Abstract
Pedigree analysis is a useful tool in the study of speciation. It can reveal trans-generational influences on the choice of mates. We examined mating patterns in a population of Darwin's medium ground finches (Geospiza fortis) on Daphne Major Island to improve our understanding of how a barrier to the exchange of genes between populations arises in evolution. Body sizes of mates were weakly correlated. In one year, the smallest females were paired non-randomly with the males of similar size, and in another year the largest males were paired with the largest females. An influence of parental morphology on the choice of mates, as expected from sexual imprinting theory, was found; the body size of mates was predicted by the body sizes of both parents, and especially strongly by the father's. These associations imply that the seeds of reproductive isolation between species are present within a single variable population. The implication was subject to a natural test: two exceptionally large birds of the study species, apparently immigrants, bred with each other, as did their offspring, and not with the members of the resident population. The intense inbreeding represents incipient speciation. It parallels a similar phenomenon when another species, the large ground finch, immigrated to Daphne and established a new population without interbreeding with the resident medium ground finches.
Keywords: beak and body size, Geospiza fortis, inbreeding, mating patterns, reproductive isolation, sexual imprinting
1. Introduction
Speciation is the fundamental process in evolution that generates biological diversity. Most recent advances in understanding of speciation have come from genetic analyses of closely related species of a few model organisms (e.g. Presgraves et al. 2003; Kronforst et al. 2006; Masly et al. 2006; Dettman et al. 2007). Less is known from the studies in nature about the ecological circumstances and microevolutionary forces responsible for speciation (Schluter 2001; Nosil et al. 2007). Consequently, there is much debate on how it occurs and what the responsible mechanisms are (Turelli et al. 2001; Coyne & Orr 2004; Ryan et al. 2007). Since the essence of a species is an inability to exchange genes with other species, a critical question for many organisms is how barriers arise that prevent or hinder interbreeding. To address this question, we have studied the populations of Darwin's finches on the Galápagos Islands.
Fourteen species have been derived from a common ancestor in the last 2–3 Myr (Grant & Grant 2007). They are at a relatively early stage of speciation and diversification as shown by the fact that they hybridize, albeit rarely, and the F1 hybrids and backcrosses are at no obvious fitness disadvantage under benign conditions of a plentiful and suitable food supply for the individuals with intermediate beak sizes (Grant & Grant 1992, 1998). Experiments with museum specimens, and other experiments involving the playback of tape-recorded song, show that the individuals of the six species of the ground finch genus (Geospiza) can discriminate between members of the same and a closely related species solely on the basis of visual (morphological) or acoustic (song) cues (Ratcliffe & Grant 1983, 1985). Moreover, the mating patterns of interbreeding species, hybrids and backcrosses implicate both song and morphology in the species-specific choice of mates (Grant & Grant 1997a,b, 1998). The mating patterns suggest, as do laboratory experiments with Darwin's finches (Bowman 1983), that the cues are learned early in life from parents and other adults, i.e. through sexual imprinting (Immelmann 1975; ten Cate et al. 1993; Irwin & Price 1999; Price 2007). Plumage colour and pattern, shown to be important in the choice of mates in a variety of bird species elsewhere (Dale & Slagsvold 1996; Kimball 1996; Hunt et al. 1999; Collins & Luddem 2002; Price 2007; Pryke 2007), do not differ among the six ground finch species.
Some cues that are used in the choice of a particular mate, such as age-related courtship vigour, are not necessarily the same as those used in discriminating between members of the same and related species (Ryan & Rand 1993; Boake et al. 1997; Ryan et al. 2003; Phelps et al. 2005). It is therefore an open question for Darwin's finches whether an individual mate is chosen on the basis of the particular signal value of its song, if it is a male, and morphology. Acoustic cues have been examined in several investigations and found to vary among individuals within populations (Bowman 1983; Millington & Price 1985; Gibbs 1990; Grant & Grant 1989, 1996; Podos 2001; Podos et al. 2004; Huber & Podos 2006). Here, we focus on morphological cues that might be involved in the choice of mates.
Ideally, the stimulus value of variants of the salient cues should be tested experimentally to determine preference functions in relation to signal variation, as is done more easily with fish (Boughman et al. 2005), frogs (Höbel & Gerhardt 2003; Phelps et al. 2005), crickets (Shaw 1996) and flies (Ritchie 1996) than with birds (but see Patten et al. 2004). Instead, we use pedigree data from a long-term field study of a population of medium ground finches (Geospiza fortis) to test for non-random patterns that are indicative of mate choice based on morphology. We first ask whether pairs are formed assortatively with respect to overall size; body size is the principal axis of morphological variation (Grant et al. 2004). We test for correlations between mates (i) across the population as a whole and (ii) at the size extremes. Assortative mating is an expected consequence of sexual imprinting on parental morphology when the offspring (the choosers) and their parents resemble each other genetically and phenotypically. In fact, body size in this population of G. fortis is highly heritable (Keller et al. 2001). We test directly for parental influence on offspring using multiple regression analysis to predict the morphological trait values of their first mates from the values of the parents. Since finches may have greater opportunity to exercise choice at the second time of pairing than at the first, and more in some years than others, we repeat the test with the second mates.
Finally, we describe and interpret a pattern of non-random mating in a family of unusually large G. fortis (figure 1). They are treated separately because they are considered to have originated from two immigrants. Like the extreme members of the resident population, their pairing pattern gives insights into the morphological variation that is necessary for discrimination among potential mates. They also give insights into the origin of reproductive isolation.
Figure 1.
Medium ground finches (G. fortis) on Daphne Island. (a) An immigrant offspring (immigrant, F2 generation) and (b) a typical resident (adult male). Photos by B. R. Grant.
