Abstract
In a number of primate species, females utter loud and distinctive calls during mating. Here we aim to clarify the information content and function of Barbary macaque (Macaca sylvanus) copulation calls by testing (i) whether or not copulation calls advertise the female fertile phase and (ii) whether and how copulation calls influence male ejaculatory behaviour. In order to do this, we combined hormone measurements with acoustic analysis and behavioural observations. In contrast to a previous study implying that the structure of copulation calls indicates the timing of the fertile phase, our results, using objective endocrine criteria for assessing ovulation, provide evidence that the structure of copulation calls of female Barbary macaques does not reveal the timing of the fertile phase. More importantly, females seem to influence the likelihood of ejaculation by calling versus remaining silent and by adjusting the timing of call onset. Females make use of this ability to influence mating outcome to ensure ejaculatory matings with almost all males in the group. In addition, calls given during ejaculatory copulations differ from those during non-ejaculatory copulations, providing information about mating outcome for listeners. We conclude that in this species, copulation calls apparently serve to enhance sperm competition and maximize paternity confusion.
Keywords: acoustic analysis, Barbary macaque, copulation calls, hormones, ovulation, sexual selection
1. Introduction
Female copulation calls are loud and distinctive vocalizations uttered immediately before, during or after copulation (Semple 1998b, 2001; Nikitopoulos et al. 2004). They occur in a wide range of non-human primate species, particularly Old World monkey species with multi-male–multi-female social systems, and also in humans (for an overview see Semple 1998a). A number of non-exclusive hypotheses have been put forward to explain their functional significance (reviewed in Maestripieri & Roney 2005; Pradhan et al. 2006), but the empirical evidence is still sketchy and the subject continues to be controversially discussed.
At the proximate level, female copulation calls may serve to influence mating outcome, as originally proposed by Todt & Pohl 1984 (see also Todt et al. 1995). These authors reported that females’ use of specific signals such as the mating call, the mating grasp and the mating stare influenced the likelihood of male ejaculation. Although interesting, the information is limited to a single study published in abstract form only. Neither the nature of the salient acoustic parameters nor the detailed temporal relationship of female and male behaviours was investigated. Furthermore, it is not clear whether and, if so, how males change their mating effort in response to the timing and structure of the call.
Of the various hypotheses that exist to explain the ultimate function of copulation calls, the two most prominent suggest that copulation calls are used either to facilitate sperm competition and achieve paternity confusion (O'Connell & Cowlishaw 1994; Semple 1998b), or, alternatively, to function as a signal of post-copulatory female choice (Maestripieri et al. 2005). While the former hypothesis predicts that females should conceal their fertile phase and mate with multiple males, the latter predicts a completely different scenario in which females should advertise their fertile phase by calling to encourage male mate guarding by preferred males. Separation of these two contrasting hypotheses has been complicated, at least in part, owing to the technical difficulties in reliably determining the female fertile phase in primates living in social groups under natural conditions. Semple & McComb (2000) reported clear structural differences (spectral: dominant frequency; temporal: number of call units, call length, duration of call units) between copulation calls given at the onset compared to mid-cycle, thereby implying a potential for copulation calls to be used to indicate reproductive status, but the degree to which the calls varied over a time scale useful for advertising the transient window of fertility around ovulation could not be assessed. Given the highly promiscuous mating behaviour of this species, together with the low level of male monopolization (Brauch 2007) and the extensive male care behaviour in this species (Paul et al. 1992; Reichelmann et al. 1994), we consider it unlikely that females would use copulation calls to advertise their fertile phase in this way.
Thus, in this study, we set out to clarify the information content and functional significance of Barbary macaque (Macaca sylvanus) copulation calls by testing (i) whether or not copulation calls advertise the female fertile phase and (ii) whether and how copulation calls influence male ejaculatory behaviour. In order to do this, we combined acoustic analysis with hormone measurements in faeces, enabling us to determine the time of ovulation from which we could estimate the fertilization window or fertile phase. A fine-tuned analysis of call characteristics as well as the temporal relationship between copulation and calling behaviours was used to test whether calling influences the likelihood of ejaculation. In the light of the promiscuous mating behaviour of this species, we predict that female copulation calls do not advertise the fertile phase and, extrapolating from Todt & Pohl's (1984) earlier findings, we further expected that the use, timing and structure of these calls may affect the likelihood of male ejaculation.
2. Material and methods
(a) Study subject and study site
The study was conducted on a group (‘Middle Hill’) of free-ranging Barbary macaques living in the Upper Rock Nature Reserve, Gibraltar (for details of study site see Möhle et al. 2005). Animals are habituated and individually recognizable. The data presented here were collected during the mating seasons 2003/2004 and 2004/2005 during which the group consisted of 46 (including 6 adult males and 14 adult females) and 50 (5 adult males and 13 adult females) individuals, respectively. We collected acoustic recordings and faecal samples from eight females. In total we included data from 15 ovarian cycles in our analysis, with 4 females being represented once in each mating season, 3 females being represented in one mating season and 1 female represented twice in each of the two mating seasons.
