Abstract
Sexual dimorphism in coloration is a taxonomically widespread phenomenon often attributed to sexual selection on visual signals. However, the ambush bug Phymata americana exhibits sexual dimorphism in coloration that has no apparent signalling function. Here we provide evidence that colour pattern in this species influences male mating success indirectly through its effect on thermoregulation. We demonstrate, using experimental manipulation, that individuals with dark colour pattern achieve higher thoracic temperatures under illumination. We also show that dark colour pattern predicted mate-searching success but only under thermally challenging conditions (i.e. cool ambient temperature). As far as we are aware, this is the first study to provide evidence that sexual dimorphism can be accounted for by sexual selection on thermoregulatory performance.
Keywords: sexual selection, thermoregulation, colour pattern, sexual dimorphism, mate searching
1. Introduction
In ectotherms, physiological processes and behavioural activity are strongly influenced by body temperature (e.g. foraging, mating patterns; Larsson 1989; Heinrich 1993; Seymour et al. 2003). Temperature has been shown to directly affect various components of fitness including viability, survival, fecundity and fertility (Rodriguez-Saona & Miller 1999; Forsman 2001; Hazel 2002; Fischer et al. 2003; Stillwell & Fox 2005). As a consequence, many ectothermic species have evolved specific behaviours to facilitate thermoregulation (e.g. Kingsolver 1987; Rutowski et al. 1994; Forsman et al. 2002; Kemp & Krockenberger 2002). In addition to its importance for the evolution of behavioural adaptations, thermoregulatory demands have also shaped the evolution of morphology—particularly colour pattern. Since the absorption of light energy by an object is partly determined by its reflectance, hue and saturation, colour pattern can be especially important for regulating body temperature (e.g. Brakefield & Willmer 1985; de Jong et al. 1996; Forsman 1997; Hazel 2002). As a result, thermoregulation has been invoked to explain seasonal polyphenism in insects, where dark phenotypes are more prevalent during cooler periods (e.g. Watt 1968, 1969; Shapiro 1976; Kingsolver & Wiernasz 1987; Holloway 1993; Kingsolver 1995; Hazel 2002). Similarly, temperature has been linked to melanic polymorphisms in a number of ectotherms (e.g. Benham et al. 1974; Jones et al. 1977; Brakefield 1985; de Jong & Brakefield 1998; Forsman et al. 2002).
Some indirect evidence suggests that selection on thermoregulatory performance can also potentially account for the evolution of a different form of intraspecific colour pattern variation, i.e. sexual dimorphism. Although the evolution of sexually dimorphic coloration is commonly attributed to sexual selection on visual signals (reviewed by Andersson 1994), phenotypic divergence between the sexes may also occur when sexes occupy different habitats or ecological niches (Slatkin 1984). Differences in sex roles can result in quite different thermal demands and, subsequently, sexually divergent selection on traits that influence thermoregulation. Previous work has shown that the expression of sexually dimorphic melanic coloration in Colias butterflies increases along an altitudinal gradient (Kingsolver 1983; Ellers & Boggs 2002) and that this can be attributed to the thermal effects of melanization on female egg maturation rates and flight capability (Ellers & Boggs 2004).
In addition to its thermal effects on viability and fecundity, colour pattern might confer thermal properties that have important consequences for mating success. Microclimate (e.g. ambient temperature) is known to influence mating patterns in insects (e.g. Larsson 1989; Jann et al. 2000), suggesting that temperature may affect the strength of sexual selection. In scramble mating systems, where variance in mate-searching ability accounts for a good proportion of the variance in total mating success (e.g. Sutherland 1985; Hubbell & Johnson 1987; Carroll & Salamon 1995), intrasexual selection on a thermoregulatory trait seems particularly plausible. Previous work by Pivnick & McNeil (1986) showed that the skipper Thymelicus lineolus exhibits sexual dimorphism in size (but not colour) and this might be the result of selection favouring small male size owing to thermal constraints on flight (i.e. smaller individuals can achieve minimum flight temperatures sooner). In species exhibiting male colour pattern polymorphism, different morphs are associated with particular behaviours/tactics and these differences are assumed to be related to the thermal costs and benefits of melanism (e.g. O'Neill & Evans 1983; Van Dyck et al. 1997). However, direct evidence linking colour pattern to mating success via thermoregulatory performance has, thus far, been lacking.
In the current study, we asked whether thermoregulation could account for the sexual colour pattern dimorphism observed in the ambush bug Phymata americana. Males of this species have a darker head and thorax than their female counterparts. Previous studies (Punzalan 2007; Punzalan et al. submitted) have shown that males expressing increased pigmentation in these areas achieve elevated coupling success (a surrogate of mating success) in the wild, yet this trait plays neither an apparent role in mediating direct male–male competition, nor is it subject to direct female mating biases (Punzalan 2007). Males actively search for females and spend the majority of their time in search of prospective mates (Mason 1986). Mate guarding allows males to effectively monopolize access to females but direct male–male competition (e.g. agonistic interactions) is not common in this species (D. Punzalan 2002, personal observation). Furthermore, successfully coupled males are nearly always successful in achieving copulation, at least, in laboratory conditions (Punzalan 2007). These lines of evidence suggest that male fitness is primarily limited by the ability to find mates and that sexual selection on coloration may be primarily the result of variance in male mate-searching success.
We tested the hypothesis that male coloration is a sexually selected thermal adaptation in two parts. First, we measured the effect of manipulated pigmentation on thoracic heating rates to test the prediction that dark thoracic coloration would lead to higher thoracic temperatures. Second, we asked whether male coloration predicted mate-searching performance and determined the extent to which the effects of coloration were contingent upon ambient temperature. We predicted that relatively dark males would experience a mate-searching advantage only in a challenging thermal environment (i.e. relatively cool ambient temperature) but not in a benign environment (i.e. relatively warm ambient temperature).
