Abstract
The ecological role of interference competition through toxin production is not well understood. In particular, it is unclear under what conditions the benefits of toxic killing outweigh the metabolic costs involved. A killer advantage has been suggested to rely on local competitive interactions where the benefits of killing accrue to the toxin producer preferentially, but this notion has little empirical support. In addition, contrasting predictions exist about the effect of resource abundance on the benefits of toxin production; this benefit should either be highest when resources are abundant and metabolic costs are relatively low or when resources are scarce and toxic killing is a ‘last resort strategy’ to obtain nutrients. Here, we test these predictions for one aspect of competitive ability, that is, the ability of toxin producers to invade a population of sensitive non-producers from a low initial frequency. We use competition experiments between isogenic K1 toxin-producing and non-producing strains of Saccharomyces cerevisiae, where we manipulate dispersal under two extreme nutrient conditions: one environment with and the other without replenishment of nutrients. We find that toxin production is beneficial when dispersal is limited under both nutrient conditions, but only when resources are abundant these outweigh its cost and allow invasion of the producer.
Keywords: interference competition, spatial structure, dispersal, toxin production, yeast
1. Introduction
Interference competition through toxin production is a widespread phenomenon in the microbial world. Toxin-producing strains of Escherichia coli constitute on average approximately 30% of the natural populations (Riley & Gordon 1999), while in some environments (e.g. among human isolates) their frequency can be 50% (Riley & Gordon 1992). Ninety-nine per cent of all bacteria are thought to produce at least one bacteriocin (Klaenhammer 1988). Toxin-producing yeasts have been isolated from a wide range of habitats, including fruits, trees and ant nests, where they make up 5–30% of the populations (Starmer et al. 1987, 2003; Carreiro et al. 2002; Gulbiniene et al. 2004). These toxins are generally aimed at killing sensitive individuals of the same or closely related species. Despite its ubiquity, the ecological and evolutionary significance of toxin production is not well understood (Starmer et al. 1987; Riley & Gordon 1999; Czaran et al. 2002). Toxin production usually incurs metabolic costs which make producers inferior resource competitors relative to their non-producing-sensitive counterparts (Pintar & Starmer 2003). In order for producers to invade a population of sensitive non-producers, these costs must therefore be compensated by benefits resulting from killing. Both the costs and benefits of toxin production are known to depend on environmental conditions (Frank 1994; Dykes & Hastings 1997; Pintar & Starmer 2003). We are interested in the effect of two environmental factors in particular on the ability of toxin producers (killers) to invade a population of non-producers (sensitives): the level of dispersal allowed by the spatial structure of the environment, and the availability of nutrients.
With limited dispersal, the competitive interactions between killer and sensitive individuals are localized, with two possible advantages for the killer: access to nutrients that would have otherwise been consumed by the sensitives, and disproportional feedback of nutrients from killed individuals to the killer (Chao & Levin 1981; Ganter & Starmer 1992; Czaran et al. 2002; Kerr et al. 2002). With dispersal, these two benefits are experienced more equally by killers and non-killed sensitives. In a classical study with toxin-producing and non-producing non-motile E. coli growing either in shaken liquid cultures or in soft agar, Chao & Levin (1981) showed that the lack of dispersal was a prerequisite for the successful invasion of killer bacteria from low initial frequencies. In shaken liquid cultures, where dispersal was necessarily high, the killer cells could not invade, except when their frequency became more than 2%, while no threshold for invasion was found with low dispersal in soft agar. However, the liquid and agar environment are likely to present different selective conditions (Habets et al. 2006), and hence the metabolic costs of toxin production may be different in both the environments.
Opposing views exist about the effect of nutrient availability on the invasion success of a killer. Killers may have an advantage in productive habitats, because more resources are available for the production of toxins and the costs of production may be reduced (Starmer et al. 1987), especially under conditions where killing occurs only at high toxin production rates (Brown et al. 2006). However, toxin production may also serve as a ‘last resort’ strategy to obtain nutrients from killed competitors when no other nutrients are available (Fabrizio & Longo 2003; Ivanovska & Hardwick 2005). Although toxins are optimally produced under growth conditions (Starmer et al. 1987), low toxin production may be effective in inducing apoptosis in sensitive cells (Ivanovska & Hardwick 2005; Reiter et al. 2005; Schmitt & Breinig 2006), possibly allowing a competitive advantage under these conditions. Which view is more accurate depends on the actual costs and benefits of toxic killing and how these depend on the spatial scale of competitive interactions and nutrient availability in any given environment.
