Abstract
Signals used in mate attraction are predicted to be highly condition dependent, and thus should be sensitive to environmental contributions to condition. However, the effects of temporal fluctuations in the environment on sexual selection in long-lived animals have been largely ignored. Female superb fairy-wrens, Malurus cyaneus, use the time that males moult into nuptial plumage prior to the onset of the breeding season to distinguish between the extra-group sires that dominate paternity. Although moult varies predictably with age, and shows marked differences between males, the phenotypic distribution also changes radically with climate; so after dry summers few males can attempt early moult. We use the recently introduced de-lifing technique to examine sexual selection gradients over 15 years of selection. Overall, there was strong evidence of directional sexual selection for early moult. However, sexual selection was much stronger when the conditions were favourable (rainfall was high), and selection was undetectable in some years. The contribution of early moulting males to population growth increased when many males moulted early, decreased when early moulting males suffered disproportionate mortality and decreased when females lacked subordinate helpers, forcing them to cede paternity to their social partner. These data suggest that short-term and laboratory studies of mate choice and sexual selection may misrepresent or underestimate the complexity of the sexual selection landscape.
Keywords: condition-dependent signal, sexual selection, mate choice, de-lifing, cooperative breeding, Malurus
1. Introduction
Theory suggests that where there is a conflict of interest over communication between a signaller and a receiver, the signal may need to have characteristics that enforce its intrinsic honesty (Zahavi 1975; Grafen 1990). Hence, such signals should initially be costly and condition dependent. Thereafter, further evolution should increase the extent of condition dependence as new genes and regulatory sequences are co-opted to affect the trait (Rowe & Houle 1996). Although these propositions have become almost axiomatic, empirical support for both the ideas remains weak (Kotiaho 2001; Cotton et al. 2004; Tomkins et al. 2004). One obvious but often ignored consequence of condition dependence will be a high level of sensitivity to environmental conditions. Several lines of evidence support this proposition. For example, spatial variation in the risk of predation affects both male secondary sexual characters and female preference functions (Stoner & Breden 1988; Houde & Endler 1990). Similarly, sustained temporal change driven by human-induced climate change or pollution affects both signalling and sexual selection (Järvenpää & Lindström 2004; Møller 2004; Spottiswoode et al. 2006; Candolin et al. 2007). In addition, the signal can depend more on the early environmental conditions than the underlying genotype (Griffith et al. 1999; Qvarnström 1999; Jensen et al. 2006). However, examination of how the selective landscape might change during the lifetime of an individual in response to environmental change have thus far been confined to short-lived species (Griffith & Sheldon 2001; Kruuk et al. 2001; Garant et al. 2004). This is unfortunate as recent studies of sexual signalling have drawn attention to a much richer range of possibilities when life-history trade-offs affect the amount of signalling effort (Hunt et al. 2004; Getty 2006).
The effects of environmental variation on mate choice and sexual selection in long-lived species are likely to be complex and difficult to predict (Charmantier & Garant 2005). To examine a simple but biologically plausible case, imagine a gradient of environmental quality that affects male expression, and a female choice function where females prefer to mate with males that exceed a certain level of sexual display, because the rarity of better males imposes costs of sampling (Jennions & Petrie 1997), or because female reluctance to mate is overcome at that threshold (Holland & Rice 1998). When conditions are poor, it is possible that selection pressure will be heightened, because most females will converge on the few males that can still exceed the threshold, or lessened, as such males are no longer available to most females. The predictions are also ambiguous when the conditions are highly favourable. Although benign conditions might allow some males to achieve exceptional values of the sexually selected character, if many males are capable of exceeding the threshold, selection could be reduced.
In this contribution, we use data from a long-term study of mate choice in a bird to explore how the environmental variation affects the expression of male display and the fitness surfaces that result from female choice. Our study species is the superb fairy-wren Malurus cyaneus, where male sexual advertisement is directed towards acquisition of the extra-group fertilizations that dominate paternity (Mulder et al. 1994). Male display to extra-group females has a variety of components, such as song (Dalziell & Cockburn in press), and striking dichromatic plumage and flower carrying (Mulder 1997). However, overwhelmingly the best predictor of female choice is a display of endurance (sensu Payne & Pagel 1996), the amount of time prior to the start of the breeding season that males are adorned in nuptial plumage, which in turn predicts the time those males have spent in frequent extra-territorial excursions to display to the neighbouring females (Dunn & Cockburn 1999; Green et al. 2000; Double & Cockburn 2003). The trait has been demonstrated to have adverse physiological consequences, and all available data support a hypothesis of strong condition dependence (Peters 2000; Peters et al. 2000, 2001). The trait varies dramatically between individuals, as some males can bear nuptial plumage and display throughout the winter, while most acquire nuptial plumage only in the late winter or early spring, months after their early moulting counterparts. Like many displays of endurance, the trait also varies within individuals, as males moult progressively earlier and retain nuptial plumage longer as they grow older (Dunn & Cockburn 1999). Here, we explore the observation that males are forced to reduce the duration they carry the nuptial plumage when the environmental conditions are adverse.
Specifically, we pose three questions. First, how do the changes in the environment affect male signalling? Second, is there any evidence of cost of sexual display that could influence the selective landscape? Third, is it possible to predict variation in the selective landscape under different environmental conditions? We discuss the relevance of our results to the theory pertaining to the lek paradox, the use of displays of endurance, the use of multiple cues in sexual selection and the limitations that arise from the short-term and laboratory-based studies of mate choice.