2. Material and methods
(a) Study organism and measurement of traits
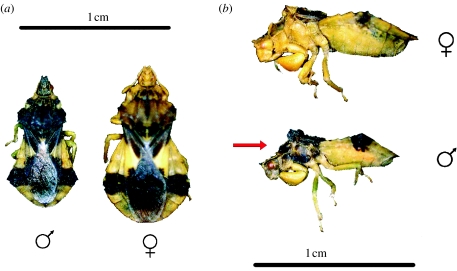
The ambush bug P. americana Melin (Heteroptera: Phymatidae) is a sit-and-wait predator of flower-visiting insects (Balduf 1939, 1941). Its colour pattern is complex, consisting of a mostly yellow integument with dark (black or brown) patches (figure 1). Although the coloration has typically been attributed to disruptive coloration related to camouflage (Dodson & Marshall 1984; Schuh 1995), P. americana exhibits striking sexual dimorphism in the expression of dark colour patches. Males are generally darker than females but also exhibit patches of dark coloration on the lateral surface of the head and thorax that are absent in females (figure 1). Males exhibit considerable phenotypic variation in lateral coloration (figure 2). We quantified two components of male colour pattern: dorsal coloration (defined as the total area of black colour patches, i.e. pixels with a value of 200 or greater) visible from the dorsal aspect and lateral coloration (defined as the area of black colour pattern on the head and thorax visible from the lateral aspect). Previous work has established that lateral colour pattern predicts mating success in the wild, suggesting sexual selection as a prominent agent in the evolution of sexual dimorphism in this species (Punzalan 2007). Colour pattern and body size were measured from digital images using Scion Image analysis software (http://www.scioncorp.com). Detailed methods describing the measurement of colour pattern are reported elsewhere (Punzalan 2007). All bugs used in the following studies were originally collected from a natural population occurring at the Koffler Scientific Reserve at Joker's Hill, King, Ontario, Canada (44°03′ N, 79°29′ W).
Figure 1.
(a) Dorsal and (b) lateral view of male and female P. americana, illustrating the sex differences in size and coloration. Notably, males express dark ‘lateral coloration’ on the head and thorax that is absent in females.
Figure 2.
Natural variation in lateral colour pattern among male P. americana. Depicted are a composite of digital photographs of a subset of individuals collected from a single population on a single date.
(b) The effect of colour pattern on thoracic temperature
To investigate the effects of colour pattern on absorption of incident (light) radiation, we measured thoracic temperatures of individuals with manipulated colour patterns under standardized lighting conditions in the laboratory. The 26 wild-caught females were euthanized by freezing for 24 hours at −20°C. For these manipulations, we did not use males because a thick application of paint was required to successfully augment colour pattern—dark coloration in males required a thicker application to achieve the ‘pale’ treatment. Instead, we used females because the absence of dark lateral colour pattern in females meant that minimal amounts of paint had to be applied. We chose 11 size-matched pairs of females, differing by less than or equal to 0.1 mm in pronotum width (a reliable measure of overall body size, Mason (1973)), and for each pair randomly assigned one individual to the ‘dark’ treatment and the other to the pale treatment. For individuals in the dark treatment, a thin layer of black acrylic paint (Liquitex, no. 1045–244) was applied to cover the entire lateral surface of the right side of the thorax. The same procedure was performed for individuals in the pale treatment except that yellow paint (Liquitex, no. 1045–830) was applied. Paint was allowed to dry for 2 hours, after which individuals were separately stored in 0.5 ml centrifuge tubes at −20°C. Just prior to measuring body temperatures, a matched pair of bugs was randomly selected and each bug carcass was decapitated using dissecting scissors and allowed to thaw on a Petri dish at room temperature for 20 min. The Petri dish was then moved into an 18°C environmentally controlled room illuminated by fluorescent full-spectrum lights and each bug was mounted (via the thorax) on one of two (30 gauge, Type-T copper-constantan) thermocouple probes spaced 2.5 cm apart and suspended 3 cm above a polystyrene board (4.7 cm in thickness). Using rubber-tipped forceps, bugs were quickly mounted on a contact such that their right lateral surface was facing upward. The bugs were covered with a brown paper towel for an additional 10 min before beginning trials to minimize premature exposure to incident light. Trials began by first turning on a 60 W incandescent lamp positioned 65 cm directly above the mounted bugs. The paper towel was removed and we began recording temperature. Paired temperature readings over the next 10 min were automatically logged every 10 s using a Digi-Sense DuaLogR digital thermometer/logger. This procedure was performed for a total of 11 matched pairs and, in each replicate, the dark and pale treatments were randomly assigned to either probe.
To evaluate treatment differences in heating rates and maximum body temperatures achieved, we calculated temperature excesses (ΔT) at each time interval as body temperature at any given time (Tt)−body temperature at the beginning of each trial (T0). Following the method used by Pivnick & McNeil (1986), we estimated thermal heating constants (k) for each individual by first calculating (for each time interval) the value X by subtracting ΔT from the maximum temperature achieved by the end of the trial (i.e. T10). Plotting X against time results in decay curves that can be described by the expression
| (2.1) |
where Xfinal corresponds to the estimated value of X at the end of the measured interval t. For each individual, we estimated the thermal warming constant k during the first minute of heating (where the most rapid temperature change occurred; see figure 3) using linear regression (after loge transformation of equation (2.1)). We tested for treatment differences between matched pairs of dark and pale bugs in k using (two-tailed) Wilcoxon signed-rank tests. Similarly, we evaluated treatment differences in temperature excess at 1 min (ΔT1) and at 10 min (ΔT10) using Wilcoxon signed-rank tests.
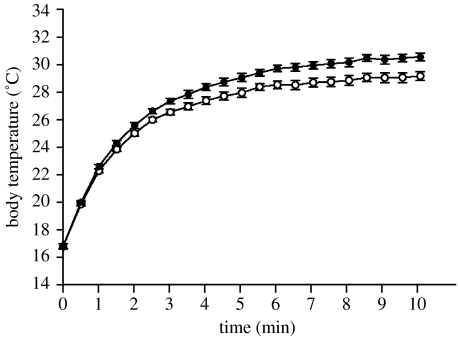
Figure 3.
Thoracic heating rates for P. americana with experimentally manipulated dark (filled circles) and pale (open circles) lateral colour pattern. Depicted are the mean (±s.e.) temperatures for 10 min under incandescent illumination.