In the present study, we seek to test the effect of dispersal and nutrient availability on the ability of Saccharomyces cerevisiae K1 killers to invade populations of sensitive cells. Toxin production and secretion in yeast killer strains is not suicidal (as in most bacteria) and relies on the cytoplasmic presence of two viruses, one encoding toxin and immunity proteins and the other encoding the genes for the encapsulation of the viruses (Magliani et al. 1997). Depending on its concentration, toxin K1 can induce both necrosis, by forming ion channels in the cytoplasmic membrane, and apoptotic cell death of the ScV-M-deprived cells (Ivanovska & Hardwick 2005; Reiter et al. 2005; Schmitt & Breinig 2006). We constructed isogenic killer and sensitive strains carrying a different genetic marker, which were allowed to compete on agar medium for 80 days (i.e. 400 generations) along with control competitions involving a killer strain cured from its viruses. To manipulate dispersal and nutrient availability, we used a two-by-two design: populations were either transferred to fresh nutrient agar every 2 days (allowing growth) or were left on the original plates (causing starvation), and populations were either mixed every 2 days (high dispersal) or the population structure was left intact as much as possible (low dispersal). Our results indicate a fitness cost of toxin production of approximately 3% when dispersal is allowed and resources are abundant, and confirm the importance of limited dispersal for killer invasion from a low initial frequency, while they refute the hypothesis that killing is a last resort strategy.
2. Material and methods
(a) Construction of isogenic killer, sensitive and control strains
All strains used in the experiment originate from a collection of haploid baker yeast S. cerevisiae BY4741 and BY4742, Open Biosystems YSC 1063 and YSC 1064 (Winzeler et al. 1999). Susceptibility of the sensitive (S) strain (MATa/MATα ΔHO:HphMX4/ΔHO:HphMX4, LYS2/lys2 MET15/met15) was confirmed by standard eclipse assay (Kishida et al. 1996). Transfer of the ScV-M1 virus and helper virus L-A from S. cerevisiae strain Y55 into the BY background to obtain a K1 killer (K) strain (MATa/MATα ΔHO:KanMX4/ΔHO:NatMX4, LYS2/lys2, MET15/met15 ScV-M1) involved mating with BY kar1 ScV-M1. For this purpose, we performed the following manipulations. First, strain Y55 MATa ScV-M1 (killer strain) was mated with the strain BY MATα kar1 cyh2r ρ−. A mutation in kar1 prevents the nuclei from fusing, but allows cytogamy (Conde & Fink 1976). The presence of killer viruses ScV-M1 and L-A in the haploid strain BY MATα ura3 kar1 cyh2r from this cross was checked by standard eclipse assay. Second, strain BY MATα kar1 cyh2r ScV-M1 was mated with BY MATa ΔHO:KanMX4 ρ− leading to BY MATa ΔHO:KanMX4 ScV-M1. Third, from the obtained killer, colonies with a ‘petite phenotype’ were selected, and crossed with BY MATα ΔHO:NatMX4 to obtain the diploid strain used as killer strain in the experiment, MATa/MATα (ΔHO:KanMX4/ΔHO:NatMX4, LYS2/lys2, MET15/met15 ScV-M1). The presence of the killer viruses was confirmed by its isolation from the cells (Schmitt & Tipper 1990). A copy of the K strain was cured from the presence of the ScV-M1 virus by growing it at an increased temperature of 38°C (Wickner 1974) in order to obtain a strain (C) to control for the effect of the antibiotic marker. The absence of killing ability of strain C was confirmed by standard eclipse assay. Finally, by crossing strains from the haploid collection (Winzeler et al. 1999), a 100% resistant strain (R) to killer toxin (BY ΔHO:KanMX4/ΔHO:Kan/MX4, LYS2/lys2, MET15/met15 kre1/kre1) (Page´ et al. 2003) was obtained. This strain served as reference strain for competition assays of resource competitive ability of the ancestral K, S and C strains. Molecular inserts ΔHO:KanMX4, ΔHO:NatMX4 and ΔHO:HphMX4 provide cell resistance to the antibiotics, such as geneticin, nourseothricin and hygromycin B, respectively, (Goldstein & McCusker 1999) and are markers which are easy to screen for (Wach et al. 1994; Goldstein & McCusker 1999) and presumably neutral with respect to fitness (Baganz et al. 1997).
(b) Experimental conditions
Experimental strains S, K and C were spread to single colonies on standard YPD agar; for contents of all the media used in the experiment, see Rose et al. (1990). To 200 μl of liquid YPD in microtitre plates, 126 random clones each of K and C, and 252 colonies of S were transferred and incubated while shaking (260 rev min−1) at 30°C for 24 hours to approach final cell density (approx. 1×108 cells ml−1). Next, K and S, as well as C and S clonal populations were mixed 1 : 104, and approximately 106 cells of each mixture were evenly spread on 10 ml of low-pH YPD agar buffered with phosphate-citrate buffer to pH 4.7 in a 6 cm Petri dish and incubated at 25°C. These conditions are favourable for K1 toxin production and activity (Schmitt & Breinig 2002). Populations were allowed to interact and evolve for 80 days in four experimental conditions differing in nutrient availability and the degree of dispersal allowed.
(i) Starvation environment (Nut−)
One hundred and twenty mixed populations of the combination K/S and C/S each were initiated on the same day on low-pH YPD agar and incubated at 25°C for a maximum of 80 days until they were harvested without transfer to fresh nutrient medium.
