Abstract
Social insects and insects that provision nests are well known to have complex foraging behaviour involving repeated visits to learned locations. Other insects do not forage from a central location and are generally assumed to respond to resources by simple attraction without spatial memory. This simple response to resource cues is generally taken as giving rise to patterns of resource use that correspond directly to resource distribution. By contrast, the solitary parasitoid wasp Hyposoter horticola monitors the locations of multiple potential hosts (butterfly eggs) for up to several weeks, until the hosts become susceptible to parasitism. Essentially all hosts in the landscape are found, and one-third of them are parasitized, independent of host density. Here, we show that the wasps do not relocate hosts using odour markers previously left by themselves or other foragers, nor do they find the eggs anew repeatedly. Instead, the wasps relocate host eggs by learning the position of the eggs relative to visual landmarks. The anticipatory foraging behaviour presented here is a key to the wasp's exceptionally stable population dynamics.
Keywords: foraging behaviour, Hyposoter horticola, Melitaea cinxia, population dynamics, spatial memory
1. Introduction
Social bees and ants use memorized visual cues to navigate between a central nest and the resources within a foraging landscape (von Frisch 1967; Capaldi et al. 2000; Collett & Collett 2002; Menzel et al. 2006). Similarly, predatory Hymenoptera that visit their nests repeatedly while building and provisioning them also rely on visual memory (Baerends 1941; Tinbergen 1972; Wcislo 1992). However, most insects, even most Hymenoptera, are solitary, do not have a central home or nest to provision and are assumed to forage by means of chance encounters with odours and other resource-associated cues (Nicholson & Bailey 1935; Vet et al. 1990; Papaj & Lewis 1993; Hassell 2000). One large class of such insects is the parasitoid wasps.
Adult female parasitoids forage for host arthropods to lay eggs in or upon, and their offspring grow to adulthood at the expense of the host (Godfray 1994). Parasitoids are important for biological control of insect pests (van Driesche & Bellows 1996), and are the basis of much theory about the dynamics of predator–prey interactions. These theories predict parasitism of a fraction of hosts that is dependent on parasitoid and host densities (Walde & Murdock 1988; Hassell 2000). An implicit assumption is that parasitoid foraging behaviour is based on simple sensory responses. The study presented here challenges this picture.
The parasitoid wasps are very well known to search for hosts by responding to odours associated with hosts. The wasps respond to some odours innately, and learn to respond to others in association with successful encounters (Papaj & Lewis 1993; Turlings et al. 1995; Smid et al. 2007). Parasitoids have also been shown to use odour to avoid previously visited hosts or patches, either by recognizing the odours of parasitized host individuals (Ueno & Tanaka 1996) or by recognizing odour markers left during a previous visit (Nufio & Papaj 2001). While it is known that parasitoids also use visual cues when foraging (Arthur 1966; Wäckers & Lewis 1994), their response to visual stimuli has not been well studied, and reliance on learned visual cues is even less known (Turlings et al. 1993). There is some previous laboratory evidence concerning the ability, or inability, of parasitoid wasps to learn spatial locations while foraging over small spatial and temporal time scales. On the one hand, in a laboratory flight chamber some species clearly do not keep track of where they have been (Hoffmeister & Gienapp 2001). On the other hand, the parasitoid wasp Microplitis croceipes (Braconidae) briefly remembers where it has foraged in a flight chamber, allowing it to avoid revisiting recently searched empty patches, and to avoid nearby hosts that it has already parasitized (Sheehan et al. 1993; Wäckers & Lewis 1994).
In the studies described above and many others, parasitoids that learned did so by associating sensory stimuli with finding suitable hosts, or with the avoidance of unproductive patches. This learning presumably increases the parasitoid's future foraging efficiency. Foraging efficiency of a parasitoid could be further increased by its remembering the locations of resources that are not yet productive, as this would increase the amount of time available for foraging, and broaden the pool of resources worth finding. The possibility of such anticipatory foraging by arthropods has been little studied. As far as we know, the ability of an insect to learn the spatial locations of potential resources, as opposed to resources it has already used, has been reported previously just once. In that study, Rosenheim (1987) showed that the cleptoparasitic wasp Argochrysis armilla (Chrysididae) locates ground nesting wasps as they build nests, and attends the nests during excavation, which takes a few minutes. The cleptoparasite then leaves while the mother provisions the nests with prey, returning within a few hours to oviposit into the host nests. Thus, the predator is able to locate the potential resources while they are conspicuous but unproductive, and use them when they have become rewarding but also inconspicuous.
The subject of our study is the parasitoid Hyposoter horticola (Gravenhorst) (Ichneumonidae: Campopleginae), which is an endoparasitoid of the Glanville fritillary butterfly, Melitaea cinxia (Nymphalidae). In a 50×70 km region of the Finnish Åland Islands in the Baltic Sea, the butterfly inhabits several hundred small (102–104 m2) meadows, where it lays clusters of 100–200 eggs on its larval food plants (Nieminen et al. 2004). In each annual generation, the wasp can only oviposit (figure 1) during the single several hour period when the host has developed into a larva but has not yet broken out of the egg shell (van Nouhuys & Ehrnsten 2004). Although the host occupies only 10–20% of the suitable meadows in Åland each year, H. horticola finds and parasitizes hosts in virtually every egg cluster (van Nouhuys & Hanski 2002). For example, of the 115 host egg clusters we placed in 38 different local host populations over 4 years, at least 113 clusters were parasitized. Although multiple wasps find each egg cluster, each cluster is parasitized by only one wasp. That wasp parasitizes 30–40% of the host larvae in the cluster, as described in van Nouhuys & Ehrnsten (2004).
Figure 1.