(c) The interaction between ambient temperature and sexual selection
To test whether variation in colour pattern translates into differences in performance, we evaluated the extent to which male colour pattern predicted mate-searching success in contrasting thermal environments. Owing to the difficulty associated with direct manipulation of colour pattern traits on live bugs (discussed below), we instead partitioned the effects of colour pattern statistically, estimating multivariate phenotypic selection on these traits in each environment. This was done by evaluating male mate-searching success in groups of males allowed to search for mates in replicate arenas housed in a laboratory environmental chamber maintained either at a cool ambient temperature (18°C) or at a relatively warm ambient temperature (27°C). These temperatures corresponded roughly to the morning and midday temperatures recorded in field conditions during the peak of P. americana abundance at the collection site. The study was performed in blocks; on three separate days (roughly a week apart), we assayed male mating success in the low ambient temperature (‘cool’) environment and the high ambient temperature (‘warm’) environment. In total, we measured mate-searching success of 96 wild males (48 in each treatment, combined across the three blocks). The protocol used in each block is as follows. From the field, 32 males were collected and transported to the laboratory. An equal number of females were randomly drawn from a laboratory colony composed of approximately 100 males and females. Males and females were then photographed and were individually marked with a unique combination of dots of coloured (Liquitex) acrylic paints. These marks were very small and were positioned on the thorax (left and right anterior tubercules) and abdomen (the right connexiva) so as to be visible during the trials while minimizing their interference with the natural phenotype. Bugs were housed overnight (approx. 12 hours; 27°C, 40% RH and 14 L : 10 D) in same-sex groups in two separate 0.25 l glass jars containing cardboard substrate and provisioned with an ad libitum supply of live Drosophila melanogaster.
On the following day, females were randomly introduced into one of the two transparent plastic arenas (32×21×22 cm3). Within each arena was a polystyrene base supporting an array of wooden applicator sticks (14 cm in length and 4 mm in diameter), distributed in a uniformly spaced 8×5 arrangement. An arena was placed into each of the two environmentally controlled rooms with identical lighting conditions (full-spectrum fluorescent lights approx. 15 cm above the top of the cage, plus a 60 W incandescent lamp positioned 40 cm above the top of the cage) but maintained at different temperatures. The males were weighed and randomly assigned/placed into one of the two separate Petri dishes where they were maintained at room temperature. One hour after the females were introduced into the cages, the males were introduced; 16 males were placed in the cool temperature arena and the other 16 males were placed in the warm temperature arena. Every 30 min thereafter, we recorded whether individuals were mating (i.e. copulating or coupled with a member of the opposite sex; described below) and recorded their identity. After 12 hours, trials were ended and males were individually stored and frozen at −20°C. Females were returned to the laboratory colony but were not reused in subsequent trials.
We estimated two measures of male mate-searching performance: coupling latency and coupling frequency. ‘Coupling’ refers to a pre- (or post-) copulatory position where males are positioned atop of the female, clasping her dorsal surface. Coupling typically lasts for several hours (Balduf 1941; Dodson & Marshall 1984) and has been shown to be highly correlated with copulation (Punzalan 2007). In the present study, we operationally defined coupling to include events where males were observed to be in the position described above as well as observed instances of actual copulation. For each male, coupling latency (in min) was defined as the duration that elapsed from the beginning of the trial until the male was found coupled. This was calculated for each male by multiplying the number of sampling intervals observed prior to first coupling by 30 min. We recorded coupling frequency as the number of different females a male successfully coupled with during a trial.
To be consistent with a previous field study estimating selection on the same suite of traits (Punzalan 2007), we included four male traits in the subsequent analyses: body size, measured as pronotum width (in mm; see Mason 1973), weight (in mg) and dorsal and lateral coloration (in mm2). Although we were specifically interested in the effect of ambient temperature for mediating selection on lateral coloration, we measured selection on the other traits for a number of reasons. First, despite an apparent lack of sexual selection favouring dorsal coloration in the wild (Punzalan 2007), this colour pattern trait could have strong effects on mate-searching performance via thermoregulation. Efficiency in thermoregulation may also be related to both overall body size and shape because the ratio of surface area-to-volume can influence the net gain and loss of heat from radiation and convection (e.g. Brakefield & Willmer 1985; Bittner et al. 2002). The size of colour patches may also simply scale with body size and, therefore, body size may experience selection for thermoregulatory reasons (Pivnick & McNeil 1986). Furthermore, components of body size and shape may also affect other aspects of locomotory performance, contributing to variance in mate-searching success (e.g. Carroll & Salamon 1995). Finally, weight (after controlling for body size) is often considered a measure of condition (e.g. fat reserves) and, thus, a correlate of male quality or vigour (e.g. Jakob et al. 1996; but see Kotiaho 1999). Since coloration in P. americana is condition dependent (Punzalan 2007), it is difficult to separate the effects of condition and male colour pattern without directly manipulating colour pattern. However, due to the nature and location of the trait (i.e. on the head and thorax), we were unable to physically manipulate lateral pigmentation without severely affecting male behaviour and health. Therefore, including weight in the analyses provided some measure of statistical control of the effects of variance in male condition.
Weight was cube root transformed and colour pattern measures were square root transformed prior to analysis. Image data for one individual in the cool temperature treatment was lost and, subsequently, this individual was omitted from the analyses. All traits were loge transformed and standardized (mean=0, s.d.=1) prior to analyses.
We estimated the effect of treatment (ambient temperature), trial date and male traits on coupling latency using a censored proportional hazard (Cox regression) model because not all males successfully coupled and the distribution of coupling latency was not normally distributed. The independent variables in the model included treatment (coded as dummy variables), trial date (i.e. block effects), the four male traits (pronotum width, weight, dorsal and lateral coloration) as well as the four treatment×trait interaction terms. The response variable in the model was coupling latency and we included a binary censor status variable (0, unsuccessful; 1, successful) indicating whether or not individuals had successfully coupled at least once during the trial.
In terms of coupling frequency, the distribution was approximately normal and we estimated the effect of treatment, trial date and male traits on coupling frequency, using a mixed model linear regression with coupling frequency as a continuous response variable. The independent variables in the model were treatment (categorical variable), trial date (block effect), the four male traits (continuous variables) and the treatment×trait interactions. Trial date was treated as a random effect.
To facilitate interpretation, and because the full statistical model did not reveal a significant block (trial date) effect, we pooled the data across days. From these data, we calculated the phenotypic selection gradients describing selection acting on male traits in each ambient temperature environment (i.e. cool versus warm) separately. Following Lande & Arnold (1983), variance-standardized linear selection gradients were estimated from multiple linear regression of relative fitness (i.e. absolute coupling frequency divided by mean coupling frequency) on the four measured male traits. Relative fitness and trait standardizations were performed for each treatment separately. Similarly, we used these treatment-specific values of fitness to calculate ‘the opportunity for selection’, I, the variance in relative fitness (Arnold & Wade 1984).
Statistical analyses were performed using Systat software (v. 10.0) except for the survival analysis, where parameters were estimated using the Cox proportional hazards (coxph) function in the base package in ‘R’: a language and environment for statistical computing, v. 2.4.1 (available at: http://www.r-project.org).
