertight Perspex tubes, were placed in the experimental compartment 20 cm apart from one another and at the end of the tank opposite to the neighbour fish. No chemical communication was possible between stimulus and test fish. The test females perceived the stimulus females as intruders, and typically responded aggressively to both stimulus females, while the neighbouring P. nyererei female was entirely neglected. We recorded the number of attacks of the test female to each of the stimulus females for 5 min starting directly after introduction of the stimulus females. An attack was defined as biting or butting at the walls of the tube containing the stimulus female. An attack was terminated with a display event, or when the test female turned away from the stimulus female (Dijkstra et al. 2006, 2007). Across trials we randomly assigned stimulus females to left and right positions. The sequence in which the two stimulus pair combinations were presented was also randomized. After a female had been tested, she was used as a stimulus female to test other females. The interval between testing a female and using her as a stimulus was at least one day.
(iv) Analysis experiment I
A female's aggression level was estimated by the total number of attacks she launched in 5 min averaged over all trials of that female. We tested for differences in the level of aggression between morphs using ANOVAs.
Aggression biases were expressed in two ways. The attack ratio towards females of a particular morph was calculated as the number of attacks launched against the stimulus females of that morph divided by the total number of attacks launched in the same trial. The aggression bias can also be expressed as the attack ratio towards females of own morph calculated as the number of attacks launched against the stimulus females of their own morph divided by the total number of attacks launched in the same trial.
Own-morph aggression biases predict different aggression biases between female morphs tested with the same stimulus pair combination (e.g. OB and P are predicted to respond differently to an OB–P stimulus pair). We tested for this by comparing the attack ratio towards females of a particular morph using two-sample t-tests. We then tested whether females had a significant aggression bias towards females of their own morph, using a doubly nested repeated-measure ANOVA (RM-ANOVA), with ‘morph of test female’ (three levels: OB, P and WB) as the explanatory variable and attack ratio towards females of own morph as the response variable. The first repeat (referred to as ‘stimulus pair’) consisted of attack ratios towards females of own morph for the two stimulus female pairs with which a test female was tested. To test whether females had a significant aggression bias towards females of their own morph, we used a second repeat (referred to as ‘bias’) nested within the stimulus pair repeat; the bias repeat tested whether the attack ratio deviated from the no-bias ratio of 0.5.
One wild-caught WB female launched no attacks at all and was not included in the analysis of aggression bias. To meet assumptions of parametric testing, we arcsine-square-root transformed the attack ratio data and square-root transformed the aggression level data. All reported probabilities are for two-tailed tests. Statistical analyses were performed in SPSS v. 12.0.1.
(d) Experiment II: aggression bias in laboratory-bred morph crosses
(i) Subjects and housing
We generated plain and blotched full sib sisters by crossing a blotched with a P parent (blotch is X-linked; Seehausen et al. 1999b). The laboratory crossings are summarized in table 1. With one exception, we used different wild-caught fish for each cross (table 1). We made two cross types: OB female with P male (OB×P), and WB female with P male (WB×P). We also made one cross between a P female and a WB male. All five OB×P families contained P (henceforth Pob) and OB females, and P males (table 1). All five WB×P families contained P (henceforth Pwb) and WB females, and P males, consistent with an existing model of inheritance (Seehausen et al. 1999b). In two of the WB×P families, we also had one WB male each (table 1).
Table 1.
Summary of the crosses to generate laboratory-bred plain and blotched females. (Shown are the family code, morph of mother, father, the number of plain (P) and blotched (OB or WB) sons and daughters. Note that these numbers represent the moment when females were transferred from sib-groups to the pre-experimentation housing, and that numbers at the moment of fry release were different due to mortality. Also shown are the number of plain and blotched daughters that we tested. All parents were wild-caught, unless indicated otherwise (F1=mother bred from wild-caught parents).)