Hyposoter horticola exploring an egg cluster of M. cinxia. The back of the thorax is marked with a colour paint, in this case red, to identify the individual wasp.
We previously showed that this remarkably time-constrained parasitoid achieves such a uniform rate of parasitism by lengthening the time it has to forage. In natural (van Nouhuys & Ehrnsten 2004) and artificial (S. van Nouhuys 2005, unpublished data) habitat patches, individually marked H. horticola find host egg clusters in the weeks before they are ready to be parasitized, and continue to visit them until they are susceptible to parasitism. In a field experiment, van Nouhuys & Ehrnsten (2004) found that host egg clusters that were available for parasitoids to visit as the eggs developed were mostly parasitized, whereas host clusters that were only available briefly before becoming vulnerable to parasitism were mostly missed. This showed that wasps do not simply rediscover the same hosts repeatedly, but that they find their way back to hosts using the information gained during previous visits. Two possibilities are that the wasps relocate potential hosts by following an odour marker left during a previous visit or that they use memorized visual landmarks. Here, we first present a pair of experiments demonstrating that female H. horticola do not use odour markers as their primary cues for revisiting hosts. Second, we present two experiments showing that the wasps do use visual landmarks to help keep track of the spatial locations of host egg clusters.
2. Material and methods
The experiments were conducted in Åland, Finland in the summers of 2003 and 2005. Potted host plants with and without M. cinxia egg clusters on them were used in each experiment. The host plants, Plantago lanceolata and Veronica spicata (Plantaginaceae), were transplanted from natural patches into 12 cm diameter pots in early May. They were kept outdoors in an area with no M. cinxia butterflies. In order to get single egg clusters on a subset of the plants, individual mated female butterflies were caged with a single plant for 24 hours. The butterflies were from a colony maintained in the laboratory for two to three generations. Once a plant received an egg cluster, it was kept outside under the same conditions as the plants that had no eggs on them, covered by screen to assure no visitation by H. horticola.
(a) The use of odour: rate of parasitism
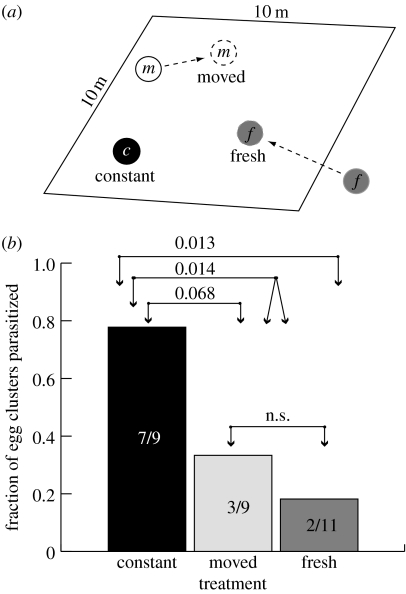
In this experiment, the rate of parasitism of host egg clusters under three conditions was compared in order to distinguish between reliance on (i) innate odour of eggs or induced odour of egg occupied plants, (ii) odour markers left by foraging wasps and (iii) visual memory of wasps. In late June 2003, a square grid of 25 squares (2×2 m each) was marked in each of the three habitat patches naturally occupied by H. horticola. Twelve potted P. lanceolata plants with one-week-old M. cinxia egg clusters on them were each randomly assigned to one of three treatments, and to a square in each plot (figure 2a). The ‘constant’ and ‘moved’ treatments were placed in the plots immediately. The ‘fresh’ treatment plants with eggs on them were kept outdoors under screen for 10–14 days and put in the habitat patch when the eggs were nearly ready to be parasitized (within 12 hours). The eggs change from yellow to creamy to dark before hatching. The wasps cannot parasitize the hosts until the eggs are dark, so we put them in the field when they were creamy (van Nouhuys & Ehrnsten 2004). Eggs in the moved treatment were checked each morning, and the plant with the eggs on it was moved to a new random (but unoccupied) square in the plot at the same developmental stage as the fresh eggs treatment. There were four replicates of each treatment in each of the three plots.
Figure 2.
(a) Schematic of constant (c), moved (m) and fresh (f) egg clusters in one replicate in one plot of the field experiment on rate of parasitism. There are three plots in the experiment with four replicates of each treatment in each plot. (b) The fraction of egg clusters parasitized by H. horticola in each of the three treatments. The numbers above the bars are the p values of post hoc comparisons among treatments.
Upon hatching (just after parasitism), the plants with larvae on them were brought to the laboratory where the host larvae were reared to third instar and then dissected to check for the presence of H. horticola larvae. During the experiment, several egg clusters were desiccated in the extremely warm weather or eaten by predators. Additionally, two of the moved and one of the fresh treatment egg clusters were excluded because they were accidentally moved or placed in the field one to several days before egg hatching. The fraction of egg clusters that were parasitized by H. horticola was analysed using logistic regression, with parasitism (yes/no) as the response variable and treatment (constant, moved or fresh) and plot as the explanatory variables. Post-estimation linear comparisons adjusted for multiple comparisons were made among treatments.
(b) The use of odour markers: rate of visitation
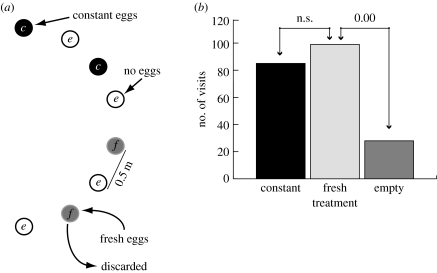
In this experiment, we distinguished revisitation based on odour markers left by the wasp, from spatial memory and from the innate odour of the host or induced plant odour. Four potted P. lanceolata with host eggs on them were arranged in a semicircle interspersed with four plants that had no eggs on them. The plants were 50 cm apart (figure 3a). There were two replicate arrays, 10 m apart in an open meadow. In total, 66 female H. horticola were released: 20 at the start of the experiment, 24 on day 6 and 22 on day 11. The wasps were not individually marked, but those released on each date had a different colour marker on the back of the thorax. The experiment took place on a small isolated island with suitable habitat patches but no natural M. cinxia butterflies or H. horticola.
