Abstract
While sexual selection is generally assumed to quickly cause or strengthen prezygotic barriers between sister species, its role in causing postzygotic isolation, through the unattractiveness of intermediate hybrids, is less often examined. Combining 24 years of pedigree data and recently developed species-specific molecular markers from collared (Ficedula albicollis) and pied (Ficedula hypoleuca) flycatchers and their hybrids, we were able to quantify all key components of fitness. To disentangle the relative role of natural and sexual selection acting on F1 hybrid flycatchers, we estimated various fitness components, which when combined represent the total lifetime reproductive success of F1 hybrids, and then compared the different fitness components of F1 hybrids to that of collared flycatchers. Female hybrid flycatchers are sterile, with natural selection being the selective force involved, but male hybrids mainly experienced a reduction in fitness through sexual selection (decreased pairing success and increased rate of being cuckolded). To disentangle the role of sexual selection against male hybrids from a possible effect of genetic incompatibility (on the rate of being cuckolded), we compared male hybrids with pure-bred males expressing intermediate plumage characters. Given that sexual selection against male hybrids is a result of their intermediate plumage, we expect these two groups of males to have a similar fitness reduction. Alternatively, hybrids have reduced fitness owing to genetic incompatibility, in which case their fitness should be lower than that of the intermediate pure-bred males. We conclude that sexual selection against male hybrids accounts for approximately 75% of the reduction in their fitness. We discuss how natural and sexual selection against hybrids may have different implications for speciation and conclude that reinforcement of reproductive barriers may be more likely when there is sexual selection against hybrids.
Keywords: postzygotic isolation, hybrid fitness, speciation, reinforcement, extra-pair paternity, assortative mating
1. Introduction
Understanding the selective forces involved in the evolution of reproductive isolation between populations can give us important insights into the origin and maintenance of the large diversity of species found in nature. Despite the widespread implications of sexual selection and sexual conflict for the evolution of prezygotic isolation (e.g. Price 1998; Rice 1998; Owens et al. 1999; Arnqvist et al. 2000; Gavrilet 2000), the role of selection in postzygotic isolation is typically dominated by discussions of genetic incompatibilities (e.g. Noor 1997; Howard et al. 1998; Brown & Eady 2001) or hybrids failing to use parental niches (e.g. Grant & Grant 1997; Hatfield & Schluter 1999; Naisbit et al. 2001). However, the postzygotic isolation can also result from sexual selection. For instance, intermediate phenotypes may make hybrids unattractive to potential partners of either parental species (e.g. Wiernasz & Kingsolver 1992).
Sexual selection against hybrids can have important consequences for the process of reinforcement in secondary contact between diverged populations. According to theory, the evolution of prezygotic isolation may result from natural selection against hybridization (Dobzhansky 1937, 1940). However, a small amount of gene flow between two populations can inhibit this type of divergence by causing recombination between alleles that cause assortative mating and those that cause hybrid inviability (e.g. Servedio & Kirkpatrick 1997; Kirkpatrick & Servedio 1999). When selection against hybrids is driven by their unattractiveness, the same trait is involved in both pre- and postzygotic isolation and recombination is therefore less of a problem.
Several previous studies have identified sexual selection against hybrids (e.g. Vamosi & Schluter 1999; Gray & Cade 2000; Bridle et al. 2006), but the relative importance of sexual selection for postzygotic isolation is rarely investigated, due to it being a rather complicated undertaking. The most informative approach for estimating effects of hybridization is to measure the relative difference in fitness between hybrid and parental species under natural conditions. However, the typically low frequency of hybridization, and the difficulty of following individuals in the wild throughout their lifetime and measuring all components of fitness, have generally precluded such studies in the past.
By combining 24 years of pedigree data and recently developed species-specific molecular markers, we investigate here the selection processes acting on F1 hybrid Ficedula flycatchers, at different stages of their life cycle, to infer the roles of natural and sexual selection in causing postzygotic isolation in a natural hybrid zone. Collared (Ficedula albicollis) and pied (Ficedula hypoleuca) flycatchers are two closely related passerine bird species that probably came into secondary contact since the last glacial period (Sæther et al. 2001). They hybridize where they co-occur in central and eastern Europe and on the two Baltic islands Öland and Gotland off the east coast of Sweden (Alatalo et al. 1990). It was on these two islands that the current study took place. The Baltic hybrid zone is relatively young, where the two Ficedula species have been in contact for ca 150 years (F. hypoleuca being the predecessor; Lundberg & Alatalo 1992). Pied and collared flycatchers generally mate species assortatively but hybridization occurs at a moderate frequency and heterospecific pairs constitute 3–4% of the breeding pairs that we have monitored. Approximately 4 and 2% of the individuals in the population are hybrids (percentages refer to Öland and Gotland, respectively). Both species are sexually dimorphic in plumage and males of the two species differ in plumage characters (Svensson 1992) and song (e.g. Haavie et al. 2004), both of which are important in species recognition (Sæther et al. 1997; Wiley et al. 2005; Qvarnström et al. 2006). Males generally arrive at the breeding grounds a week earlier than females (Pärt & Gustafsson 1989) and start to defend a territory. Males attract females by song and courtship displays. Females then visit several males before choosing a partner and settling (Dale et al. 1990). Females of both Ficedula species have been shown, by controlled mate choice experiments, to discriminate against heterospecific males (Sæther et al. 1997), an important precondition for the existence of sexual selection against hybrids. The main aim of the present study is to investigate the relative importance of sexual selection in causing postzygotic isolation. Since the two species use very similar foraging niches (Wiley et al. 2007), natural selection against hybrids probably results from genetic incompatibilities. We use pure-bred males with intermediate morphological phenotype (similar to hybrid males in appearance but without hybrid gametes) to disentangle sexual selection against hybrid males from a fertility reduction caused by genetic incompatibility. Given that sexual selection against male hybrids is a result of their intermediate plumage we expect these two groups of males to have a similar fitness reduction. Alternatively, hybrids have reduced fitness owing to genetic incompatibility, in which case their fitness should be lower than that of the intermediate pure-bred males. Previous studies have estimated the reproductive success of Ficedula hybrids (Alatalo et al. 1990; Veen et al. 2001). For example, Veen et al. 2001 used the reproductive success of hybrids to be able to incorporate this in the predicted reproductive success of heterospecific pairs. The main conclusion in this latter paper was, from a hybridizing female's point of view, that the low fitness of hybrid offspring was counteracted by elevated levels of extra-pair offspring sired by conspecific males. Our present study aims to tease apart which components (sexual versus natural selection) determine hybrid reproductive success, thus here we focus on the ‘next level’ (reproductive success of hybrids and not heterospecific species pairings).
