Abstract
During natal dispersal, young animals leave their natal area and search for a new area to live. In species in which individuals inhabit different types of habitat, experience with a natal habitat may increase the probability that a disperser will select the same type of habitat post-dispersal (natal habitat preference induction or NHPI). Despite considerable interest in the ecological and the evolutionary implications of NHPI, we lack empirical evidence that it occurs in nature. Here we show that dispersing brush mice (Peromyscus boylii) are more likely to search and settle within their natal habitat type than expected based on habitat availability. These results document the occurrence of NHPI in nature and highlight the relevance of experience-generated habitat preferences for ecological and evolutionary processes.
Keywords: dispersal, habitat selection, natal habitat preference induction, habitat imprinting, Peromyscus
1. Introduction
Biologists have long assumed that experience with a natal habitat might increase a disperser's level of preference for the same type of habitat (Thorpe 1945; Wecker 1963); a phenomenon defined as natal habitat preference induction (NHPI; Davis & Stamps 2004). NHPI is an ‘umbrella concept’ that covers a number of related terms, including habitat imprinting, Hopkin's host selection principle, habitat preference induction and natal habitat-biased dispersal. Recently, biologists have become interested in the implications of NHPI for problems in behaviour, ecology and evolution. For instance, NHPI may generate individual differences in habitat preferences (Davis & Stamps 2004), impact metapopulation dynamics by influencing habitat patch colonization rates (Hanski & Singer 2001), help maintain genetic variation across landscapes (Sacks et al. 2005; Tonnis et al. 2005), facilitate extra-genetic inheritance of phenotypically plastic traits (West-Eberhard 2003; Slagsvold & Wiebe 2007) and contribute to sympatric speciation (Beltman & Haccou 2005; Forbes et al. 2005; Slagsvold & Wiebe 2007).
Despite the considerable interest in the broader implications of NHPI, the vast majority of empirical studies of this phenomenon have been conducted in the laboratory and used insects as the experimental subjects (Davis & Stamps 2004; Davis 2006; Stamps & Davis 2006), although a few field studies have reported a population genetic structure consistent with NHPI (Sacks et al. 2005; Tonnis et al. 2005; Pilot et al. 2006). Recently, Haughland & Larsen (2004) found that red squirrels (Tamiasciurus hudsonicus) born in either logged or intact coniferous forests were likely to recruit to new sites that were structurally similar to their natal site. Similarly, Selonen et al. (2007) found that flying squirrel (Pteromys volans) natal dispersers tended to recruit to new patches of spruce forest of the same size as their natal patch. However, neither of these studies demonstrated NHPI in the context that is most relevant to problems in ecology and evolution: when natal dispersers originating from distinctly different types of natural habitat within the same landscape search for and select a new habitat. In addition, these studies relied on statistical models which did not explicitly control for individual differences in distances moved during the dispersal process. As we show below, the assumption that exploratory distances are similar for all individuals is problematic, and field studies of NHPI which ignore individual differences in disperser movements are likely to overestimate the range of habitat types that are available to individual dispersers, potentially leading to inflated estimates of the chances that individuals prefer new habitats comparable to their natal habitat.
NHPI makes the straightforward, but still untested, prediction that dispersers should search and settle within their natal habitat type more frequently than expected based on availability of that habitat. However, although the question seems simple, the methods required to answer it are not. This is because field studies of NHPI, like many other studies of habitat selection under natural conditions, require estimates of the range of habitats that are available to each of the individuals in the study. It has been obvious for some time that the habitat available to dispersers varies both spatially and temporally (Arthur et al. 1996; Muller et al. 1997; Buskirk & Millspaugh 2006). Less widely appreciated is that habitat availability may also vary as a function of individual differences in the distances that natal dispersers travel away from their natal site. Previous studies of habitat selection in general, and NHPI in particular, have calculated available habitat using a single estimate of movement distance for all animals within a population, despite growing evidence of substantial variation in the distances dispersers venture away from their natal site prior to selecting a new site (Haughland & Larsen 2004; Doerr & Doerr 2005; Selonen & Hanski 2006). Such individual differences in movement behaviour may affect the amount and composition of the habitat available to dispersers, and should be considered in statistical tests for the occurrence of NHPI.