Figure 3.
(a) The arrangement of potted P. lanceolata plants in the field rate of visitation experiment. The constant (c) plants with host egg clusters on them that were left in place throughout the experiment; the (f), fresh plants with host eggs on them that were replaced each day if visited by wasps; the empty (e), plants without host eggs. Two arrays 10 m apart were observed simultaneously. (b) The number of visits by H. horticola to plants in each of the three treatments described above. The numbers above the bars represent the p values of post hoc comparisons among treatments.
An observer sat in the centre of each semicircle recording the visitation to each plant by the wasps while the wasps were active (approx. 09.30 to 18.00, depending on weather). The experiment lasted 17 days in the one replicate and 15 days in the other replicate. The two constant plants with eggs on them were left in place during the experiment. The two fresh plants with eggs on them were replaced each day that the egg cluster was observed being visited by H. horticola. In order to avoid potential bias due to plant position in the array, the locations of the constant and the fresh egg clusters (figure 3a) were switched in the middle of the experiment.
The number of times each treatment (constant, fresh and empty) was visited by wasps was analysed as a generalized linear model (GLM), using a Poisson distribution (StataCorp 2005). The dependent variable was the number of visits, and treatment and replicate were the explanatory variables. Post-estimation linear comparisons with corrections for multiple comparisons were made among treatments (c, f and e). The visits were not treated as independent because the wasps were not identified individually. We know from the studies of individually marked wasps (below; van Nouhuys & Ehrnsten 2004) that multiple wasps visit each egg cluster multiple times. In this experiment wasps from all three release dates were observed on multiple days, and on both arrays, and there were no naturally occurring H. horticola.
(c) Visual landmark experiments
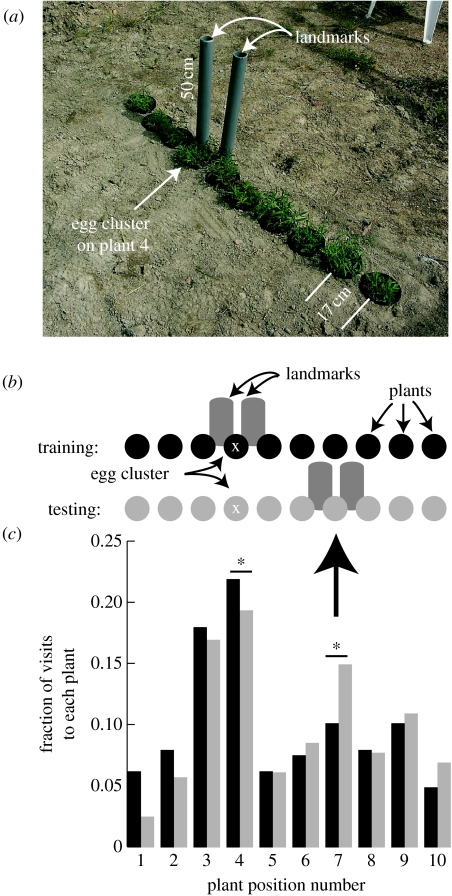
Two experiments (egg-flanked and plant-flanked) were designed to detect the use of visual landmarks by H. horticola revisiting previously found egg clusters. They took place in July 2005 in a 32×26×3 m outdoor mesh-enclosed habitat patch (figure 4) suitable for the butterfly M. cinxia (Hanski et al. 2006). Individually marked H. horticola were presented with a configuration of several host food plants with eggs on one of them. Plant-scale cylindrical landmarks were placed nearby (figure 5a). The visitation of plants by wasps was observed continuously for several days of wasp training. Then, the landmarks were moved and observation continued. Our primary data were the change in the distribution of visits to plants after the landmarks were moved. We conducted two different experiments of this type and two replicates of each experimental set-up were conducted simultaneously by two different observers.
Figure 4.
Inside of the 32×26×3 m3 mesh enclosure used for the landmark experiments. Photo by A. Ruina.
Figure 5.
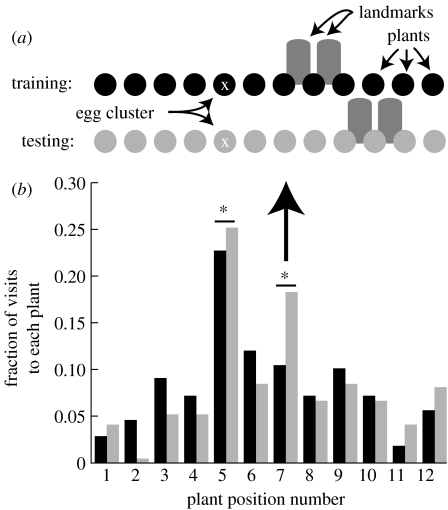
The egg-flanked landmark experiment. (a) An array of V. spicata plants during the training portion of the experiment. The two grey cylinders (landmarks) are shifted between the wasp training and testing periods. (b) Schematic of the experiment. Filled circles, plants during training (black) and testing (grey); White X, the plant with host eggs on it; grey bars, the landmarks. The thick arrow points to the plant expected to receive increased visitation during testing. (c) The fraction of visits to each plant by H. horticola during training (black bars) and testing (grey bars). The asterisk indicates a significant difference between the number of visits between the last day of training and the first day of testing. Altogether, there were 1060 observed visits to plants.