2. Material and methods
(a) Fitness of male hybrids
(i) Study area and species identification
The study was performed on the two Baltic islands, Öland (57°10′ N, 16°58′ E) and Gotland (57°10′ N, 18°20′ E), off the east coast of Sweden. The densities of the two species are greatly skewed, with collared flycatchers constituting 85 and 95% of the flycatcher populations on Öland and Gotland, respectively. On Gotland, nest-box areas have been established and monitored from 1980 to present, while on Öland nest-boxes have been monitored during two separate periods, between 1981–1985, and 2001 to present. Field identification of males of the two species was based on their distinct plumage characters as well as species-specific song and alarm calls (Svensson 1992). F1 hybrids have intermediate plumage (Sæther et al. 2003), but overlap with extremes in plumage characteristics present in the parental species. This makes identification of hybrids based on morphology alone unreliable. Based on plumage characters, we categorized all males in the field into three groups; collared, pied and intermediate. Hybrid status of intermediate males was confirmed if pedigree data showed that they were reared as nestlings by a heterospecific pair, or if molecular diagnostic markers supported their F1 hybrid status (see below).
To determine hybrid status genetically, blood samples (5 μl) were taken from the brachial vein and stored in ethanol, and later DNA from all birds was extracted and purified using standard phenol–chloroform extraction. Birds were genotyped at nuclear sites containing species-informative single nucleotide polymorphisms, using tag-array based mini sequencing assays as described by Sæther et al. (2003). Several markers (N=40) were used in 29 different genes (see Borge et al. 2005). Assignment tests were carried out by applying the Bayesian cluster method of Pritchard et al. (2000) as implemented in the software Structure. Individuals were identified either as collared, pied or F1 hybrids. Combined, the markers have a very high power of differentiating between collared, pied and F1 hybrid genotypes (more than 99% accuracy). It is even possible to identify first- and second-generation backcrosses using this technique (C. Wiley, A. Qvarnström, G. Andersson, T. Borge & G.-P. Sætre 2008, unpublished results).
We investigated the phenotypic overlap between collared (CF), pied (PF), F1 hybrid (HY) and non-hybrid intermediate (INTER) flycatcher males by measuring the following plumage characters: % grey within the black upperparts, sum of the length of white on the outer six primaries (mm), area of the white forehead patch (mm2) and area of white on the outer two feathers on each side of the tail (mm2). In addition, we measured tarsus length (mm) and wing length (mm). Morphological differences between the four male groups were examined by generating two principal components explaining the majority of variation in the six characters measured.
(ii) Pairing success (mate attraction)
The probability of breeding in any one year is an essential component of fitness and is mainly an indication of success at competition for females (i.e. establishing good territories and possessing preferred ornaments). We estimated pairing success using two different methods. In the first method, on Öland in April–May 2005, we caught a large number of males, newly arrived to the breeding sites but before pair formation. This was done as males inspected vacant nest-boxes soon after arrival. Males that were successful in attracting a female were recaptured and identified when feeding their nestlings later in the season. Since egg hatchability might influence the probability of individuals being recaptured, and failing in recapturing some individuals might lead to a bias when estimating reproductive success, we swapped three newly hatched chicks into all nests containing sterile eggs until the male was caught. Because this fitness (i.e. pairing success) component was a priori considered to reflect sexual selection against intermediate hybrid phenotypes, we combined hybrids and intermediate non-hybrids into a single group (which was then compared to collared and pied flycatchers).
In the second method, we estimated pairing success by calculating (from long-term breeding data) the number of years that each male was found breeding, divided by their lifespan (described later). Because intermediate non-hybrids can have normal plumage in other years of their life (N. Svedin et al., unpublished data), and therefore cannot be used in comparison of reproductive success, only true hybrids were used in this analysis. Because lifespan is likely to be an underestimate (as lifespan is inferred from their last recorded breeding event, see below), the estimates of pairing success using this technique are likely to overestimate actual pairing success. However, the reduction in pairing success of hybrids when compared with the parental species is a relative measure and should, therefore, be robust against such biases. Because it was not possible to classify immigrants of intermediate plumage as either hybrids or intermediate non-hybrids (given lack of knowledge on their parents), the dataset for both hybrids and purebreds was restricted to birds hatched in the study area. When we calculate lifetime reproductive success (below), we present two estimates each based on one of the two methods of estimating pairing success.
(iii) Amount of extra-pair young within nests
As in other birds, female flycatchers sometimes engage in extra-pair copulations (e.g. Griffith et al. 2002; Westneat & Stewart 2003). This is a potentially strong component of sexual selection against hybrid males. To investigate this, we analysed the ratios of extra-pair paternity in broods reared by the four male groups (CF, PF, HY and INTER). We compared the alleles of nestlings at five microsatellite loci (Fhu1, Fhu2, Fhu3, Fh4 and Pdu5) with those of the female and male attending the nest, to determine the proportion of nestlings sired by extra-pair males (PCR method and protocol from Haavie et al. (2000)). However, the occurrence of high ratios of extra-pair paternity need not only to stem from sexual selection, but also could be a result of failure of hybrid male sperm to fertilize eggs (e.g. Howard et al. 1998) or it might be that offspring from hybrid males suffer from higher rates of embryonic death. Neither sperm incompatibility nor embryonic death can be considered as sexual selection against male hybrids (although sexual selection may have driven the evolution of incompatibility in the first place). However, the existence of intermediate non-hybrid males (with similar phenotype to hybrids but without hybrid gametes) provides an unique opportunity to infer the importance of sexual selection against hybrids when controlling for effects of genetic incompatibility on increased levels of extra-pair paternity. We considered the levels of extra-pair paternity in nests of male F1 hybrids, beyond that in nests of intermediate non-hybrid males to stem from genetic incompatibility (i.e. reduced intrinsic fertility of the males that does not depend on the number, quality or reproductive investment of their mates). Thus, we assume that F1 hybrids experience higher extra-pair paternity caused by both genetic incompatibility and a reduction in sexual attractiveness while intermediate males solely experience a reduction in paternity caused by decreased sexual attractiveness.