2. General methods and study species
Superb fairy-wrens form socially monogamous pairs that live on year-round territories. The pairs can breed alone, but in about half of all breeding efforts are assisted in rearing young by as many as four male subordinates, which are generally living on their natal territory (Cockburn et al. in press a,b). Although females are often unrelated to males on the territory (Cockburn et al. 2003), fertilizations are dominated by extra-group sires, which are themselves dominants or subordinates on the territory on which they live (Mulder et al. 1994; Dunn & Cockburn 1999). Females control access to extra-group mating, which they initiate via pre-dawn visits to the territory of their preferred sire (Double & Cockburn 2000). Males show seasonal dichromatism, usually moulting to an eclipse female-like dull brown plumage at the end of each breeding season. Then, they show considerable variation in the time at which they moult into nuptial plumage, which involves brilliant sky blue feather patches set against a velvet black background. Males commence extra-group courtship as soon as they complete the nuptial moult (Mulder 1997), and females prefer to mate with those extra-group males that have acquired blue plumage and commenced courtship months before the start of the breeding season (Dunn & Cockburn 1999; Green et al. 2000; Double & Cockburn 2003).
We have studied a population of superb fairy-wrens at the Australian National Botanic Gardens continuously since 1988, though because the age of many males was not known precisely at the start of the study, here we report data from mid-1991 to mid-2006, which represents 15 years of selection. We determine parentage through microsatellite-based genotyping (Double et al. 1997a,b; Double & Cockburn 2000) and gender of offspring using the CHD method (Griffiths et al. 1998). Because males are highly philopatric and live and die on their natal or an immediate neighbouring territory (Cockburn et al. in press a), and because we monitor all nesting attempts (Cockburn et al. in press b), almost all males are initially colour ringed as nestlings. The time at which males acquire and lose nuptial plumage each year is determined by a rolling census, in which we try and observe every individual each week. As a consequence, the birth and death dates of most males are known precisely. Our sample is drawn from 50 to almost 90 territories, as the number of territories changes over time due to fusion and fission (Cockburn et al. 2003).
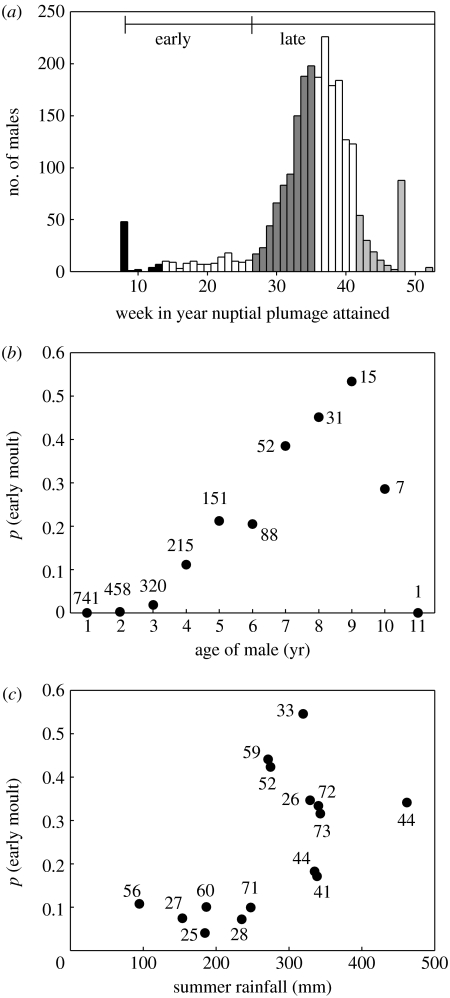
In some analyses, it proved informative to separate the time nuptial plumage was achieved into five time classes informed by biological detail, and hence of unequal length (figure 1a). (i) Stayed in nuptial plumage—achieved nuptial plumage via the post-breeding moult or by rapidly initiating a nuptial moult before achieving full eclipse plumage. (ii) Winter—achieved full eclipse but returned to nuptial plumage before the end of week 26 (2 July). These birds completed moult before the main bell-shaped part of the distribution. (iii) Pre-breeding—before the fertile period of the earliest female to initiate reproduction. (iv) Intermediate—before 90% of females had initiated reproduction. (v) Breeding—the period after 90% of females had initiated reproduction, and ending when reproduction ceased, which was on average on week 7 of the new year. For some analyses, we also aggregated the first two classes into the category early moult, and the latter classes into late moult.
Figure 1.
Variation in the time of completion of the moult into nuptial plumage. (a) Frequency distribution across all years. The shading depicts five classes of males used in statistical analysis. The peak at eight weeks refers to males that have used the post-breeding moult to renew their nuptial plumage. The peak at 48 weeks refers to males that failed to achieve full nuptial plumage. Only a very small number of males (at 52 weeks) never moulted. (b) The effect of age of the male on the probability of early moult (N=sample size for each age class). (c) The effect of summer rain on the probability that birds at least 4 years of age will moult early (N=sample size of older birds in each season).