(ii) Growth environment (Nut+)
Six mixed populations of the combination K/S and C/S each were initiated on low-pH YPD agar, incubated at 25°C, and every 48 hours 0.1% of each mixed population was transferred to fresh medium. In both the nutrient treatments, half of the populations were mixed every 48 hours to allow dispersal (Dis+); of the other half, the spatial structure was left intact and no dispersal was allowed (Dis−). For the Nut+ populations, Dis+ treatment involved washing the cells from the agar and transferring 0.1% by pipette, while Dis− treatment involved transferring approximately 0.1% by means of a sterile velvet cloth. For the Nut− populations, Dis− treatment meant leaving the populations untouched, while for the Dis+ treatment the population structure was destroyed by adding 0.5 ml sterile water and mixing with a glass rod. At regular intervals, the frequency of K, C and S of three replicate populations of each of the four treatments was estimated by plating dilutions on YPD agar. After 3 days of incubation at 25°C, colonies were replicated on two distinct YPD plates, containing geneticin and hygromycin B, respectively, to distinguish K and C from S colonies, and incubated for 2 days, after which colonies were counted.
(c) Fitness of K, S and C
Relative fitness of each of the three strains (K, S and C) in the Nut+ Dis+ environment was measured by competing randomly chosen clones against strain R which is fully resistant to the K1 toxin (Page´ et al. 2003) and assuming transitivity of fitness interactions. Monocultures of 1% of the stationary phase cultures were grown for 24 hours on the low-pH YPD plates to adjust them physiologically to the experimental conditions. The cells were washed off with 10 ml of water, equal proportions of the competitors were mixed and 10 μl of this mix was spread on 10 ml of low-pH YPD agar. At the beginning and after 48 hours of competition, the frequency of both competitors was estimated by plating dilutions on non-selective YPD agar, and replicating colonies after 3 days with a velvet cloth on YPD medium with the antibiotic corresponding to each marker. Fitness of each of the three strains (K, S and C) relative to reference strain R was computed as the ratio of the Malthusian parameters of both the strains (Lenski et al. 1991). At least 20 replicate competition experiments per strain combination were performed.
(d) Killing assay during starvation
We distinguished natural mortality of the ageing population from mortality caused by the killer under conditions of starvation (Nut−) by checking the frequency of monocultures and 1 : 10 mixtures of K and S with threefold replication after 2, 4, 10, 15 and 21 days of incubation.
(e) Statistical analyses
Repeated-measures ANOVA was used to test the effect of strain (C versus K), dispersal and time (and their interactions) on the log ratio of the strain densities (C/S and K/S) in the Nut+ environment, because there the same three populations were repeatedly assayed. We restricted this analysis to 11 equally dispersed time points during the first 50 days due to restrictions in the statistical software (Systat, SPSS, Inc. 1998). To measure the frequency of both strains from the Nut− environment, populations were destroyed, and hence conventional three-way ANOVA was used to test the effect of the above factors for these populations. To test changes in the ratio of the frequency of both strains in competition and in monoculture in the 21-day growth experiment under Nut− conditions, we used the jackknife procedure (Sokal & Rohlf 1981, pp. 795–799). This yielded 12 pseudovalues (two time points per interval×two culture conditions (mix or monoculture)×three replicates), which were used to calculate the s.e.s in figure 3 and for testing the effect of period, dispersal and strain, as well as their interactions in ANOVA (table 1).
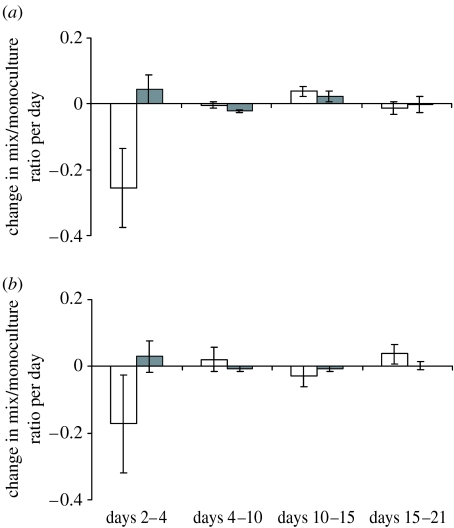
Figure 3.
Assay of toxic killing in the Nut− environment. The change in frequency of both the strains (S, white bars and K, grey bars) in competition relative to monoculture in four constitutive intervals during the 21 days of the experiment is shown. (a) Dis+ environment and (b) Dis− environment. Error bars reflect the s.e. based on pseudovalues from the jackknife procedure (see §2).
Table 1.