(iv) Breeding success and lifespan
All nest-boxes were visited at regular intervals throughout the breeding season (May and June) and data on pair formation, onset of egg laying, clutch size, number of hatchlings and fledglings was recorded. All adults and nestlings were ringed with individually marked metal rings, allowing later identification. Fledglings that recruited back to the breeding grounds in later years were inferred from long-term breeding records. To allow for the fact that certain fledglings may not have bred for the first time in the final year of this study (2005), we excluded data from years 2002 to 2005. Our long-term dataset consisted of repeated observations of some individuals but we treat each breeding/pairing event as independent. This is because very few pairings involved the same pair of individuals in different years, and also since the within-individual repeatability of life-history traits, such as number of fledglings or recruits, is very low (Merilä & Sheldon 2000; Przybylo et al. 2000). For analyses of variables of breeding success (rate of extra-pair young, hatch rate, fledgling rate and recruitment rates), we used generalized linear models (GLMs) with binominal error (logit link) and appropriate denominators (McCullagh & Nelder 1989). Sexual selection arises through variation in both the number and the quality of mates obtained, and because breeding success depends on the quality of both parents, components of natural and sexual selection partly blend. We have chosen to assign the components of breeding success that are primarily determined by the quality of the females as sexually selected fitness components from the hybrid male perspective. By contrast, we assume that components of breeding success that largely depend on the intrinsic quality of the hybrid male are naturally selected fitness components. Thus, we expected variation in clutch size to primarily reflect sexual selection (i.e. variation in female quality, Darwin 1871; Burley 1986; Kirkpatrick et al. 1990). It probably also reflects pairing speed (earlier broods are larger, e.g. Howe 1978), and a female's investment into the brood, which is potentially affected by attractiveness of her mate (e.g. Cunningham & Russell 2000). Estimations of sexually selected fitness components of hybrids come from combining data on all intermediate looking birds (F1 hybrids and intermediate non-hybrids). All other components of breeding success (hatching rate, fledgling rate and recruitment rate) were considered a priori to primarily reflect natural selection, and were therefore estimated using only data from true hybrids.
The age of individuals can be estimated from the year they hatch or from plumage characters if caught for the first time as a 1-year-old (Ojanen 1987). In this study, lifespan was estimated as the age at which individuals were last recorded breeding. We excluded individuals breeding in 2002 or later to account for the fact that they might still be alive at the conclusion of this study.
(b) Fitness of female hybrids
In addition to the detailed examination of male hybrid fitness, we investigated the breeding success of female hybrids to confirm previous suggestions that they are sterile. The genetic markers used in this study allow a much more powerful tool to identify hybrids accurately than was available in previous studies of the fitness of female hybrid flycatchers (e.g. Alatalo et al. 1990; Veen et al. 2001). Here, we only used females where diagnostic molecular markers supported their true identity as either collared, pied or F1 hybrid females.
Scaling parameters were adjusted when the data were over-dispersed (dispersal parameters=2.02, 1.48 and 3.41 for the analysis on ratio of extra-pair paternity, hatching rate and proportion of young fledged, respectively).
3. Results
(a) Fitness of male hybrids
In total, 5440 male collared flycatchers, 315 male pied flycatchers and 319 intermediate-looking males (i.e. 5.2% of all males) were monitored on Gotland and Öland throughout the study. Out of the 319 intermediate-looking males, 191 males were confirmed as hybrid or non-hybrid through pedigrees or genetic data. The remaining males were immigrants without pedigree information or blood sampled. Of these 191 males with confirmed species identity, 63 individuals were identified as F1 hybrids and the remaining 128 males were not F1 hybrids. From these 128 males, 105 males were confirmed through genetics to have collared (103) or pied (2) genotypes. The 23 remaining males were hatched from pure pairs, and because extra-pair mating with heterospecific males are very rare (Veen et al. 2001; this study) we assume that these were non-hybrids (all collared flycatchers). In no case (when comparison was possible) did the pedigree data deviate from the molecular identification.
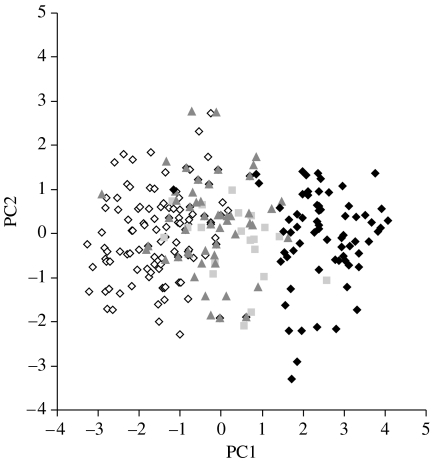
We analysed morphological variation in a subset of males for which we confirmed genotype through molecular analysis or pedigrees, and for which we had all morphological measurements (CF=111, PF=82, HY=27 and INTER=50). Intermediate non-hybrids closely resembled F1 hybrids in plumage and other morphological characters (figure 1 and tables 1 and 2).
Figure 1.
Principal component analysis incorporating six morphological characters (tarsus length, wing length, forehead patch area, white in the wing, white on tail and % grey of the head and back) of the four male groups (collared flycatcher males, white diamonds; F1 hybrid males, grey squares; intermediate non-hybrid males, grey triangles; and pied flycatcher males, black diamonds).
Table 1.
Mean values and s.d. (sample size in brackets) of morphological traits measured in a subset of collared (CF), F1 hybrids (HY), non-hybrid intermediates (INTER) and pied (PF) flycatcher males with genotypes confirmed through molecular analysis or pedigrees.
| morphological traits | CF (N=111) | HY (N=27) | INTER (N=50) | PF (N=82) |
|---|---|---|---|---|
| tarsus length (mm) | 19.2±0.6 | 19.2±0.5 | 19.4±0.5 | 19.3±0.5 |
| wing length (mm) | 83.1±1.7 | 80.8±2.0 | 81.6±2.0 | 79.5±1.9 |
| forehead patch area (mm2) | 77.8±13.7 | 62.7±17.5 | 62.5±15.6 | 28.4±11.3 |
| % grey on back and head | 6.3±6.3 | 19.0±21.0 | 16.7±22.6 | 36.9±35.7 |
| white in tail feathers (mm2) | 31.3±50.5 | 86.9±98.8 | 61.1±78.6 | 166.4±101.3 |
| white on wing patch (mm) | 24.6±15.8 | 20.6±12.4 | 22.8±12.2 | 5.0±3.8 |
Table 2.