We investigated the influence of natal habitat type on dispersal and habitat selection behaviour of the brush mouse (Peromyscus boylii) under unmanipulated field conditions. The brush mouse is a common small mammal (average adult mass approx. 25 g) in many parts of western North America, where it occurs in multiple habitat types (Kalcounis-Rüppell & Spoon submitted). At our study site, brush mice are equally abundant in woodland and chaparral, two distinct habitat types that often occur in close proximity (Mabry 2007). In habitat similar to that at our study site, home ranges were 0.11–0.15 ha, and no between-sex difference in adult home range size was detected (Kalcounis-Rüppell 2000). Space use patterns and observations of multiple paternity within litters suggest that brush mice have a promiscuous mating system (Ribble & Stanley 1998; Kalcounis-Rüppell 2000; Kalcounis-Rüppell & Spoon submitted); however, there is no evidence of a sex-bias in natal dispersal distance, which averages 19.02±3.71 m (mean±1 s.e., N=132; K.E. Mabry & M. C. Kalcounis-Rüppell 2006, unpublished manuscript).
The objectives of this study were twofold. First, we tested the prediction that dispersing brush mice would be more likely to search and settle within their natal habitat type than expected based on availability of that habitat. Second, we considered how statistical methods which controlled for individual differences in movement patterns would affect levels of support for NHPI, as compared with traditional methods of estimating habitat availability. Throughout this study, we use ‘dispersal’ to refer to the complete process of dispersal (including departure from the natal area, search and settlement in an adult home range; Bowler & Benton 2005; Doerr & Doerr 2005), rather than a movement of a particular distance. We apply this terminology due to the increasing recognition that in many species (including brush mice), dispersal is not necessarily a single movement between natal and breeding sites (Bowler & Benton 2005; Doerr & Doerr 2005). Rather, dispersal is a process that may occur over a period of days, weeks or even longer periods, and includes the movements of juveniles prior to selecting a place to settle.
2. Material and methods
(a) Study site
This study was conducted at the Quail Ridge Reserve in Napa County, CA, USA (38°49′04″ N, 122°14′28″ W) between June 2004 and October 2006. We established three study sites, each of which contained a 0.79 ha trapping grid that covered equal areas of woodland and chaparral habitat. Woodland habitat was dominated by three tree species, interior live oak (Quercus wizlizenii), California bay-laurel (Umbellularia californica) and buckeye (Aesculus californica), while chaparral was dominated by a shrub, chamise (Adenostoma fasciculatum). Despite these pronounced between-habitat differences in dominant plant species and habitat structure, brush mouse population density, population growth rate, survival, adult body mass, the proportion of adults reproductive and the number of juveniles recruited per reproductive female were comparable between the two habitat types (Mabry 2007). The mean per capita probability of moving between habitats each night was 0.09 (pooled data from both juveniles and adults), suggesting that gene flow should not be restricted (Mabry 2007), and minimizing the possibility of genetic differences between habitats.
(b) Radiotracking
Animals were captured and radiocollared on trapping grids, but were free to move across the landscape after collaring. We fitted pre-dispersal juveniles with BD-2NC radiocollars (Holohil, Inc., Carp, Ontario, Canada) in the field, allowing mice to acclimate to collars for at least 24 hours before data collection began. We then radiotracked the movements of collared juveniles until they settled at a new nest. Because animals were initially trapped on a grid rather than at nest sites, we had no knowledge of potential relationships among individuals until they were collared and tracked back to nests. However, only two mice shared a natal nest site and were born at approximately the same time, suggesting that they might be littermates. Therefore, potential non-independence of dispersal behaviour of siblings should not confound our analyses.
We used standard radiotracking methods (White & Garrott 1990) to follow individuals. We determined diurnal nest locations by following a radiocollar's signal until directly above it (White & Garrott 1990), then recorded nest coordinates using a handheld GPS unit with submetre accuracy (Trimble GeoExplorer 3; Trimble Navigation Limited, Sunnyvale, CA, USA). We located mice during their nocturnal activity period using triangulation from known coordinates (White & Garrott 1990). The triangulation allowed us to locate animals from a distance, without approaching or disturbing them. Triangulation locations were estimated using Lenth's technique for maximum-likelihood estimates (MLE) in the computer program Locate III (Nams 2006).