(b) Acoustic recordings and analysis
We recorded copulation calls from females throughout their ovarian cycle at a distance of 2–4 m and with a sampling rate of 44.1 kHz ad libitum using a SONY TCDD100 DAT recorder and Sennheiser directional microphone (K6 power module with Rycote Modular Windshield System and Rycote Windjammer). Over a total of 15 ovarian cycles, we collected 1027 calls, of which 751 were of sufficient quality (e.g. not disturbed by wind or background noise, such as construction work or other animals calling) to perform acoustic analyses.
To describe the acoustic call characteristics, we conducted temporal and spectral analyses. For the temporal analysis, the program Avisoft SASLab Pro v. 3.92 (Avisoft, Berlin, Germany) was used. The start and end of each call unit was visually identified and marked by cursor placement based on the spectrogram. Using these marks, the program automatically calculates various temporal parameters: total number of call units; call duration; average unit duration; and unit interval, that is, the time between the end of a given unit and the onset of the subsequent unit. The intra-observer reliability for determining these temporal parameters was very high (rS=1.0, p<0.001, N=196). For the spectral analysis, we randomly selected three call units from the middle part of each call. Their sampling frequency was reduced to 22.05 kHz in order to achieve a better frequency resolution. Subsequently, we ran a fast Fourier transformation (FFT length: 1024 pt, time step: 5 ms, frequency range: 11.025 kHz, frequency resolution: 21.6 Hz). We submitted the resulting frequency time spectra to the custom software program LMA v. 8.4 that extracts different sets of call parameters from acoustic signals (Hammerschmidt 1990). Because units are short and reveal a little to no frequency modulation, we focused on variables that describe the distribution of the amplitudes in the frequency spectrum (DFA) and the location of the dominant frequency bands (DFB). This was done by first determining the overall amplitude (energy) for each time segment. Subsequently, we assessed the mean frequency at which the distribution of the amplitude in the frequency spectrum reaches the first (DFA1) and second quartiles (DFA2), respectively, of the total value. Second, we calculated parameters that describe the first two DFBs. The DFBs are characterized by amplitudes that exceed a given threshold in a consecutive number of frequency bins. Note that the numbers of DFBs count from the lowest frequency up; the first DFB is not necessarily the DFB with the highest amplitude. In addition to the mean and maximum values for these structural parameters, we determined the peak frequency (PF, corresponding to ‘dominant frequency’ as defined in Semple & McComb 2000), which is the frequency of the highest amplitude in a certain time segment. For a description of the algorithms, see Hammerschmidt (1990) and Schrader & Hammerschmidt (1997). A schematic illustration describing DFA, DFB and PF can be found in Pfefferle et al. 2007.
Owing to high degrees of multicollinearity, the acoustic variables were collapsed into five factors using a principal component analysis (PCA). Factor loadings indicate that the different factors mainly represent (i) the distribution of the amplitude in the frequency spectrum (DFA), (ii) the peak frequency (PF), (iii) the call duration, (iv) the location of the dominance frequency bands, and (v) the interval between units.
(c) Behavioural recordings and analysis
Parallel to the acoustic recordings, mating-related behaviour was spoken online into the second channel of the recorder. Besides information regarding the identity of the mating partners and the outcome of the copulation (ejaculatory or non-ejaculatory; definition see below), we noted the time the male started mounting the female, the time intromission, the start of the ejaculatory pause (if one occurred), the time of the male dismounting the female and each occurring pelvic thrust performed by the male before and after the start of the copulation call. All behavioural parameters were spoken online into the second audio channel. A copulation was considered ejaculatory if an ejaculatory pause and fresh sperm (in 96% of all cases) or one of both was observed during or after the mating. Subsequently the timing of the different behaviour patterns was measured using the wave-form representation of the according word in the second audio channel. The test of inter-observer reliability revealed a high accuracy of repetition of measurements (rS=0.998, p<0.001, N=55). By superimposing the audio channel with the copulation call onto the corresponding time segment of the behavioural recording (second channel), we calculated the call latency, the overall duration of the copulation, the time of intromission and the time to the ejaculatory pause. In order to check whether copulation call structure reflects the intensity of a copulation, we calculated the thrust rate at the beginning of the mating (thrust rate before call) and after (thrust rate after call) the onset of the copulation call.
Male rank was assessed by transcribing submissive and aggressive behaviours as well as the outcome of dyadic interactions into an agonistic interaction matrix. Males were classified according to their position in the hierarchy as high-ranking (top half of the hierarchy) and low-ranking (bottom half of the hierarchy) individuals.
To assess how many males each female mated with in the different phases of their ovarian cycle, we calculated the Simpson's diversity index D (, where S is the total number of males and Pi is the proportion of the ith individual), as well as the equitability index E, indicating how evenly matings are distributed among different males (E=D/Dmax; Begon et al. 1990). We calculated the mean D and E values for each female and phase across cycles and observation years, and used a repeated measures with missing values analysis (based on a permutation approach) to examine differences across the different cycle phases.