Eigenvectors of morphological variables and the first two components (PC1 and PC2) obtained in the principal component analysis. (The PC1 explains 43.3% of the variation and PC2 an additional 15.3%.)
| variable | PC1 | PC2 |
|---|---|---|
| tarsus length | −0.08447 | 0.94860 |
| wing length | 0.43980 | 0.30598 |
| forehead patch area | 0.49924 | −0.4860 |
| % grey on back and head | −0.40681 | 0.04694 |
| white in tail feathers | −0.45132 | 0.04092 |
| white in wing patch | 0.42546 | 0.01734 |
Analysis of variance (ANOVA) indicated that these groups significantly differed along the first principal component (PC1, F3,269=263.23, p<0.0001). There was no significant difference in PC2. Tukey's pairwise comparisons indicated that F1 hybrids and intermediate non-hybrid males did not significantly differ in PC1 from each other, but that both were significantly different from collared or pied groups. The PC1 is based primarily on variation in plumage characters, while PC2 is based primarily on body size (table 2). The PC1 explained 43.3% of the variation and PC2 an additional 15.3% of the variation. The high similarity between hybrid and intermediate non-hybrid males validates the use of intermediate non-hybrid males to disentangle the effect of natural and sexual selection against hybrid males.
(i) Male pairing success
On Öland in 2005, we caught 277 males prior to pairing (collared=223, pied=35 and intermediates=19). Overall, intermediate birds had a pairing rate of 0.263, which was only half the pairing rate of collared (0.498) and pied (0.486) males. However, this difference was not statistically different (Χ22=4.143, p=0.1260), owing to the small samples of intermediate males obtained.
Using the second method of analysing pairing success of males (using the pedigree data), the analysis from their number of breeding attempts during their lifetime (on Gotland) revealed the similar pattern that males in the three groups (CF, PF and HY) had different pairing success (ANOVA, F2,1574=14.616, p<0.0001). Hybrids had significantly lower paring success than collared flycatchers (table 3). Pied flycatchers, however, also had a low pairing success that was lower than male collared flycatchers but not significantly different from that of hybrid males (table 3). This is probably a reflection of the fact that this dataset came from birds on Gotland (where pied flycatchers are rarer) while the first analysis of early caught male flycatchers were based on data from Öland, where there is a greater abundance of female pied flycatchers. Lifespan is likely to be underestimated, while pairing success therefore is probably overestimated. However, their product, the lifetime number of breeding events, is well estimated from our long-term breeding data.
Table 3.
Summary of fitness components assessed at different stages in the life cycle/breeding cycle of male collared flycatchers (CF), F1 male hybrids (HY), male pied flycatchers (PF) and pure-bred males with intermediate plumage (INTER) estimated from box-breeding populations on Öland and Gotland, Sweden. The fitness of male collared flycatchers and hybrid males are compared because these two groups of males do not breed outside our study areas. Intermediate males do not stay intermediate throughout their lives. Values that are significantly different between CF and HY males are indicated in italics (standard errors in brackets). Lifetime reproductive success is the product of all fitness components and the calculation of relative fitness of hybrids is based on these estimates. Two estimates of lifetime reproductive success are given according to the two different estimates of pairing success (method 1 is based on absent years in the total breeding record during the male's lifespan, while method 2 is based on catching of males before and after pairing). Results from method 2 were obtained solely from an experiment performed on Öland in 2005.
| fitness component | main selective force | CF | HY | PF | INTER |
|---|---|---|---|---|---|
| prop. pairing (method 1) | sexual | 0.729±0.006 (N=223) | 0.552±0.045 (N=19) | 0.509±0.050 (N=35) | |
| prop. pairing (method 2) | sexual | 0.498 (N=1519) | 0.263 (N=36) | 0.486 (N=20) | |
| clutch size | sexual | 6.098±0.010 (N=1793) | 6.156±0.119 (N=25) | 6.290±0.044 (N=25) | 6.088±0.102 (N=13) |
| prop. eggs hatched | natural | 0.925±0.002 (N=5634) | 0.849±0.023 (N=34) | 0.935±0.009 (N=291) | 0.896±0.021 (N=48) |
| prop. within-pair young | sexual/natural | 0.921±0.026 (N=58) | 0.371±0.082 (N=6) | 0.962±0.042 (N=23) | 0.737±0.054 (N=16) |
| prop. hatchlings fledged | natural | 0.811±0.004 (N=5067) | 0.898±0.051 (N=28) | 0.876±0.019 (N=238) | 0.832±0.044 (N=44) |
| prop. fledgling recruited | natural | 0.111±0.002 (N=3568) | 0.137±0.080 (N=16) | 0.064±0.011 (N=387) | 0.087±0.023 (N=38) |
| lifespan | natural | 2.308±0.035 (N=1518) | 2.792±0.280 (N=24) | 2.750±0.307 (N=20) | |
| lifetime reproductive success (method 1) | 0.787 | 0.368 | 0.444 | ||
| relative fitness (method 1) | 1 | 0.467 | 0.564 | ||
| lifetime reproductive success (method 2) | 0.538 | 0.175 | 0.424 | ||
| relative fitness (method 2) | 1 | 0.325 | 0.788 |
(ii) Ratios of extra-pair young within nests
We analysed the paternity of a total of 103 broods reared by the four male groups (CF=58, PF=23, HY=6 and INTER=16). Overall, we detected extra-pair young in 26.2% of flycatcher broods and extra-pair young comprised 12.8% of 585 nestlings. The proportions of extra-pair young strongly differ between the four male groups (GLM with binominal error, F3,102=18.47, p<0.001). The difference was caused by the much higher rates of extra-pair paternity within the nests of hybrid males than in the nests of collared and pied males (table 3).
(iii) Breeding success and lifespan
We compared several components of breeding success between F1 male hybrids and males belonging to the two parental species (table 3). Intermediate non-hybrid males were used to disentangle a possible reduction in reproductive success, either caused by reduced quality/investment of the females due to sexual unattractiveness of male hybrids (sexual selection) or caused by genetic incompatibility (natural selection). Females paired to male hybrids did not lay significantly smaller clutches when compared with the two parental species but their hatching rates were significantly lower (GLM, F3,6003=5.38, p<0.001). We assume that this reduction in hatching success is caused by genetic incompatibility because intermediate non-hybrid males did not experience a similar reduction in hatching rate. There was no significant reduction in the fledging rates of hatched offspring in the nests reared by male hybrids and no significant reduction in the probability that these offspring recruited back to the breeding population.