On average, individual mice were observed over a period of 33.83±3.52 days (range: 15–70), during which movements were tracked on 7.61±0.85 nights (range: 3–15) and nests were located on 16.75±1.84 days (range: 6–31). We began nocturnal tracking approximately 30 min after sunset, corresponding to the beginning of the brush mouse activity period (Kalcounis-Rüppell & Millar 2002); tracking typically ended between 01.00 and 03.00 hours. The mean time interval between subsequent nocturnal locations of an individual was 92.29±2.19 min (range: 31–254).
(c) Pre- and post-dispersal criteria
We categorized P. boylii (weighing 18g or less) with juvenal pelage at first capture as pre-dispersal juveniles (McCabe & Blanchard 1950; Jameson 1953). The first diurnal location of an individual was its natal nest. Peromyscus disperse from their natal range just before reaching sexual maturity (Howard 1949); therefore, we tracked dispersers until adulthood (completion of the post-juvenal moult, external evidence that the animal was in reproductive condition or body mass greater than 20 g). The nest at which an animal was first observed as an adult was its post-dispersal nest. If an animal was not mature when radiotracking ended, but was later re-trapped as an adult, we used the first adult trap location as its post-dispersal location. We radiocollared 33 juvenile brush mice out of which 18 animals met all of the criteria for identification of both pre- and post-dispersal locations and were included in habitat selection analyses. The remaining 15 individuals were lost during tracking due to several causes: six animals lost their collars, two animals were predated, two radiocollar batteries died and five individuals disappeared due to unknown causes.
(d) Movement during dispersal
To determine if dispersers travelled more within their natal habitat type than expected, we re-sampled each animal's observed movements during the dispersal process to generate 100 simulated paths. The simulated paths were constrained to be the same total length, and to begin and end at the same points as actual movement paths; movement steps were sampled from each individual's observed distribution of move lengths and turning angles. We used the alternate animal movements extension (Jenness 2004) for ArcView v. 3.2 (ESRI, Redlands, CA, USA) for these analyses. We determined the length of each simulated path that intersected each habitat type, and took a mean of 100 simulated paths to obtain an individual's expected travel distance through each habitat type. We standardized path length measurements as the proportion of each path that intersected each habitat type, and arcsine-square root-transformed data before comparing observed and expected values using a paired t-test in SPSS v. 15 (SPSS, Inc., Chicago, IL, USA).
(e) Habitat availability
We conducted the habitat selection analysis using two different methods to calculate habitat availability: ‘individual’ and ‘landscape’ (described below). Under each method, we used a logistic regression model to test whether dispersing brush mice were more likely to settle in their natal habitat type than expected based on habitat availability. The proportion of natal-type habitat available to an individual was considered equal to its expected probability of settling in the natal habitat type. We used PROC GENMOD with a binomial distribution and the logit of habitat availability as an offset variable in SAS v. 9.1 (SAS Institute, Cary, NC, USA) to conduct logistic regressions. The offset variable controlled for individual variation in natal habitat availability. For example, consider two hypothetical individuals with natal habitat availabilities of 0.98 and 0.40, respectively. Even if both individuals settled within the natal habitat, the habitat choices made by these two individuals do not contain equal amounts of information about the effects of natal experience on habitat preference. This is because the animal with natal availability 0.98 has a 0.98 a priori expectation of settling in natal habitat; it would not be surprising if that animal did settle in the natal habitat. On the other hand, it is much more meaningful if an animal with natal habitat availability 0.40 chooses to settle within its natal habitat type. Our analysis would weight these two observations based on their differences in habitat availability.
For the individual method, we first defined an ‘exploration radius’, calculated as the distance between the natal nest and the farthest point from the natal nest at which an individual was located during dispersal. We considered all habitat within the exploration radius of an individual's natal nest to be available to that individual. For the landscape method, we followed many habitat selection studies (reviewed by Buskirk & Millspaugh 2006) in computing habitat availability based on the proportion of woodland and chaparral present within the study area (53% woodland, 46% chaparral, 1% other). Under the landscape method, all of the dispersers in the study were assumed to have natal habitat availability equal to the per cent landscape covered by that habitat type. All means are presented ±1 s.e.