(d) Faecal sample collection, hormone analysis and definition of female's cycle phase
We collected 30–74 (mean: 47.6) faecal samples per study female (mean: one sample every 2.3 days). The faecal bolus was gently homogenized and a portion (3–5 g) was placed in a plastic tube containing 10 ml of 96% ethanol. The samples were stored for two to five months and transported to the endocrine laboratory at ambient temperature. Samples were extracted (Ziegler et al. 2000) and extracts were measured for levels of immunoreactive 5α-reduced-20-oxo pregnanes (5-P-3OH) using a previously validated enzyme immunoassay which has been shown to provide reliable information on female ovarian function and timing of ovulation in the Barbary macaque (Möhle et al. 2005). Sensitivity of the assay at 90% binding was 19.6 pg. Intra- and inter-assay coefficients of variation, calculated from replicate determinations of high- and low-value quality controls, were 8.2% (N=16) and 16.4% (N=47) (high) and 10.4% (N=16) and 13.4% (N=47) (low). For the generation of hormone profiles, we aimed to use only faecal samples without urine contamination, despite the fact that the latter had no influence on measured hormone levels (urine: rS=0.962, p<0.001, N=20). A potential effect of storage duration on faecal hormone levels was not tested because the hormonal data were only used to time ovulation, with each animal serving as its own control. Furthermore, no comparisons of absolute hormone levels were made between animals and conditions. We used the faecal 5-P-3OH profiles to determine the time of ovulation in each ovarian cycle. Since faecal samples were collected on average every second day and given a time lag in faecal hormone excretion of 1–2 days in macaques (Shideler et al. 1993), the most likely days of ovulation were defined as days −2/−3 relative to the defined rise in faecal 5-P-3OH (day 0) (Heistermann et al. 2007). Sperm can be fertile for up to 72 hours (Behboodi et al. 1991; Wilcox et al. 1995), therefore copulations occurring before ovulation may be fertile. Thus, we defined the fertile phase (the period in which a female can conceive) as comprising the 2 most likely days of ovulation plus the 3 preceding days (approx. sperm lifespan). The 5 days preceding and following the fertile phase are termed pre-fertile and post-fertile phases, respectively. In addition to these three phases, we distinguished the ‘−10 day’ and ‘more than −16 day’ phase. While the phase −10 day combines copulation calls uttered between 10 and 15 days before the fertile phase, the more than −16 day phase includes the first given copulation calls, comprising a time period of 25–16 days before the fertile phase (analagous to the ‘early oestrus call’ phase in Semple & McComb 2000).
(e) Data and statistical analysis
We used a general linear mixed model (GLMM) to analyse the effect of categorical variables (e.g. cycle phase, male rank) and continuous variables (copulation intensity, intromission time, copulation length) on a dependent continuous variable (e.g. call latency, acoustic structure). GLMM is an extension of the general linear model and allows accounting for repeated observations of the same subjects by including subject (female ID) as a random factor in the model (Deschner et al. 2004). As a covariate structure for the random factor, we selected compound symmetry. For data analysis, we used the restricted maximum-likelihood method. Following pre-selection based on the mating time course (figure 1a), we used the Akaike-information criterion (AIC) to select the model that best explained the variation in the dependent variable. We used a step-up-Hochberg correction to adjust p-values for multiple testing (Westfall & Young 1993). For significant effects, we conducted further pairwise comparisons.
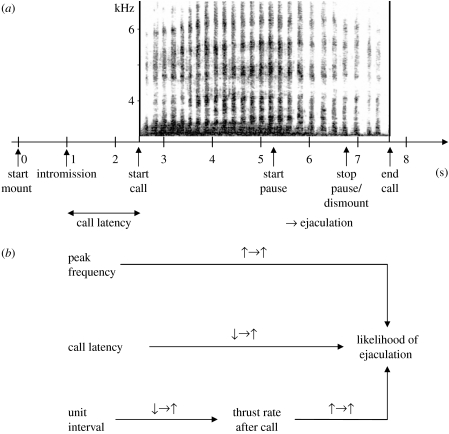
Figure 1.
Time course of an ejaculatory mating and relationship between factors affecting mating outcome. (a) Average time course of an ejaculatory copulation in Barbary macaques. (b) Interaction of factors influencing the likelihood of ejaculation. Arrows indicate the direction of interaction; upward arrow, signifying increase; downward arrow, decrease; and rightward arrow leads to.
The effect of copulation calls on the occurrence of an ejaculation was tested by calculating the Mantel–Haenszel chi-square (Sprent & Smeeton 2001). Whether all females showed the same pattern in the occurrence of copulations with or without a call and with or without ejaculation was checked by calculating the Breslow–Day chi-square (Sprent & Smeeton 2001). A non-significant result of the Breslow–Day chi-square states that females do not differ in the pattern of distribution. All described statistical analyses were done using SPSS v. 12.0.
To identify parameters influencing the success of a copulation (ejaculation versus non-ejaculation), we performed a binary logistic regression for stratified data using the program ‘LogXaxt’ (Cytel software Corporation 2005). The model explaining the distribution of ejaculation best was selected by calculating the AIC coefficient. Since cycle phase influences some of the acoustic parameters (see §3 main text) as well as ejaculation, the test of determining factors influencing ejaculation was performed while controlling for cycle phase. All statistical tests were two sided with an alpha level of 0.05.