There were some significant differences between the two parental species. Pied flycatchers have larger clutch sizes (ANOVA, F3,4241=5.831, p<0.0006) and a higher proportion of their nestlings fledged (GLM, F3,5335=5.45, p<0.001) but a lower proportion of their fledged offspring returned back to the breeding population (GLM, F3,3715=30.06, p<0.001).
The lifespan did not significantly differ between male groups (ANOVA, F3,1570=2.14, p=0.093). However, the proportion of yearling males was slightly higher among male pied flycatchers (44%) and non-hybrid intermediate males (44%) when compared with male collared flycatchers (30%) and male hybrids (29%, Χ32=45.809, p<0.001).
(b) Fitness of female hybrids
In total, 6058 female collared flycatchers, 322 female pied flycatchers and 110 suspected female hybrids (i.e. 1.7% of all females) were monitored. In a subset of 230 females, we assigned species identity based on the 40 genetic markers (CF=199, PF=17, HY=26). The genetic classification corresponded to the phenotypic classification. Analysis of hatching rates of females that had been assigned species identity by the use of molecular markers showed a significant difference between female groups (GLM, F2,201=28.486, p<0.0001) explained by the complete sterility of F1 hybrid females (means±s.e. for CFN=176=5.676±0.205; PFN=14=5.785±0.727; HYN=14=0.000±0.000).
(c) Relative importance of sexual selection against male hybrids
We estimated lifetime reproductive success as the product of all fitness components (table 3) and the calculation of relative fitness of hybrids is based on these estimates. We found that the fitness of male hybrids was 47 or 32% of the fitness of male collared flycatchers depending on which of our two estimate of pairing success that we used. In order to single out the effect of sexual selection acting against male hybrids, we constructed an additional estimate of lifetime reproductive success where all naturally selected fitness components were kept equal to those of collared flycatchers (all values from table 3). Furthermore, the sexual selection component of ‘proportion of within-pair young’ of male hybrids was estimated using pure-bred males with intermediate phenotype (0.737, see results above) to control for a possible effect of genetic incompatibility. According to this estimate (where we use the most conservative estimate of pairing success and when the effect of natural selection is being controlled for), the fitness of male hybrids was 61% of the fitness of male collared flycatchers. Thus, 74% of the overall reduction in fitness of hybrid males was attributable to sexual selection.
4. Discussion
Our results confirm, through the use of molecular markers, that female hybrids between pied and collared flycatchers are sterile (e.g. Alatalo et al. 1992), and that male hybrids experience a reduction in fitness (e.g. Tegelström & Gelter 1990; Veen et al. 2001). We also observed low local fitness of pied flycatchers (compared to collared flycatchers) that probably stems from the fact that they are being out competed by collared flycatchers from most of our study areas on the two islands and that they in general display a higher dispersal (Lundberg & Alatalo 1992). This means that we cannot follow the reproductive success of pied flycatchers throughout their lives. Since there are allopatric populations of pied flycatchers surrounding the Swedish Ficedula hybrid zone, any pied flycatcher that disperse stands a good chance of finding a mate. Male hybrids, on the other hand, generally do not breed outside the hybrid zone and we therefore compare their lifetime fitness to that of collared flycatchers. The main goal of our study was to disentangle the effect of natural and sexual selection on male hybrids. We found that male hybrids are approximately half as fit as male collared flycatchers (based on our most conservative estimate). The reduced fitness of hybrid males largely resulted from a poor ability to acquire a mate and from larger ratios of extra-pair young within their nests. Both these features are important components of sexual selection. However, the increased levels of extra-pair young may partly be caused by hybrid sperm inviability or sperm incompatibility (e.g. Wade et al. 1994; Price 1997; Birkhead 1998; Hellberg & Vacquier 1999; Palumbi 1999), or perhaps an early embryonic death of backcross offspring. We did not detect any reduction in male hybrid fitness through sexual selection through female fecundity (here estimated by clutch size).
The existence of sexual selection against hybrids is known from other hybrid zones (e.g. West-Eberhard 1983; Wiernasz & Kingsolver 1992, Vamosi & Schluter 1999, Bridle et al. 2006). However, few studies have attempted to estimate the relative importance of sexual selection in causing postzygotic isolation in natural populations. In this study, we found that the major reduction in fitness of male hybrids is caused by a disadvantage in competition over mates. Selection against hybrid males accounts for approximately one-third of the total postzygotic isolation between the two species, and approximately 75% of this selection is attributable to sexual selection. Thus, the reduced ability of hybrid males to compete for mates explains approximately one-quarter of the total postzygotic isolation between collared and pied flycatchers. Our estimate of the relative strength of sexual selection against male hybrids partly depends on the assumption that non-hybrid males with intermediate phenotype provide a reasonable control for natural selection against hybrids. Given that selection against male hybrids is a result of a disadvantage in competition over mates resulting from their intermediate plumage, we would expect these two groups of males to have a similar fitness reduction. Alternatively, hybrids have reduced fitness owing to genetic incompatibility, in which case their fitness should be lower than that of the intermediate pure-bred males. Thus, we assume that any fitness reduction of hybrid males beyond the fitness reduction of intermediate pure-bred males reflects genetic incompatibility. Such an assumption might not be valid. The intermediate characteristics of certain individuals may be a result of developmental stress (Badyaev et al. 2005; Spencer et al. 2005) and hence reflect males that are of poorer quality. Although we did not detect a reduced lifespan of non-hybrid males with intermediate phenotype, we cannot exclude the possibility that these males had a decreased ejaculate quality, hence increasing the rate of extra-pair young through natural selection (i.e. through a reduction in fertility of the males). This could suggest that the relative importance of sexual selection, in influencing ratios of extra-pair young in hybrid nests, might be overestimated using intermediate non-hybrids in our comparisons. Alternatively, estimates using intermediate non-hybrid males might produce an underestimate of sexual selection against hybrids. While our analyses demonstrate that hybrids and intermediate non-hybrids are morphologically similar, hybrid males also frequently possess intermediate vocalizations, where they incorporate song elements from both parental species (Gelter 1987). This intermediate singing is lacking in intermediate non-hybrids, suggesting that the pairing success of hybrids may be overestimated. Thus, our estimates of the relative importance of sexual selection in reducing the fitness of male hybrid flycatchers should be considered as approximate. Nevertheless, overall our results show that sexual selection constitutes an important selective force against male hybrids.