Because the fairy-wrens have overlapping generations, the appropriate measure of fitness is the relative contribution to population growth rather than absolute reproductive success (Lande 1982), which will depend on both viability and fecundity. Coulson et al. (2006) have introduced a powerful but simple method (de-lifing) that explicitly solves this problem and allows measurement of variation in selection pressure over ecological time frames. They provide detailed explanation of the relevant calculations, which involve determining the parameter pt(i), which is the fitness of an individual measured as its contribution to population growth over 1 year relative to the average contribution of the population excluding that individual. For each male alive at the end of week 26 in each year, we determined whether it survived until week 26 the following year, the number of males that it sired within the study area during the ensuing breeding season, which survived until week 26, and used these to calculate pt(i) for each individual. We related these values to the week in which moulting was completed by each individual in the year in which the fitness calculations commenced.
Our data have a number of limitations that require more detailed discussion than those allowed by space constraints. We discuss these limitations fully in the electronic supplementary material. All statistical analyses were performed with Genstat for Windows v. 9.1, except for a proportional hazards analysis in §4, which was fitted with JMP v. 5.
3. Environmental influences on sexual signalling
The distribution of moult dates is strongly left skewed (figure 1a). While most males acquire nuptial plumage in a classically bell-shaped distribution centred on the start of the breeding season, some moult earlier and can acquire nuptial plumage during the post-breeding moult or at any time during the winter. Earlier studies of female preference functions have consistently identified these males as most successful in obtaining extra-group fertilizations (Dunn & Cockburn 1999; Green et al. 2000; Double & Cockburn 2003). Generally, only males in their fourth or subsequent winter attempt early acquisition of nuptial plumage, and thereafter the proportion of early moulters increases linearly with age until the age of 9 years (figure 1b; generalized linear mixed model with binomial error and logit link and the identity of the male and the year of sampling as random effects; effect of age on early moult: Χ12=429.2, p=0.001; analysis based on 826 males whose age was known precisely when sampled, which were sampled on average in 2.5±1.9 s.d. years). The decline thereafter is supported by small sample sizes. In addition, the proportion of males 4 years or older, which attempt early moult, varied between the years from 4 to 55% (figure 1c). This proportion only reaches high levels when the rainfall is high in the months preceding and during the post-breeding moult (November–March, hereafter summer rainfall) (GLMM: Χ12=8.2, p=0.004).
4. Costs of sexual signalling
Early moult imposes two distinct costs on males: sustaining the blue plumage and engaging in courtship for a long time, and moult during unfavourable conditions in the winter.
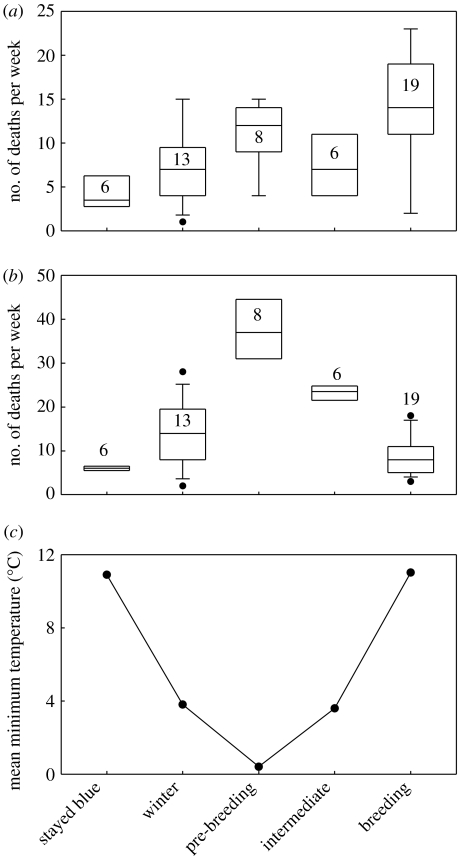
Previous studies have shown that the acquisition and maintenance of nuptial plumage in fairy-wrens is dependent on elevated testosterone levels (Peters et al. 2000, 2001), which compromise immunocompetence; though consistent with honest signalling theory, high-quality males are better able to regulate immunocompetence (Peters 2000). Whether sustaining blue plumage for a long time imposes viability costs is extremely difficult to test, as a male can only be defined as not having moulted by virtue of its survival in eclipse plumage, biasing analysis to conclude that early moulting imposes a cost. A partial solution is to contrast the survival of those males that were alive at a particular point according to whether they had or had not acquired nuptial plumage. We chose the end of early moult as the starting point of this analysis, as this time point distinguishes all those males with extravagant early moult from those that moulted in the Gaussian part of the distribution (figure 1a), yet precedes a period when males are particularly susceptible to mortality (figure 2b). There was no difference in survival between the late and early moulters from week 26 until the end of the year (proportional hazard model: Χ12=0.13, p=0.71; hazard for late relative to early moulters=0.97; 95% CIs=0.83–1.12).
Figure 2.
Temporal distribution of death dates of adult fairy-wrens according to the moult phase and temperature. Sample sizes refer to the number of weeks in each phase, and the boxplots reflect the 10th, 25th, 50th, 75th and 90th percentiles of deaths in those weeks. (a) Females, (b) males and (c) average minimum temperature.