3. Results
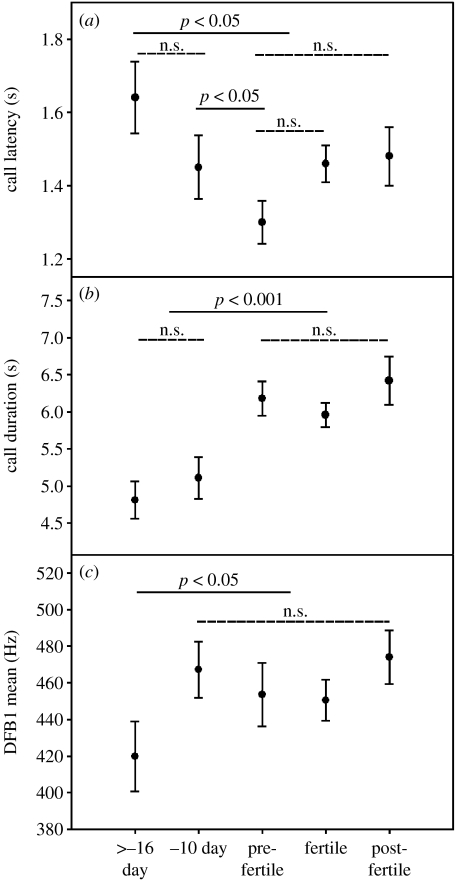
Acoustic analysis of the copulation calls of female Barbary macaques indicate that neither call use (call latency, figure 2a), nor temporal (unit interval, call duration, figure 2b) or spectral acoustic variables (DFA, PF, DFB, figure 2c) provides circumstantial evidence for the timing of the fertile phase. Significant structural changes were only found when comparing calls given at the beginning (more than −16 days before ovulation) with those given at later stages of the ovarian cycle ((pre-fertile, fertile, post-fertile phase) PF, call duration, DFB p<0.05; unit interval p<0.001; DFA n.s., see figure 2). In contrast, none of the call parameters tested differed significantly between any of the defined phases around the time of ovulation (pre-fertile, fertile, post-fertile). Male ID, rank and age also did not influence the call structure (table 1).
Figure 2.
Distribution of call characteristics throughout the female ovarian cycle. Mean and s.e.m. of (a) call latency, (b) call duration and (c) frequency of the DFB across the different cycle phases (n.s., non-significant.)
Table 1.
Results of the GLMM representing the influence of cycle phase, male rank, thrust rate before call, thrust rate after call, time to intromission and duration of copulation on call latency, frequencies of the dominant amplitude (DFA), peak frequency (PF), frequencies of the dominant frequency bands (DFB), call duration and unit interval. (AIC model selection was used to decide which combination of parameters provided the best explanation of the variance of the depending variable. The p-values were adjusted using Step-Up-Hochberg procedure (Adj. p, adjusted p value). Values below the alpha level of 0.05 are marked in italic.)
| d.f. denominator | d.f. numerator | F | adj. p | |
|---|---|---|---|---|
| call latency | ||||
| overall cycle phase | 4 | 405 | 3.8 | 0.012 |
| male rank | 1 | 407 | 3.0 | 0.08 |
| DFA | ||||
| overall cycle phase | 4 | 249 | 2.2 | 0.23 |
| male rank | 1 | 249 | 3.2 | 0.23 |
| thrust rate before call | 1 | 248 | 0.3 | 0.58 |
| thrust rate after call | 1 | 248 | 0.4 | 0.58 |
| PF | ||||
| overall cycle phase | 4 | 254 | 2.6 | 0.12 |
| thrust rate before call | 1 | 253 | 1.8 | 0.35 |
| thrust rate after call | 1 | 253 | 0.3 | 0.60 |
| DFB | ||||
| overall cycle phase | 4 | 251 | 9.1 | <0.001 |
| thrust rate before call | 1 | 250 | 1.8 | 0.51 |
| thrust rate after call | 1 | 250 | 3.0 | 0.35 |
| male rank | 1 | 252 | 0.4 | 1.00 |
| call duration | ||||
| overall cycle phase | 4 | 258 | 4.6 | 0.004 |
| thrust rate before call | 1 | 257 | 3.0 | 0.16 |
| thrust rate after call | 1 | 257 | 0.7 | 0.39 |
| copulation duration | 1 | 257 | 104.1 | <0.001 |
| unit interval | ||||
| overall cycle phase | 4 | 255 | 1.5 | 0.19 |
| thrust rate before call | 1 | 254 | 1.8 | 0.19 |
| thrust rate after call | 1 | 254 | 6.6 | 0.03 |
Females called during 86% of all copulations (N=1478); of these, 59% were ejaculatory. Of the copulations without a call, 98.2% were non-ejaculatory. In all cases copulation calls began before ejaculation occurred. The occurrence of a copulation call positively correlated with the likelihood of an ejaculation (Mantel–Haenszel: Χ12=180.6, p<0.001, eβ=76.8), with all females showing the same pattern (Breslow–Day: Χ72=7.8, n.s.).