Observers stationed in front of two identical arrays of potted V. spicata host plants recorded the frequencies and durations of wasp visits to each plant from approximately 09.30 to 18.00 each day, spanning the active period of the wasps on most days. The plants were arranged in lines to create a simple foraging environment that was similar in scale and structure to those commonly used by the host butterfly. Four observers took turns sitting on the ground 0.6–1 m from the array in front of the centre plants. The arrays were rows of potted V. spicata in the areas of bare soil (figure 5a). The plants were similar in size and colour. The landmarks were 50 cm grey plastic cylinders (5 cm diameter PVC pipe) placed upright in the soil (figure 5a). For each experiment, there were two arrays, 18 m apart and offset by 90°. Between the experiments, the arrays were shifted 3 m and rotated 90°, and a new set of plants was used. The host eggs were approximately 10 days old at the start of each experiment and did not mature enough to be parasitized during the experiment. Sixty-eight female H. horticola were individually marked on the back of the thorax with a coloured paint. Forty-eight were released at the start of the egg-flanked experiment, and 20 more added at the start of the plant-flanked experiment. For 97% of the visits to plants, we were able to identify the wasp individual making the visit (2440 out of 2502 observed visits).
Visitation to the plants in the arrays during training and testing was compared using ANOVA and the statistical software Stata (StataCorp 2005). The dependent variable was the number of visits to a plant in a day. Arrays (2), plant positions (1–10 in the egg-flanked and 1–12 in the plant-flanked) and days (1–6 in the egg-flanked and 1–5 in the plant-flanked) were fixed factors, with plant position nested in the array. The interaction between day and plant position was also in the model. Only data from the wasps observed in both the training and the testing were used. This excluded 45 and 30% of the observations from the egg- and plant-flanked experiments, respectively. The excluded wasps were a small fraction of individuals that did not become active until several days after release, and a larger fraction that were eaten by predators (spiders), died or became inactive after the first few days. For each landmark experiment, the hypothesis that during testing wasps would take a disproportionate interest in plant 7 relative to the other plants was tested by constructing specific post hoc contrasts separately comparing the number of visits to each plant on the last day of training with the first day of testing.
Individual wasp visits were treated as independent because loss of statistical power due to the identification of individuals, and bouts of visits, was large compared with the small amount of information gained from each. Additionally, the hypotheses are based on average differences, not individual variation among the wasps. The results obtained were the consequence of many different wasps (figure 6) making many trips to the plant arrays. The two replicates (arrays) of each experiment were also not independent because both the arrays were visited by 66% of the same wasps. Even the experiments themselves were not entirely independent because, though additional wasps were added before the second experiment, about half the wasps were present in both the experiments. Initial statistical analyses identified no difference in the rate of visitation between the wasps observed in only one or the other array, nor did wasps observed in both the experiments visit the plants with and without eggs at a different rate than the wasps observed in only one. Consequently, we did not account for the lack of independence in the statistical analyses presented.
Figure 6.
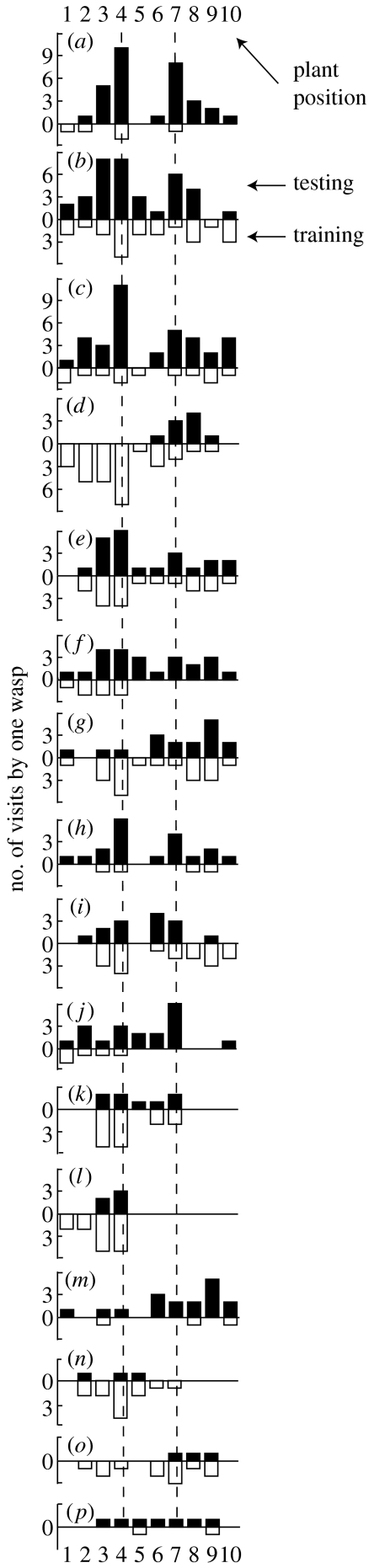
The number of visits to each plant by 16 H. horticola in one replicate of the egg-flanked landmark experiment. Each bar plot (a–p) shows visitation by a single wasp. Inverted white bars, visits during training; black bars, visits during testing. The wasps shown are those that visited the array at least twice during both training and testing. Dashed lines, plant 4 (with the eggs and landmarks during training) and plant 7 (which received the landmarks during testing). Of these 16 wasps, 13 increased their visits to plant 7 when the landmarks were moved.
3. Results
(a) The use of odour by H. horticola
In the first experiment, we compared the rate of parasitism of host egg clusters by naturally occurring wasps under three different conditions. Constant plants with eggs on them were put into the field and left in place for the entire development time of the eggs. Moved plants with eggs on them were also left in the field, but were shifted several metres just prior to vulnerability to parasitism. Fresh plants with eggs on them were put into the field only when the eggs were soon to be ready for parasitism (figure 2a).