However, direct evidence of costs comes from a number of episodes where a large number of males in the early moult category died at approximately the same time, in the weeks immediately prior to the breeding season. We identified five such events (where at least four males died at the same time) in 4 years. Although the annual mortality of males is less than 30% (Cockburn et al. in press b), in the 1996 winter, 12 of 27 (44%) early moulting males died in just two mortality events, which occurred on two nights separated by five weeks. The proximate cause of these abrupt mortality events will be explored elsewhere, but rather than being associated with absolute measures of climatic conditions, they appear to be predicted by abrupt transition from unusually warm, moist conditions to nights of heavy frost (≤5°C).
Evidence that the process of moult is costly comes from the timing of mortality. We analysed the weekly incidence of deaths of each sex during the five phases described in figure 1. While female mortality is bimodal, with a peak in winter and during the breeding season (figure 2a; analysis of variance; F4,47=6.7, p<0.001), mortality of adult males during breeding is negligible, and the peak in mortality when temperatures are at their minimum is extremely pronounced (figure 2b; F4,47=40.7, p=0.001), beyond any effect of the mortality events describe above. The cost of moulting during the coldest part of the year is implicated in this susceptibility, as some of the deaths at this time are of males known to have initiated the nuptial moult.
5. Selection gradients and their variation
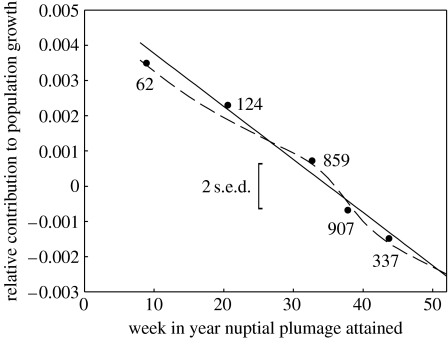
Across all 15 years of data, contribution to population growth (pt(i)) decreased strongly as the moult date advanced (figure 3; linear mixed model evaluated with REML with the identity of male and the year of sampling as random variables; Χ12=60.0, p=0.001; regression coefficient=−0.00013±0.00002 s.e.; analysis based on 902 males which were sampled on average in 2.5±1.9 s.d. years), implicating directional sexual selection for earlier moult. However, the ability of this regression to depict the selection gradient must be viewed with some caution. First, the strong skew in the phenotypic distribution could allow rare extreme values to exert leverage on the regression, and there is no a priori reason to assume a linear selection function (Schluter 1988). We assessed leverage and nonlinearity by fitting generalized additive models using a cubic spline function to explore the shape of the selection gradient, and fitting categorical models treating moult date as a factor broken into the classes previously identified. Both these approaches support the assumption of linearity (figure 3). The second problem arises because the moult date is correlated with other aspects of male phenotype, such as male age, and whether the bird is dominant or a subordinate; so it is necessary to disentangle the role of these variables (Dunn & Cockburn 1999). We addressed this difficulty by repeating the analysis just for dominant birds that were 4 years of age or older. While the breeding class is essentially absent (N=2) for these older birds, and the sample sizes are much smaller, very similar results were recovered for a linear model (Χ12=8.0, p=0.005; regression coefficient=−0.00010±0.00004 s.e.; analysis based on 296 males which were sampled on average in 2.6±1.6 s.d. years), and the categorical analysis (Χ42=13.5, p=0.009), supporting an influence of moult date regardless of age and social status.
Figure 3.
The relationship between the completion of the nuptial moult and the relative contribution to population growth, pt(i). The curves were estimated using a conventional linear model using REML (continuous line), a generalized additive model (GAM) based on a cubic spline (broken line), and by dividing the explanatory variable into the five categories from figure 1 (filled circles). For the latter case, sample sizes are the number of males in each category for this analysis, the value of nuptial plumage is the average for that class of individuals, and the vertical bar denotes twice the average standard error of the difference (s.e.d.) in the REML analysis.
The final caveat to our ability in describing selection arose because the estimate of the random term for year in the original model was highly significant, being eight times the standard error. Therefore, we repeated the analysis of the full dataset by treating year as a fixed effect and found a highly significant interaction between year and moult date (linear mixed model estimated with REML: Χ142=53.3, p=0.001). Notably, the regression coefficients in several years were indistinguishable from zero (range of estimates from −0.00045 to +0.00001; s.e.d.=0.00008). Therefore, we investigated the basis for the difference between years. Unfortunately, it is inappropriate to follow conventional practice and use standardized regression coefficients to compare inter-annual differences in the selection gradient (Arnold & Wade 1984a,b), because while the regression method is robust to changes in the distribution of fitness, its assumptions are violated by large changes in the variance and skewness of the phenotypic distribution (Lande & Arnold 1983; Arnold & Wade 1984a). There are two problems. First, if the earliest males to moult dominate population growth and they all moult just a couple of standard deviations earlier than the mean, then the selection gradient can actually be steeper than where the best males moult many standard deviations earlier than the mean, owing to leverage. Second, in some years only one or two males achieve early moult, which will exert strong influence on the regression in a way that is not easily solved by data transformation. We partly circumvented these difficulties by calculating the contribution of the earliest 10% of males to complete moult to population growth (pt10). Following Coulson et al. (2006), we calculated this as , where the individuals have a rank (i) according to the moult date; Nt is the number of competing males; and x is the rank of the individual at the 10th percentile (x averaged 14.8±4.3 s.d., N=15, range=8–21). Although more individuals contribute to this measure in some years, absolute values of pt(i) decrease with increased Nt; so the pt10 estimate is comparable. While pt10 is highly correlated with the selection gradient (F1,13=26.1, p=0.0002, R2=67%), it proved less sensitive to the high leverage that occurred when just one or two males achieve extremely early moult.