We also examined whether the timing and the structure of the calls correlates with mating outcome. We found that the earlier the female began to call after the male mounted, the more likely it was that ejaculation occurred (likelihood ratio test: Χ32=34.5, p<0.001; call latency: eβ=0.36; see figure 1a for the time course of a typical mating). Peak frequency and thrust rate after call onset also predicted the likelihood of an ejaculation (mean peak frequency: eβ=1.01, p<0.01; thrust rate after call: eβ=8.2, p<0.01).
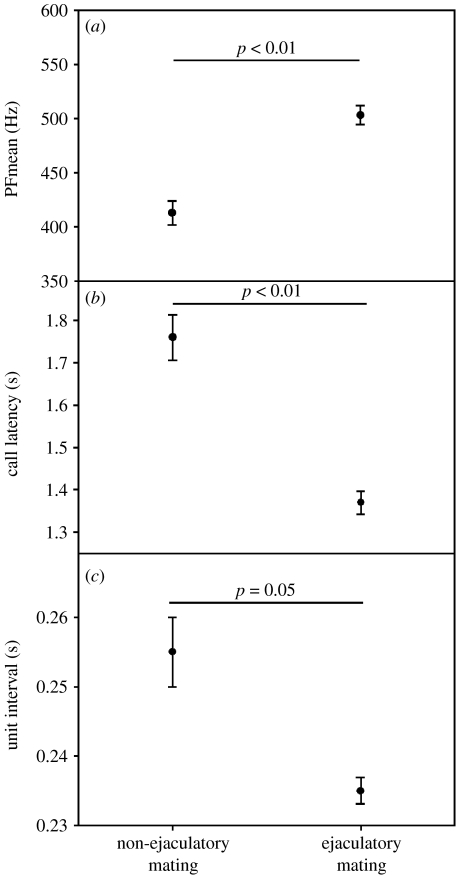
We controlled for the argument that variation of calling in ejaculatory versus non-ejaculatory mating could be an epiphenomenon of a more intense copulation (i.e. that onset and characteristics of calling were influenced by male pelvic thrust rate). There was no relationship between the unit interval and the thrust rate before the call, but a relationship between the unit interval and the thrust rate after call onset did exist, suggesting that unit interval influences thrust rate, and not vice versa (GLMM: unit interval F1,101=4.8, p=0.03; all others p>0.05; controlling for cycle phase). Unit interval, which actually reflects calling effort, varied between ejaculatory and non-ejaculatory copulations, but did not change throughout the duration of mating. At the beginning of a copulation, the thrust rate did not differ between ejaculatory (mean±s.e.m. 2.56±0.03) and non-ejaculatory copulations (2.48±0.04; GLMM with female ID as a random factor F1,410=2.1, p=0.15), while after call onset, the thrust rate was significantly lower in non-ejaculatory (2.15±0.05) than in ejaculatory copulations (2.45±0.02; F1,355=24.5, p<0.001). Thus, it seems warranted to assume that unit interval affected the thrust frequency after call onset, and consequently the likelihood of ejaculation. There was no relationship between thrust rate at the beginning of the mating and after call onset (Pearson r=0.05, p=0.413, N=358). Finally, we found that call latency correlated with the timing of ejaculation as indicated by the onset of the ejaculatory pause: the earlier the female began to call the faster the male ejaculated (partial correlation r=0.35, p<0.001, N=201, controlling for cycle phase and female ID). Taken together, our results suggest that the earlier, faster and more intensely the female called, the higher the likelihood that the male ejaculated (see figure 1b). The usage (call latency) and structure of copulation calls (peak frequency and unit interval) differed between ejaculatory and non-ejaculatory matings (mean values for females, paired t-test for call latency: t=3.55, d.f.=7, p=0.009; peak frequency: t=−3.88, d.f.=7, p=0.006; unit interval: t=2.31, d.f.=7, p=0.05; see figure 3).
Figure 3.
Call characteristics during ejaculatory and non-ejaculatory matings. Mean and s.e.m. of (a) mean peak frequency, (b) call latency and (c) unit interval in ejaculatory and non-ejaculatory matings.
To shed some light on the possible function of this influence on mating outcome, we examined whether females became more selective during the fertile phase, i.e. whether they had ejaculatory matings with fewer males during the fertile phase than during other stages of the cycle. Females tended to have ejaculatory matings with more males, and they distributed their mating effort slightly more evenly during the fertile phase compared with the pre- or post-fertile phase, but these difference were not significant (repeated measures with missing values test (Mundry 1999): N=8, for D: test statistic M=1.6, p=0.19; for E: M=0.14; p=0.2).
4. Discussion
By using hormone measurements to provide a more precise and objective assessment of female cycle stage, we found no evidence that acoustic changes during the Barbary macaque ovarian cycle are sufficiently precise to provide any reliable information on the timing of ovulation. At the same time, we were able to confirm Semple & McComb's (2000) earlier results indicating that calls given at the very beginning of the cycle differ from those given around peak swelling. We consider it unlikely that female copulation calls serve to advertise the fertile phase of the female cycle or to communicate information useful to males in timing their mating effort. Accordingly, we interpret our findings as favouring the sperm competition/paternity confusion hypothesis (rather than the post-copulatory female choice), as an explanation for the function of female copulation calls.