| OB×P family code | mother | father | sons | daughters | number of daughters tested | |||
|---|---|---|---|---|---|---|---|---|
| P | OB | P | OB | P | OB | |||
| 1 | OB | P | 4 | 0 | 3 | 12 | 3 | 4 |
| 2 | OB (F1) | P | 5 | 0 | 3 | 11 | 3 | 5 |
| 3 | OB | P | 6 | 0 | 1 | 4 | 1 | 3 |
| 4 | OB | P | 7 | 0 | 5 | 8 | 1 | 1 |
| 5 | OBa | P | ?b | 0 | 11 | 13 | 5 | 4 |
| — | — | |||||||
| total | 13 | 17 | ||||||
| WB×P family code | mother | father | sons | daughters | number of daughters tested | |||
|---|---|---|---|---|---|---|---|---|
| P | WB | P | WB | P | WB | |||
| 1 | WB | P | 4 | 0 | 3 | 1 | 1 | 1 |
| 2 | WB | P | 3 | 0 | 16 | 14 | 6 | 5 |
| 3 | WB | P | 9 | 0 | 3 | 18 | 2 | 5 |
| 4 | WB(F1) | P | 2 | 0 | 5 | 8 | 2 | 2 |
| 5 | WBc | P | 5 | 1 | 22 | 12 | 4 | 4 |
| 6 | P | WB | 8 | 1 | 4 | 27 | 1 | 4 |
| — | — | |||||||
| total | 16 | 21 | ||||||
This female bred twice with two different males—broods were raised together in a single aquarium.
We could not count males because they were accidentally lost upon removal from the brood tank; all males were plain.
This is a combined brood of two females and a single male—broods were raised together in a single aquarium.
Females grew up in sib groups, guarded by their mother for the first four weeks post-hatching in 100 l aquaria. Fish were thereafter raised in 100 or 170 l aquaria. We removed males as soon as the sexes differentiated at approximately six months of age. When individuals began to mature at an age of four to five months, we added 10–15 juvenile Pundamilia sp. to each family to disperse aggression. Pundamilia are less aggressive than N. omnicaeruleus and are commonly used as dither fish in our breeding aquaria. We randomly selected females for measuring aggression bias. In total, we tested 18 OB, 13 Pob, 22 WB and 16 Pwb laboratory-bred females between October 2004 and January 2005. The number of females tested per sib group is shown in table 1 (note that not all females were included in the analysis, see below).
(ii) Pre-experimentation housing
The pre-experimentation housing was similar to that described for experiment I, except that laboratory-bred females were visually exposed only to females of their own morph and that of their sisters. All females reached territorial condition.
(iii) Simulated intruder choice test
We used the same simulated intruder choice test to estimate aggression biases as described for experiment II. To test for differences in aggression bias between blotched and plain sisters, we tested laboratory-bred Pob and OB females with P–OB stimulus pairs, and laboratory-bred Pwb and WB females with P–WB stimulus pairs. Stimulus pairs could contain sister(s), but individual separation of at least one week reduced any potential effects of prior experience and individual recognition on aggression.
(iv) Analysis experiment II
The statistical analysis was similar to experiment I unless stated otherwise. We first tested for each cross type (WB×P versus OB×P) separately as to whether family explained any significant fraction of the variance in the attack ratio. To this end, we used linear hierarchical models in MlWin v. 2.0, using a two-level model with individual fish nested within families. The response variable was the attack ratio towards P morphs. Family explained none of the variances in attack ratio in any of the cross types, also when morph of test female (plain versus blotched) was included as an explanatory variable (family effect in both cross types: p>0.9). We therefore treated each female as an independent data point in subsequent data analysis. We tested for linkage or pleiotropy between colour and aggression bias against the alternative of independent inheritance of both traits by comparing the attack ratio of plain and blotched females towards P using two-sample t-tests. A significant difference in attack ratio towards P would support linkage or pleiotropy. We also tested whether females had a significant aggression bias towards females of their own morph, using a RM-ANOVA, with morph of test female (plain versus blotched) and cross type (WB×P versus OB×P) as explanatory variables. To test whether females had a significant aggression bias towards females of their own morph, we used a repeat (referred to as bias) to test whether the attack ratio deviated from the no-bias ratio of 0.5.