3. Results
(a) The effect of colour pattern on thoracic temperature
The thoracic temperature of bugs of both colour patterns increased under illumination, initially exhibiting a rapid increase followed by a slow, asymptotic increase (figure 3). Dark and pale bugs showed similar heating rates (mean±s.d.: kdark=1.004±3.35×10−4; kpale=1.004±2.22×10−4; Wilcoxon signed-rank test, z=1.156, n=11, p=0.248) but dark bugs achieved higher body temperatures (dark: ΔT1=5.95±0.535, pale: mean ΔT1=5.65±0.499; Wilcoxon signed-rank test, z=2.149, n=11, p=0.032) during the first minute of heating and this difference was equally apparent at the end of the trials (ΔT10: Wilcoxon signed-rank test, z=2.671 n=11, p=0.008).
(b) The interaction between ambient temperature and sexual selection
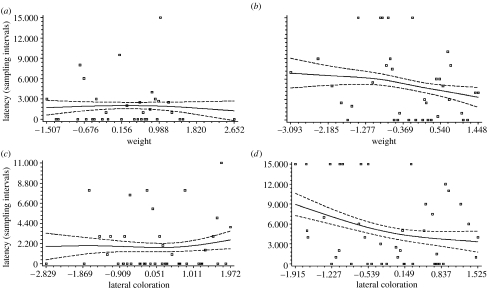
As expected, ambient temperature had a significant effect on mating patterns. Males in the warm ambient temperature treatment had shorter coupling latencies than males in the cool ambient temperature treatment. Trial date had no significant effect on coupling latency. Both weight and lateral colour pattern were predictors of mate-searching success but the strength of these effects were more apparent in the cool ambient temperature treatment (table 1; figure 4).
Table 1.
The effects of ambient temperature treatment, trial day (block) and four male traits for predicting mate-searching success (coupling latency) in P. americana. (Coefficients were estimated from Cox proportional hazards regression. The p<0.05 are indicated by asterisks. Full model: likelihood-ratio test Χ102=23.2, p<0.001, n=95.)
| coefficient | s.e. | z | p | |
|---|---|---|---|---|
| treatment | 0.655 | 0.233 | 2.82 | 0.005* |
| trial day | −0.323 | 0.208 | −1.55 | 0.120 |
| pronotum width | −0.428 | 0.261 | −1.64 | 0.100 |
| weight | 0.607 | 0.261 | 2.33 | 0.020* |
| dorsal | −0.389 | 0.264 | −1.47 | 0.140 |
| lateral | 0.580 | 0.274 | 2.12 | 0.034* |
| treatment×pronotum width | 0.646 | 0.348 | 1.86 | 0.063 |
| treatment×weight | −0.683 | 0.328 | −2.08 | 0.037* |
| treatment×dorsal | 0.483 | 0.345 | 1.40 | 0.160 |
| treatment×lateral | −0.919 | 0.337 | −2.73 | 0.006* |
Figure 4.
Cubic splines (solid lines) ±s.e. (dashed lines) depicting the relationship between searching latency (in 30-min sampling intervals) plotted against residual weight in (a) warm versus (b) cool ambient temperatures and searching latency plotted against residual lateral colour pattern in (c) warm versus (d) cool ambient temperatures for male P. americana. Plotted values for (cube root) weight and (square root) lateral colour pattern were calculated as residuals from separate regressions on pronotum width. For all splines, smoothing parameter (λ)=1 and standard errors were calculated from 1000 bootstraps using Fortran (Schluter 1988).
Coupling frequency was significantly affected by ambient temperature; males had higher coupling success in the warm ambient temperature (median coupling frequency=2, range=0–4) than in the cool ambient temperature (mean coupling frequency=1, range=0–4; treatment: F1,83=12.129, p<0.001, table 2). There was also a significant interaction between ambient temperature and the strength of selection on lateral colour pattern (treatment×lateral colour interaction: F1,83=7.398, p=0.008, table 2). Examination of plots of coupling frequency versus residual lateral colour pattern (after regression on pronotum width) revealed that one particular individual in the cool treatments had an unusually high coupling frequency compared with the other males in the same treatment. We reanalysed the data with the full model (with the potentially influential observation removed) but the results were qualitatively similar; there remained a significant treatment effect (F1,82=17.651, p<0.001) and significant treatment×lateral colour pattern effect (F1,82=6.175, p=0.015).
Table 2.
The effects of ambient temperature treatment, trial day (block) and four male traits for predicting coupling frequency in P. americana. (The effects of each model term were estimated using a mixed model linear regression. The p<0.05 are indicated by asterisks. Full model: multiple R2=0.505, n=95.)
| SS | d.f. | MS | F-ratio | p | |
|---|---|---|---|---|---|
| treatment | 8.871 | 1 | 8.871 | 13.129 | 0.001* |
| trial day | 0.599 | 2 | 0.300 | 0.443 | 0.643 |
| pronotum width | 0.479 | 1 | 0.479 | 0.709 | 0.402 |
| weight | 0.140 | 1 | 0.140 | 0.207 | 0.650 |
| dorsal | 0.229 | 1 | 0.229 | 0.339 | 0.562 |
| lateral | 0.011 | 1 | 0.011 | 0.017 | 0.897 |
| treatment×pronotum width | 1.331 | 1 | 1.331 | 1.970 | 0.164 |
| treatment×weight | 0.233 | 1 | 0.233 | 0.345 | 0.559 |
| treatment×dorsal | 0.680 | 1 | 0.680 | 1.007 | 0.319 |
| treatment×lateral | 4.999 | 1 | 4.999 | 7.398 | 0.008* |
| error | 56.078 | 83 | 0.676 |
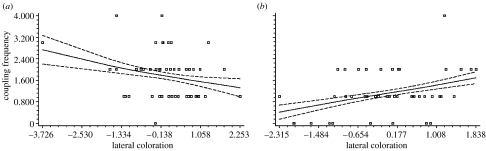
In the currency of phenotypic selection gradients (with coupling frequency as the measure of fitness), we detected significant positive selection on lateral coloration but only in the cool ambient temperature treatment, indicating that, in relatively low temperatures, relatively dark individuals had higher coupling success than relatively pale individuals (table 3; figure 5a). In contrast, we observed a trend towards negative linear selection on lateral colour pattern in the warm ambient temperature treatment but this was not statistically significant (table 3; figure 5b). We found no evidence for direct selection on the other three traits we measured: dorsal coloration, body weight and pronotum width. The opportunity for selection was approximately twice as great in the cool ambient temperature treatment (I=0.502) than in the warm ambient temperature treatment (I=0.247).