3. Results
We observed a great deal of individual variation in movement behaviour during the dispersal process, including a wide range of exploration radii. The maximum distance at which animals were detected from their natal nests ranged from 24.67 to 186.68 m (mean: 77.43±11.56). The differences in exploration radii were not attributable to differences in the amount of data gathered on different individuals (one-tailed Spearman's correlation between exploration radius and number of nights tracked: rs=0.190, p=0.225). Combining exploration radii and unique natal nest locations on the landscape generated substantial variation in natal habitat type availabilities among individuals (0.423–0.998); therefore, some animals had a much higher a priori expectation of settling in natal habitat than did other individuals. Dispersal distances (distance between natal and settlement nests) were shorter than exploration radii, ranging from 0 to 179.23 m (mean: 28.08±9.87). Owing to a low sample size, we were unable to test for a difference in dispersal distances between the sexes.
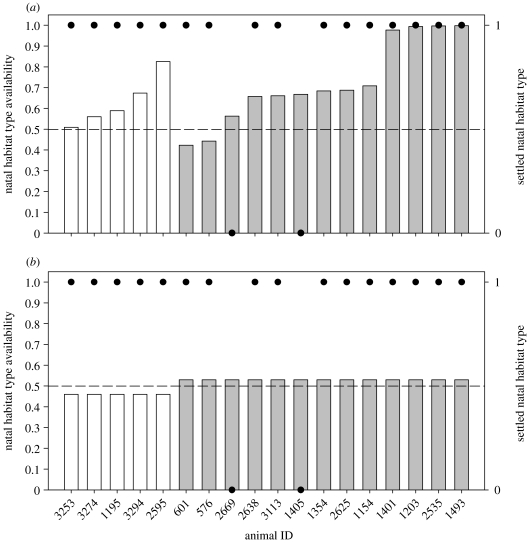
Consistent with the predictions made by NHPI, dispersers were more likely to both search and settle within their natal habitat type than expected based on habitat availability. The total distance travelled within the natal habitat type was farther than expected based on simulated movement paths (mean observed proportion of travel path in natal habitat type=0.81±0.06, mean expected proportion=0.66±0.04; paired t-test, t(17)=3.435, p=0.003). Brush mice were also more likely to settle in their natal habitat type than expected under both the methods of computing habitat availability (logistic regression, ‘individual’: Χ2=5.94, p=0.015, figure 1a; ‘landscape’: Χ2=7.73, p=0.005, figure 1b). Ninety per cent of tracked individuals ventured into the non-natal habitat type at least once, indicating that they actively chose a habitat, rather than settling within their natal habitat because they never encountered the other habitat type.
Figure 1.
Dispersing brush mice were more likely to settle in their natal habitat type than expected based on habitat availability. Bars show natal habitat availability for animals originating in chaparral (white) and woodland (grey) habitats under two methods of defining available habitat: (a) individual and (b) landscape. Filled circles denote whether an individual settled within its natal habitat type (in logistic regression analysis, ‘1’ corresponded to settling in natal habitat, ‘0’ to settling in non-natal habitat). Dashed reference lines show natal habitat availability of 0.5 (representing an equal chance of selecting either habitat type).
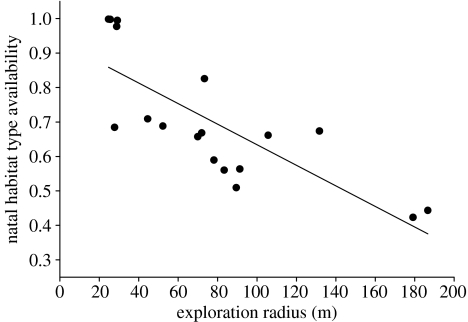
Results from the two methods of calculating available habitat were qualitatively similar, but the difference in the p values generated by the two methods illustrates the importance of considering variation among dispersers in both points of origin and movement patterns when studying habitat choice. Many of the brush mice in our study confined all of their exploratory movements to areas adjacent to their natal home range, but most individuals had to travel considerably further to encounter areas of non-natal habitat. As a result, for this species, there was a very strong negative relationship between an individual's exploration radius and the availability of natal habitat for that individual (one-tailed Spearman correlation, rs=−0.835, p<0.001, figure 2). That is, animals whose movements were confined to the vicinity of their natal home range during the dispersal process had an extremely high chance of settling within their natal habitat, while those individuals that ventured farther away had a much greater chance of encountering, and potentially settling in, non-natal habitat.