If the wasps found eggs anew each visit by following the odours produced by the eggs themselves, or by the occupied plants, then the rate of parasitism of all the three treatments should have been equal, which was not the case (logistic regression, Χmodel2=7.91, p=0.02; figure 2b). This confirms a previous study in which the parasitism of fresh and constant host egg clusters were compared (van Nouhuys & Ehrnsten 2004). If the wasps relied on an odour mark left by themselves or another wasp during a previous visit, then the moved and constant eggs should be parasitized at equal and high rates, because within the habitat patch the wasps could locate their odour markers equally well. Few of the fresh egg clusters should be parasitized because they were not present ahead of time for the wasps to mark. We found that fewer of the egg clusters on both the fresh and the moved plants were parasitized than were egg clusters that were left in place throughout their development (post-estimation contrasts, Χm=f2=0.59, p=n.s.; Χc=(m+f)/22=6.01, p=0.014; figure 2b). Though the difference between the moved and the constant eggs was only marginally statistically significant (Χc=m2=3.31, p=0.068), these results in combination with the following experiment strongly suggest that the wasps were not relying on the odour but had memorized the spatial locations of the egg clusters.
In the second experiment, an array of potted plants (figure 3a) was placed in the field and wasp visitation was observed over 17 days. Plants without (empty) and with (constant) egg clusters on them were left in place. Another set of plants that had eggs on them (fresh) was replaced each day by new plants with eggs on them that had not been exposed to wasps. If wasps used marking odours to relocate host eggs, then the fresh plants should be visited at a lower frequency than the constant plants because they were not present during previous visits to be marked. Instead both the constant and fresh plants were visited with equal frequency (Poisson regression, Χmodel2, p=0.00; Χc=f2=1.06, p=n.s.; Χc=e2=25.97, p=0.00; Χf=e2=34.81, p=0.00; figure 3b), revealing that the wasps could not revisit hosts over days by relying on deposited olfactory cues. This, along with the results of the first experiment and previously published work (van Nouhuys & Ehrnsten 2004), showing that the wasps were not primarily following induced plant odour or odour of the eggs, indicates that the wasps must learn the spatial locations of the egg clusters.
(b) Landmark use by H. horticola
We could now reasonably infer that the wasps were using visual landmarks, so we set out to document this directly, in an outdoor area the size of a typical host-occupied meadow enclosed by a mesh cage (figure 4). During the training period in the first of two experiments (the egg-flanked experiment), the fourth plant from the left in a row of 10 had host eggs on it and was flanked by a pair of landmarks (figure 5a,b). After 3 days of training, the landmarks were moved to plant 7. The eggs remained at plant 4 (figure 5b) and the human observer did not change seating position. Over 6 days of the experiment, 38 different wasps were observed to make an average of 5.76 (±5.19) visits (each) to the arrays. Individuals visited on multiple days, not always consecutively. Once at an array, wasps visited multiple plants, leading to a total of 1060 visits to the individual plants (figure 6). The wasps also flew along the array of plants, occasionally landing very briefly on the human observer or one of the landmarks. During the training period, the plant with eggs on it and the adjacent plant (plant 3), which was close to the eggs in one of the replicates, received the most visits. When the landmarks were shifted to plant 7, there was a significant increase in the number of visits to plant 7, suggesting that the two cylinders were used by the wasps to recognize the appropriate plant (ANOVA F69,119=7.17, p=0.0001; post hoc contrast comparing visits to plant 7 on day 4 with day 5: F1,50=16.28, p=0.004). There was also a corresponding decrease in the visitation to the plant with the eggs on it (F1,50=16.28, p=0.02), and no significant change to the visitation to other plants (figures 5c and 6).
A second experiment (the plant-flanked experiment) eliminated the possibility that the wasps were simply responding innately to attractive landmarks, and then encountering nearby eggs by chance. This experiment also demonstrated that the wasps learned the spatial position, both distance and direction, of the host eggs relative to the landmarks. During training, the fifth position plant had eggs on it, and landmarks flanked the eighth plant in a row of 12. After 3 days of training, the landmarks were shifted to position 10, and the eggs left in place (figure 7a). The wasps were observed for 2 days of testing. Over 5 days, 37 wasps were observed to make a total of 325 visits to the arrays, and 1442 visits to individual plants in the arrays. As in the first landmark experiment, during training wasps visited the plant with eggs on it with the highest frequency; but in this second experiment they visited the plant flanked by landmarks with low frequency (figure 7b). After training, when the landmarks were shifted from plant 8 to plant 10, the wasp's visitation to plant 7 dramatically increased (ANOVA F70,119=5.82, p=0.000; post hoc contrast, F1,50=16.28, p=0.001) (figure 7b). There was not a significant change in the rate of visitation to other plants without eggs on them. This showed that at least some of the wasps had learned to visit the plant 2 away from the landmarks in a specific direction.
Figure 7.
The plant-flanked landmark experiment. The symbols and shades are as shown in figure 5. (a) Schematic of the experiment. (b) The fraction of visits to each plant by H. horticola during training and testing. The asterisk indicates a statistically significant difference between the number of visits between the last day of training and the first day of testing. Altogether, there were 1442 visits to plants.
In both the experiments, the plant with eggs on it was still visited with high frequency during the testing periods (figures 5c and 7b). In the second experiment, the visitation to the plant with the eggs significantly increased (F1,50=11.57, p=0.001). Probably, this is because some of the wasps quickly learned the new configuration, and also surely used cues other than our landmarks, including the stationary human observers. Furthermore, the eggs themselves may have a short-range attractive odour (Castelo et al. 2005) that could arrest the wasps once they arrive at the right plant. The experiments demonstrated the response of the wasps to a change in only one of perhaps many available visual and olfactory cues.