Our data also provide evidence that copulation calls directly affect mating outcome. Here, we not only confirm Todt's (Todt & Pohl 1984; Todt et al. 1995) original proposal that calling during copulation influences male ejaculation, but significantly extend it by indicating that not just the occurrence but also the timing (latency) and structure (peak frequency, unit interval) of female copulation calls are salient parameters involved in this cascade. While we cannot disprove that the timing and/or structure of the call may be influenced by aspects of the male's mating effort, we do show that pelvic thrust rate before call onset did not predict the likelihood to ejaculate, supporting our contention that female calling plays a significant role in influencing mating outcome. Other variables that we did not integrate into our analysis, such as pre-copulatory interactions, long-term relationships or odour, may also potentially impact on the usage of copulation calls or/and mating outcome, although we can discount mating partner identity as a factor in this respect.
Although our data indicate that female calling may influence mating outcome (ejaculation), we have no evidence that females use this to regulate either the number or identity of ejaculates received, and there is no reduction in number of mating partners as ovulation approaches. Thus, females continue to mate promiscuously throughout the fertile phase and the number of both total and ejaculatory matings during this phase is in fact, higher than during any other period of the cycle (Heistermann et al. 2007). Since female proactive behaviours, such as presenting the hindquarters, active solicitations, etc., do not show a peak around the time of ovulation (Brauch et al. 2007) and our current data show that copulation calls do not advertise the fertile phase, some other cue appears to be available that indicates the probability of ovulation and leads to changes in male mating behaviour. Anogenital swelling size, which increases progressively throughout the ovarian cycle reaching peak values around the time of ovulation, is highly correlated with frequencies of male ejaculatory copulations and may provide such a cue (Brauch et al. 2007).
While the standard scenario of sexual selection depicts a situation where males compete over access to females, our data imply that in Barbary macaques, the situation may be reversed: the high degree of promiscuity, and the fact that sperm in species living in multi-male groups with a promiscuous mating system can become a limited resource (Dewsbury 1982; Preston et al. 2001; Wedell et al. 2002), suggests that female Barbary macaques may be in a situation where they are competing for high-quality sperm. Although our results indicate that females may have some control over male ejaculation, they apparently do not use this to select certain males as mating (ejaculation) partners. Since male vertebrates are known to allocate their sperm between matings (Packer & Pusey 1982; Wedell et al. 2002) and since high frequencies of ejaculations can lead to a decrease in sperm quality (Synott et al. 1981; Marson et al. 1989; Preston et al. 2001), calling may enable females to (at least partially) influence the allocation of this limited resource. Whether or not cryptic female choice mechanisms operate to influence sperm transport or selection at a post-ejaculatory level (Birkhead & Pizzari 2002) in this species is not yet known. Finally, since copulation calls are loud, distinctive vocalizations and because the mating success (ejaculation) is encoded in its structure (via peak frequency, unit interval), its occurrence and/or structure might be used by other group members, particularly males, to distinguish a successful mating from an unsuccessful one.
Overall, our findings support the view that in Barbary macaques, the function of copulation calls can be best explained by the sperm competition/paternity confusion hypothesis. In contrast to other primate species where paternity confusion serves as a shield against infanticidal males (van Schaik et al. 2004), paternity confusion may confer different advantages in the Barbary macaque, in which males provide extensive care for infants, irrespective of their degree of relatedness (Paul et al. 1992). Therefore, when females confuse paternity during mating they may be setting the stage for care from multiple males. This does not discount other hypotheses explaining the occurrence of copulation calls in species with a different mating system such as for instance in the harem-like structure of Guinea baboons, Papio papio, in which copulation calls occur after copulation and appear to lead to increased male mate guarding (Maestripieri et al. 2005). Thus, although it is unlikely that a single hypothesis can account for the occurrence of vocalizations given during mating across the diverse mating systems found in primates, we should nevertheless expect some predictable patterns in relation to factors such as the number of available males, risk of infanticide and extent of male care.
Acknowledgments
This study was conducted as part of the Gibraltar Barbary macaque project (GBMP). We thank Eric Shaw and Dr John Cortes of the Gibraltar Ornithological and Natural History Society for their support and cooperation, the Royal Air Force Gibraltar for permission to enter the study area, Kurt Hammerschmidt for discussion and making his sound analysis program available, Daniel Stahl for statistical advice, Andrea Heistermann and Jutta Hagedorn for support with the hormone analyses; Nina Stobbe and Brian Gomilla for assistance during data collection, Friederike Jansen for help with the data analysis, Ralf Brockhausen for discussion, and Meredith Small and Dietmar Zinner for comments on the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (to J.F. and J.K.H.).