Figure 2.
Dispersing brush mice with shorter exploration radii have a higher a priori chance of settling within their natal habitat type. Habitat availability of 1 indicates that all available habitat was of the natal type; by definition, an animal with natal availability 1 must settle in natal habitat.
In contrast to the individual method, the landscape method implied that nearly all of the dispersers in the study area were highly likely to encounter non-natal habitat. As a result, estimates of natal habitat availability were significantly lower under the landscape than the individual method (Wilcoxon signed-rank test, Z=−3.375, p=0.001). These results suggest that ignoring individual differences in exploratory behaviour during dispersal may increase the chances of incorrectly rejecting the null hypothesis (type I error), in this case, inflating the chances of detecting NHPI.
4. Discussion
NHPI has long been suspected to occur in nature, and its potential to influence ecological and the evolutionary processes has been recognized for some time (i.e. Thorpe 1945). However, a lack of robust evidence that NHPI occurs under natural conditions in the field has led some to question its relevance (reviewed by Davis & Stamps 2004; Davis 2006). The current study presents evidence of NHPI for free-living natal dispersers in a species whose members live in different types of natural habitat (chaparral versus woodland), using statistical methods that explicitly controlled for individual differences in movement patterns when estimating habitat availability. We found that dispersers were more likely to both search and settle within their natal habitat type than expected based on availability of that habitat type. Together with previous studies suggestive of the occurrence of NHPI, our work demonstrates that experience in a particular natal habitat type may have a substantial effect on the habitat choices made by natal dispersers.
We have also shown that ignoring inter-individual variation in movement behaviour when estimating habitat availability may lead to overestimates of the probability that NHPI occurs in a given species. For example, when we followed a practice common in habitat selection studies, of assuming that all of the habitat within the study area was available to all of the dispersers (landscape method), the p value for the test of NHPI was much lower than when we defined habitat availability for a given individual based on the distance that individual actually travelled away from its natal nest during the dispersal process (individual method). This discrepancy in results occurred because animals that did not venture far from home had a much higher a priori probability of settling within natal habitat than did animals that moved across long distances prior to selecting a new habitat; as a result, the individual test was much more conservative than the landscape test. Given growing evidence of considerable inter-individual variation in movement behaviour in a wide range of species (Fraser et al. 2001; Diffendorfer et al. 2005; Doerr & Doerr 2005; Sheppard et al. 2006), our results imply that researchers who do not consider individual differences in movement patterns when estimating habitat availability may inadvertently find stronger support for NHPI than is warranted.
Other researchers have investigated NHPI in free-living mammals (i.e. Haughland & Larsen 2004; Selonen et al. 2007); however, earlier studies have suffered from two issues which weaken their results. First, ours is the first study of NHPI conducted in a landscape composed of different types of naturally occurring suitable habitat, which varied with respect to vegetation community, habitat structure, available food resources, and many other biotic and abiotic features (Mabry 2007). Haughland & Larsen (2004) studied red squirrels (T. hudsonicus) in a highly modified coniferous forest landscape in which different ‘habitats’ were defined based on whether or not they were logged, and whether they were close to the edge between logged and unlogged areas. Logged and unlogged areas are different in many ways, but logged habitat is fairly novel in the evolutionary history of red squirrels. Selonen et al. (2007) studied Siberian flying squirrels (P. volans) living in a landscape that contained only one type of habitat suitable for residency (spruce forest), and compared geometric features (patch size, edge versus centre) of the natal site and the post-dispersal site selected by natal dispersers in this species. Both of these studies present valuable information on the impact of natal surroundings on post-dispersal habitat choices, but do not address the fundamental question of whether habitat preferences are influenced by experience in different naturally occurring natal habitat types with which the focal species has an evolutionary history.