According to Haldane's rule (1922), hybrid sterility and inviability evolve relatively faster in the heterogametic sex, and this pattern is clearly followed in flycatchers where females constitute the heterogametic sex. The two major and generally best-supported explanations for Haldane's rule are the dominance theory and the faster-male theory (Coyne & Orr 2004). We can directly exclude the faster-male theory since that theory is applicable only to taxa in which males are heterogametic. The dominance theory is an extension of the Dobzhansky–Muller model, which assumes that hybrid dysfunction is caused by epistatic effects between genes that have not co-evolved (i.e. by genes that have evolved in isolated populations and therefore interact poorly when co-occurring in hybrids). According to the dominance theory, all incompatible Z-linked genes will affect female hybrids whereas only incompatible genes with dominant effects influence male hybrid dysfunction (Muller & Pontecorvo 1942). This is consistent with the apparently minor reduction in male hybrid intrinsic fertility when compared with female hybrid intrinsic fertility (this study) and with the observation of introgression of autosomal genes but not of Z-linked genes in these flycatchers (Sæther et al. 2003). At present, the identity of the Z-linked genes that potentially leads to incompatibility between the two flycatcher species remains unknown but an ongoing genetic mapping project (e.g. Backström et al. 2006) opens novel possibilities for revealing details about the genetic bases of reproductive isolation barriers.
The build-up of genetic incompatibilities that cause complete postzygotic reproductive isolation (i.e. non-viable hybrids) is a very slow process (e.g. Price & Bouvier 2002) and speciation is therefore believed to also rely on the evolution of assortative mating (i.e. individuals mate within their own subgroup). This is because divided populations are likely to come into secondary contact before they are completely incompatible. Partial incompatibilities will then cause selection that favour individuals that mate assortatively in accordance with their population of origin (Dobzhansky 1937, 1940). Disruptive selection for assortative mating is, however, problematic as a form of reinforcement of population subdivision, because the selection is not directly targeting the traits/genes that cause hybrid dysfunction. A low level of gene flow between two populations can then inhibit the speciation process by causing recombination between alleles that cause assortative mating and those that cause hybrid inviability (e.g. Servedio & Kirkpatrick 1997; Kirkpatrick & Servedio 1999). However, when hybrids experience a disadvantage in competition over mates due to their unattractive intermediate phenotype, the same trait(s) is involved in both pre- and postzygotic isolation and recombination becomes less problematic for the speciation process (Servedio & Kirkpatrick 1997). Thus, if postzygotic isolation is driven through sexual selection, reinforcement becomes much more likely (Servedio 2004). A previous study on flycatchers implies that species-specific plumage characters are Z-linked (Sæther et al. 2003), and we have recently demonstrated that species-specific mate preferences are also determined by this sex chromosome (Sæther et al. 2007). These findings, together with the demonstrated sexually selected disadvantage of hybrid males (this study) imply that conditions for reinforcement are very favourable in the flycatcher system. The detected sexual selection against male hybrids (accounting for the main reduction in their fitness) may therefore constitute an important force that facilitates the evolution of complete reproductive isolation. Furthermore, as the combined forces of natural and sexual selection against hybrids reinforce assortative mating, we anticipate that sexual selection against male hybrids will become even stronger as females of the two species become more discriminatory.
Both sexual selection and ecological factors are likely to reduce hybrid fitness before genetic incompatibilities evolve. Price (2007) outlined two important differences between ecological versus sexual selection against hybrids in addition to the effect on reinforcement outlined above. First, reduced fitness of hybrids caused by sexual selection means that hybrid unfitness would not be affected by fluctuations in environmental conditions. When the fitness of hybrids depends on ecological factors they may, in fact, experience an advantage compared with their parental species in certain environments. Second, the build-up of hybrid unfitness caused by sexual selection is likely to occur along multiple dimensions (due to plumage, song and courtship behaviours). In this study, we have focused on the role of intermediate plumage and are therefore likely to underestimate the strength of sexual selection against male hybrids (see discussion above).
To our knowledge, this is the first study attempting to disentangle components of natural and sexual selection in postzygotic isolation in a natural hybrid zone. We summarize different components of natural and sexual selection acting on F1 hybrids and found that natural selection against female hybrids is the primary source of postzygotic isolation in flycatchers, but there is also substantial amount of sexual selection against male hybrids. Male hybrids have approximately half the fitness of male collared flycatchers and this reduction primarily arises through a disadvantage in competition over mates. This role of sexual selection in causing hybrid unfitness has important implications for speciation.
Acknowledgments
The study was approved by the Swedish Research Animals Ethical Committee.
The authors would like to thank many people who helped in collecting data from Öland and Gotland over the years. They would also like to thank K. Hjernquist-Thuman, R. Dufva, S. Adilagic, G. P. Saetre and G. Andersson for carrying out some of the molecular analyses and M. Hjernquist and Tomas Pärt for their comments on previous version of the manuscript. They are grateful to D. Eriksson, R. Alatalo and A. Lundberg for allowing us to use their pedigree data from the 1980s. The work was funded by Zoologiska stiftelsen and Anna-Marias Lundins stipendiefond to N.S., The Netherlands Organization for Scientific Research (grant NOW-ALW 812.04.001) to T.V., and the Swedish Research Council and FORMAS to A.Q., who is as Royal Swedish Academy of Sciences Research Fellow supported by Knut and Alice Wallenberg Foundation.
Footnotes
One contribution of 18 to a Special Issue ‘Evolutionary dynamics of wild populations’.