ANOVA of the change in frequency of S and K when in competition relative to the corresponding monoculture in the Nut− environment during the first time interval (days 2–4) versus three later intervals combined (days 4–10, 10–15 and 15–21). Values used in ANOVA are 12 pseudovalues from jackknife for each period (see §2) and expressed as change in relative frequency per day.
| source | d.f. | MS | F | p |
|---|---|---|---|---|
| strain | 1 | 0.5211 | 16.037 | 0.000090 |
| dispersal | 1 | 0.0096 | 0.295 | 0.587 |
| period | 1 | 0.2976 | 9.158 | 0.00283 |
| strain×dispersal | 1 | 0.0239 | 0.740 | 0.392 |
| strain×period | 1 | 0.6157 | 18.948 | 0.000022 |
| dispersal×period | 1 | 0.0105 | 0.325 | 0.570 |
| strain×dispersal×period | 1 | 0.0187 | 0.575 | 0.449 |
| error | 184 | 0.0325 |
3. Results
(a) Cost of toxin production
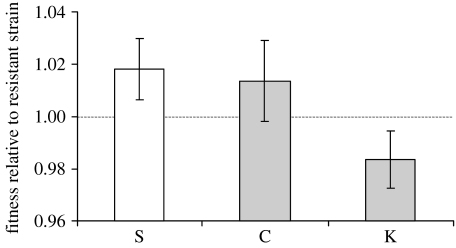
The three ancestral strains (K, S and C) were competed against the resistant (R) reference strain with at least 20-fold replication under conditions identical to the Nut+ Dis+ environment to estimate the fitness cost of toxin production. Relative fitness varies among the three strains (figure 1; F2,75=9.73, p=0.00018). Post hoc Tukey's tests indicate that the lower fitness of K is not due to a marker effect, because S and C have similar fitness (p=0.86), but due to toxin production, because K's fitness is not only lower than that of S (p=0.00024) but also than that of C (p=0.0044), which carries the same antibiotic marker and was derived from K by curing it from its toxic viruses. We estimate the metabolic cost of toxin production in the Nut+ Dis+ environment from the difference in fitness of K and S relative to R, which is approximately 3.4%.
Figure 1.
Fitness of the killer (K), sensitive (S) and control (C) strains relative to a resistant reference strain measured in direct competition in the Nut+ Dis+ environment with at least 20-fold replication. C and K carry the same genetic marker (grey bars), which is different from that of S (white bar). Error bars represent 95% CIs.
(b) Condition-dependent benefit of toxin production
To test whether the benefit of killing can outweigh the fitness cost of toxin production, we studied the competitive dynamics of three replicate 1 : 10 000 mixtures of K and S (allowing killing and invasion) over the course of 80 days, and compared those with control mixtures of C and S where no killing is involved. Because our interest is the effect of dispersal and nutrient availability on the competitive ability of the toxin producer, competition experiments were done in four different environments: allowing growth (Nut+) or causing starvation (Nut−), and with (Dis+) or without (Dis−) mixing to force dispersal every 2 days.
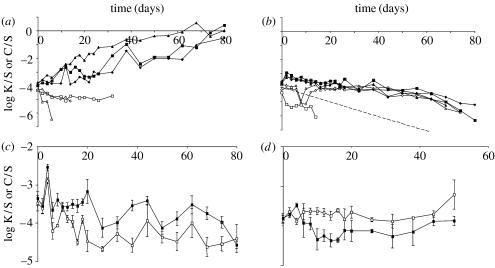
In the Nut+ environments, the benefit of toxin production depends on the rate of dispersal allowed. Only when dispersal is limited (figure 2a), K repeatedly invades the population of S from its initial frequency of 10−4 (linear regression of average log strain ratio versus time, Dis−: slope=0.0452 per day, F1,20=296.7, p<0.0001), while C suffers a non-significant competitive disadvantage (slope=−0.0192, F1,11=1.605, p=0.23), causing the extinction of C in two of the three replica populations within 16 days (figure 2a). When tested using repeated-measures ANOVA of the log strain ratio, K's overall frequency is higher than that of C (F1,3=35.78, p=0.0094), while the difference in response of both the strains in time (i.e. K invading and C declining) is marginally significant as well (time×strain combination interaction: F10,30=2.11, p=0.056). When dispersal is allowed (figure 2b), K slowly but consistently declines in frequency (Dis+: slope=−0.0258 per day, F1,21=182.8, p<0.0001), as does C (slope=−0.0120, F1,20=10.34, p=0.0043) at a rate indistinguishable from that of K (time×strain combination interaction of repeated-measures ANOVA: F10,20=1.54, p=0.20). In this environment, where we estimated the cost of toxin production to be approximately 3.4%, we expect log (K/S) to decline at a rate of 0.0515 per day without the benefit of toxic killing (dashed line in figure 2b; see Lenski et al. 1991). Although the benefit of killing does not outweigh its cost when dispersal is allowed, the rate of decline of K is slower than this expected rate (one-sample t-test: t2=11.94, two-tailed p=0.0069), indicating some benefit of killing also under these conditions.
Figure 2.