One laboratory-bred WB and one OB female launched no attacks at all and were not included in the analysis of aggression bias.
3. Results
(a) Experiment I: aggression bias in wild-caught females
The aggression level of OB females was 43.9±21.9 attacks per 5 min (mean±s.d.), that of P females was 46.4±23.2 and WB females 38.7±18.2. These differences were not statistically significant (ANOVA: F2,43=0.65, p=0.53).
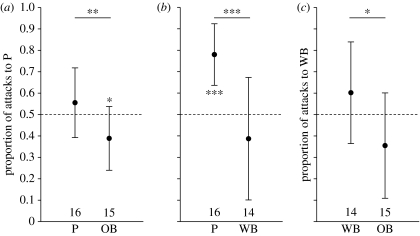
The mean aggression bias of wild-caught females of each morph towards P or WB females is shown in figure 1. Own-morph aggression biases predict differences in attack ratios between females of the different morphs. This was indeed the case. P and OB differed significantly in aggression bias when tested with a P–OB stimulus pair (two-sample t-test: t29=−2.84, p<0.01); P and WB females differed when tested with a P–WB stimulus pair (t30=−4.23, p<0.001); and WB and OB females differed when tested with a WB–OB stimulus pair (t29=2.28, p<0.05). Significance for the first two comparisons (P–OB and P–WB) was retained after a sequential Bonferroni correction to account for multiple testing.
Figure 1.
The attack ratio (mean±s.d.) of wild-caught females of each colour morph ((a), P and OB; (b), P and WB; (c), WB and OB) for each stimulus pair combination ((a), P–OB; (b), P—WB; (c), WB–OB). The sample sizes are indicated at the bottom. The attack ratio is expressed as the proportion of attacks towards females of a particular morph (P in the P–OB and P–WB combination, and WB in the WB–OB combination) relative to the total number of attacks. An attack ratio of 0.5 represents identical numbers of attacks to both stimuli (dashed line). Indicated are differences in attack ratio between females of different morphs using two-sample t-tests and significant deviations of the attack ratio from 0.5 using one-sample t-tests. *p<0.05, **p<0.01, ***p<0.001.
The average value of all ratios of each morph differed in the direction predicted by own-morph aggression bias, although the bias significantly differed from 0.5 in only two out of six cases (figure 1). After a sequential Bonferroni correction to account for multiple testing, this remained significant for only the P morph when presented with a P–WB stimulus pair. Overall, the attack ratio towards females of own morph deviated significantly from 0.5, indicating that females preferentially attacked their own morph (see table 2). The magnitude of this own-morph bias was not different between morphs (see table 2).
Table 2.
The results of the RM-ANOVA in experiment I testing whether wild-caught females biased aggression towards their own morph. (The response variable is the attack ratio towards own morph; the explanatory variable is ‘morph of test female’ (OB, P and WB). The first repeat (‘stimulus pair’) controls for the fact that each test female was presented with two stimulus pairs (results not presented); for example, an OB female was tested with an OB–P and OB–WB pair. The second repeat (‘bias’) was nested within the ‘stimulus pair’ repeat; the ‘bias’ repeat tested whether the attack ratio deviated from the no-bias ratio of 0.5.)
| source | d.f. | F | p |
|---|---|---|---|
| experiment I | |||
| ‘bias’ repeat | 1 | 23.24 | <0.001 |
| morph of test female | 2 | 0.68 | 0.51 |
| error | 42 | ||
(b) Experiment II: lack of aggression bias in laboratory-bred morph crosses
The aggression level of Pob females was 32.6±22.7 attacks per 5 min (mean±s.d.), that of OB females 33.2±26.1, of Pwb females 34.6±23.9 and WB females 27.3±20.4. There was no difference in aggression levels either between females from OB×P families and those from WB×P families (ANOVA: cross type (OB×P versus WB×P), F1,65=0.084, p=0.77) or between blotched and plain individuals (ANOVA: morph of test female (blotched versus plain), F1,65=0.639, p=0.43).