The conclusions of previous studies of NHPI are also complicated by the fact that they did not consider individual variation in movement distances when estimating habitat availability for different dispersers in the same study area. For example, Haughland & Larsen (2004) used the maximum distance moved by any individual between subsequent locations to estimate the habitat available to all animals in their study, based on the implicit assumption that all individuals in the population were likely to move on a comparable scale. Similarly, Selonen et al. (2007) used a single distance (500 m from an individual's settlement patch) to estimate the habitat that was available to that individual. The landscape method used in the current study relied on a more extreme definition of available habitat than that used by either Haughland & Larsen (2004) or Selonen et al. (2007); however, our results do raise the possibility that studies that ignore individual variation in movement may be vulnerable to incorrectly rejecting the null hypothesis of no effect of natal habitat experience on subsequent habitat preferences.
Any demonstration that NHPI occurs in nature raises questions about the potential adaptive significance of this phenomenon (Stamps & Davis 2006). At this point, two sets of mutually non-exclusive hypotheses explain why experience in a natal habitat might increase a natal disperser's level of preference for that type of habitat (Stamps 2001; Davis & Stamps 2004; Stamps & Davis 2006). ‘Habitat training’ hypotheses argue that as a result of adaptive developmental plasticity, dispersers that have adjusted to conditions in their natal habitat are more efficient at using new post-dispersal habitats of the same type as their natal habitat. In contrast, ‘habitat cuing’ hypotheses argue that experiences in the natal habitat increase disperser estimates of the quality of that type of habitat at the current time in the current landscape. The habitat training hypotheses predict that animals will perform better (e.g. higher foraging success, greater efficiency at avoiding predators, higher growth or fecundity) if their post-dispersal patch is of the same type as their natal patch, while habitat cuing hypotheses predict that by selecting a new post-dispersal habitat of the same type as their natal habitat, dispersers improve their chances of making the right decision when choosing a new habitat, and (correctly) selecting a type of habitat that is of relatively high quality at the current time in the current landscape. However, at this point, neither of these hypotheses for NHPI has been explicitly tested for any animal.
While the fitness consequences of NHPI for individual dispersers are still unknown, it is clear that by generating intraspecific variation in habitat preferences, NHPI leads to a situation in which the perceived quality of a given habitat is not necessarily the same for all individuals of a species. When the results of the current study are added to those of previous studies on this topic (Haughland & Larsen 2004; Sacks et al. 2005; Tonnis et al. 2005; Pilot et al. 2006; Selonen et al. 2007), evidence is accumulating that natal experience may play an important role in determining habitat choice for free-living dispersers in natural landscapes. In light of this evidence, it seems likely that NHPI may impact ecological and evolutionary processes in a variety of species under natural conditions.
Acknowledgments
This research adhered to the Association for the Study of Animal Behaviour/Animal Behavior Society Guidelines for the Use of Animals in Research. All procedures were approved by the University of California, Davis Animal Use and Care Administrative Advisory Committee, and fieldwork was carried out under a scientific collecting permit from the California Department of Fish and Game.
We thank Jeremy Davis, Tom Hahn, Doug Kelt, Sarah Karlen, Nick Haddad and two anonymous referees for their comments on previous versions of the manuscript, and Jeremy Davis, James Wilson, Terry Ord and Matina Kalcounis-Rüppell for discussions. Mallory Bello, Manuel Breuer, Aaron Corcoran, Michelle Early, Rachael Handley, Mo Li, Nobu Nameta, Leilani Roser, Bill Ruggles and Glenda Yenni assisted in the field. We thank Neil Willits for statistical consulting, and Virginia Boucher, Dan Tolson, Shane Waddell and Kenny Walker for their logistical support at the Quail Ridge Reserve. Funding provided by the US National Science Foundation (Graduate Research Fellowship to K.E.M. and Doctoral Dissertation Improvement Grant to J.A.S. and K.E.M.), the US Environmental Protection Agency (STAR Fellowship to K.E.M.), the American Society of Mammalogists (K.E.M.), the Animal Behavior Society (K.E.M.), the University of California Natural Reserve System (Mildred Mathias Grant to K.E.M.), the UC Davis Animal Behavior Graduate Group (K.E.M.) and the UC Davis Center for Population Biology (K.E.M.).