2. Material and methods
The study was conducted on the small, undisturbed and uninhabited island of Daphne Major (0.34 ha) situated in the centre of the Galápagos archipelago. The focal species is the medium ground finch (G. fortis). Additional information on morphology was obtained from a second species, the large ground finch (Geospiza magnirostris). Finches were captured in mist nets, measured, given a unique combination of coloured leg bands and released. Six body size and beak traits were measured, as described in detail elsewhere (Boag & Grant 1984) and illustrated by Grant & Grant (2007). For an index of G. fortis body size, we use the first component of a principal components analysis (PCA) of mass (weight), wing length and tarsus length (n=884). Original and synthetic traits in most samples were normally distributed. In each breeding year, the range of body sizes (PC scores) of potential mates approximated the range of body sizes of parents.
The hypothesis of assortative pairing was tested with parametric correlations between PC scores of the members of a mated pair, identified as a male and a female attending a nest with eggs or nestlings. Parametric correlations for the combined data across the whole 35 years of the study, 1973–2007, are expected to be positive as a spurious consequence of fluctuating changes in morphological means due to natural selection (Grant & Grant 2002, 2006). Therefore, we corrected sex specifically for annual variation in the mean body size among years, and then combined data prior to analysis.
For a more restricted test of assortative mating among individuals in the tails of a frequency distribution of the body sizes, we applied t-tests to the difference in means between the males mated with the most extreme 10% of the females, either the smallest or the largest 10%, and all other mated males. The criterion of 10% and a minimum sample size of 50 were chosen to ensure a minimum number of five extreme females in each test. We repeated the test with the mates of the smallest and the largest 10% of males. Mate choice is not one sided; males hold territories and females visit several of these territories where they are either courted or chased away (Grant 1999). Sample size requirements were met by pairs breeding in 3 years: 1983 (n=108), 1987 (n=96) and 1993 (n=77). The same pairs did not breed in more than one of these years. Each test is directional, justifying one-sided tests, but we applied two-sided tests because each frequency distribution of mate sizes was tested twice. Apart from this, we made no correction for multiple testing because the samples were discretely different, being drawn from three different populations (in time) composed almost entirely of different adults.
We used pedigrees constructed from the observations of breeding birds to examine a possible influence of parental morphology on mate choice. If, according to sexual imprinting theory, offspring learn the appearance and behaviour of their parents in early life and use the information when choosing mates in adult life, a statistical association is expected between the morphology of one or both parents and the mates of the offspring. We used multiple regression analyses to predict the trait values (PC scores) of the first mates from the trait values of the choosers' parents. Sons and daughters may be influenced in their choice of a mate mostly by the parent of opposite sex (ten Cate et al. 2006). Taking this possibility into account, we performed regression analyses separately for the mates of sons and daughters. The four largest cohorts, produced in 1978 (n=37), 1983 (n=151), 1987 (n=147) and 1991 (n=107), were chosen for the analysis; little or no breeding occurred in several of the other years (Grant 1999). Testing in different years is needed because the opportunity for finches to express preferences varies among years, according to several factors including age structure and degree of breeding synchrony as determined by rainfall (Grant 1999). The cohorts are not the same as those in the assortative mating analyses because the years of high production did not always coincide with the years of large samples of measured parents. Extra-pair paternity has been documented in some cases (Keller et al. 2002), but this does not affect the sexual imprinting hypothesis that applies to social parents regardless of whether they are biological parents or not. For a test of parental influences on the choice of second mates, we used annually corrected data in a single analysis.
To construct the pedigree of the family of large birds (§1), we used allele lengths at 15 at the 16 microsatellite loci developed by Ken Petren at the Princeton University (Petren 1998). Genotyping for the present study was performed by Ecogenics GmbH, Switzerland. For standardizing allele length determinations between laboratories, correction factors (1–4 bp) were calculated and applied. One-third of the loci required no adjustments. As a test of repeatability of allele scores, the Ecogenics Laboratory determined allele lengths twice at a combined total of 1671 loci in G. fortis, G. magnirostris and Geospiza scandens (cactus finch) samples. Second samples were coded so that they were anonymous. Only one allele at one locus differed between samples. Of the original 16 loci, Gf.15 was not used in our analyses owing to a biologically unreasonable excess of homozygotes. This was probably caused by dropout of one allele at a heterozygous locus (Bonin et al. 2005). For an assessment of parentage, we used the criterion of a minimum 4 bp difference between pair members at a locus to declare it to be a mismatch, and allowed 2 bp differences as being within the range of scoring variation (Keller et al. 2001, 2002).
The original father of the family of large birds is suspected to have been an unmeasured large male that was observed in 2000–2003. It sang the same G. fortis song type III (Millington & Price 1985; Gibbs 1990), as did all four of the other large males. Song is inherited paternally through learning (Gibbs 1990; Grant & Grant 1996), although copying errors do occur. The alternative possibility, that the father was a large ground finch, G. magnirostris, can be effectively excluded with genetic data. The presumptive mother (19669) and daughter (19798) were homozygous (123/123) at locus Gf.8 for an allele that was not present in any one of 41 genotyped G. magnirostris individuals present in 2002 (the presumptive year of hatch). The genotype of the presumptive son (19228) was 123/139. In the total dataset accumulated over 20 years, the frequency of allele 123 is less than 0.01 in G. magnirostris (8/876), in contrast to 0.19 in G fortis (465/2400). Phenotypic data are also inconsistent with a hybridization hypothesis. The large birds are not intermediate in beak proportions between those of G. fortis and G. magnirostris, as they should have been if they were F1 and F2 hybrids. Instead, their morphology is close to a least-squares line of best fit to the Daphne G. fortis beak width and beak length measurements (figure 2) or beak depth and beak length (not shown). The points for G. magnirostris, in contrast, fall well above the line. Therefore, we conclude that the parents of all of the exceptionally large birds were immigrant G. fortis.
Figure 2.
Beak morphology of the six large G. fortis (crosses) on Daphne compared with 319 resident G. fortis (circles) and 70 resident G. magnirostris (diamonds). Only those hatched on the island and/or bred there in the years 2002–2007 have been used. A line calculated by the least-squares method has been fitted to the G. fortis data, excluding the large birds, and extended.