The mean attack ratio towards P females is shown in figure 2. Linkage or pleiotropy between colour and aggression bias predicts a difference in aggression bias between blotched and plain females. Neither Pob nor OB from OB×P families (two-sample t-test: t28=1.38, p=0.18) nor Pwb and WB from WB×P families (t35=−0.293, p=0.77) differed in the attack ratio towards P.
Figure 2.
The attack ratio (mean ± s.d.) for (a) laboratory-bred Pob and OB females derived from OB×P families and (b) laboratory-bred Pwb and WB females derived from WB×P families. The morph and sample sizes are indicated at the bottom, the stimulus pair combination at the top. The attack ratio is expressed as the proportion of attacks to females of P relative to the total number of attacks. An attack ratio of 0.5 represents identical number of attacks to both stimuli (dashed line). We tested for differences in attack ratio between females of different morphs using two-sample t-tests. None of these was significant. The attack ratios never deviated significantly from 0.5 using one-sample t-tests. n.s., not significant.
The attack ratio towards females of own morph did not deviate from 0.5 (see figure 2), suggesting that laboratory-bred females lacked an own-morph aggression bias (see table 3). The effects of cross type and morph of test female were not significant (see table 3).
Table 3.
The results of the RM-ANOVA in experiment II testing whether laboratory-bred female sisters biased aggression towards their own morph (experiment I). (The response variable is the attack ratio towards own morph; explanatory variables are ‘morph of test female’ (blotched versus plain) and cross type (OB×P versus WB×P). The repeat (‘bias’) tested whether the attack ratio deviated from the no-bias ratio of 0.5.)
| source | d.f. | F | p |
|---|---|---|---|
| experiment II | |||
| ‘bias’ repeat | 1 | 0.605 | 0.44 |
| morph of test female | 1 | 1.333 | 0.25 |
| cross type | 1 | 2.089 | 0.15 |
| error | 64 | ||
4. Discussion
Using a simulated intruder choice test and wild-caught fish, we found aggression biases towards own morph among three female colour morphs of N. omnicaeruleus. Although this was only significant in two out of six cases, all three morphs exhibited aggression biases that were in the direction predicted by own-morph aggression biases. Moreover, in the overall model we found a significant own-morph aggression bias when the data of the different morphs were combined. These results suggest that in competition for territories, females of all three morphs may receive fewer attacks, and thereby may experience elevated fitness when their morph is locally the least abundant of the three. Elevated fitness may come about through an elevated probability to access limiting food resources or shelter from predators. Both are important for females themselves and for their fry. The negative frequency-dependent selection that is expected to arise from own-morph aggression biases may both facilitate the invasion of a novel colour type and stabilize existing colour polymorphisms (Mikami et al. 2004; Seehausen & Schluter 2004).
Ecological niche partitioning between the three morphs of N. omnicaeruleus is absent or at least not known—the morphs have fully overlapping microdistributions and trophic morphologies (Seehausen & Bouton 1997; Seehausen et al. 1999b; Maan et al. submitted). Hence, ecological resource competition is unlikely to have led to the evolution of female intrasexual aggression bias towards own-coloured individuals. In N. omnicaeruleus, mutual mate choice has been described. Males (and not females) of the blotched morph exhibit mating preferences against the plain morph. Females and some males of the plain morph exhibit strong mating preferences against the blotched morphs, whereas other males of the plain morph exhibit preferences for one or the other blotched morph (Seehausen et al. 1999b; Pierotti & Seehausen 2007). Mutual mate choice leaves females of the same morph stronger competitors for males than females of different morphs (see also Lande et al. 2001; van Doorn et al. 2004). Such intrasexual competition might have led to the evolution of the observed aggression biases.