We tested five explanatory variables on pt10 as follows. (i) The proportion of males 4 years of age or older, which moulted early. It is possible to develop a priori hypotheses that predict opposite results. If poor conditions mean that only a few males achieve early moult, then these males might be expected to dominate reproduction. By contrast, if good conditions allow many males to achieve early moult, comparison might be facilitated, or differences between the best males accentuated. Because the proportion of early moulting males is correlated with the summer rainfall (figure 1c), we also contrasted the performance of models containing the summer rainfall and the proportion of early moulting males, though we did not use both in the same model owing to collinearity. (ii) Whether mortality events occur. Not only do these events reduce the number of early moulting males but also frustrate female choice if her preferred extra-group male dies just before the start of the breeding season. (iii) The proportion of territories that have helpers. Once again, the direction of any effect is uncertain. Females with helpers have greater ability to cuckold their social partner completely (Mulder et al. 1994); so the presence of helpers should accentuate sexual selection. However, helpers should reduce sexual selection because they parasitize the success of the early moulting males that attract extra-group females (Double & Cockburn 2003). (iv) The number of territories, which influences population size, and hence the extent to which any one individual can contribute to growth (Coulson et al. 2006). (v) The average production of independent offspring per territory. Fecundity fluctuates much more than viability in response to climate (Cockburn et al. in press b), so the relative importance of fecundity and viability to population growth should change as productivity increases.
We contrasted the ability of these parameters to explain pt10, using the 15 annual estimates of each explanatory variable. Using stepwise deletion, there was no effect of the number of territories (least-squares multiple regression analysis; F1,10=0.7, p=0.43) or the number of independent offspring produced (F1,10=0.1, p=0.78), but the other three terms remained in the model, explaining 79% of the variance between years. pt10 increased with the proportion of early moulting males (figure 4a; F1,11=14.7, p=0.003; regression estimate=0.152±0.040 s.e.), though was reduced sharply when the mortality events occurred (figure 4b; F1,11=20.5, p<0.001; effect=−0.312±0.007 s.e.). In addition, pt10 increased with the proportion of territories with helpers (figure 4c; F1,11=9.4, p=0.011; regression estimate=0.140±0.046 s.e.). Substitution of summer rainfall for the proportion of early moulting males reduced the explanatory power (R2=55%), perhaps because our measure of rainfall does not explain the variation when the rainfall is high (figure 1c).
Figure 4.
Variables that explain the difference between years in the contribution of the earliest 10% of males to moult to population growth. The graphs depict the capacity of each of the three explanatory variables to explain the residuals of the models containing just the other two variables. (a) The probability that males 4 years or older moulted early. (b) Whether early moulting birds were affected by one or more pre-breeding mortality events. (c) The proportion of territories with at least one helper.
6. Conclusions
Our data support the hypotheses that sexual selection in superb fairy-wrens is heightened when there are many early moulting males available to females, and when helpers are common, so females have a greater opportunity to cuckold their social partner completely. All of these variables in turn represent a response to inter-annual differences in climate. More males moult early when the summer rainfall is high (figure 1c), and helper number increases in response to the spring rainfall (Cockburn et al. in press b).
Our results have a number of general implications. First, if the female choice that drives intersexual selection focuses on condition-dependent traits and augments their condition dependence, environmental effects on condition will lead to changes in the phenotypic distribution and possibly also its variance, changing the adaptive landscape. These effects are likely to be general and non-trivial. For example, in many species the ability to produce courtship signals improves with age (Manning 1985), presumably as the ability of older individuals to convert condition into display is enhanced (sensu Getty 2006). While there is clear evidence of the differences in performance among age classes in the fairy-wrens, we were unable to identify any aspect of age-related improvement that is likely to rival the dramatic changes in food availability, which occur between droughts and wet conditions (Cockburn et al. in press b). Perhaps unsurprisingly, drought precludes extreme sexual signalling in most males. Sexual selection in fairy-wrens is accentuated in favourable conditions, but we see no cogent theoretical reason why this should generally be the case, and a plausible case can be made that the opposite will often be true, as only a few males reach a level of display that suffices to attract females. Only many more empirical studies of this question will resolve whether environmental amelioration generally increases or decreases sexual selection.
Second, it has been argued that displays of endurance are more likely to reveal the differences in male condition than temporary displays or traits that are acquired or displayed in a narrow window of time (Sullivan 1990; Payne & Pagel 1996; Kokko et al. 1999). While the importance of early moult in fairy-wrens supports that argument, our data also suggest that extreme displays of endurance have a peculiar source of unreliability. In extreme systems such as fairy-wrens, males can die between the formation of mate preference and its consummation.
Third, female fairy-wrens continue to mate with extra-group sires despite the weakening of sexual selection in some years, which could obscure any benefits that females obtain from choice (Qvarnström 2001; Welch 2003). In addition, episodes where choice is ineffectual could reduce the depletion of genetic variation that is predicted under the lek paradox (Kirkpatrick & Ryan 1991), and hence contribute to its explanation (Kokko & Heubel 2008). Alternatively, if the primary cue used in choice is unreliable under some conditions, then females may redirect their attention to alternative cues (Greenfield & Rodriguez 2004; Cotton et al. 2006). There remains little consensus on why sexual signalling often involves multiple ornaments (Johnstone 1996; Candolin 2003), but the use of backup signals when the main cue is rendered unreliable represents one possibility. Unfortunately, we lack comparable long-term sampling of such cues, and such data will prove difficult and expensive to obtain (Dalziell & Cockburn in press).