3. Results
(a) Assortative mating
Across the whole study period, the body sizes of mates were weakly correlated in the annually corrected data (r=0.123, n=243 pairs, one-tailed p=0.028) (figure 3). In 2 of the 3 years when the sample sizes of measured breeding pairs were sufficiently large, extreme individuals paired non-randomly. In 1987, the smallest 10% of females paired with unusually small males (t95=3.024, p=0.0032), and in 1993 the extreme large males paired with unusually large females (t87=−2.309, p=0.0233). Of the remaining 10 non-significant tests with extreme individuals, 7 were in the direction expected from the assortative mating hypothesis.
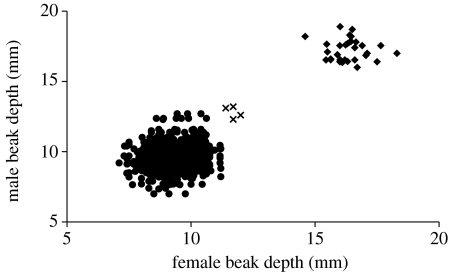
Figure 3.
Pairing patterns in relation to beak morphology of 759 G. fortis pairs (circles) and 32 G. magnirostris pairs (diamonds). Members of the family of large birds are indicated by crosses. The average of the beak depths of the two known females has been used for the two unknown females (see text). The total dataset for the years 1976–2007 has been used. Three male G. fortis with large beaks were paired with a total of 10 females with beaks of average size in the years up to but not beyond 1994.
(b) Parental influence on mate choice
For birds hatched in 1991, parental body size predicted the body size of the first mate for both sons and daughters (table 1). Sons mated with females of similar body size to their fathers but not to their mothers. Successful prediction was associated with exceptional social and environmental conditions. Mate changes were rare in 1991 (17.9%, n=106), and much less frequent than in 1987 (37.8%, n=98), a breeding season of comparable length (four to five broods). Extensive rain in each of the 2 years following 1991 fostered early recruitment to the breeding population. This had not happened in any of the preceding 15 years.
Table 1.
Parental influence on mate choice inferred from the results of multiple regression analyses. (Morphological traits of first mates of the 1991 cohort (above) were predicted from the same traits in fathers and mothers. Predictions differed for those members of the 1991 cohort breeding for the first time in 1992 or 1993 (below). Significant values for the overall regression (F-tests) and the partial regression coefficients for each parent (t-tests) are indicated in italics.)
| parental contribution | ||||||||
|---|---|---|---|---|---|---|---|---|
| regression | father | father | mother | mother | ||||
| trait | F | d.f. | p | R2 | t | p | t | p |
| cohort 91 | ||||||||
| sons | 3.423 | 2,43 | 0.0417 | 0.061 | 2.547 | 0.0145 | 0.315 | 0.7546 |
| daughters | 4.290 | 2,65 | 0.0178 | 0.111 | 1.763 | 0.0827 | 1.400 | 0.1662 |
| bred 92 | ||||||||
| sons | 2.125 | 2,14 | 0.1564 | 0.233 | 1.842 | 0.0868 | −1.412 | 0.1798 |
| daughters | 6.744 | 2,29 | 0.0039 | 0.317 | 1.853 | 0.0741 | 1.727 | 0.0948 |
| bred 93 | ||||||||
| sons | 4.198 | 2,23 | 0.0279 | 0.267 | 2.271 | 0.0328 | 1.859 | 0.0758 |
| daughters | 0.018 | 2,32 | 0.9820 | 0.001 | 0.036 | 0.9712 | −0.190 | 0.8501 |
Most of the 1991 cohort that survived to breed (total sample, n=272) bred for the first time in 1992 (38.2%) or 1993 (47.8%). Parental values predicted the size of mates of sons and daughters differently in the two main years of breeding, reflecting the fact that a large proportion of daughters bred for the first time in 1992 (0.48, n=67) and a large proportion of males bred for the first time in 1993 (0.60, n=43). In 1992, parental values of daughters breeding for the first time jointly predicted mate values (table 1). Contributions of father and mother morphologies (partial regression coefficients) were each close to statistical significance. However, uniquely in this year, the morphological values of the parents were strongly correlated (r=0.492, p=0.0037), and correspondingly single regression coefficients were significant for both fathers (r=0.445, p=0.0084) and mothers (r=0.486, p=0.0048). By contrast, parental values of sons breeding for the first time jointly predicted mate values only in 1993. The prediction was influenced by paternal but not the maternal morphological values (table 1).
(c) Second mates
Change of mates occurred frequently, allowing altered expression of mate preferences. Most changes occurred within the first (29.0%) or in the next year of breeding (54.8%, n=228 pairs). For those sons that changed mates, parental morphology predicted the body size of the second mates (F2,81=8.9292, p=0.0005), but not the size of their first mates (F2,81=0.381, p=0.6844). Paternal morphology contributed strongly to the significant prediction (b′=0.400±0.095 s.e., p=0.0003), whereas maternal morphology did not (b′=0.049±0.095 s.e., p=0.6433). For daughters, parental morphology did not predict the body size of either first (F2,99=1.395, p=0.2527) or second mates (F2,99=1.494, p=0.2295).
(d) Pairing of the exceptionally large birds
Figure 3 shows the pairing of the large G. fortis in 2005–2007. They comprise two pairs of measured birds and two pairs of measured males and unmeasured females. We observed the two unmeasured females, for which we lack genotypes, in the company of their mates close to their nests and unobstructed by vegetation at minimal distances of 1 and 2 m, respectively. Like their mates, they were unusually large. To include them in the figure, we assigned them the average measurements of the large females. Even without them, it is clear that the large size of four males and two females sets them apart from all other G. fortis on the island. The measured females are larger than all other measured females that bred on Daphne during the preceding 30 years.