The selective mating among colour morphs led Seehausen et al. (1999b) to suggest that the N. omnicaeruleus system has properties of an incipient stage of sympatric speciation by sexual selection. The species has inspired models of sympatric speciation by sexual selection (Lande et al. 2001; Kocher 2004, see also Seehausen et al. 1999b; Maan et al. 2006; Pierotti & Seehausen 2007). Forces that can account for the maintenance of a colour polymorphism, such as own-morph aggression biases, are essential to the process of sympatric speciation (e.g. van Doorn et al. 2004; Dijkstra et al. 2006). Such forces, also among females, would allow time for the development of reproductive barriers and phenotypic divergence between colour morphs.
Given the evidence for considerable gene flow among the N. omnicaeruleus morphs at Makobe Island (Seehausen et al. 1999b), the question has to be asked how the association between colour and aggression bias is maintained. If own-morph biases in aggression express pleiotropically with colour, the emergence and coexistence of incipient species would be greatly facilitated. Such pleiotropy or tight genetic linkage between colour and preference, even though perhaps unlikely at first sight, has recently been demonstrated in Heliconius butterflies (Kronforst et al. 2006). We tested for linkage or pleiotropy by examining aggression biases of laboratory-bred plain and blotched sisters from plain×blotched crosses. In these laboratory crosses, we found no difference in aggression bias between plain and blotched, and no own-morph bias. Thus, we found no support for linkage or pleiotropy. Yet, failure to reject the null hypothesis is weak evidence of no difference when sample sizes are relatively low. We therefore tentatively conclude that linkage or pleiotropy is unlikely to explain the observed own-morph biases in aggression of wild-caught females. The association between colour and aggression bias has, it seems, to be then maintained by assortative mating and/or selection against recombinant phenotypes. While consistent with the hypothesis of Seehausen et al. (1999b) that morphs are partially reproductively isolated by male and female mating preferences, it seems that gene flow between the morphs would have to be very low or disruptive selection very strong. Several forces can contribute to disruptive selection. For example, there might be selection against spawnings between different morphs because these can result in broods with a female-biased sex ratio and may in theory also yield unfit YY males (Seehausen et al. 1999b; Lande et al. 2001). Selection against morph hybrids due to impaired growth, immunocompetence, etc. seems unlikely in Lake Victoria cichlids (van der Sluijs et al. in press), but is still untested in our study species. At any rate, the data of our laboratory-bred females suggest that morph hybrids experience reduced fitness, because they lack an aggression bias towards their most direct competitors for mates.
Recent work indicates that in the Lake Victoria cichlid fish Pundamilia learning may shape mate preferences (Verzijden & ten Cate 2007) as well as aggression biases (Dijkstra et al. in press b; Verzijden et al. in press). Can learning explain the association between colour and aggression bias? We consider this unlikely. Imprinting on the colour of the mother during the fry care phase could explain aggression bias in blotched morphs since most were raised by a blotched mother. However, this cannot explain the own-morph bias for plain morphs, because they not only come from plain but also from blotched mothers. Later in life females forage in dense mixed-morph aggregations, making it unlikely that morph-specific encounter bias affects the development of preferential attack. Can learning effects explain the discrepancy between experiments I and II? It is obvious that laboratory-bred and wild-caught females experienced a different rearing environment. However, the attack ratio comparison of laboratory-bred sister morphs is not confounded by the rearing background because sisters grew up in the same aquarium.
In conclusion, our study shows that wild-caught females of the polymorphic Lake Victoria cichlid N. omnicaeruleus bias aggression towards females of their own colour morph. Such an aggression bias in competition among males has been hypothesized to cause negative frequency-dependent selection on male colour (Mikami et al. 2004; Seehausen & Schluter 2004; van Doorn et al. 2004; Dijkstra et al. 2006). Here we suggest and show evidence that it might also operate in females. The colour polymorphism observed in N. omnicaeruleus is widely distributed among cichlids in several African Lakes (Lande et al. 2001; Kocher 2004). Many other animal species exist in which conspicuous coloration is combined with aggressive behaviour among female morphs (for review see Amundsen & Pärn 2006), such as the Gouldian finch (Pryke 2007) and the white-throated sparrow (Kopachena & Falls 1993). It will be interesting to know how widely spread female aggression biases are.