Supplementary Material
Loadings of acoustic parameters on the corresponding factors
Definition of acoustic parameters used in the analysis
References
- Begon M, Harper J.L, Townsend C.R. Blackwell Scientific Publications; Oxford, UK: 1990. Ecology: individuals populations and communities. [Google Scholar]
- Behboodi E, Katz D.F, Samuels S.J, Tell L, Hendrickx A.G, Lasley B.L. The use of a urinary estrone conjugates assay for detection of optimal mating time in the cynomolgus macaque (Macaca fascicularis) J. Med. Primatol. 1991;20:229–234. [PubMed] [Google Scholar]
- Birkhead T.R, Pizzari T. Postcopulatory sexual selection. Nat. Rev. Genet. 2002;3:262–273. doi: 10.1038/nrg774. doi:10.1038/nrg774 [DOI] [PubMed] [Google Scholar]
- Brauch, K. 2007 Male and female reproductive strategies in relation to paternity outcome in Barbary macaques (Macacasylvanus). PhD thesis, University of Münster. Göttingen: Cuvellier Verlag.
- Brauch K, Pfefferle D, Hodges K, Möhle U, Fischer J, Heistermann M. Female sexual behavior and sexual swelling size as potential cues for males to discern the female fertile phase in free-ranging Barbary macaques (Macaca sylvanus) of Gibraltar. Horm. Behav. 2007;52:375–383. doi: 10.1016/j.yhbeh.2007.06.001. doi:10.1016/j.yhbeh.2007.06.001 [DOI] [PubMed] [Google Scholar]
- Deschner T, Heistermann M, Hodges K, Boesch C. Female sexual swelling size, timing of ovulation, and male behavior in wild West African chimpanzees. Horm. Behav. 2004;46:204–215. doi: 10.1016/j.yhbeh.2004.03.013. doi:10.1016/j.yhbeh.2004.03.013 [DOI] [PubMed] [Google Scholar]
- Dewsbury D.A. Ejaculate cost and male choice. Am. Nat. 1982;119:601–610. doi:10.1086/283938 [Google Scholar]
- Hammerschmidt, K. 1990 Individuelle Lautmuster bei Berberaffen (Macaca sylvanus): Ein Ansatz zum Verständnis ihrer vokalen Kommunikation. PhD thesis, Freie Universität Berlin.
- Heistermann M, Brauch K, Möhle U, Pfefferle D, Dittami J, Hodges J.K. Female ovarian cycle phase affects the timing of male sexual activity in free-ranging Barbary macaques (Macaca sylvanus) of Gibraltar. Am. J. Primatol. 2007;69:1–15. doi: 10.1002/ajp.20455. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Roney J.R. Primate copulation calls and postcopulatory female choice. Behav. Ecol. 2005;16:106–113. doi:10.1093/beheco/arh120 [Google Scholar]
- Maestripieri D, Leoni M, Raza S.S, Hirsch E.J, Whitham J.C. Female copulation calls in Guinea baboons: evidence for postcopulatory female choice? Int. J. Primatol. 2005;26:737–758. doi:10.1007/s10764-005-5306-6 [Google Scholar]
- Marson J, Gervais D, Cooper R.W, Jouannet P. Influence of ejaculation frequency on semen characteristics in chimpanzees (Pan troglodytes) J. Reprod. Fertil. 1989;85:43–50. doi: 10.1530/jrf.0.0850043. [DOI] [PubMed] [Google Scholar]
- Möhle U, Heistermann M, Dittami J, Reinberg V, Hodges J.K. Patterns of anogenital swelling size and their endocrine correlates during ovulatory cycles and early pregnancy on free-ranging Barbary macaques (Macaca sylvanus) of Gibraltar. Am. J. Primatol. 2005;66:351–368. doi: 10.1002/ajp.20161. doi:10.1002/ajp.20161 [DOI] [PubMed] [Google Scholar]
- Mundry R. Testing related samples with missing values: a permutation approach. Anim. Behav. 1999;58:1143–1153. doi: 10.1006/anbe.1999.1246. doi:10.1006/anbe.1999.1246 [DOI] [PubMed] [Google Scholar]
- Nikitopoulos E, Arnhem E, van Hooff J.R.A.M, Sterck E.H.M. Influence of female copulation calls on male sexual behavior in captive Macaca fascicularis. Int. J. Primatol. 2004;25:659–677. doi:10.1023/B:IJOP.0000023579.30595.ae [Google Scholar]
- O'Connell S.M, Cowlishaw G. Infanticide avoidance, sperm competition and mate choice: the function of copulation calls in female baboons. Anim. Behav. 1994;48:687–694. doi:10.1006/anbe.1994.1288 [Google Scholar]
- Packer C, Pusey A.E. Cooperation and competition within coalitions of male lions—kin selection or game-theory. Nature. 1982;296:740–742. doi:10.1038/296740a0 [Google Scholar]
- Paul A, Kuester J, Arnemann J. DNA fingerprinting reveals that infant care by male Barbary macaques (Macaca sylvanus) is not paternal investment. Folia Primatol. 1992;58:93–98. doi: 10.1159/000156613. [DOI] [PubMed] [Google Scholar]
- Pfefferle D, West P.W, Grinnell J, Packer C, Fischer J. Do acoustic features of lion, Panthera leo, roars reflect sex and male condition? J. Acoust. Soc. Am. 2007;121:3947–3953. doi: 10.1121/1.2722507. doi:10.1121/1.2722507 [DOI] [PubMed] [Google Scholar]