Genetically, the large birds constitute one family. The pair (19228×19798) that bred in 2005 and 2006 were probably a brother and sister as they had at least one allele in common at all 15 loci and both in common at six of them (three homozygotes and three heterozygotes). The chance occurrence of at least one allele in common at all 15 loci is estimated to be approximately 1% on the basis of 2 out of 235 pairs in the total G. fortis dataset (excluding 19228×19798 and one known brother–sister pair). An older female (19669) that died in 2003 matched 19228 and 19798 at all loci, and is therefore presumed to have been the mother. The genotypes of four younger adults also matched 19228 and 19798 at all loci and are presumed to be their offspring that hatched in 2005. One additional bird (figure 1) that did not survive to adulthood, and therefore was not measured, was fed as a fledgling in 2006 by both 19228 and 19798. It also matched the two presumptive parents at all loci.
A pedigree for this family (figure 4b) is constructed on the basis of the observations and inferences from the genetic data. It resembles the pedigree of a population of G. magnirostris (figure 4a) that became established on Daphne in late 1982 (Gibbs & Grant 1987; Grant et al. 2001). Both display close inbreeding in the first couple of generations: the ultimate in assortative mating. Close inbreeding is rare in the G. fortis population on Daphne (0.8%; Keller et al. 2002). In the absence of additional immigration, it is expected to give rise to intense inbreeding depression (Keller et al. 2002), after a delay of a couple of generations as occurred in the G. magnirostris population (Grant & Grant 1995; Grant et al. 2001).
Figure 4.
Pedigrees of (a) the first three generations of G. magnirostris following establishment of a breeding population on Daphne in late 1982 (from Grant & Grant 1995) compared with (b) the family of large G. fortis. Genealogical relationships in both species were inferred from genetic (microsatellite) data. Filled symbols are genotyped birds (circles, females; squares, males; diamond, sex unknown). Open symbols are unknown or known but not genotyped individuals. Grey symbols refer to two birds whose inclusion is hypothesized from their phenotypes (see text). Double lines connect the breeding of close relatives (brother and sister or parent and offspring).
4. Discussion
Pedigree analysis is a useful tool in the study of speciation. It has the potential to reveal trans-generational influences on the choice of mates in natural populations (e.g. Qvarnström et al. 2006). Experimental manipulations, in the laboratory (Shaw 1996) and the field (Slagsvold et al. 2002), can be used to separate these influences into genetic and non-genetic components. In combination, the methods of field observation and experimentation can reveal how mates are chosen, i.e. precisely how and why courting individuals are either accepted or rejected as mates. The resulting information can then be used as a basis for constructing an evolutionary theory for the origin and maintenance of pre-mating isolation between members of coexisting populations in the early stages of speciation.
The use of pedigrees for investigating the causes of speciation is rare, however, principally owing to the difficulty in establishing genealogical relationships between successive generations in many taxonomic groups. In this context, terrestrial vertebrates are exceptional rather than typical. Birds, mammals and lizards are well characterized taxonomically and phylogenetically, and numerous examples of incipient speciation are known. Many of the species are conspicuous, parentage can be established, and breeding of the offspring of known parents can be determined as in the study reported here. Occasional hybridization, and hence the strength of the barrier to interbreeding, can be assessed and interpreted directly by the observation of pairs, supplemented by genetic information, instead of being inferred solely from the genetic or phenotypic data. Despite these advantages, the genetic basis of variation in mate choice is almost unknown (Qvarnström et al. 2006; Price 2007; Sæther et al. 2007). How genetic factors interact with early experience in governing mate choice is poorly understood, yet such interaction may be very important in those birds and mammals whose mate choice is guided by imprinting in early life (e.g. Payne et al. 2000, 2002). In fact, there may be no genetic divergence in mate recognition and response systems in the early stages of speciation. Reproductive isolation may arise solely from learning, becoming consolidated by genetic factors only later. Furthermore, it is not known whether learning distinguishes birds and mammals from the numerous invertebrate taxa in which mate choice is known or presumed to be governed primarily if not entirely by genetic factors (Rice & Hostert 1993; Coyne & Orr 2004). There is a gap between laboratory knowledge and field ignorance, with few bridges between them (Etges 1998; Hebets 2003). Pedigree analyses can help to build more.
To the extent that pairing patterns reflect the expression of choice of mates, the Daphne population of G. fortis exhibits weak assortative mating on the basis of size. There is no evidence of the alternative that assortative pairing arises as a passive consequence of selection of a breeding site by microhabitat selection (Boag & Grant 1984). In 1992 and 1993, the size of an individual's mate was predicted from the sizes of the individual's mother and father. This implies either a genetic association between the body size in parents and preference for the same size in mates or, as seems more likely from the mating patterns of hybrids (Grant & Grant 1997b), a behavioural influence of parental morphology on the choice of mates in agreement with sexual imprinting theory (Immelmann 1975; Grant & Grant 1997a; Irwin & Price 1999; ten Cate & Vos 1999). In zebra finches, there is evidence for a parental influence of the opposite sex on mate choice based on plumage colour (Witte & Sawka 2003; ten Cate et al. 2006). Our results with Darwin's finches are mainly consistent with this. The evidence suggests that both sons and daughters are influenced by the imprinting experience, but that the father's influence predominates in both sexes, which is consistent with field observations on the feeding of fledglings. A brood of fledglings is divided between the parents, each parent is as likely to feed sons as daughters, but overall the social father takes on more of the feeding of fledglings than the mother (Price & Gibbs 1987; Grant & Grant 1989).
In view of the strong annual variation in rainfall and food supply and, consequently, the number of breeding attempts, degree of breeding synchrony, breeding density, age structure and sex ratio, we expected, and observed, annual variation in morphological associations between mates. The expressions of mate preferences are likely to be promoted under some conditions and suppressed under others: promoted by strong recruitment, for example, as happened in the early 1990s, and suppressed by high survival of adults coupled with limited opportunities for young birds to breed. Re-pairing within a breeding season gives finches the opportunity to express a mate preference that was suppressed at first breeding. For those sons that changed mates, we found that parental morphology predicted the body size of the second mates but not the size of the first mates.