Table 3.
Linear selection gradients (β) and their standard errors (s.e.) estimating the relationship between male traits and coupling frequency in P. americana assayed in cool versus warm ambient temperature treatments. (Partial regression coefficients and their statistical significance were estimated from separate multiple linear regressions. Mean and coefficient of variation (CV) for traits (prior to loge transformation) for males in each treatment are also included. The p<0.05 are denoted by an asterisk. The opportunity for selection (I) is also reported for each treatment. (Cool treatment, n=47; warm treatment, n=48).)
| cool ambient temperature (18°C) | warm ambient temperature (27°C) | |||||
|---|---|---|---|---|---|---|
| traits | mean (CV) | β (s.e.) | p | mean (CV) | β (s.e.) | p |
| pronotum width | 3.059 mm (0.054) | −0.065 (0.174) | 0.710 | 3.106 mm (0.047) | 0.149 (0.095) | 0.126 |
| weight | 1.8 mg (0.129) | 0.122 (0.150) | 0.423 | 1.9 mg (0.112) | −0.002 (0.084) | 0.983 |
| dorsal coloration | 16.16 mm2 (0.124) | −0.239 (0.171) | 0.170 | 16.78 mm2 (0.108) | 0.031 (0.102) | 0.765 |
| lateral coloration | 4.72 mm2 (0.172) | 0.315 (0.148) | 0.039* | 4.90 mm2 (0.195) | −0.191 (0.099) | 0.060 |
| opportunity for selection | ||||||
| I=0.502 | I=0.247 | |||||
Figure 5.
Cubic splines (solid lines) ±s.e. (dashed lines) depicting the relationship between lateral colour pattern on coupling frequency in male P. americana under (a) warm and (b) cool ambient temperature treatments. Plotted values for lateral colour pattern were calculated as residuals from a regression of lateral coloration on dorsal colour pattern. For both splines, smoothing parameter (λ)=1 and standard errors were calculated from 1000 bootstraps using Fortran (Schluter 1988).
4. Discussion
We tested the hypothesis that dark lateral colour pattern in P. americana is a thermoregulatory trait that mediates mate-searching success. We found evidence to support our prediction that dark colour pattern confers higher rates of thoracic heat gain and that this translates into enhanced mate-searching performance. We show that, consistent with previous evidence, microclimate variables can exert tremendous effects on mating patterns and the opportunity for sexual selection in insects (e.g. Larsson 1989; Blanckenhorn et al. 1999; Jann et al. 2000; also see Twiss et al. (2007) for an example in endotherms). Specifically, we demonstrate that lateral colour pattern had a strong influence on thoracic temperatures and was associated with mate-searching success. Males with relatively dark lateral colour pattern generally had shorter mate-searching times (latency) but this advantage was more pronounced in cool ambient temperatures. Similarly, dark lateral coloration was associated with higher coupling frequency (i.e. the number of females found) and this advantage was manifested only in cool ambient temperatures.
Overall, our results suggest that male lateral coloration plays an important role in mediating mate-searching success through thermoregulatory function. Dark lateral coloration may be selectively advantageous by allowing males to achieve/maintain higher body temperatures such that the wing and leg muscles are at the optimum required for locomotion, thus, allowing mate searching even in relatively cool ambient temperatures. This might be especially important in the morning when low ambient temperatures limit the activity of P. americana. Early activity is probably advantageous since mate guarding in this species appears to be particularly effective in allowing males to monopolize access to females (D. Punzalan 2002, unpublished data). Males that succeed in finding a prospective mate are unlikely to be displaced by other males and, as a result, mating success is primarily limited by mate searching.
This role of lateral colour pattern in mate searching may explain our previous findings that male lateral colour pattern predicts coupling success in the wild despite having no apparent role in intersexual selection via female mating preferences or in intrasexual selection through direct male–male competition. This trait appears to be strongly condition dependent (Punzalan 2007), suggesting that costs of production and/or maintenance of this trait are responsible for the maintenance of phenotypic (and possibly genetic) variance in male colour pattern. Although dark lateral colour pattern may be favoured in some environmental conditions, it could also be selectively disfavoured under other ecological conditions. For example, in the present study, we observed positive selection on lateral colour pattern in cool ambient temperatures but a non-significant trend towards negative selection in warm ambient temperatures, possibly indicating that excessive heat gain (i.e. overheating) has detrimental effects on male mate-searching success.
There is also indirect evidence supporting the link between environmental temperature and colour pattern in P. americana. A study by Mason (1975) showed that cooler (i.e. spring) environmental temperatures during egg development were correlated with darker male coloration in wild populations. This may be indicative of adaptive plasticity exhibited by this species in response to anticipated environmental conditions in a manner similar to that reported for some hoverflies (Holloway 1993) and butterflies (e.g. Watt 1969; Kingsolver & Wiernasz 1987; Kingsolver 1995). The effects of temperature during ontogeny have also long been recognized as important in determining colour pattern development in a wide range of insects (e.g. Hazel 2002).
An alternative explanation for the apparent importance of lateral coloration for mate-searching success is that the trait is only correlated with another trait that truly underlies variance in male mate-searching success. For example, male condition could potentially affect all aspects of male mating effort and performance (i.e. males in good condition may have more total resources to allocate to all components of fitness/performance, including lateral coloration). It is important to note, however, that although residual weight (often considered a surrogate of condition) did indeed predict one component of mate-searching success (i.e. relatively short coupling latency) in cool ambient temperatures, lateral colour pattern predicted searching success (latency and coupling frequency) even after correction for the effect of residual weight. Thus, even though lateral colour pattern is itself a strongly condition-dependent trait, lateral colour pattern appears to be a target of direct selection independent of selection favouring males of high body condition.