Acknowledgments
The research was carried out with an animal experiment licence (DEC 2812) from Groningen University and complied with current laws in The Netherlands.We thank Martine Maan, Mhoja Kayeba, Mohamed Haluna, Machteld Verzijden, Inke van der Sluijs, Marcel Häsler, Kees Hofker and John Mrosso for help with the collection of live fish. Sander van Dijk, Iris Bakker and Anneke Procee are sincerely acknowledged for help with experiments. Serge Daan, Albert Ros, Nicolaus Baron von Engelhardt and three anonymous reviewers gave useful comments on the manuscript. The research was financed by a NWO (SLW) grant 810.64.013.
References
- Amundsen T, Pärn H. Female coloration: review of functional and non-functional hypotheses. In: Hill G.E, McGraw K.J, editors. Bird coloration. Function and evolution. vol. 2. Harvard University Press; Cambridge, MA: 2006. pp. 280–348. [Google Scholar]
- Bouton N, Seehausen O, van Alphen J.J.M. Resource partitioning among rock-dwelling haplochromines (Pisces: Cichlidae) from Lake Victoria. Ecol. Freshw. Fish. 1997;6:225–240. doi:10.1111/j.1600-0633.1997.tb00165.x [Google Scholar]
- Chase I.D, Tovey C, Spangler-Martin D, Manfredonia M. Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proc. Natl Acad. Sci. USA. 2002;99:5744–5749. doi: 10.1073/pnas.082104199. doi:10.1073/pnas.082104199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase I.D, Tovey C, Murch P. Two's company, three's a crowd: differences in dominance relationships in isolated versus socially embedded pairs of fish. Behaviour. 2003;140:1193–1217. doi:10.1163/156853903771980558 [Google Scholar]
- Danley P.D, Kocher T.D. Speciation in rapidly diverging systems: lessons from Lake Malawi. Mol. Ecol. 2001;10:1075–1086. doi: 10.1046/j.1365-294x.2001.01283.x. doi:10.1046/j.1365-294X.2001.01283.x [DOI] [PubMed] [Google Scholar]
- Dijkstra P.D, Seehausen O, Gricar B.L.A, Maan M.E, Groothuis T.G.G. Can male–male competition stabilize speciation? A test in Lake Victoria cichlids. Behav. Ecol. Sociobiol. 2006;59:704–713. doi:10.1007/s00265-005-0100-1 [Google Scholar]
- Dijkstra P.D, Seehausen O, Pierotti M.E.R, Groothuis T.G.G. Male–male competition and speciation: aggression bias towards differently coloured rivals varies between stages of speciation in a Lake Victoria cichlid species complex. J. Evol. Biol. 2007;20:496–502. doi: 10.1111/j.1420-9101.2006.01266.x. doi:10.1111/j.1420-9101.2006.01266.x [DOI] [PubMed] [Google Scholar]
- Dijkstra, P. D., van der Zee, E. M. & Groothuis, T. G. G. In press a Territory quality affects female preference in a Lake Victoria cichlid fish. Behav. Ecol. Sociobiol (doi:10.1007/s00265-007-0500-5)
- Dijkstra, P. D., Seehausen, O., Fraterman, R. E. & Groothuis, T. G. G. In press b Learned aggression biases in males of Lake Victoria cichlid fish. Anim. Behav
- Fryer G, Iles T.D. Oliver and Boyd; London, UK: 1972. The cichlid fishes of the great lakes of Africa: their biology and evolution. [Google Scholar]
- Genner M.J, Turner G.F. The mbuna cichlids of Lake Malawi: a model for rapid speciation and adaptive radiation. Fish Fish. 2005;6:1–34. [Google Scholar]