Finally, our data emphasize that short-term studies or those in the laboratory environments where environmental variation is constrained could potentially either underestimate the complexity or misrepresent the nature of sexual selection. Most research is conducted in the narrow window imposed by the 3-year cycle of a doctoral program or research grant. In the case of the data reported here, it is possible a posteriori to identify 3-year periods where a short-term study could have led to the conclusion that strong and consistent directional sexual selection is the primary influence on moult date (1992–1994; Χ12=82.9, p=0.001), or just one decade later, and that there is no sexual selection at all (2002–2004; Χ12=0.2, p=0.65). Environmental variation may explain much of the inconsistency between the short-term studies of sexual signalling, and cannot be ignored in the theoretical and empirical analyses of sexual selection.
Acknowledgments
This research was supported by several grants from the Australian Research Council. We are grateful to the Australian National Botanic Gardens and the ANU Animal Experimentation Ethics Committee for permits. Tim Coulson, Loeske Kruuk and Michael Jennions provided helpful comments, and Hanna Kokko and Anja Heubel allowed early access to the manuscript.
Footnotes
One contribution of 18 to a Special Issue ‘Evolutionary dynamics of wild populations’.
Supplementary Material
‘Constraints imposed by the biology of the study species mean that some data are inevitably incomplete. These problems are described in some detail in the Supplementary Material’
References
- Arnold S.J, Wade M.J. On the measurement of natural and sexual selection: applications. Evolution. 1984a;38:720–734. doi: 10.1111/j.1558-5646.1984.tb00345.x. doi:10.2307/2408384 [DOI] [PubMed] [Google Scholar]
- Arnold S.J, Wade M.J. On the measurement of natural and sexual selection: theory. Evolution. 1984b;38:709–819. doi: 10.1111/j.1558-5646.1984.tb00344.x. doi:10.2307/2408383 [DOI] [PubMed] [Google Scholar]
- Candolin U. The use of multiple cues in mate choice. Biol. Rev. Camb. Phil. Soc. 2003;78:575–595. doi: 10.1017/s1464793103006158. doi:10.1017/S1464793103006158 [DOI] [PubMed] [Google Scholar]
- Candolin U, Salesto T, Evers M. Changed environmental conditions weaken sexual selection in sticklebacks. J. Evol. Biol. 2007;20:233–239. doi: 10.1111/j.1420-9101.2006.01207.x. doi:10.1111/j.1420-9101.2006.01207.x [DOI] [PubMed] [Google Scholar]
- Charmantier A, Garant D. Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B. 2005;272:1415–1425. doi: 10.1098/rspb.2005.3117. doi:10.1098/rspb.2005.3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn A, Osmond H.L, Mulder R.A, Green D.J, Double M.C. Divorce, dispersal and incest avoidance in the cooperatively breeding superb fairy-wren Malurus cyaneus. J. Anim. Ecol. 2003;72:189–202. doi:10.1046/j.1365-2656.2003.00694.x [Google Scholar]
- Cockburn, A., Osmond, H. L., Mulder, R. A., Double, M. C. & Green, D. J. In press a Demography of male reproductive queues in cooperatively breeding superb fairy-wrens Malurus cyaneus J. Anim. Ecol [DOI] [PubMed]
- Cockburn, A., Sims, R. A., Osmond, H. L., Green, D. J., Double, M. C. & Mulder, R. A. In press b Can we measure the benefits of help in cooperatively breeding birds: the case of superb fairy-wrens Malurus cyaneus? J. Anim. Ecol [DOI] [PubMed]
- Cotton S, Fowler K, Pomiankowski A. Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc. R. Soc. B. 2004;271:771–783. doi: 10.1098/rspb.2004.2688. doi:10.1098/rspb.2004.2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton S, Small J, Pomiankowski A. Sexual selection and condition-dependent mate preferences. Curr. Biol. 2006;16:R755–R765. doi: 10.1016/j.cub.2006.08.022. doi:10.1016/j.cub.2006.08.022 [DOI] [PubMed] [Google Scholar]
- Coulson T, Benton T.G, Lundberg P, Dall S.R.X, Kendall B.E, Gaillard J.-M. Estimating individual contributions to population growth: evolutionary fitness in ecological time. Proc. R. Soc. B. 2006;273:547–555. doi: 10.1098/rspb.2005.3357. doi:10.1098/rspb.2005.3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziell, A. H. & Cockburn, A. In press. Dawn song in superb fairy-wrens: a bird that seeks extra-pair copulations during the dawn chorus. Anim. Behav (doi:10.1016/j.anbehav.2007.05.014)