It is unclear why lateral coloration is a target of selection while dorsal coloration did not experience significant selection. The dorsal surface of the thorax surely experiences considerable exposure to sunlight and might be expected to have similar effects on thoracic temperature; yet, mate-searching performance did not covary with dorsal coloration. One possibility is that, because male dorsal coloration exhibits relatively low phenotypic variance compared with lateral colour pattern based on coefficients of phenotypic variation measured here and in our previous studies (Punzalan 2007; Punzalan et al. submitted), the absolute effects of variation in dorsal colour on body temperature are relatively small and statistically difficult to detect. Ambush bugs also express dark colour patches on the abdominal sternites (not measured in this study), the adaptive significance of which is unclear. It is possible that dorsal and abdominal coloration have different functions (e.g. stabilizing selection on crypsis), potentially accounting for why both of these components of coloration are also expressed in females of this species, albeit to a lesser extent. Being sit-and-wait predators, females probably maximize fitness by spending most of their time motionless and hidden from prey and potential predators (Mason 1986). If dorsal and abdominal coloration enhances crypsis, then these components are unlikely to experience directional selection at equilibrium. Males too may benefit from disruptive coloration when hunting or when coupled, as males often guard females for extended periods of time, during which colour pattern may be subject to considerable selection imposed by visually orienting predators and prey. Thus, both sexes may benefit to some extent from dorsal and abdominal coloration, while lateral coloration confers fitness advantages only to males. In light of the sex differences in habits and reproductive strategy (Mason 1977, 1986) and the apparent resource limitations to producing dark pigmentation, dark lateral coloration could be selectively disfavoured in females.
The absence of dark lateral coloration in females may also be partially explained by the costs of excessively high body temperatures. In addition to the obvious negative effects of overheating on survival, excessively high temperature has also been shown to result in reduced fecundity in insects (e.g. Carroll & Quiring 1993; Wang & Tsai 1996; Fischer et al. 2003). Further experimental work is required to fully understand the adaptive significance of dorsal and abdominal colour pattern in this species.
Invoking thermoregulatory function to account for the evolution of lateral coloration implies that the lateral thorax must be exposed to enough solar radiation to effect changes in thoracic temperature. Although the lateral surface of the thorax is probably exposed to ample incident solar radiation under natural conditions, one might expect correlated selection for behaviours that can mediate the amount of light energy absorbed. Males may actively adjust their location or position in their microhabitat to increase absorption under low ambient temperatures or to decrease absorption under high temperatures. Stereotyped posturing (e.g. basking) is common in many ectotherms (e.g. Rawlins 1980; Kingsolver 1985; Rutowski et al. 1994; Kemp & Krockenberger 2002) but whether this occurs in P. americana has yet to be investigated.
We should point out that because we estimated the effects of dark coloration by measuring heating curves of manipulated female phenotypes, the effects of lateral coloration on passive heating curves could differ somewhat in males. Females are larger than males (approx. 53% wider in pronotum width and about twice as heavy), potentially providing greater (approx. 28%) surface area for absorption. On the other hand, large size is also associated with greater thermal inertia, resulting in slower rates of passive heating. Assuming that heating rates scale with surface area (e.g. pronotum width2) and inversely with mass (Punzalan 2007), the thermal constants estimated in our study are approximately 18% higher than expected for smaller (i.e. male) sizes (based on average measures of male and female size). However, this does not qualitatively alter the conclusion that dark colour pattern resulted in higher temperature excesses and that coloration is an important thermal trait that mediates the strength and direction of sexual selection.
The pattern of selection observed in the cool ambient temperature treatment of the present study is qualitatively very similar to the patterns of multivariate phenotypic selection for the same four male traits estimated from natural populations (Punzalan 2007). Though selection in the wild varied temporally, lateral coloration consistently surfaced as the only trait subject to significant positive sexual selection although male weight tended to show a weak positive covariance with coupling success. In contrast, in the field, dorsal coloration and body size (pronotum width) tended to be selectively disfavoured. Because our laboratory study detected patterns of selection similar to those we observed in the field, it suggests that our study was successful in capturing some of the important factors that govern mating dynamics and selection in the wild.
While the idea that thermal performance might mediate mating success has been suggested in the past (e.g. Rawlins 1980; O'Neill & Evans 1983; Pivnick & McNeil 1986; Rutowski et al. 1994), empirical demonstrations of this potential mechanism for sexual selection are scarce. Furthermore, in many of the species studied by previous authors invoking the importance of thermoregulation for sexual selection, sexual dimorphism (in the direction of sexual selection) is notably absent. Curiously, the sexual colour dimorphism exhibited by P. americana is not atypical for this family of bugs (Phymatidae) but is rare in this order (Heteroptera; G. G. E. Scudder 2003, personal communication). If this mechanism of sexual selection functions in other species of ambush bugs as well, it may reflect a mechanism of selection that is particularly important under certain ecological conditions. Whether sexual colour pattern dimorphism driven by selection for thermally advantageous traits is a widespread phenomenon, and under what conditions it is favoured, will be an interesting avenue for future research.
In conclusion, we have provided evidence that a thermoregulatory trait mediates a component of mating success in P. americana. To our knowledge, this is the first documented case of sexual dimorphism in coloration that can be accounted for by sexual selection via thermoregulatory performance. Although thermoregulation has been implicated in the evolution and maintenance of colour polymorphism and polyphenism in other species, its potential importance for explaining sexual colour dimorphism has, until now, been largely overlooked.
Acknowledgments
We are grateful to M. Cooray and B. Turner for assistance in insect collection, maintenance and conducting experiments. The author would like to thank to A. Budden, J. Perry, M. Kasumovic G. Tattersall, D. Gwynne and three anonymous reviewers for their comments on previous drafts of the manuscript and also the University of Toronto Statistical Consulting Services. D.P. was supported by funds from the Natural Sciences and Engineering Research Council and the Premier's Research Excellence Award to L.R. and F.H.R., and by funds from the University of Toronto. This work was also supported by a W. John D. Eberlie Research Travel Award (Toronto Entomologists Association) to D.P.