- Genner M.J, Turner G.F, Barker S, Hawkins S.J. Niche segregation among Lake Malawi cichlid fishes? Evidence from stable isotope signatures. Ecol. Lett. 1999a;2:185–190. doi:10.1046/j.1461-0248.1999.00068.x [Google Scholar]
- Genner M.J, Turner G.F, Hawkins S.J. Resource control by territorial male cichlid fish in Lake Malawi. J. Anim. Ecol. 1999b;68:522–529. doi:10.1046/j.1365-2656.1999.00301.x [Google Scholar]
- Gray S.M, McKinnon J.S. Colour polymorphism and implications for speciation. Trends Ecol. Evol. 2007;22:71–79. doi: 10.1016/j.tree.2006.10.005. doi:10.1016/j.tree.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Greenwood P.H. Species flocks and explosive speciation. In: Greenwood P.H, Forey P.L, editors. Chance, change and challenge—the evolving biosphere. Cambridge University Press; London, UK: 1981. pp. 61–74. [Google Scholar]
- Hutchinson G.E. Paradox of the plankton. Am. Nat. 1961;95:137–145. doi:10.1086/282171 [Google Scholar]
- Huxley J. Morphism in birds. Proc. Int. Ornithol. Congr. 1955;11:309–328. [Google Scholar]
- Knight M.E, Turner G.F. Laboratory mating trials indicate incipient speciation by sexual selection among populations of the cichlid fish Pseudotropheus zebra from Lake Malawi. Proc. R. Soc. B. 2004;271:675–680. doi: 10.1098/rspb.2003.2639. doi:10.1098/rspb.2003.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher T.D. Adaptive evolution and explosive speciation: the cichlid fish model. Nat. Genet. 2004;5:289–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- Kopachena J.G, Falls J.B. Aggressive performance as a behavioural correlate of plumage polymorphism in the white-throated sparrow (Zonotrichia albicollis) Behaviour. 1993;124:249–266. [Google Scholar]
- Kronforst M.R, Young L.G, Kapan D.D, McNeely C, O'Neill R.J, Gilbert L.E. Linkage of butterfly mate preference and wing colour preference cue at the genomic location of wingless. Proc. Natl Acad. Sci. USA. 2006;103:6575–6580. doi: 10.1073/pnas.0509685103. doi:10.1073/pnas.0509685103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Seehausen O, van Alphen J.J.M. Mechanisms of rapid sympatric speciation by sex reversal and sexual selection in cichlid fish. Genetica. 2001;112-113:435–443. doi:10.1023/A:1013379521338 [PubMed] [Google Scholar]
- Maan M.E, Seehausen O, Soderberg L, Johnson L, Ripmeester A.P, Mrosso H.D.J, Taylor M.I, van Dooren T.J.M, van Alphen J.J.M. Intraspecific sexual selection on a speciation trait, male colouration, in the Lake Victoria cichlid Pundamilia nyererei. Proc. R. Soc. B. 2004;271:2445–2452. doi: 10.1098/rspb.2004.2911. doi:10.1098/rspb.2004.2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maan M.E, Haesler M.P, Seehausen O, van Alphen J.J.M. Heritability and heterochrony of polychromatism in a Lake Victoria cichlid fish: stepping stones for speciation? J. Exp. Zool. B: Mol. Dev. Evol. B. 2006;306B:168–176. doi: 10.1002/jez.b.21083. doi:10.1002/jez.b.21083 [DOI] [PubMed] [Google Scholar]
- Maan, M. E., Eshuis, B., Haesler, M. P., Schneider, M. V., van Alphen, J. J. M. & Seehausen, O. Submitted. Color polymorphism and predation in a Lake Victoria cichlid fish.