References
- Arthur S.M, Manly B.F.J, McDonald L.L, Garner G.W. Assessing habitat selection when availability changes. Ecology. 1996;77:215–227. doi:10.2307/2265671 [Google Scholar]
- Beltman J.B, Haccou P. Speciation through the learning of habitat features. Theor. Popul. Biol. 2005;67:189–202. doi: 10.1016/j.tpb.2005.01.001. doi:10.1016/j.tpb.2005.01.001 [DOI] [PubMed] [Google Scholar]
- Bowler D.E, Benton T.G. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 2005;80:205–225. doi: 10.1017/s1464793104006645. doi:10.1017/S1464793104006645 [DOI] [PubMed] [Google Scholar]
- Buskirk S.W, Millspaugh J.J. Metrics for studies of resource selection. J. Wildl. Manage. 2006;70:358–366. doi:10.2193/0022-541X(2006)70[358:MFSORS]2.0.CO;2 [Google Scholar]
- Davis, J. M. 2006 The influence of natal experience on habitat selection. PhD dissertation, University of California, Davis.
- Davis J.M, Stamps J.A. The effect of natal experience on habitat preferences. Trends Ecol. Evol. 2004;19:411–416. doi: 10.1016/j.tree.2004.04.006. doi:10.1016/j.tree.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Diffendorfer J.E, Rochester C, Fisher R.N, Brown T.K. Movement and space use by coastal rosy boas (Lichanura trivirgata roseofusca) in coastal Southern California. J. Herpetol. 2005;39:24–36. doi:10.1670/212-03A [Google Scholar]
- Doerr E.D, Doerr V.A.J. Dispersal range analysis: quantifying individual variation in dispersal behaviour. Oecologia. 2005;142:1–10. doi: 10.1007/s00442-004-1707-z. doi:10.1007/s00442-004-1707-z [DOI] [PubMed] [Google Scholar]
- Forbes A.A, Fisher J, Feder J.L. Habitat avoidance: overlooking an important aspect of host-specific mating and sympatric speciation? Evolution. 2005;59:1552–1559. doi: 10.1554/04-740. [DOI] [PubMed] [Google Scholar]
- Fraser D.F, Gilliam J.F, Daley M.J, Le A.N, Skalski G.T. Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am. Nat. 2001;158:124–135. doi: 10.1086/321307. doi:10.1086/321307 [DOI] [PubMed] [Google Scholar]
- Hanski I, Singer M.C. Extinction–colonization dynamics and host-plant choice in butterfly metapopulations. Am. Nat. 2001;158:341–353. doi: 10.1086/321985. doi:10.1086/321985 [DOI] [PubMed] [Google Scholar]
- Haughland D.L, Larsen K.W. Exploration correlates with settlement: red squirrel dispersal in contrasting habitats. J. Anim. Ecol. 2004;73:1024–1034. doi:10.1111/j.0021-8790.2004.00884.x [Google Scholar]
- Howard, W. E. 1949 Dispersal, amount of inbreeding, and longevity in a local population of prairie deermice on the George Reserve, Southern Michigan Contributions from the laboratory of vertebrate biology, no. 43, pp. 1–52. Ann Arbor, MI: University of Michigan.
- Jameson E.W., Jr Reproduction of deer mice (Peromyscus maniculatus and P. boylei) in the Sierra Nevada, California. J. Mammal. 1953;34:44–58. doi:10.2307/1375943 [Google Scholar]
- Jenness, J. 2004 Alternate animal movement routes (altroutes.avx) extension for ArcView 3.x, v. 2.1. Flagstaff, AZ: Jenness Enterprises.
- Kalcounis-Rüppell, M. C. 2000 Breeding systems, habitat overlap, and activity patterns of monogamous and promiscuous mating in Peromyscus californicus and P. boylii PhD dissertation, University of Western Ontario.
- Kalcounis-Rüppell M.C, Millar J.S. Partitioning of space, food, and time by syntopic Peromyscus boylii and P. californicus. J. Mammal. 2002;83:614–625. doi:10.1644/1545-1542(2002)083<0614:POSFAT>2.0.CO;2 [Google Scholar]
- Kalcounis-Rüppell, M. C. & Spoon, T. R. Submitted. Peromyscus boylii
- Mabry, K. E. 2007 Habitat selection by dispersing brush mice (Peromyscus boylii). PhD dissertation, University of California, Davis.