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Arnold S.J, Wade M.J. On the measurement of natural and sexual selection: theory. Evolution. 1984;38:709–719. doi: 10.1111/j.1558-5646.1984.tb00344.x. doi:10.2307/2408383 [DOI] [PubMed] [Google Scholar]
- Balduf W.V. Food habits of Phymata pennsylvanica americana Melin (Hemip.) Can. Entomol. 1939;71:66–74. [Google Scholar]
- Balduf W.V. Life history of Phymata pennsylvanica americana Melin (Phymatidae, Hemiptera) Ann. Entomol. Soc. Am. 1941;34:204–214. [Google Scholar]
- Benham B.R, Lonsdale D, Muggleton J. Is polymorphism in two-spot ladybird an example of non-industrial melanism? Nature. 1974;249:179–180. doi: 10.1038/249179a0. doi:10.1038/249179a0 [DOI] [PubMed] [Google Scholar]
- Bittner T.D, King R.B, Kerfin J.M. Effects of body size and melanism on the thermal biology of garter snakes (Thamnophis sirtalis) Copeia. 2002;2002:477–482. doi:10.1643/0045-8511(2002)002[0477:EOBSAM]2.0.CO;2 [Google Scholar]
- Blanckenhorn W.U, Morf C, Mulhauser C, Reusch T. Spatiotemporal variation in selection on body size in the dung fly Sepsis cynipsea. J. Evol. Biol. 1999;12:563–576. doi:10.1046/j.1420-9101.1999.00050.x [Google Scholar]
- Brakefield P.M. Differential winter mortality and seasonal selection in the polymorphic ladybird Adalia bipunctata in The Netherlands. Biol. J. Linn. Soc. 1985;24:189–206. [Google Scholar]
- Brakefield P.M, Willmer P.G. The basis of thermal melanism in the ladybird Adalia bipunctata: differences in reflectance and thermal properties between the morphs. Heredity. 1985;54:9–14. [Google Scholar]
- Carroll A.L, Quiring D.T. Interactions between size and temperature influence fecundity and longevity of a tortricid moth, Zeiraphera canadensis. Oecologia. 1993;93:233–241. doi: 10.1007/BF00317676. doi:10.1007/BF00317676 [DOI] [PubMed] [Google Scholar]
- Carroll S.P, Salamon M.H. Variation in sexual selection on male body size within and between populations of the soapberry bug. Anim. Behav. 1995;50:1463–1474. doi:10.1016/0003-3472(95)80003-4 [Google Scholar]
- de Jong P.W, Brakefield P.M. Climate and change in clines for melanism in the two-spot ladybird, Adalia bipunctata (Coleoptera, Coccinellidae) Proc. R. Soc. B. 1998;265:39–43. doi:10.1098/rspb.1998.0261 [Google Scholar]
- de Jong P.W, Gussekloo S.W.S, Brakefield P.M. Differences in thermal balance, body temperature and activity between non-melanic and melanic two-spot ladybird beetles (Adalia bipunctata) under controlled conditions. J. Exp. Biol. 1996;199:2655–2666. doi: 10.1242/jeb.199.12.2655. [DOI] [PubMed] [Google Scholar]
- Dodson G, Marshall L. Mating patterns in an ambush bug Phymata fasciata (Phymatidae) Am. Midl. Nat. 1984;112:50–57. doi:10.2307/2425456 [Google Scholar]
- Ellers J, Boggs C.L. Evolution of wing color in Colias butterflies: heritability, sex-linkage, and population divergence. Evolution. 2002;56:836–840. doi: 10.1111/j.0014-3820.2002.tb01394.x. [DOI] [PubMed] [Google Scholar]
- Ellers J, Boggs C.L. Functional ecological implications of intraspecific differences in wing melanization in Colias butterflies. Biol. J. Linn. Soc. 2004;82:79–87. doi:10.1111/j.1095-8312.2004.00319.x [Google Scholar]
- Fischer K, Eenhorn E, Bot A.N.M, Brakefield P.M, Zwaan B.J. Cooler butterflies lay larger eggs: developmental plasticity versus acclimation. Proc. R. Soc. B. 2003;270:2051–2056. doi: 10.1098/rspb.2003.2470. doi:10.1098/rspb.2003.2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman A. Thermal capacity of different color morphs in the pygmy grasshopper Tetrix subulata. Ann. Zool. Fenn. 1997;34:145–149. [Google Scholar]
- Forsman A. Clutch size versus clutch interval: life history strategies in the colour-polymorphic pygmy grasshopper Tetrix subulata. Oecologia. 2001;129:357–366. doi: 10.1007/s004420100743. [DOI] [PubMed] [Google Scholar]
- Forsman A, Ringblom K, Civantos E, Ahnesio J. Coevolution of color pattern and thermoregulatory behavior in polymorphic pygmy grasshoppers Tetrixundulata. Evolution. 2002;56:349–360. doi: 10.1111/j.0014-3820.2002.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Hazel W.N. The environmental and genetic control of seasonal polyphenism in larval color and its adaptive significance in a swallowtail butterfly. Evolution. 2002;56:342–348. doi: 10.1111/j.0014-3820.2002.tb01344.x. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Harvard University Press; Cambridge MA: 1993. The hot-blooded insects: strategies and mechanisms of thermoregulation. [Google Scholar]
- Holloway G.J. Phenotypic variation in color pattern and seasonal plasticity in Eristalis hoverflies (Diptera, Syrphidae) Ecol. Entomol. 1993;18:209–217. [Google Scholar]
- Hubbell S.P, Johnson L.K. Environmental variance in lifetime mating success, mate choice and sexual selection. Am. Nat. 1987;130:91–112. doi:10.1086/284700 [Google Scholar]
- Jakob E.M, Marshall S.D, Uetz G.W. Estimating fitness: a comparison of body condition indices. Oikos. 1996;77:61–67. doi:10.2307/3545585 [Google Scholar]
- Jann P, Blanckenhorn W.U, Ward P.I. Temporal and microspatial variation in the intensities of natural and sexual selection in the yellow dung fly Scathophaga stercoraria. J. Evol. Biol. 2000;13:927–938. doi:10.1046/j.1420-9101.2000.00230.x [Google Scholar]
- Jones J.S, Leith B.H, Rawlings R. Polymorphism in Cepaea: a problem with too many solutions? Annu. Rev. Ecol. Syst. 1977;8:109–143. doi:10.1146/annurev.es.08.110177.000545 [Google Scholar]
- Kemp D.J, Krockenberger A.K. A novel method of behavioural thermoregulation in butterflies. J. Evol. Biol. 2002;15:922–929. doi:10.1046/j.1420-9101.2002.00470.x [Google Scholar]
- Kingsolver J.G. Thermoregulation and flight in Colias butterflies: elevational patterns and mechanistic limitations. Ecology. 1983;64:534–545. doi:10.2307/1939973 [Google Scholar]