- Pradhan G.R, Engelhardt A, van Schaik C.P. The evolution of female copulation calls in primates: a review and a new model. Behav. Ecol. Sociobiol. 2006;59:333–343. doi:10.1007/s00265-005-0075-y [Google Scholar]
- Preston B.T, Stevenson I.R, Pemberton J.M. Dominant rams lose out by sperm depletion—a waning success in siring counters a ram's high score in competition for ewes. Nat. Rev. Genet. 2001;409:681–682. doi: 10.1038/35055617. doi:10.1038/35055617 [DOI] [PubMed] [Google Scholar]
- Reichelmann C, Hultsch H, Todt D. Early development od social telationship in Barbary macaques (Macaca sylvanus): trajectories of alloparental behaviour during an infant's first three month of life. In: Roeder J.J, Thierry B, Anderson J.R, Hammerschmidt N, editors. Current primatology. Université Louis Pasteur; Strasbourg, France: 1994. pp. 268–279. [Google Scholar]
- Schrader L, Hammerschmidt K. Computer-aided analysis of acoustic parameters in animal vocalisations: a multi-parametric approach. Bioacoust. Int. J. Anim. Sound Record. 1997;7:247–265. [Google Scholar]
- Semple, S. 1998a Female copulation calls in primates. PhD thesis, University of Sussex.
- Semple S. The function of Barbary macaque copulation calls. Proc. R. Soc. B. 1998b;265:287–291. doi: 10.1098/rspb.1998.0294. doi:10.1098/rspb.1998.0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple S. Individuality and male discrimination of female copulation calls in the yellow baboon. Anim. Behav. 2001;61:1023–1028. doi:10.1006/anbe.2001.1692 [Google Scholar]
- Semple S, McComb K. Perception of female reproductive state from vocal cues in a mammal species. Proc. R. Soc. B. 2000;267:707–712. doi: 10.1098/rspb.2000.1060. doi:10.1098/rspb.2000.1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shideler S.E, Shackleton C.H.L, Moran F.M, Stauffer P, Lohstroh P.N, Lasley B.L. Enzyme immunoassays for ovarian steroid metabolites in the urine of Macaca fascicularis. J. Med. Primatol. 1993;22:301–312. [PubMed] [Google Scholar]
- Sprent P, Smeeton N.C. Chapman & Hall/CRC London; London, UK: 2001. Applied nonparametric statistical methods. [Google Scholar]
- Synott A.L, Fulkerson W.J, Lindsay D.R. Sperm output by rams and distribution amongst ewes under conditions of continual mating. J. Reprod. Fertil. 1981;65:355–361. doi: 10.1530/jrf.0.0610355. [DOI] [PubMed] [Google Scholar]
- Todt D, Pohl R. Communicative strategies in estrous Barbary ape females (Macaca sylvanus) during copulation behaviour: advertising, triggering, affiliating. Verhandlungen der Deutschen Zoologischen Gesellschaft. 1984;7:225. [Google Scholar]
- Todt D, Hammerschmidt K, Ansorge V, Fischer J. The vocal behaviour of Barbary macaques: call features and their performance in infants and adults. In: Zimmermann E, Newman J.D, Jürgens U, editors. Current topics in primate vocal communication. Plenum Press; New York, NY: 1995. pp. 141–160. [Google Scholar]
- van Schaik C.P, Pradhan G.R, van Noordwijk M.A. Mating conflict in primates: infanticide, sexual harassment and female sexuality. In: Kappeler P, van Schaik C, editors. Sexual selection in primates new and comparative perspectives. Cambridge University Press; Cambridge, UK: 2004. pp. 131–150. [Google Scholar]
- Wedell N, Gage M.J.G, Parker G.A. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 2002;17:313–320. doi:10.1016/S0169-5347(02)02533-8 [Google Scholar]
- Westfall P.H, Young S.S. Wiley; New York, NY: 1993. Resampling-based multiple testing: examples and methods for p-value adjustment. [Google Scholar]
- Wilcox A.J, Weinberg C.R, Baird D.D. Timing of sexual intercourse in relation to ovulation. N. Engl. J. Med. 1995;333:1517–1522. doi: 10.1056/NEJM199512073332301. doi:10.1056/NEJM199512073332301 [DOI] [PubMed] [Google Scholar]
- Ziegler T, Hodges J.K, Heistermann M. Hormonal correlates of reproductive seasonality in wild female hanuman langurs (Presbytes entellus) Am. J. Primatol. 2000;51:119–134. doi: 10.1002/(SICI)1098-2345(200006)51:2<119::AID-AJP2>3.0.CO;2-O. doi:10.1002/(SICI)1098-2345(200006)51:2<119::AID-AJP2>3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Loadings of acoustic parameters on the corresponding factors
Definition of acoustic parameters used in the analysis