4. Discussion
Parasitoids have generally been observed to search for hosts that can be parasitized upon discovery (van Alphen & Vet 1986). Such a direct relationship between finding a resource and using it is assumed by most foraging theory (e.g. Hubbard & Cook 1978; Stephens & Krebs 1986). However, in most studies of parasitoids, observations have been in the laboratory where only a limited range of foraging behaviours can reasonably be observed. Furthermore, while there have been many detailed studies of parasitoid foraging behaviour, only a few key species have been used, representing only a few life-history types. Thus, it is possible that other more complex foraging behaviours might be common but not yet documented.
Hyposoter horticola, for instance, does have a more complex foraging behaviour. The wasp monitors multiple potential hosts, repeatedly visiting them over a period of weeks until the host larvae become susceptible to parasitism. Here, we have shown that a foraging H. horticola does not simply discover the same potential hosts repeatedly, nor does it follow odour markers to return to known host egg clusters. Instead, the wasp uses spatial memory to keep track of the locations of the host egg clusters over time. Such anticipatory foraging has rarely been observed in insects and has not been addressed in insect foraging theory.
(a) Visual memory rather than olfactory cues
As mentioned previously, while parasitoids are known to use some host-associated visual cues (Wäckers & Lewis 1999), most studies of parasitoid foraging concerns their ability to perceive, learn and, to a lesser extent, produce odour (Vet et al. 1990; Turlings et al. 1995; Nufio & Papaj 2001; Smid et al. 2007). There are nevertheless compelling benefits for a parasitoid if it could use visual spatial memory. First, visual landmarks are likely to persist longer than odour markers, so should be useful when a resource must be relocated on a scale of weeks rather than hours. This longer time scale of visual landmarks is appropriate for foraging by H. horticola because the wasp depends on foraging for hosts that are not yet vulnerable. Second, a memorized visual landmark does not make the prey apparent to conspecific foragers, whereas an odour marker would. Revealing hosts to conspecific wasps would be especially detrimental for H. horticola because there is a strong intraspecific competition for a limiting number of host egg clusters. Thus, there are good reasons for visual rather than olfactory-based memory to be used by this wasp, and perhaps others.
Hyposoter horticola monitors the locations of potential hosts as the host eggs develop for up to three weeks. The monitoring might be achieved using long-term memory of a type that fruit flies (Drosophila; Tully et al. 1994), honeybees (Apis mellifera; Wuestenberg et al. 1998) and more recently parasitoid wasps (Muller et al. 2006; Smid et al. 2007) have been shown to possess. However, an individual H. horticola visits a host egg cluster frequently, sometimes several times in a day in both the enclosed habitat as shown here and under natural conditions (van Nouhuys & Ehrnsten 2004). Therefore, we cannot tell whether H. horticola uses long-term memory spanning many days or a few weeks, or short-term memory lasting only on the order of a day or less, but is reinforced at each visit.
(b) Anticipatory foraging
Some solitary arthropods that are not nest builders are known to remember locations of places or resources, but unlike H. horticola, these insects have already benefited from the memorized site. For instance, the jumping spider Phidippus clarus learns to recognize beacons marking their night-time retreats (Hoefler & Jakob 2006), and cockroaches integrate visual and olfactory information to return to the previously used shelters (Blatella germanica; Rivault & Durier 2004). Insects that forage by traplining, such as some butterflies, must also use spatial memory to learn the locations of, or distances between, productive resources (nectar flowers; Bell 1990).
Although not well documented in the literature, other insects probably also benefit from anticipatory foraging, or memorizing the locations of resources that cannot yet provide a tangible reward. Though only some of the potential resources monitored will ultimately be beneficial, the ability to forage for resources that are not yet useful increases the resource pool beyond what is usually appreciated in the studies of insect foraging behaviour.
As discussed in the §1, the cleptoparasitoid A. armilla clearly benefits from foraging for a resource before the resource is usable (Rosenheim 1987), as H. horticola does, but for a somewhat different reason. Similarly, male Heliconius (Nymphalidae) butterflies that compete to mate with females that have not yet eclosed (Deinert et al. 1994) may keep track of the locations of multiple young female pupae, as might male Synagris vespid wasps that patrol conspecific nests in anticipation of the appearance of newly emerged adult females (Longair 2004).
Parasitoids other than H. horticola use hosts that are only briefly vulnerable, such as some egg parasitoids, as well as parasitoids of teneral pupae could, and maybe do, benefit from locating potential hosts ahead of time. More generally, parasitoids that forage for rare, widely dispersed or spatially unpredictable hosts would benefit from an increased amount of time to forage. Additionally, parasitoids, like H. horticola, that experience strong competition among foraging females may benefit from anticipatory foraging because the chance of finding a susceptible host that has not yet been parasitized is low. The possibility of spatial memory in these insects has not yet been investigated.
(c) Host–parasitoid population dynamics
A striking feature of this predator–prey system is that essentially all host egg clusters in the landscape are found, and one-third of each one is parasitized. This pattern is independent of natural host density, either as it varies over the landscape or from year to year. Thus, the large-scale population dynamics are not of the classical ‘density-dependent’ type typically invoked in the models of predator–prey dynamics (Nicholson & Bailey 1935; Walde & Murdock 1988; Hassell 2000; van Nouhuys & Hanski 2002; Kankare et al. 2005). Instead, the wasp population size is a fixed fraction of the total butterfly population size (van Nouhuys & Hanski 2002).