References
- Alatalo R, Eriksson D, Gustafsson L, Lundberg A. Hybridization between pied and collared flycatchers-sexual selection and speciation theory. J. Evol. Biol. 1990;3:375–389. doi:10.1046/j.1420-9101.1990.3050375.x [Google Scholar]
- Alatalo R, Gustafsson L, Lundberg A. Hybridization and breeding success of collared and pied flycatchers on the island of Gotland. Auk. 1992;99:285–291. [Google Scholar]
- Arnqvist G, Edvardsson M, Friberg U, Nilson T. Sexual conflicts promote speciation in insects. Proc. Natl Acad. Sci. USA. 2000;97:10 460–10 464. doi: 10.1073/pnas.97.19.10460. doi:10.1073/pnas.97.19.10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backström N, Brandström M, Gustafsson L, Qvarnström A, Cheng H, Ellegren H. Genetic mapping in a natural population of collared flycatchers (Ficedula albicollis): conserved synteny but gene order rearrangements on the avian Z chromosome. Genetics. 2006;174:377–386. doi: 10.1534/genetics.106.058917. doi:10.1534/genetics.106.058917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A.V, Foresman K.R, Young R.L. Evolution of morphological integration: developmental accommodation of stress-induced variation. Am. Nat. 2005;166:382–395. doi: 10.1086/432559. doi:10.1086/432559 [DOI] [PubMed] [Google Scholar]
- Birkhead T.R. Sperm competition in birds. Rev. Reprod. 1998;3:123–129. doi: 10.1530/ror.0.0030123. doi:10.1530/ror.0.0030123 [DOI] [PubMed] [Google Scholar]
- Borge T, Webster M.T, Andersson G, Saetre G.-P. Contrasting patterns of polymorphism and divergence on the Z chromosome and autosomes in two Ficedula flycatcher species. Genetics. 2005;171:1861–1873. doi: 10.1534/genetics.105.045120. doi:10.1534/genetics.105.045120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle J.R, Saldamando C.I, Koning W, Butlin R.K. Assortative preferences and discrimination by females against hybrid male song in the grasshoppers Chorthippus brunneus and Chorthippus jacobsi (Orthoptera: Acrididae) J. Evol. Biol. 2006;19:1248–1256. doi: 10.1111/j.1420-9101.2006.01080.x. doi:10.1111/j.1420-9101.2006.01080.x [DOI] [PubMed] [Google Scholar]
- Brown D.V, Eady P.E. Functional incompatibility between the fertilization systems of two allopatric populations of Callosobrucus maculatus (Coleoptera: Bruchidae) Evolution. 2001;55:2257–2262. doi: 10.1111/j.0014-3820.2001.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Burley N. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 1986;127:415–445. doi:10.1086/284493 [Google Scholar]
- Cunningham E.J, Russel A.F. Egg investment is influenced by male attractivness in the mallard. Nature. 2000;404:74–75. doi: 10.1038/35003565. doi:10.1038/35003565 [DOI] [PubMed] [Google Scholar]
- Coyne J.A, Orr A.C. Sinauer Associates, Inc; Sunderland, MA: 2004. Speciation. [Google Scholar]
- Dale S, Amundsen T, Lifjeld J.T, Slagsvold T. Mate sampling behavior of female pied flycatchers: evidence for active mate choice. Behav. Ecol. Sociobiol. 1990;27:97–91. doi:10.1007/BF00168450 [Google Scholar]
- Darwin C. J. Murray; London, UK: 1871. The descent of man, and selection in relation to sex. [Google Scholar]
- Dobzhansky T. Genetic nature of species differences. Am. Nat. 1937;71:404–420. doi:10.1086/280726 [Google Scholar]
- Dobzhansky T. Speciation as a stage in evolutionary divergence. Am. Nat. 1940;74:312–321. doi:10.1086/280899 [Google Scholar]
- Gavrilet S. Rapid evolution of reproductive barriers driven by sexual conflict. Nature. 2000;403:886–889. doi: 10.1038/35002564. doi:10.1038/35002564 [DOI] [PubMed] [Google Scholar]
- Gelter H.P. Song differences between the pied flycatcher Ficedula hypoleuca, the collared flycatcher F. albicollis, and their hybrids. Ornis Scand. 1987;18:205–215. doi:10.2307/3676768 [Google Scholar]
- Grant P.R, Grant R.B. Hybridization, sexual imprinting and mate choice. Am. Nat. 1997;149:1–28. doi:10.1086/285976 [Google Scholar]
- Gray D.A, Cade W.H. Sexual selection and speciation in field crickets. Proc. Natl Acad. Sci. USA. 2000;97:14 449–14 454. doi: 10.1073/pnas.97.26.14449. doi:10.1073/pnas.97.26.14449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith S.C, Owens I.P.F, Thuman K.A. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. doi:10.1046/j.1365-294X.2002.01613.x [DOI] [PubMed] [Google Scholar]
- Haavie J, Saetre G.-P, Moum T. Discrepancies in population differentiation at microsatellites, mitochondrial DNA and plumage color in the pied flycatcher—inferring evolutionary processes. Mol. Ecol. 2000;9:1137–1148. doi: 10.1046/j.1365-294x.2000.00988.x. doi:10.1046/j.1365-294x.2000.00988.x [DOI] [PubMed] [Google Scholar]
- Haavie J, Borge T, Bures S, Garamszegi L.Z, Lampe H.M, Moreno J, Qvarnström A, Török J, Saetre G.-P. Flycatcher song in allopatry and sympatry—convergence, divergence and reinforcement. J. Evol. Biol. 2004;17:227–237. doi: 10.1111/j.1420-9101.2003.00682.x. doi:10.1111/j.1420-9101.2003.00682.x [DOI] [PubMed] [Google Scholar]
- Haldane J.B.S. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 1922;12:101–109. [Google Scholar]
- Hatfield T, Schluter D. Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution. 1999;53:866–873. doi: 10.1111/j.1558-5646.1999.tb05380.x. doi:10.2307/2640726 [DOI] [PubMed] [Google Scholar]
- Hellberg M.E, Vacquier V.D. Rapid evolution of fertilization selectivity and lysin cDNA sequences in teguline gastropods. Mol. Biol. Evol. 1999;16:839–848. doi: 10.1093/oxfordjournals.molbev.a026168. [DOI] [PubMed] [Google Scholar]
- Howard D.J, Gregory P.G, Chu J, Cain M.L. Conspecific sperm precedence is an effective barrier to hybridization between closely related species. Evolution. 1998;52:511–516. doi: 10.1111/j.1558-5646.1998.tb01650.x. doi:10.2307/2411086 [DOI] [PubMed] [Google Scholar]
- Howe H.F. Initial investment, clutch size, and brood reduction in the common grackle (Quiscalus quiscula L.) Ecology. 1978;59:1109–1122. doi:10.2307/1938226 [Google Scholar]