Positive assortative mating of extreme individuals, as observed in 2 years, implies that mate choice occurs only when the difference between potential mates is at a maximum, in other words when the candidates can be clearly discriminated. The pairing pattern of the extremely large birds of the immigrant family is consistent with this interpretation. Although they are not much larger than the largest members of the G. fortis population, they are very much larger than the smallest. The pairing pattern of these birds reflects a degree of reproductive isolation from the rest of the population unmatched by any other finch family in our experience. Two members of the pedigree have not been characterized genetically, and therefore the degree of reproductive isolation is uncertain. It could be complete. If so, the situation has a strong parallel in the establishment of a breeding population of the large ground finch, G. magnirostris, on Daphne in 1982. This species is much larger than G. fortis on Daphne, and the two have never hybridized there (Grant & Grant 2007). Thus, reproductive isolation of both G. magnirostris and large G. fortis (even if transitory) on Daphne is an exaggerated form of discrimination between mates of the same population, which differ substantially in size. We conclude that the seeds of reproductive isolation between species may be present within a single variable population in the form of a tendency towards assortative mating when the extremes can be clearly discriminated (cf. Ryan & Rand 1993; Phelps et al. 2005).
The exceptionally large birds appear to be a rare example of an important step in speciation: the establishment of coexistence of two non-interbreeding populations related by descent. According to the allopatric model of speciation, two populations derived from a common ancestor diverge in allopatry, and upon encountering each other later they are reproductively isolated to some degree, and may diverge further (Coyne & Orr 2004; Grant & Grant 2007; Price 2007). G. magnirostris are known to have arrived on Daphne from another island (Grant et al. 2001), and the original large G. fortis probably did so as well because they and their offspring are distinctly larger than all of their contemporaries (figures 1–3). There are 13 other populations of G. fortis in the archipelago (Grant & Grant 2007), and the mean body size is larger in all of them (Grant et al. 1985). A more compelling argument for an allopatric origin of the large birds could potentially be made by applying assignment tests (Pritchard et al. 2000) to genetic data from G. fortis on Daphne and other islands. Assignment tests were successfully applied to immigrant G. magnirostris on Daphne (Grant et al. 2001) to identify their island(s) of origin. Unfortunately, G. fortis populations are too weakly differentiated for a reliable test to be made (Grant et al. 2004).
Assortative mating has been found in another population of G. fortis, on the south side of Santa Cruz Island, and is associated with pronounced variation and a unique bimodal distribution of beak sizes (Ford et al. 1973; Hendry et al. 2006; Huber et al. 2007). Pairing occurs within each modal size group, and genetic data show that mating and breeding are also likely to be assortative in this size-restricted manner (Huber et al. 2007). Thus, the population appears to be in the process of splitting into two, possibly through disruptive selection (Hendry et al. 2006) and non-random mating. Alternative or additional factors responsible for the bimodality are introgressive hybridization with G. magnirostris, or immigration from a differentiated population of G. fortis on another island (Grant 1999; Huber et al. 2007). Pedigree analysis, combined with measurement of food supply, would be especially valuable to throw light on the maintenance of the unusual mating structure of the population. The origin of incipient speciation is unknown, however, because bimodality, or at least a strong skew in the distribution of the Santa Cruz population, was present more than 100 years ago when the first extensive series of specimens were collected for museums (Grant & Grant 2007). In contrast, our observations on Daphne have been made close to the origin of the non-random mating. The abrupt appearance of exceptionally large birds beyond the range of morphological variation in the Daphne G. fortis population cannot be attributed to disruptive selection; they have been added to the population rather than split off from it. The possibility of a hybrid origin was tested with genetic and phenotypic data and effectively ruled out. This leaves immigration as the most likely hypothesis. It could have given rise to the unique situation on Santa Cruz Island as well.
Although we have focused on morphological cues, other influences on the choice of mates include behaviour and features of the territory, especially nest sites, and the particular availability of mates when chosen. Therefore, not surprisingly, the predictive equations we used to account for mate choice in terms of parental morphology explained relatively little of the total morphological variation among mates. Another important cue is advertising song (Grant & Grant 1996; Podos 2001, 2006; Huber & Podos 2006). Song carrier frequency correlates with body size (Bowman 1983). Non-random pairing with respect to body size might be a correlated effect of female choice of mates that sing at a particular carrier frequency. Similarly, song note repetition rate correlates with beak size and skull musculature (Podos et al. 2004), and repetition rate may be the important trait in the choice of mates (but see Huber & Podos 2006). The body and beak sizes are strongly correlated with each other. Future studies that employ pedigree data, as well as experiments, may be able to disentangle the correlations in order to identify the particular cues that birds use when choosing a mate. Perhaps they use all of them, each strengthening the influence of the others (Slabbekorn & Smith 2002; Grant & Grant 2007).
Acknowledgements
We thank the Charles Darwin Foundation and the Galápagos National Parks for permission and support of our research, and Loeske Kruuk, Bill Hill and two referees for their valuable comments on the manuscript. The research has been funded by the grants from the Canadian National Science and Engineering Research Council, the US National Science Foundation and Class of 1877 funds from the Princeton University.
Footnotes
One contribution of 18 to a Special Issue ‘Evolutionary dynamics of wild populations’.