The anticipatory foraging behaviour of H. horticola, achieved through learning the locations of potential hosts using visual landmarks, is central to these absolutely stable dynamics. Without anticipatory foraging, few egg clusters would be found during the very brief period of vulnerability of the hosts. With it, the location of each host is known, usually by several individuals, by the time the hosts become vulnerable to parasitism. The wasps then compete for the egg clusters, which are a limiting resource, but leave two-thirds of each cluster unparasitized. An equal fraction of the entire M. cinxia population in Åland is parasitized each generation owing to individual wasp behaviour at the egg cluster (van Nouhuys & Ehrnsten 2004) in combination with the anticipatory foraging described here.
Acknowledgments
We thank S. Ikonen, S. Kuokkanen, K. Lindqvist and N. Revankar for field and laboratory assistance; M. Collett for discussion of the landmark experiments; F. Vermeylen for statistical consultation; and A. Agrawal, M. Collett, T. S. Collett, I. Hanski, R. Menzel, J. L. Osborne, J. Rosenheim, A. Ruina, P. Woodbury and three anonymous reviewers for their useful comments on the manuscript. The Academy of Finland Centre of Excellence program (grant no. 20286) funded this work.
References
- Arthur A.P. Associative learning in Itoplectis conquisitor (Say) (Hymenoptera: Ichneumonidae) Can. Entomol. 1966;98:213–223. [Google Scholar]
- Baerends G.P. Fortpflanzungsverhalten und Orientierung der Grabwespe Ammophila campestris. Tijdschrift voor Entomol. 1941;84:68–275. [Google Scholar]
- Bell W.J. Searching behavior patterns in insects. Annu. Rev. Entomol. 1990;35:447–467. doi:10.1146/annurev.en.35.010190.002311 [Google Scholar]
- Capaldi E.A, et al. Ontogeny of orientation flight in the honeybee revealed by harmonic radar. Nature. 2000;403:537–540. doi: 10.1038/35000564. doi:10.1038/35000564 [DOI] [PubMed] [Google Scholar]
- Castelo, M. K., van Nouhuys, S. & Corley, J. 2005 Respuesta olfatoria del parasitoide Hyposoter horticola (Ichneumonidae: Campoplaginae) hacia claves asociadas con su hospedador, Melitaea cinxia (Lepidoptera: Nymphalidae). In Congreso Argentino de Entomologia San Miguel de Tucumán, Argentina.
- Collett T.S, Collett M. Memory use in insect visual navigation. Nat. Rev. Neurosci. 2002;3:542–552. doi: 10.1038/nrn872. doi:10.1038/nrn872 [DOI] [PubMed] [Google Scholar]
- Deinert E.I, Longino J.T, Gilbert L.E. Mate competition in butterflies. Nature. 1994;370:23–24. doi:10.1038/370023a0 [Google Scholar]
- Godfray H.C.J. Princeton University Press; Princeton, NJ: 1994. Parasitoids: behavioural and evolutionary ecology. [Google Scholar]
- Hanski I, Saastamoinen M, Ovaskainen O. Dispersal-related life-history trade-offs in a butterfly metapopulation. J. Anim. Ecol. 2006;75:91–100. doi: 10.1111/j.1365-2656.2005.01024.x. doi:10.1111/j.1365-2656.2005.01024.x [DOI] [PubMed] [Google Scholar]
- Hassell M.P. Oxford series in ecology and evolution. Oxford University Press; London, UK: 2000. The spatial and temporal dynamics of host–parasitoid interactions. [Google Scholar]
- Hoefler C, Jakob E. Jumping spiders in space: movement patterns, nest site fidelity and the use of beacons. Anim. Behav. 2006;71:109–116. doi:10.1016/j.anbehav.2005.03.033 [Google Scholar]
- Hoffmeister T.S, Gienapp P. Discrimination against previously searched, host free patches by a parasitoid wasp. Ecol. Entomol. 2001;26:487–494. doi:10.1046/j.1365-2311.2001.00349.x [Google Scholar]
- Hubbard S.F, Cook R.M. Optimal foraging by parasitoid wasps. J. Anim. Ecol. 1978;47:593–604. doi:10.2307/3803 [Google Scholar]
- Kankare M, van Nouhuys S, Gaggiotti O, Hanski I. Metapopulation genetic structure of two coexisting parasitoids of the Glanville fritillary butterfly. Oecologia. 2005;143:77–84. doi: 10.1007/s00442-004-1782-1. doi:10.1007/s00442-004-1782-1 [DOI] [PubMed] [Google Scholar]
- Longair R.W. Tusked males, male dimorphism and nesting behavior in a subsocial afrotropical wasp, Synagris cornuta, and weapons and dimorphism in the genus (Hymenoptera: Vespidae: Eumeninae) J. Kans. Entomol. Soc. 2004;77:528–557. doi:10.2317/E-38.1 [Google Scholar]
- Menzel R, De Marco R.J, Greggers U. Spatial memory, navigation and dance behaviour in Apis mellifera. J. Comp. Physiol. A. 2006;192:889–903. doi: 10.1007/s00359-006-0136-3. doi:10.1007/s00359-006-0136-3 [DOI] [PubMed] [Google Scholar]
- Muller C, Collatz J, Wieland R, Steidle J.L.M. Associative learning and memory duration in the parasitic wasp Lariophagus distinguendus. Anim. Biol. (Leiden) 2006;56:221–232. doi: 10.1101/lm.192506. doi:10.1163/157075606777304195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, A. J. & Bailey, V. A. 1935 The balance of animal populations. In Proc. Zoological Society of London, Part 1, pp. 551–598.