- Double M.C, Cockburn A. Pre-dawn infidelity: females control extra-pair mating in superb fairy-wrens. Proc. R. Soc. B. 2000;267:465–470. doi: 10.1098/rspb.2000.1023. doi:10.1098/rspb.2000.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Double M.C, Cockburn A. Subordinate superb fairy-wrens (Malurus cyaneus) parasitize the reproductive success of attractive dominant males. Proc. R. Soc. B. 2003;270:379–384. doi: 10.1098/rspb.2002.2261. doi:10.1098/rspb.2002.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Double M.C, Cockburn A, Barry S.C, Smouse P.E. Exclusion probabilities for single-locus paternity analysis when related males compete for matings. Mol. Ecol. 1997a;6:1155–1166. doi:10.1046/j.1365-294X.1997.00291.x [Google Scholar]
- Double M.C, Dawson D, Burke T, Cockburn A. Finding the fathers in the least faithful bird: a microsatellite-based genotyping system for the superb fairy-wren Malurus cyaneus. Mol. Ecol. 1997b;6:691–693. doi:10.1046/j.1365-294X.1997.00228.x [Google Scholar]
- Dunn P.O, Cockburn A. Extrapair mate choice and honest signaling in cooperatively breeding superb fairy-wrens. Evolution. 1999;53:938–946. doi: 10.1111/j.1558-5646.1999.tb05387.x. doi:10.2307/2640733 [DOI] [PubMed] [Google Scholar]
- Garant D, Sheldon B.C, Gustafsson L. Climatic and temporal effects on the expression of secondary sexual characters: genetic and environmental components. Evolution. 2004;58:634–644. [PubMed] [Google Scholar]
- Getty T. Sexually selected signals are not similar to sports handicaps. Trends Ecol. Evol. 2006;21:83–88. doi: 10.1016/j.tree.2005.10.016. doi:10.1016/j.tree.2005.10.016 [DOI] [PubMed] [Google Scholar]
- Grafen A. Biological signals as handicaps. J. Theor. Biol. 1990;144:517–546. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- Green D.J, Osmond H.L, Double M.C, Cockburn A. Display rate by male fairy-wrens (Malurus cyaneus) during the fertile period of females has little influence on extra-pair mate choice. Behav. Ecol. Sociobiol. 2000;48:438–446. doi:10.1007/s002650000258 [Google Scholar]
- Greenfield M.D, Rodriguez R.L. Genotype-environment interaction and the reliability of mating signals. Anim. Behav. 2004;68:1461–1468. doi:10.1016/j.anbehav.2004.01.014 [Google Scholar]
- Griffith S.C, Sheldon B.C. Phenotypic plasticity in the expression of sexually selected traits: neglected components of variation. Anim. Behav. 2001;61:987–993. doi:10.1006/anbe.2000.1666 [Google Scholar]
- Griffith S.C, Owens I.P.F, Burke T. Environmental determination of a sexually selected trait. Nature. 1999;400:358–360. doi:10.1038/22536 [Google Scholar]
- Griffiths R, Double M.C, Orr K, Dawson R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. doi:10.1046/j.1365-294x.1998.00389.x [DOI] [PubMed] [Google Scholar]
- Holland B, Rice W.R. Chase-away sexual selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. doi:10.2307/2410914 [DOI] [PubMed] [Google Scholar]
- Houde A.E, Endler J.A. Correlated evolution of female mating preferences and male color patterns in the guppy Poecilia reticulata. Science. 1990;248:1405–1408. doi: 10.1126/science.248.4961.1405. doi:10.1126/science.248.4961.1405 [DOI] [PubMed] [Google Scholar]
- Hunt J, Brooks R, Jennions M.D, Smith M.J, Bentsen C.L, Bussiere L.F. High-quality male field crickets invest heavily in sexual display but die young. Nature. 2004;432:1024–1027. doi: 10.1038/nature03084. doi:10.1038/nature03084 [DOI] [PubMed] [Google Scholar]
- Järvenpää M, Lindström K. Water turbidity by algal blooms causes mating system breakdown in a shallow-water fish, the sand goby Pomatoschistus minutus. Proc. R. Soc. B. 2004;271:2361–2365. doi: 10.1098/rspb.2004.2870. doi:10.1098/rspb.2004.2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennions M.D, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. Camb. Phil. Soc. 1997;72:283–327. doi: 10.1017/s0006323196005014. doi:10.1017/S0006323196005014 [DOI] [PubMed] [Google Scholar]
- Jensen H, Svorkmo-Lundberg T, Ringsby T.H, Saether B.-E. Environmental influence and cohort effects in a sexual ornament in the house sparrow, Passer domesticus. Oikos. 2006;114:212–224. doi:10.1111/j.2006.0030-1299.14423.x [Google Scholar]
- Johnstone R.A. Multiple displays in animal communication: backup signals and multiple messages. Phil. Trans. R. Soc. B. 1996;351:329–338. doi:10.1098/rstb.1996.0026 [Google Scholar]
- Kirkpatrick M, Ryan M.J. The evolution of mate preferences and the paradox of the lek. Nature. 1991;350:33–38. doi:10.1038/350033a0 [Google Scholar]
- Kokko H, Heubel K. Condition-dependence, genotype-by-environment interactions, and the lek paradox. Genetica. 2008;132:209–216. doi: 10.1007/s10709-007-9166-1. doi:10.1007/s10709-007-9166-1 [DOI] [PubMed] [Google Scholar]
- Kokko H, Rintamäki P.T, Alatalo R.V, Höglund J, Karvonen E, Lundberg A. Female choice selects for lifetime lekking performance in black grouse males. Proc. R. Soc. B. 1999;266:2109–2115. doi:10.1098/rspb.1999.0895 [Google Scholar]