References
- Boag P.T, Grant P.R. Darwin's finches (Geospiza) on Isla Daphne Major. Galápagos: breeding and feeding ecology in a climatically variable environment. Ecol. Monogr. 1984;54:463–489. doi:10.2307/1942596 [Google Scholar]
- Boake C.R.B, DeAngelis M.P, Andreadis D.K. Is sexual selection and species recognition a continuum? Mating behavior of the stalk-eyed fly Drosophila heteroneura. Proc. Natl Acad. Sci. USA. 1997;94:12 442–12 445. doi: 10.1073/pnas.94.23.12442. doi:10.1073/pnas.94.23.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin A, Bellemain E, Bronken Eidesen P, Pompanon F, Brochmann C, Taberlet P. How to track and assess genotyping errors in population genetic studies. Mol. Ecol. 2005;13:3261–3273. doi: 10.1111/j.1365-294X.2004.02346.x. doi:10.1111/j.1365-294X.2004.02346.x [DOI] [PubMed] [Google Scholar]
- Boughman J.W, Rundle H.D, Schluter D. Parallel evolution of sexual isolation in sticklebacks. Evolution. 2005;59:361–373. [PubMed] [Google Scholar]
- Bowman R.I. The evolution of song in Darwin's finches. In: Bowman R.I, Berson M, Leviton A.E, editors. Patterns of evolution in Galápagos organisms. American Association for the Advancement of Science, Pacific Division; San Francisco, CA: 1983. pp. 237–537. [Google Scholar]
- Collins S.A, Luddem C. Degree of male ornamentation affects female preference for conspecific versus heterospecific males. Proc. R. Soc. B. 2002;269:111–117. doi: 10.1098/rspb.2001.1864. doi:10.1098/rspb.2001.1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J.A, Orr A.R. Sinauer Associates; Sunderland, MA: 2004. Speciation. [Google Scholar]
- Dale S, Slagsvold T. Mate choice on multiple cues, decision rules and sampling strategies in female pied flycatchers. Behaviour. 1996;133:903–944. [Google Scholar]
- Dettman J.R, Sirjusingh C, Kohn L.M, Anderson J.B. Incipient speciation by divergent adaptation and antagonistic epistasis in yeast. Nature. 2007;447:585–588. doi: 10.1038/nature05856. doi:10.1038/nature05856 [DOI] [PubMed] [Google Scholar]
- Etges W.J. Pre-mating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. IV. Correlated responses in behavioral isolation to artificial selection on a life history trait. Am. Nat. 1998;152:129–144. doi: 10.1086/286154. doi:10.1086/286154 [DOI] [PubMed] [Google Scholar]
- Ford H.A, Parkin D.T, Ewing A.W. Divergence and evolution in Darwin's finches. Biol. J. Linn. Soc. 1973;5:289–295. [Google Scholar]
- Gibbs H.L. Cultural evolution of male song types in Darwin's medium ground finches, Geospiza fortis. Anim. Behav. 1990;39:253–263. doi:10.1016/S0003-3472(05)80869-3 [Google Scholar]
- Gibbs H.L, Grant P.R. Ecological consequences of an exceptionally strong El Niño event on Darwin's finches. Ecology. 1987;68:1735–1741. doi: 10.2307/1939865. doi:10.2307/1939865 [DOI] [PubMed] [Google Scholar]
- Grant P.R. Princeton University Press; Princeton, NJ: 1999. Ecology and evolution of Darwin's finches. [Google Scholar]
- Grant B.R, Grant P.R. University of Chicago Press; Chicago, IL: 1989. Evolutionary dynamics of a natural population. The large cactus finch of the Gálapagos. [DOI] [PubMed] [Google Scholar]
- Grant B.R, Grant P.R. Cultural inheritance of song and its role in the evolution of Darwin's finches. Evolution. 1996;50:2471–2487. doi: 10.1111/j.1558-5646.1996.tb03633.x. doi:10.2307/2410714 [DOI] [PubMed] [Google Scholar]
- Grant B.R, Grant P.R. Hybridization and speciation in Darwin's finches: the role of sexual imprinting on a culturally transmitted trait. In: Howard D.J, Berlocher S.H, editors. Endless forms: species and speciation. Oxford University; New York, NY: 1998. pp. 404–422. [Google Scholar]
- Grant P.R, Grant B.R. Hybridization of bird species. Science. 1992;256:193–197. doi: 10.1126/science.256.5054.193. doi:10.1126/science.256.5054.193 [DOI] [PubMed] [Google Scholar]
- Grant P.R, Grant B.R. The founding of a new population of Darwin's finches. Evolution. 1995;49:229–240. doi: 10.1111/j.1558-5646.1995.tb02235.x. doi:10.2307/2410333 [DOI] [PubMed] [Google Scholar]
- Grant P.R, Grant B.R. Hybridization, sexual imprinting and mate choice. Am. Nat. 1997a;149:1–28. doi:10.1086/285976 [Google Scholar]
- Grant P.R, Grant B.R. Mating patterns of Darwin's finch hybrids determined by song and morphology. Biol. J. Linn. Soc. 1997b;60:317–343. doi:10.1006/bijl.1996.0103 [Google Scholar]
- Grant P.R, Grant B.R. Unpredictable evolution in a 30-year study of Darwin's finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. doi:10.1126/Science.1070315 [DOI] [PubMed] [Google Scholar]
- Grant P.R, Grant B.R. Evolution of character displacement in Darwin's finches. Science. 2006;313:224–226. doi: 10.1126/science.1128374. doi:10.1126/science.1128374 [DOI] [PubMed] [Google Scholar]
- Grant P.R, Grant B.R. Princeton University Press; Princeton, NJ: 2007. How and why species multiply. The radiation of Darwin's finches. [Google Scholar]
- Grant P.R, Abbott I, Schluter D, Curry R.L, Abbott L.K. Variation in the size and shape of Darwin's finches. Biol. J. Linn. Soc. 1985;25:1–39. [Google Scholar]