Trajectories of the log ratio of K versus S and C versus S densities during the 80 days of competition in three replicate populations in four environments: (a) Nut+ Dis−, (b) Nut+ Dis+, (c) Nut− Dis− and (d) Nut− Dis+ environment. Closed symbols are for K/S and open symbols for C/S. The dashed line in (b) shows the expected trajectory based on the resource competitive difference between K and S only (i.e. without the effect of toxic killing). To estimate the frequency of K and S in the Nut− environment, populations had to be destroyed, and hence the trajectories reflect the average and s.e. of three different replicate populations for each time point.
In the environment where nutrients were not replenished after depletion of the first batch (Nut−), competitive dynamics are much slower (figure 2c,d). Irrespective of dispersal, K cannot invade under these conditions and rather declines in frequency (Dis−: slope=−0.0100, F1,19=11.87, p=0.0027; Dis+: slope=−0.0031, F1,14=0.510, p=0.49). However, the relative benefit of K compared with C when dispersal is limited (figure 2c,d; dispersal×strain combination interaction of three-way ANOVA using data until day 50: F1,123=89.91, p<0.0001) is consistent with a benefit of toxic killing also under these conditions. This is supported by comparing K's frequency directly between the Dis− and Dis+ environment: it is higher without than with dispersal (two-way ANOVA of log (K/S) until day 50: F1,61=58.91, p<0.0001). Therefore, although these results support some benefit of toxin production also under conditions of starvation, they refute the ‘last resort hypothesis’.
(c) Killing during starvation
The relative lack of competitive dynamics in the starvation environment (Nut−) either reflects physiological changes in killing ability (e.g. due to a downregulation of toxin production; Gray et al. 2004) or toxin resistance induced by the starvation conditions, or a roughly stable balance between the cost and benefit of toxin production under these conditions of metabolic constraint. To distinguish between these alternatives, we tested whether killing is happening during a 21-day growth experiment under Nut− conditions by comparing the densities of K and S in monoculture with those in 1 : 10 mixtures of K and S. Killing is expected to show a decline in the density of S in the mixture relative to its density in monoculture, associated by an increase of K in the mixture relative to the monoculture. Figure 3 shows these changes in the relative densities of both the strains per day in each of four constitutive intervals in the Nut− environment with (figure 3a) and without dispersal (figure 3b). Effective killing is only apparent during the first time interval, that is, from days 2 to 4 after inoculation of the cultures. To test the significance of this pattern, we calculated pseudovalues of each time-averaged change in relative frequency using the jackknife procedure based on the 12 frequency estimates per interval and used these values in analysis of variance (table 1). When the first period is compared with the three combined later periods, we find a significant strain×period interaction, consistent with toxic killing during the first few days only (figure 3). Dispersal has no significant effect on this pattern. These results suggest that the lack of competitive dynamics in the Nut− environment (figure 2c,d) is caused by physiological changes in toxin production and/or resistance after the nutrients have been depleted rather than a stable balance between the cost and benefit of toxin production.
4. Discussion
Despite the ubiquity of toxin production in the microbial world and beyond, the ecology and evolution of this and other forms of interference competition is not well understood. Previous theoretical work predicted the importance of local competitive interactions for the invasion of a toxin producer (Case & Gilpin 1974; Frank 1994; Czaran et al. 2002; Kerr et al. 2002; Czaran & Hoekstra 2003). In addition, some (e.g. Frank 1994) suggested that resources should be abundant for toxin producers to be successful, while others suggested a function in competition during starvation (Ivanovska & Hardwick 2005; Schmitt & Breinig 2006). Few studies have actually tested these predictions (Chao & Levin 1981; Kerr et al. 2002; Ivanovska & Hardwick 2005). We present the first study of the combined effect of dispersal and nutrient availability on the competitive advantage of toxin production using isogenic toxin-producing and non-producing strains of the yeast S. cerevisiae. Different from previous work (Chao & Levin 1981), we manipulate dispersal in the same environment, use competitions between a cured killer and sensitive strain to directly control for possible marker effects, and monitor competition over a longer time period (i.e. 80 instead of 18 days). We find that the benefit of toxic killing is greater than its metabolic cost (of approx. 3%) only when nutrients are abundant and dispersal is limited. With dispersal, a significant benefit of killing was still observed, but it was insufficient to compensate for its resource competitive cost. A benefit of toxin production was also seen under conditions of starvation, but this was again too small to allow invasion of the producing strain. Our findings, therefore, do not support the last resort hypothesis, which states that toxin production may have evolved as a strategy to release nutrients from killed cells when no other nutrients are available (Ivanovska & Hardwick 2005). We find that the reason for this is a physiological change in toxin production and/or resistance soon after nutrient depletion, rather than a stable balance between the costs and benefits involved under these conditions.