- Kotiaho J.S. Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol. Rev. Camb. Phil. Soc. 2001;76:365–376. doi: 10.1017/s1464793101005711. doi:10.1017/S1464793101005711 [DOI] [PubMed] [Google Scholar]
- Kruuk L.E.B, Merilä J, Sheldon B.C. Phenotypic selection on a heritable size trait revisited. Am. Nat. 2001;158:557–571. doi: 10.1086/323585. doi:10.1086/323585 [DOI] [PubMed] [Google Scholar]
- Lande R. A quantitative genetic theory of life history evolution. Ecology. 1982;63:607–615. doi:10.2307/1936778 [Google Scholar]
- Lande R, Arnold S.J. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. doi:10.2307/2408842 [DOI] [PubMed] [Google Scholar]
- Manning J.T. Choosy females and correlates of male age. J. Theor. Biol. 1985;116:349–354. doi:10.1016/S0022-5193(85)80273-3 [Google Scholar]
- Møller A.P. Protandry, sexual selection and climate change. Glob. Change Biol. 2004;10:2028–2035. doi:10.1111/j.1365-2486.2004.00874.x [Google Scholar]
- Mulder R.A. Extra-group courtship displays and other reproductive tactics of superb fairy-wrens. Aust. J. Zool. 1997;45:131–143. doi:10.1071/ZO96041 [Google Scholar]
- Mulder R.A, Dunn P.O, Cockburn A, Lazenby-Cohen K.A, Howell M.J. Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proc. R. Soc. B. 1994;255:223–229. doi:10.1098/rspb.1994.0032 [Google Scholar]
- Payne R.J.H, Pagel M. Escalation and time costs in displays of endurance. J. Theor. Biol. 1996;183:185–193. doi:10.1006/jtbi.1996.0212 [Google Scholar]
- Peters A. Testosterone treatment is immunosuppressive in superb fairy-wrens, yet free-living males with high testosterone are more immunocompetent. Proc. R. Soc. B. 2000;267:883–889. doi: 10.1098/rspb.2000.1085. doi:10.1098/rspb.2000.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Astheimer L.B, Boland C.R.J, Cockburn A. Testosterone is involved in acquisition and maintenance of sexually selected male plumage in superb fairy-wrens, Malurus cyaneus. Behav. Ecol. Sociobiol. 2000;47:438–445. doi:10.1007/s002650050688 [Google Scholar]
- Peters A, Astheimer L.B, Cockburn A. The annual testosterone profile in cooperatively breeding superb fairy-wrens, Malurus cyaneus, reflects their extreme infidelity. Behav. Ecol. Sociobiol. 2001;50:519–527. doi:10.1007/s002650100403 [Google Scholar]
- Qvarnström A. Genotype-by-environment interactions in the determination of the size of a secondary sexual character in the collared flycatcher (Ficedula albicollis) Evolution. 1999;53:1564–1572. doi: 10.1111/j.1558-5646.1999.tb05419.x. doi:10.2307/2640901 [DOI] [PubMed] [Google Scholar]
- Qvarnström A. Context-dependent genetic benefits from mate choice. Trends Ecol. Evol. 2001;16:5–7. doi: 10.1016/s0169-5347(00)02030-9. doi:10.1016/S0169-5347(00)02030-9 [DOI] [PubMed] [Google Scholar]
- Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. B. 1996;263:1415–1421. doi:10.1098/rspb.1996.0207 [Google Scholar]
- Schluter D. Estimating the form of natural selection on a quantitative trait. Evolution. 1988;42:849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. doi:10.2307/2408904 [DOI] [PubMed] [Google Scholar]
- Spottiswoode C.N, Tottrup A.P, Coppack T. Sexual selection predicts advancement of avian spring migration in response to climate change. Proc. R. Soc. B. 2006;273:3023–3029. doi: 10.1098/rspb.2006.3688. doi:10.1098/rspb.2006.3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner G, Breden F. Phenotypic differentiation in female preference related to geographic variation in male predation risk in the Trinidad guppy. Behav. Ecol. Sociobiol. 1988;22:285–291. doi:10.1007/BF00299844 [Google Scholar]
- Sullivan M.S. Assessing female choice for mates when the male characters vary during the sampling period. Anim. Behav. 1990;40:780–782. doi:10.1016/S0003-3472(05)80709-2 [Google Scholar]
- Tomkins J.L, Radwan J, Kotiaho J.S, Tregenza T. Genic capture and resolving the lek paradox. Trends Ecol. Evol. 2004;19:323–328. doi: 10.1016/j.tree.2004.03.029. doi:10.1016/j.tree.2004.03.029 [DOI] [PubMed] [Google Scholar]
- Welch A.M. Genetic benefits of a female mating preference in gray tree frogs are context-dependent. Evolution. 2003;57:883–893. doi: 10.1111/j.0014-3820.2003.tb00299.x. doi:10.1111/j.0014-3820.2003.tb00299.x [DOI] [PubMed] [Google Scholar]
- Zahavi A. Mate selection: selection for a handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. doi:10.1016/0022-5193(75)90111-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
‘Constraints imposed by the biology of the study species mean that some data are inevitably incomplete. These problems are described in some detail in the Supplementary Material’