- Nieminen M, Siljander M, Hanski I. Structure and dynamics of Melitaea cinxia metapopulations. In: Ehrlich P.R, Hanski I, editors. Checkerspot butterfly population ecology. Oxford University Press; Oxford, UK: 2004. pp. 63–91. [Google Scholar]
- Nufio C.R, Papaj D.R. Host marking behaviour in phytophagous insects and parasitoids. Entomol. Exp. Appl. 2001;99:273–293. doi:10.1023/A:1019204817341 [Google Scholar]
- Papaj D.R, Lewis A.C, editors. Insect learning: ecological and evolutionary perspectives. Chapman & Hall; New York, NY: 1993. [Google Scholar]
- Rivault C, Durier V. Homing in German cockroaches, Blattella germanica (L.) (Insecta: Dictyoptera): multi-channelled orientation cues. Ethology. 2004;110:761–777. doi:10.1111/j.1439-0310.2004.01018.x [Google Scholar]
- Rosenheim J.A. Host location and exploitation by the cleptoparasitic wasp Argochrysis armilla: the role of learning (Hymenoptera: Chrysididae) Behav. Ecol. Sociobiol. 1987;21:401–406. doi:10.1007/BF00299935 [Google Scholar]
- Sheehan W, Wäckers F.L, Lewis W.J. Discrimination of previously searched, host-free sites by Microplitis croceipes (Hymenoptera: Braconidae) J. Insect Behav. 1993;6:323–331. doi:10.1007/BF01048113 [Google Scholar]
- Smid H.M, Wang G, Bukovinszky T, Steidle J.L.M, Bleeker M.A.K, van Loon J.J.A, Vet L.E.M. Species-specific acquisition and consolidation of long-term memory in parasitic wasps. Proc. R. Soc. B. 2007;274:1539. doi: 10.1098/rspb.2007.0305. doi:10.1098/rspb.2007.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. StataCorp; College Station, TX: 2005. Stata statistical software. [Google Scholar]
- Stephens D.W, Krebs J.R. Princeton University Press; Princeton, NJ: 1986. Foraging theory. [Google Scholar]
- Tinbergen N. On the orientation of the digger wasp Philanthus triangulum Fabr. I. In: Tinbergen N, editor. The animal in its world. vol. 1. Harvard University Press; Cambridge, MA: 1972. pp. 103–127. [Google Scholar]
- Tully T, Preat T, Boynton S.C, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35. doi: 10.1016/0092-8674(94)90398-0. doi:10.1016/0092-8674(94)90398-0 [DOI] [PubMed] [Google Scholar]
- Turlings T.C.J, Wäckers F.L, Vet L.E.M, Lewis W.J, Tumlinson J.H. Learning of host-finding cues by hymenopterous parasitoids. In: Papaj D.R, Lewis A.C, editors. Insect learning: ecological and evolutionary perspectives. Chapman and Hall; New York, NY: 1993. pp. 51–78. [Google Scholar]
- Turlings T.C.J, Loughrin J.H, McCall P.J, Rose U.S.R, Lewis W.J, Tumlinson J.H. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl Acad. Sci. USA. 1995;92:4169–4174. doi: 10.1073/pnas.92.10.4169. doi:10.1073/pnas.92.10.4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Tanaka T. Self-host discrimination by a parasitic wasp: the role of short-term memory. Anim. Behav. 1996;52:875–883. doi:10.1006/anbe.1996.0235 [Google Scholar]
- van Alphen J.J.M, Vet L.E.M. An evolutionary approach to host finding and selection. In: Waage J, Greathead D, editors. Insect parasitoids. Academic Press; San Diego, CA: 1986. pp. 23–62. [Google Scholar]
- van Driesche R.G, Bellows T.S. Springer; Boston, MA: 1996. Biological control. [Google Scholar]
- van Nouhuys S, Ehrnsten J. Wasp behavior leads to uniform parasitism of a host available only a few hours per year. Behav. Ecol. 2004;15:661–665. doi:10.1093/beheco/arh059 [Google Scholar]
- van Nouhuys S, Hanski I. Colonization rates and distances of a host butterfly and two specific parasitoids in a fragmented landscape. J. Anim. Ecol. 2002;71:639–650. doi:10.1046/j.1365-2656.2002.00627.x [Google Scholar]
- Vet L.E.M, Lewis W.J, Papaj D.R, van Lenteren J.C. A variable response model for parasitoid foraging behavior. J. Insect Behav. 1990;31:471–490. doi:10.1007/BF01052012 [Google Scholar]
- von Frisch K. Harvard University Press; Cambridge, MA: 1967. The dance language and orientation of bees. [Google Scholar]
- Wäckers F.L, Lewis W.J. Olfactory and visual learning and their combined influence on host site location by the parasitoid Microplitis croceipes (Cresson) Biol. Control. 1994;4:105. doi:10.1006/bcon.1994.1018 [Google Scholar]
- Wäckers F.L, Lewis W.J. A comparison of color-, shape- and pattern-learning by the hymenopteran parasitoid Microplitis croceipes. J. Comp. Physiol. A. 1999;184:387–393. doi:10.1007/s003590050337 [Google Scholar]
- Walde S.J, Murdock W.W. Spatial density dependence in parasitoids. Annu. Rev. Entomol. 1988;33:441–466. doi:10.1146/annurev.en.33.010188.002301 [Google Scholar]
- Wcislo W.T. Nest location and recognition in a solitary bee, Lasioglossum (Dialictus) figueresi Wcislo (Hymenoptera: Halictidae), in relation to sociality. Ethology. 1992;92:108–123. [Google Scholar]
- Wuestenberg D, Gerber B, Menzel R. Long- but not medium-term retention of olfactory memories in honeybees is impaired by actinomycin D and anisomycin. Eur. J. Neurosci. 1998;10:2742–2745. doi: 10.1046/j.1460-9568.1998.00319.x. doi:10.1046/j.1460-9568.1998.00319.x [DOI] [PubMed] [Google Scholar]