- Mikami O.K, Kohda M, Kawata M. A new hypothesis for species coexistence: male–male repulsion promotes coexistence of competing species. Popul. Ecol. 2004;46:213–217. doi:10.1007/s10144-004-0189-5 [Google Scholar]
- Pierotti M.E.R, Seehausen O. Male mating preferences predate the origin of a female trait in an incipient species complex of Lake Victoria cichlids. J. Evol. Biol. 2007;20:240–248. doi: 10.1111/j.1420-9101.2006.01206.x. doi:10.1111/j.1420-9101.2006.01206.x [DOI] [PubMed] [Google Scholar]
- Pryke S.R. Fiery red heads: female dominance among head color morphs in the Gouldian finch. Behav. Ecol. 2007;18:621–627. doi:10.1093/beheco/arm020 [Google Scholar]
- Ribbink A.J. Distribution and ecology of the cichlids of the African Lakes. In: Keenleyside M.H.A, editor. Cichlid fishes. Behaviour, ecology and evolution. Chapman and Hall; London, UK: 1991. pp. 36–102. [Google Scholar]
- Scheffer M, van Nes E.H. Self-organized similarity, the evolutionary emergence of groups of similar species. Proc. Natl Acad. Sci. USA. 2006;103:6230–6235. doi: 10.1073/pnas.0508024103. doi:10.1073/pnas.0508024103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck-Paim C, Pompilio L, Kacelnik A. State-dependent decisions cause apparent violations of rationality in animal choice. PLoS Biol. 2004;2:1–11. doi: 10.1371/journal.pbio.0020402. doi:10.1371/journal.pbio.0020402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen O. Verduyn Cichlids; Zevenhuizen, The Netherlands: 1996. Lake Victoria rock cichlids: taxonomy, ecology and distribution. [Google Scholar]
- Seehausen O. African cichlid fish: a model system in adaptive radiation research. Proc. R. Soc. B. 2006;273:1987–1998. doi: 10.1098/rspb.2006.3539. doi:10.1098/rspb.2006.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen O, Bouton N. Microdistribution and fluctuations in niche overlap in a rocky shore cichlid community in Lake Victoria. Ecol. Freshw. Fish. 1997;6:161–173. doi:10.1111/j.1600-0633.1997.tb00159.x [Google Scholar]
- Seehausen O, Schluter D. Male–male competition and nuptial-colour displacement as a diversifying force in Lake Victoria cichlid fishes. Proc. R. Soc. B. 2004;271:1345–1353. doi: 10.1098/rspb.2004.2737. doi:10.1098/rspb.2004.2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen O, van Alphen J.J.M. The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex) Behav. Ecol. Sociobiol. 1998;42:1–8. doi:10.1007/s002650050405 [Google Scholar]
- Seehausen O, van Alphen J.J.M, Witte F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science. 1997;277:1808–1811. doi:10.1126/science.277.5333.1808 [Google Scholar]
- Seehausen O, Witte F, van Alphen J.J.M, Bouton N. Direct mate choice maintains diversity among sympatric cichlids in Lake Victoria. J. Fish Biol. 1998;53(Suppl. A):37–55. [Google Scholar]
- Seehausen O, van Alphen J.J.M, Witte F. Can ancient colour polymorphisms explain why some cichlid lineages speciate rapidly under disruptive sexual selection? Belg. J. Zool. 1999a;129:43–60. [Google Scholar]
- Seehausen O, van Alphen J.J.M, Lande R. Colour polymorphism and sex ratio distortion in a cichlid fish as an incipient stage in sympatric speciation by sexual selection. Ecol. Lett. 1999b;2:367–378. doi:10.1046/j.1461-0248.1999.00098.x [Google Scholar]
- van der Sluijs, I., van Dooren, T. J. M., Seehausen, O. & van Alphen, J. J. M. In press. A test of fitness consequences of hybridization in sibling species of Lake Victoria cichlid fish. J. Evol. Biol [DOI] [PubMed]
- van Doorn G.S, Dieckmann U, Weissing F.J. Sympatric speciation by sexual selection: a critical re-evaluation. Am. Nat. 2004;163:709–725. doi: 10.1086/383619. doi:10.1086/383619 [DOI] [PubMed] [Google Scholar]
- Verzijden M.N, ten Cate C. Early learning influences species assortative mating preferences in Lake Victoria cichlid fish. Biol. Lett. 2007;3:134–136. doi: 10.1098/rsbl.2006.0601. doi:10.1098/rsbl.2006.0601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijden, M. N., Korthof, R. E. M. & ten Cate, C. In press. Females learn from mothers, and males learn from others. The effect of mother and siblings on the development of female mate preference and male aggression biases in Lake Victoria cichlids, genus Mbipia. Behav. Ecol. Sociobiol