- McCabe T.T, Blanchard B.D. Rood Associates; Santa Barbara, CA: 1950. Three species of Peromyscus. [Google Scholar]
- Muller K.L, Stamps J.A, Krishnan V.V, Willits N.H. The effects of conspecific attraction and habitat quality on habitat selection in territorial birds (Troglodytes aedon) Am. Nat. 1997;150:650–661. doi: 10.1086/286087. doi:10.1086/286087 [DOI] [PubMed] [Google Scholar]
- Nams V.O. Pacer Computer Software; Tatamagouche, Canada: 2006. Locate III user's guide. [Google Scholar]
- Pilot M, Jedrzejewski W, Branicki W, Sidorovich V.E, Jedrzejewska B, Stachura K, Funk S.M. Ecological factors influence population genetic structure of European grey wolves. Mol. Ecol. 2006;15:4533–4553. doi: 10.1111/j.1365-294X.2006.03110.x. doi:10.1111/j.1365-294X.2006.03110.x [DOI] [PubMed] [Google Scholar]
- Ribble D.O, Stanley S. Home ranges and social organization of syntopic Peromyscus boylii and P. truei. J. Mammal. 1998;79:932–941. doi:10.2307/1383101 [Google Scholar]
- Sacks B.N, Mitchell B.R, Williams C.L, Ernest H.B. Coyote movements and social structure along a cryptic population genetic subdivision. Mol. Ecol. 2005;14:1241–1249. doi: 10.1111/j.1365-294X.2005.02473.x. doi:10.1111/j.1365-294X.2005.02473.x [DOI] [PubMed] [Google Scholar]
- Selonen V, Hanski I.K. Habitat exploration and use in dispersing juvenile flying squirrels. J. Anim. Ecol. 2006;75:1440–1449. doi: 10.1111/j.1365-2656.2006.01168.x. doi:10.1111/j.1365-2656.2006.01168.x [DOI] [PubMed] [Google Scholar]
- Selonen V, Hanski I.K, Desrochers A. Natal habitat-biased dispersal in the Siberian flying squirrel. Proc. R. Soc. B. 2007;274:2063–2068. doi: 10.1098/rspb.2007.0570. doi:10.1098/rspb.2007.0570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard J.K, Preen A.R, Marsh H, Lawler I.R, Whiting S.D, Jones R.E. Movement heterogeneity of dugongs, Dugong dugon (Muller), over large spatial scales. J. Exp. Mar. Biol. Ecol. 2006;334:64–83. doi:10.1016/j.jembe.2006.01.011 [Google Scholar]
- Slagsvold T, Wiebe K.L. Learning the ecological niche. Proc. R. Soc. B. 2007;274:19–23. doi: 10.1098/rspb.2006.3663. doi:10.1098/rspb.2006.3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamps J.A. Habitat selection by dispersers: integrating proximate and ultimate approaches. In: Clobert J, Danchin E, Dhondt A.A, Nichols J.D, editors. Dispersal. Oxford University Press; New York, NY: 2001. pp. 230–242. [Google Scholar]
- Stamps J.A, Davis J.M. Adaptive effects of natal experience on habitat selection by dispersers. Anim. Behav. 2006;72:1279–1289. doi:10.1016/j.anbehav.2006.03.010 [Google Scholar]
- Thorpe W.H. The evolutionary significance of habitat selection. Anim. Ecol. 1945;14:67–70. doi:10.2307/1385 [Google Scholar]
- Tonnis B, Grant P.R, Grant B.R, Petren K. Habitat selection and ecological speciation in Galapagos warbler finches (Certhidea olivacea and Certhidea fusca) Proc. R. Soc. B. 2005;272:819–826. doi: 10.1098/rspb.2004.3030. doi:10.1098/rspb.2004.3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecker S.C. The role of early experience in habitat selection by the prairie deer mouse, Peromyscus maniculatus bairdi. Ecol. Monogr. 1963;33:307–325. doi:10.2307/1950749 [Google Scholar]
- West-Eberhard M.J. Oxford University Press; New York, NY: 2003. Developmental plasticity and evolution. [Google Scholar]
- White G.C, Garrott R.A. Academic Press, Inc; New York, NY: 1990. Analysis of wildlife radio-tracking data. [Google Scholar]