Our results on the effects of dispersal are consistent with those from a classical study using colicin-producing and non-producing strains of the bacterium E. coli (Chao & Levin 1981). Chao & Levin also found that limited dispersal in a spatially structured environment allowing growth was crucial for the toxin producer to invade a population of isogenic-sensitive bacteria in the short term. By varying the initial ratio of producer and non-producer, they found that the toxin producer could also invade an environment with high dispersal if the producer had a sufficiently high initial frequency. Although we did not vary the initial frequency of the toxin producer, and we also observed a significant advantage from toxin production with dispersal (figure 2b), but it was too low to compensate for its cost. Chao & Levin manipulated dispersal by comparing a mixed liquid culture with populations growing in soft agar, which may present different selective conditions (Habets et al. 2006) and hence different costs of toxin production. To avoid these complications, we used a single agar environment and manipulated dispersal by either washing the cells off at transfer and mixing a sample on a fresh plate (Dis+ environment) or by replica plating a population sample onto a fresh plate using a velvet cloth (Dis− environment). Interestingly, our high-dispersal environment resembles Chao & Levin's low-dispersal environment (except that our populations grew on top of hard agar instead of inside soft agar), and hence our results do not directly support theirs. The fact that we find the toxin producer to decline where they observed its invasion shows that the balance between the costs and benefits of toxin production is different in these two systems.
Our results support the notion that toxin production has evolved as a competitive strategy under conditions where resources are abundant and growth is allowed (Frank 1994). In contrast to the survival advantage of K1 killers during starvation found by Ivanovska & Hardwick (2005), we observed no consistent killer invasion during a similar time period (figure 3), nor during a longer period (figure 2c,d). We found a relative benefit of K under starvation conditions when dispersal was limited, but this seemed to result from a short-term advantage of killing during the first few days of competition only (figure 2c,d). How can these different results be understood? First, our manipulation of nutrient availability is rather extreme, and involves differences in the number of generations, desiccation and other conditions in addition to nutrient starvation, which may affect the relative success of the toxin producer. For instance, the much lower number of generations in the Nut− environments necessitates the advantage of the killer to be large in order to be detectable. Second, the starting frequency of killer cells was much lower in our long-term competition experiment (10−4) and slightly lower in our 21-day growth assay (0.1) than in Ivanovska and Hardwick's study (0.4). If the benefit of killing is not only positively frequency dependent in the Nut+ Dis+ environment, as observed by Chao & Levin (1981) for colicin, but also in the Nut− Dis+ environment, toxin producers need a sufficiently high frequency before they can invade. Such frequency dependence could arise when toxic killing is decreased during starvation (e.g. due to a downregulation of production or increased toxin resistance) to a level where only a sufficiently large population of killer cells produces enough toxin to kill sensitive individuals, the release of whose nutrients may subsequently support killer growth and invasion. Finally, whereas Ivanovska & Hardwick used liquid cultures, we studied the invasion of a killer strain on agar surface, probably involving different selective conditions (e.g. Habets et al. 2006) and hence different consequences for the competitive ability of the toxin producer.
The production of anti-competitor toxins can be considered as spiteful behaviour, because it not only incurs a cost on the recipient but also on the producer (Gardner et al. 2004; West et al. 2006). The evolution of spite is problematic, because the benefits involved need to be returned disproportionally to the producers' kin in order to be benefited by natural selection. Spatially structured environments may be helpful by increasing the local relatedness of non-motile organisms. However, environmental structure also increases local sib competition, which reduces the realized benefits (Gardner et al. 2004; Habets et al. 2007). This conflict suggests that the advantage of toxin production should be maximal with intermediate levels of dispersal, causing sufficient local relatedness to allow kin to profit from toxin production, while competition is sufficiently global to allow these benefits to be partly realized (Gardner et al. 2004). The prediction that invasion of the toxin producer is fastest with intermediate dispersal cannot be verified with our data, because we used high and low dispersal only, but it may explain some of the discrepancies between the conditions for killer invasion in our study and that of Chao & Levin (1981).
The ecological conditions which we found to benefit toxic killing, that is, abundant resources and local competitive interactions, coincide with those that are thought to have been important for its evolution. Abundant resources and local resource competition are conditions known to severely limit adaptation by improved resource competitive ability due to the limited impact of resource competitive superiority on realized fitness (Frank 1994; Habets et al. 2007). Hence, improvement of interference competitive ability may be the only feasible adaptive strategy under these conditions (Habets et al. 2007). In order to better understand the evolution of interference competition via anti-competitor toxins, it would also be of interest to know how readily the costs and benefits of toxin production may evolve. To this effect, we are presently studying evolved changes in resource and interference competitive ability in these yeast populations.
Acknowledgments
We thank Ryszard Korona and Duncan Greig for providing yeast strains, Bertha Koomanschap for technical support and two anonymous reviewers for helpful comments. This work was supported by an innovative research grant to J.A.G.M. de V. from NWO.