- Kingsolver J.G. Thermoregulatory significance of wing melanization in Pieris butterflies: physics, posture, pattern. Oecologia. 1985;66:540–545. doi: 10.1007/BF00379348. doi:10.1007/BF00379347 [DOI] [PubMed] [Google Scholar]
- Kingsolver J.G. Evolution and coadaptation of thermoregulatory behavior and wing pigmentation pattern in pierid butterflies. Evolution. 1987;41:472–490. doi: 10.1111/j.1558-5646.1987.tb05819.x. doi:10.2307/2409250 [DOI] [PubMed] [Google Scholar]
- Kingsolver J.G. Fitness consequences of seasonally polyphenism in the western white butterfly. Evolution. 1995;49:942–954. doi: 10.1111/j.1558-5646.1995.tb02329.x. doi:10.2307/2410416 [DOI] [PubMed] [Google Scholar]
- Kingsolver J.G, Wiernasz D.C. Seasonal polyphenism and thermoregulatory adaptation in Pieris butterflies. Am. Nat. 1987;137:816–830. doi:10.1086/285195 [Google Scholar]
- Kotiaho J.S. Estimating fitness: a comparison of body indices revisited. Oikos. 1999;87:399–400. doi:10.2307/3546755 [Google Scholar]
- Lande R, Arnold S.J. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. doi:10.2307/2408842 [DOI] [PubMed] [Google Scholar]
- Larsson F.K. Insect mating patterns explained by microclimatic variables. J. Therm. Biol. 1989;14:155–157. doi:10.1016/0306-4565(89)90038-7 [Google Scholar]
- Mason L.G. The habitat and phenetic variation in Phymata americana Melin. Syst. Zool. 1973;22:271–279. doi:10.2307/2412307 [Google Scholar]
- Mason L.G. Habitat and phenetic variation in Phymata americana Melin (Heteroptera: Phymatidae). II. Climate and temporal variation in colour pattern. Syst. Zool. 1975;25:123–128. doi:10.2307/2412738 [Google Scholar]
- Mason L.G. Prey preferences and ecological size dimorphism in Phymata americana Melin. Am. Midl. Nat. 1977;97:293–299. doi:10.2307/2425095 [Google Scholar]
- Mason L.G. Free-loaders, free-lancers and bush-whackers: sexual dimorphism and seasonal changes in prey-capture behaviour of ambush bugs. Am. Midl. Nat. 1986;116:323–329. doi:10.2307/2425740 [Google Scholar]
- O'Neill K.M, Evans H.E. Alternative male mating tactics in Bembecinus quinquespinosus (Hymenoptera: Sphecidae): correlations with size and color variation. Behav. Ecol. Sociobiol. 1983;14:39–46. doi:10.1007/BF00366654 [Google Scholar]
- Pivnick K.A, McNeil J.N. Sexual differences in the thermoregulation of Thymelicus lineola adults (Lepidoptera: Hesperiidae) Ecology. 1986;67:1024–1035. doi:10.2307/1939825 [Google Scholar]
- Punzalan, D. 2007 Evolution of sexual colour pattern dimorphism in the ambush bug Phymata americana, pp. 188. PhD dissertation, University of Toronto.
- Punzalan, D., Rodd, F. H. & Rowe, L. Submitted. Contemporary sexual selection on sexually dimorphic traits in the ambush bug Phymata americana
- Rawlins J.E. Thermoregulation by the black swallowtail butterfly, Papilio polyxenes (Lepidoptera: Papilionidae) Ecology. 1980;61:345–357. doi:10.2307/1935193 [Google Scholar]
- Rodriguez-Saona C, Miller J.C. Temperature-dependent effects on development, mortality, and growth of Hippodamia convergens (Coleoptera: Coccinellidae) Environ. Entomol. 1999;28:518–522. [Google Scholar]
- Rutowski R.L, Demlong M.J, Leffingwell T. Behavioural thermoregulation at a mate encounter site by male butterflies (Asterocampa leilia, Nymphalidae) Anim. Behav. 1994;48:833–841. doi:10.1006/anbe.1994.1307 [Google Scholar]
- Schluter D. Estimating the form of natural selection on a quantitative trait. Evolution. 1988;42:849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. doi:10.2307/2408904 [DOI] [PubMed] [Google Scholar]
- Schuh R.S. Cornell University Press; New York, NY: 1995. True bugs of the world. [Google Scholar]
- Seymour R.S, White C.R, Giberneau M. A heat reward for insect pollinators. Nature. 2003;426:243–244. doi: 10.1038/426243a. doi:10.1038/426243a [DOI] [PubMed] [Google Scholar]
- Shapiro A.M. Seasonal polyphenism. Evol. Biol. 1976;9:259–333. [Google Scholar]
- Slatkin M. Ecological causes of sexual dimorphism. Evolution. 1984;38:622–630. doi: 10.1111/j.1558-5646.1984.tb00327.x. doi:10.2307/2408711 [DOI] [PubMed] [Google Scholar]
- Stillwell R.C, Fox C.W. Complex patterns of phenotypic plasticity: interactive effects of temperature during rearing and oviposition. Ecology. 2005;86:924–934. doi:10.1890/04-0547 [Google Scholar]
- Sutherland W.J. Chance can produce a sex difference in mating success and explain Bateman's data. Anim. Behav. 1985;33:1349–1352. doi:10.1016/S0003-3472(85)80197-4 [Google Scholar]
- Twiss S.D, Thomas C, Poland V, Graves J.A, Pomeroy P. The impact of climatic variation on the opportunity for sexual selection. Biol. Lett. 2007;3:12–15. doi: 10.1098/rsbl.2006.0559. doi:10.1098/rsbl.2006.0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyck H, Matthysen E, Dhondt A.A. The effect of wing colour on male behavioural strategies in the speckled wood butterfly. Anim. Behav. 1997;53:39–51. doi:10.1006/anbe.1996.0276 [Google Scholar]
- Wang K.H, Tsai J.H. Temperature effect on development and reproduction of silverleaf whitefly (Homoptera: Aleyrodidae) Ann. Entomol. Soc. Am. 1996;89:375–384. [Google Scholar]
- Watt W.B. Adaptive significance of pigment polymorphisms in Colias butterflies. I. Variation in melanin pigment in relation to thermoregulation. Evolution. 1968;22:437–458. doi: 10.1111/j.1558-5646.1968.tb03985.x. doi:10.2307/2406873 [DOI] [PubMed] [Google Scholar]
- Watt W.B. Adaptive significance of pigment polymorphisms in Colias butterflies. II. Thermoregulation and photoperiodically controlled melanin variation in Colias eurytheme. Proc. Natl Acad. Sci. USA. 1969;63:767–774. doi: 10.1073/pnas.63.3.767. doi:10.1073/pnas.63.3.767 [DOI] [PMC free article] [PubMed] [Google Scholar]