- Grant P.R, Grant B.R, Petren K. A population founded by a single pair of individuals: establishment, expansion, and evolution. Genetica. 2001;112/113:359–382. doi:10.1023/A:1013363032724 [PubMed] [Google Scholar]
- Grant P.R, Grant B.R, Markert J.A, Keller L.F, Petren K. Convergent evolution of Darwin's finches caused by introgressive hybridization and selection. Evolution. 2004;58:1588–1599. doi: 10.1111/j.0014-3820.2004.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Hebets E.A. Subadult experience influences adult mate choice in an arthropod: exposed female wolf spiders prefer males of a familiar phenotype. Proc. Natl Acad. Sci. USA. 2003;100:13 390–13 395. doi: 10.1073/pnas.2333262100. doi:10.1073/pnas.2333262100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry A.P, Grant P.R, Grant B.R, Ford H.A, Brewer M.J, Podos J. Possible human impacts on adaptive radiation: beak size bimodality in Darwin's finches. Proc. R. Soc. B. 2006;273:1887–1894. doi: 10.1098/rspb.2006.3534. doi:10.1098/rspb.2006.3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höbel G, Gerhardt H.C. Reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea) Evolution. 2003;57:894–904. doi: 10.1111/j.0014-3820.2003.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Huber S.K, Podos J. Beak morphology and song features covary in a population of Darwin's finches (Geospiza fortis) Biol. J. Linn. Soc. 2006;88:489–498. doi:10.1111/j.1095-8312.2006.00638.x [Google Scholar]
- Huber S.K, Hendry A.P, Bermingham E, Podos J. Reproductive isolation between sympatric morphs in a bimodal population of Darwin's finches. Proc. R. Soc. B. 2007;274:1709–1714. doi: 10.1098/rspb.2007.0224. doi:10.1098/rspb.2007.0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S, Cuthill I.C, Bennett A.T.D, Griffiths R. Preferences for ultraviolet partners in the blue tit. Anim. Behav. 1999;58:809–815. doi: 10.1006/anbe.1999.1214. doi:10.1006/anbe.1999.1214 [DOI] [PubMed] [Google Scholar]
- Immelmann K. Ecological significance of imprinting and early experience. Annu. Rev. Ecol. Syst. 1975;6:15–37. doi:10.1146/annurev.es.06.110175.000311 [Google Scholar]
- Irwin D.E, Price T.D. Sexual imprinting, learning and speciation. Heredity. 1999;82:347–354. doi: 10.1038/sj.hdy.6885270. doi:10.1038/sj.hdy.6885270 [DOI] [PubMed] [Google Scholar]
- Keller L.F, Grant P.R, Grant B.R, Petren K. Heritability of morphological traits in Darwin's finches: misidentified paternity and maternal effects. Heredity. 2001;87:325–336. doi: 10.1046/j.1365-2540.2001.00900.x. doi:10.1046/j.1365-2540.2001.00900.x [DOI] [PubMed] [Google Scholar]
- Keller L.F, Grant P.R, Grant B.R, Petren K. Environmental conditions affect the magnitude of inbreeding depression in survival of Darwin's finches. Evolution. 2002;56:1229–1239. doi: 10.1111/j.0014-3820.2002.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Kimball R.T. Female choice for male morphological traits in House Sparrows, Passer domesticus. Ethology. 1996;102:639–648. [Google Scholar]
- Kronforst M.R, Young L.G, Kapan D.D, McNeely C, O'Neill R.J, Gilbert L.E. Linkage of butterfly mate preference and wing color preference cue at the genomic location of wingless. Proc. Natl Acad. Sci. USA. 2006;103:6575–6580. doi: 10.1073/pnas.0509685103. doi:10.1073/pnas.0509685103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masly J.P, Jones C.D, Noor M.A.F, Locke J, Orr H.A. Gene transposition as a cause of hybrid sterility in Drosophila. Science. 2006;313:1448–1450. doi: 10.1126/science.1128721. doi:10.1126/science.1128721 [DOI] [PubMed] [Google Scholar]
- Millington S.J, Price T.D. Song inheritance and mating patterns in Darwin's finches. Auk. 1985;102:342–346. [Google Scholar]
- Nosil P, Crespi B.J, Gries R, Gries G. Natural selection and divergence in mate preference during speciation. Genetica. 2007;129:309–327. doi: 10.1007/s10709-006-0013-6. doi:10.1007/s10709-006-0013-6 [DOI] [PubMed] [Google Scholar]
- Patten M.A, Rotenberry J.T, Zuk M. Habitat selection, acoustic adaptation, and the evolution of reproductive isolation. Evolution. 2004;58:2144–2155. doi: 10.1111/j.0014-3820.2004.tb01593.x. [DOI] [PubMed] [Google Scholar]
- Payne R.B, Payne L.L, Woods J.L, Sorenson M.D. Imprinting and the origin of parasite–host species associations in brood-parasitic indigobirds, Vidua chalybeata. Anim. Behav. 2000;59:69–81. doi: 10.1006/anbe.1999.1283. doi:10.1006/anbe.1999.1283 [DOI] [PubMed] [Google Scholar]
- Payne R.B, Hustler K, Stjernstedt R, Sefc K.M, Sorenson M.D. Behavioral and genetic evidence for a recent population switch to a novel host species in brood-parasitic indigobirds Vidua chalybeata. Ibis. 2002;144:373–383. doi:10.1046/j.1474-919X.2002.00065.x [Google Scholar]
- Petren K. Microsatellite primers from Geospiza fortis and cross-species amplification in Darwin's finches. Mol. Ecol. 1998;7:1782–1784. doi: 10.1046/j.1365-294x.1998.00518.x. [DOI] [PubMed] [Google Scholar]
- Phelps S.M, Rand A.S, Ryan M.J. A cognitive framework for mate choice and species recognition. Am. Nat. 2005;167:28–42. doi: 10.1086/498538. doi:10.1086/498538 [DOI] [PubMed] [Google Scholar]