References
- Baganz F, Hayes A, Marren D, Gardner D.C.J, Oliver S.G. Suitability of replacement markers for functional analysis studies in Saccharomyces cerevisiae. Yeast. 1997;13:1563–1573. doi: 10.1002/(SICI)1097-0061(199712)13:16<1563::AID-YEA240>3.0.CO;2-6. doi:10.1002/(SICI)1097-0061(199712)13:16<1563::AID-YEA240>3.0.CO;2-6 [DOI] [PubMed] [Google Scholar]
- Brown S.P, Le Chat L, De Paepe M, Taddei F. Ecology of microbial invasions: amplification allows virus carriers to invade more rapidly when rare. Curr. Biol. 2006;16:2048–2052. doi: 10.1016/j.cub.2006.08.089. doi:10.1016/j.cub.2006.08.089 [DOI] [PubMed] [Google Scholar]
- Carreiro S.C, Pagnocca F.C, Bacci M, Jr, Bueno O.C, Hebling M.J, Middelhoven W.J. Occurrence of killer yeasts in leaf-cutting ant nests. Folia Microbiol. (Praha) 2002;47:259–262. doi: 10.1007/BF02817648. [DOI] [PubMed] [Google Scholar]
- Case T.J, Gilpin M.E. Interference competition and niche theory. Proc. Natl Acad. Sci. USA. 1974;71:3073–3077. doi: 10.1073/pnas.71.8.3073. doi:10.1073/pnas.71.8.3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L, Levin B.R. Structured habitats and the evolution of anticompetitor toxins in bacteria. Proc. Natl Acad. Sci. USA. 1981;78:6324–6328. doi: 10.1073/pnas.78.10.6324. doi:10.1073/pnas.78.10.6324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J, Fink G.R. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc. Natl Acad. Sci. USA. 1976;73:3651–3655. doi: 10.1073/pnas.73.10.3651. doi:10.1073/pnas.73.10.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaran T.L, Hoekstra R.F. Killer-sensitive coexistence in metapopulations of micro-organisms. Proc. R. Soc. B. 2003;270:1373–1378. doi: 10.1098/rspb.2003.2338. doi:10.1098/rspb.2003.2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaran T.L, Hoekstra R.F, Pagie L. Chemical warfare between microbes promotes biodiversity. Proc. Natl Acad. Sci. USA. 2002;99:786–790. doi: 10.1073/pnas.012399899. doi:10.1073/pnas.012399899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes G.A, Hastings J.W. Selection and fitness in bacteriocin-producing bacteria. Proc. R. Soc. B. 1997;264:683–687. doi: 10.1098/rspb.1997.0097. doi:10.1098/rspb.1997.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Longo V.D. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2003;2:73–81. doi: 10.1046/j.1474-9728.2003.00033.x. doi:10.1046/j.1474-9728.2003.00033.x [DOI] [PubMed] [Google Scholar]
- Frank S.A. Spatial polymorphism of bacteriocins and other allelopathic traits. Evol. Ecol. 1994;8:369–386. doi:10.1007/BF01238189 [Google Scholar]
- Ganter P.F, Starmer W.T. Killer factor as a mechanism of interference competition in yeasts associated with cacti. Ecology. 1992;73:54–67. doi:10.2307/1938720 [Google Scholar]
- Gardner A, West S.A, Buckling A. Bacteriocins, spite and virulence. Proc. R. Soc. B. 2004;271:1529–1535. doi: 10.1098/rspb.2004.2756. doi:10.1098/rspb.2004.2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A.L, McCusker J.H. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15:1541–1553. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. doi:10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- Gray J.V, Petsko G.A, Johnston G.C, Ringe D, Singer R.A, Werner-Washburne M. “Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2004;68:187–206. doi: 10.1128/MMBR.68.2.187-206.2004. doi:10.1128/MMBR.68.2.187-206.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbiniene G, Kondratiene L, Jokantaite T, Serviene E, Melvydas V, Petkuniene G. Occurrence of killer yeast strains in fruit and berry wine yeast populations. Food Tech. Biotechnol. 2004;42:159–163. [Google Scholar]
- Habets M.G.J.L, Rozen D.E, Hoekstra R.F, de Visser J.A.G.M. The effect of population structure on the adaptive radiation of microbial populations evolving in spatially structured environments. Ecol. Lett. 2006;9:1041–1048. doi: 10.1111/j.1461-0248.2006.00955.x. doi:10.1111/j.1461-0248.2006.00955.x [DOI] [PubMed] [Google Scholar]
- Habets M.G.J.L, Czaran T, Hoekstra R.F, de Visser J.A.G.M. Spatial structure inhibits the rate of invasion of beneficial mutations in asexual populations. Proc. R. Soc. B. 2007;274:2139–2143. doi: 10.1098/rspb.2007.0529. doi:10.1098/rspb.2007.0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovska I, Hardwick J.M. Viruses activate a genetically conserved cell death pathway in a unicellular organism. J. Cell Biol. 2005;170:391–399. doi: 10.1083/jcb.200503069. doi:10.1083/jcb.200503069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Riley M.A, Feldman M.W, Bohannan B.J.M. Local dispersal promotes biodiversity in a real-life game of rock–paper–scissors. Nature. 2002;418:171–174. doi: 10.1038/nature00823. doi:10.1038/nature00823 [DOI] [PubMed] [Google Scholar]