- Podos J. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature. 2001;409:185–188. doi: 10.1038/35051570. doi:10.1038/35051570 [DOI] [PubMed] [Google Scholar]
- Podos J. Discrimination of geographical song variants by Darwin's finches. Anim. Behav. 2006;73:833–844. doi:10.1016/anbehav2006.11.001 [Google Scholar]
- Podos J, Southall J.A, Rossi-Santos M.R. Vocal mechanics in Darwin's finches: correlation of beak gape and song frequency. J. Exp. Biol. 2004;207:607–619. doi: 10.1242/jeb.00770. doi:10.1242/jeb.00770 [DOI] [PubMed] [Google Scholar]
- Presgraves D, Balagopalan L, Abmayr S.M, Orr H.A. Adaptive evolution drives divergence of a hybrid inviability gene between two species of Drosophila. Nature. 2003;423:715–719. doi: 10.1038/nature01679. doi:10.1038/nature01679 [DOI] [PubMed] [Google Scholar]
- Price T.D. Roberts & Co; Greenwood Village, CO: 2007. Speciation in birds. [Google Scholar]
- Price T.D, Gibbs H.L. Brood division in Darwin's Ground finches. Anim. Behav. 1987;35:299–301. doi:10.1016/S0003-3472(87)80240-3 [Google Scholar]
- Pritchard J.K, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryke S. Fiery red heads: female dominance among head color morphs in the Gouldian finch. Behav. Ecol. 2007;18:621–627. doi:10.1093/beheco/arm020 [Google Scholar]
- Qvarnström A, Brommer J.E, Gustafsson L. Testing the genetics underlying the co-evolution of mate choice in the wild. Nature. 2006;441:84–86. doi: 10.1038/nature04564. doi:10.1038/nature04564 [DOI] [PubMed] [Google Scholar]
- Ratcliffe L.M, Grant P.R. Species recognition in Darwin's finches (Geospiza, Gould). I. Discrimination by morphological cues. Anim. Behav. 1983;31:1139–1153. doi:10.1016/S0003-3472(83)80021-9 [Google Scholar]
- Ratcliffe L.M, Grant P.R. Species recognition in Darwin's finches (Geospiza, Gould). III. Male responses to playback of different song types, dialects and heterospecific songs. Anim. Behav. 1985;33:290–307. doi:10.1016/S0003-3472(85)80143-3 [Google Scholar]
- Rice W.R, Hostert E.E. Perspective: laboratory experiments on speciation: what have we learned in forty years? Evolution. 1993;47:1637–1653. doi: 10.1111/j.1558-5646.1993.tb01257.x. doi:10.2307/2410209 [DOI] [PubMed] [Google Scholar]
- Ritchie M.G. The shape of female mating preferences. Proc. Natl Acad. Sci. USA. 1996;93:14 628–14 631. doi: 10.1073/pnas.93.25.14628. doi:10.1073/pnas.93.25.14628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M.J, Rand A.S. Species recognition and sexual selection as a unitary problem in animal communication. Evolution. 1993;47:647–657. doi: 10.1111/j.1558-5646.1993.tb02118.x. doi:10.2307/2410076 [DOI] [PubMed] [Google Scholar]
- Ryan M.J, Rand W, Hurd P.L, Phelps S.M, Rand A.S. Generalization in response to mate recognition signals. Am. Nat. 2003;161:380–394. doi: 10.1086/367588. doi:10.1086/367588 [DOI] [PubMed] [Google Scholar]
- Ryan P.G, Bloomer P, Molony C.L, Grant T.J, Delport W. Ecological speciation in South Atlantic island finches. Science. 2007;315:1420–1423. doi: 10.1126/science.1138829. doi:10.1126/science.1138829 [DOI] [PubMed] [Google Scholar]
- Sæther S.A, et al. Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science. 2007;318:95–97. doi: 10.1126/science.1141506. doi:10.1126/science.1141506 [DOI] [PubMed] [Google Scholar]
- Schluter D. Ecology and the origin of species. Trends Ecol. Evol. 2001;16:372–380. doi: 10.1016/s0169-5347(01)02198-x. doi:10.1016/S0169-5347(01)02198-X [DOI] [PubMed] [Google Scholar]
- Shaw K.L. Polygenic inheritance of a behavioral phenotype: interspecific genetics of song in the Hawaiian cricket genus Laupala. Evolution. 1996;50:256–266. doi: 10.1111/j.1558-5646.1996.tb04489.x. doi:10.2307/2410797 [DOI] [PubMed] [Google Scholar]
- Slabbekorn H, Smith T.B. Bird song, ecology and speciation. Phil. Trans. R. Soc. B. 2002;357:493–503. doi: 10.1098/rstb.2001.1056. doi:10.1098/rstb.2001.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagsvold T, Hansen B.T, Johannessen L.E, Lifjeld J.T. Mate choice and imprinting in birds studied by cross-fostering in the wild. Proc. R. Soc. B. 2002;269:1449–1456. doi: 10.1098/rspb.2002.2045. doi:10.1098/rspb.2002.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Cate C, Vos D.R. Sexual imprinting and evolutionary processes in birds: a reassessment. Adv. Stud. Behav. 1999;28:1–31. [Google Scholar]
- ten Cate C, Vos D.R, Mann N. Sexual imprinting and learning; two of one kind? Neth. J. Zool. 1993;43:34–45. [Google Scholar]
- ten Cate C, Verzijden M.N, Etman E. Sexual imprinting can induce sexual preferences for exaggerated parental traits. Curr. Biol. 2006;16:1128–1132. doi: 10.1016/j.cub.2006.03.068. doi:10.1016/j.cub.2006.03.068 [DOI] [PubMed] [Google Scholar]
- Turelli M, Barton N.H, Coyne J.A. Theory and speciation. Trends Ecol. Evol. 2001;16:330–343. doi: 10.1016/s0169-5347(01)02177-2. doi:10.1016/S0169-5347(01)02177-2 [DOI] [PubMed] [Google Scholar]
- Witte K, Sawka N. Sexual imprinting on a novel trait in the dimorphic zebra finch: sexes differ. Anim. Behav. 2003;65:195–203. doi:10.1006/anbe.2002.2009 [Google Scholar]