- Kishida M, Tokunaga M, Katayose Y, Yajima H, Kawamura Watabe A, Hishinuma F. Isolation and genetic characterization of pGKL killer-insensitive mutants (iki) from Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 1996;60:798–801. doi: 10.1271/bbb.60.798. [DOI] [PubMed] [Google Scholar]
- Klaenhammer T.R. Bacteriocins of lactic acid bacteria. Biochimie. 1988;70:337–349. doi: 10.1016/0300-9084(88)90206-4. doi:10.1016/0300-9084(88)90206-4 [DOI] [PubMed] [Google Scholar]
- Lenski R.E, Rose M.R, Simson S.C, Tadler S.C. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am. Nat. 1991;138:1315–1341. doi:10.1086/285289 [Google Scholar]
- Magliani W, Conti S, Gerloni M, Bertolotti D, Polonelli L. Yeast killer systems. Clin. Microbiol. Rev. 1997;10:369–400. doi: 10.1128/cmr.10.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page´ N, et al. A Saccharomyces cerevisiae genome-wide mutant screen for altered sensitivity to K1 killer toxin. Genetics. 2003;163:875–894. doi: 10.1093/genetics/163.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintar J, Starmer W.T. The costs and benefits of killer toxin production by the yeast Pichia kluyveri. Anton. Leeuw. Int. J. Gen. Microbiol. 2003;83:89–97. doi: 10.1023/a:0000000089097. doi:10.1023/A:1014215200360 [DOI] [PubMed] [Google Scholar]
- Reiter J, Herker E, Madeo F, Schmitt M.J. Viral killer toxins induce caspase-mediated apoptosis in yeast. J. Cell Biol. 2005;168:353–358. doi: 10.1083/jcb.200408071. doi:10.1083/jcb.200408071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M.A, Gordon D.M. A survey of Col plasmids in natural isolates of Escherichia coli and an investigation into the stability of Col-plasmid lineages. J. Gen. Microbiol. 1992;138:1345–1352. doi: 10.1099/00221287-138-7-1345. [DOI] [PubMed] [Google Scholar]
- Riley M.A, Gordon D.M. The ecological role of bacteriocins in bacterial competition. Trends Microbiol. 1999;7:129–133. doi: 10.1016/s0966-842x(99)01459-6. doi:10.1016/S0966-842X(99)01459-6 [DOI] [PubMed] [Google Scholar]
- Rose M.D, Winston F, Hieter P. Cold Spring Harbor Laboratory Press; New York, NY: 1990. Methods in yeast genetics: a laboratory course manual. [Google Scholar]
- Schmitt M.J, Breinig F. The viral killer system in yeast: from molecular biology to application. FEMS Microbiol. Rev. 2002;26:257–276. doi: 10.1111/j.1574-6976.2002.tb00614.x. doi:10.1111/j.1574-6976.2002.tb00614.x [DOI] [PubMed] [Google Scholar]
- Schmitt M.J, Breinig F. Yeast viral killer toxins: lethality and self-protection. Nat. Rev. Microbiol. 2006;4:212–221. doi: 10.1038/nrmicro1347. doi:10.1038/nrmicro1347 [DOI] [PubMed] [Google Scholar]
- Schmitt M.J, Tipper D.J. K28, a unique double-stranded RNA killer virus of Saccharomyces cerevisiae. Mol. Cell Biol. 1990;10:4807–4815. doi: 10.1128/mcb.10.9.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. 2nd edn. Freeman and company; New York, NY: 1981. Biometry. [Google Scholar]
- Starmer W.T, Ganter P.F, Aberdeen V, Lachance M.A, Phaff H.J. The ecological role of killer yeasts in natural communities of yeasts. Can. J. Microbiol. 1987;33:783–796. doi: 10.1139/m87-134. [DOI] [PubMed] [Google Scholar]
- Starmer W.T, Schmedicke R.A, Lachance M.A. The origin of the cactus–yeast community. FEMS Yeast Res. 2003;3:441–448. doi: 10.1016/S1567-1356(03)00056-4. doi:10.1016/S1567-1356(03)00056-4 [DOI] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. doi:10.1002/yea.320101310 [DOI] [PubMed] [Google Scholar]
- West S.A, Griffin A.S, Gardner A, Diggle S.P. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 2006;4:597–607. doi: 10.1038/nrmicro1461. doi:10.1038/nrmicro1461 [DOI] [PubMed] [Google Scholar]
- Wickner R.B. “Killer character” of Saccharomyces cerevisiae: curing by growth at elevated temperature. J. Bacteriol. 1974;117:1356–1357. doi: 10.1128/jb.117.3.1356-1357.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler E.A, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. doi:10.1126/science.285.5429.901 [DOI] [PubMed] [Google Scholar]