- Kirkpatrick M, Servedio M.R. The reinforcement of mating preferences. Genetics. 1999;151:865–884. doi: 10.1093/genetics/151.2.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, Price T, Arnold S.J. The Darwin–Fisher theory of sexual selection in monogamous birds. Evolution. 1990;44:180–193. doi: 10.1111/j.1558-5646.1990.tb04288.x. doi:10.2307/2409533 [DOI] [PubMed] [Google Scholar]
- Lundberg A, Alatalo R.V. T. & A. D. Poyser; London, UK: 1992. The pied flycatcher. [Google Scholar]
- McCullagh P, Nelder J. Chapman and Hall; London, UK: 1989. Generalized Linear Models. [Google Scholar]
- Merilä J, Sheldon B.C. Lifetime reproductive success and heritability in nature. Am. Nat. 2000;155:301–310. doi: 10.1086/303330. doi:10.1086/303330 [DOI] [PubMed] [Google Scholar]
- Muller H.J, Pontecorvo G. Recessive genes causing interspecific sterility and other disharmonies between Drosophila melanogaster and simulans. Genetics. 1942;27:157. [Google Scholar]
- Naisbit R.E, Jigginns C.D, Mallet J. Disruptive sexual selection against hybrids contributes to speciation between Heliconius cydno and H. melpomene. Proc. R. Soc. B. 2001;268:1849–1854. doi: 10.1098/rspb.2001.1753. doi:10.1098/rspb.2001.1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor M.A.F. Genetics of sexual isolation and courtship dysfunction in male hybrids of Drosophila pseudoobscura and Drosophila persimilis. Evolution. 1997;51:809–815. doi: 10.1111/j.1558-5646.1997.tb03663.x. doi:10.2307/2411156 [DOI] [PubMed] [Google Scholar]
- Ojanen M. A method for age determination of pied flycatchers Ficedula hypoleuca in spring. Acta Regiae Societatis Scientarium et Litterarum Gothoburgensis. Zoologica. 1987;14:95–101. [Google Scholar]
- Owens I.P.F, Bennet P.M, Harvey P.H. Species richness among birds: body size, life history, sexual selection or ecology? Proc. R. Soc. B. 1999;266:933. doi:10.1098/rspb.1999.0726 [Google Scholar]
- Palumbi S.R. All males are not created equal: fertility differences depend on gamete recognition polymorphisms in sea urchins. Proc. Natl Acad. Sci. USA. 1999;96:12 632–12 637. doi: 10.1073/pnas.96.22.12632. doi:10.1073/pnas.96.22.12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärt T, Gustafsson L. Breeding dispersal in the collared flycatcher (Ficedula albicollis): possible causes and reproductive consequences. J. Anim. Ecol. 1989;58:305–320. doi:10.2307/5002 [Google Scholar]
- Price C.S.C. Conspecific sperm precedence in Drosophila. Nature. 1997;388:663–666. doi: 10.1038/41753. doi:10.1038/41753 [DOI] [PubMed] [Google Scholar]
- Price T. Sexual selection and natural selection in bird speciation. Phil. Trans. R. Soc. B. 1998;353:251–260. doi:10.1098/rstb.1998.0207 [Google Scholar]
- Price T. Roberts & Company; Greenwood Village, CO: 2007. Speciation in birds. [Google Scholar]
- Price T.D, Bouvier M.M. The evolution of F1 postzygotic incompatibilities in birds. Evolution. 2002;56:2083–2089. doi:10.1111/j.0014-3820.2002.tb00133.x [PubMed] [Google Scholar]
- Pritchard J.K, Stephens M, Donelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybylo R, Sheldon B.C, Merilä J. Climatic influences on breeding and morphology evidence for phenotypic plasticity. J. Anim. Ecol. 2000;69:395–403. doi:10.1046/j.1365-2656.2000.00401.x [Google Scholar]
- Qvarnström A, Haavie J, Saether S.A, Eriksson D, Pärt T. Song similarity predicts hybridization in flycatchers. J. Evol. Biol. 2006;19:1202–1209. doi: 10.1111/j.1420-9101.2006.01140.x. doi:10.1111/j.1420-9101.2006.01140.x [DOI] [PubMed] [Google Scholar]
- Rice W.R. Intergenomic conflict, interlocus antagonistic coevolution, and the evolution of reproductive isolation. In: Howard D.J, Berlocher S.H, editors. Endless forms: species and speciation. Oxford University Press; New York, NY: 1998. pp. 261–270. [Google Scholar]
- Sæther G.-P, Král M, Bures S. Differential species recognition abilities of males and females in a flycatcher hybrid zone. J. Avian Biol. 1997;28:259–263. doi:10.2307/3676978 [Google Scholar]
- Sæther G.-P, Borge T, Lindell J, Moum T, Primmer C.R, Sheldon B.C, Haavie J, Johnsen A, Ellegren H. Speciation, introgressive hybridization and nonlinear rate of molecular evolution in flycatchers. Mol. Ecol. 2001;10:737–749. doi: 10.1046/j.1365-294x.2001.01208.x. doi:10.1046/j.1365-294x.2001.01208.x [DOI] [PubMed] [Google Scholar]
- Sæther G.-P, Borge T, Lindrood K, Haavie J, Sheldon B, Primmer J, Syvänen A.-C. Sex chromosome evolution and speciation in Ficedula flycatchers. Proc. R. Soc. B. 2003;270:53–59. doi: 10.1098/rspb.2002.2204. doi:10.1098/rspb.2002.2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sæther S.A, et al. Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science. 2007;318:95–97. doi: 10.1126/science.1141506. doi:10.1126/science.1141506 [DOI] [PubMed] [Google Scholar]
- Servedio M.R. The evolution of premating isolation: local adaptation and natural and sexual selection against hybrids. Evolution. 2004;58:913–924. doi: 10.1111/j.0014-3820.2004.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Servedio M.R, Kirkpatrick M. The effects of gene flow on reinforcement. Evolution. 1997;51:1764–1772. doi: 10.1111/j.1558-5646.1997.tb05100.x. doi:10.2307/2410999 [DOI] [PubMed] [Google Scholar]
- Spencer K.A, Wimpenny J.H, Buchanan K.L, Lovel P.G, Goldsmith A.R, Catchpole C.K. Developmental stress affects the attractiveness of male song and female choice in the zebra finch (Taeniopygia guttata) Behav. Ecol. Sociobiol. 2005;58:423–428. doi:10.1007/s00265-005-0927-5 [Google Scholar]
- Svensson L. Märstatryck; Stockhom, Sweden: 1992. Identification guide to European passerines. [Google Scholar]
- Tegelström H, Gelter H.P. Haldane's rule and sex biased gene flow between two hybridizing flycatcher species (Ficedula albicollis and F. hypoleuca, Aves: Muscicapidae) Evolution. 1990;44:2012–2021. doi: 10.1111/j.1558-5646.1990.tb04307.x. doi:10.2307/2409611 [DOI] [PubMed] [Google Scholar]
- Vamosi S.M, Schluter D. Sexual selection against hybrids between sympatric stickleback species: evidence from a field experiment. Evolution. 1999;53:874–879. doi: 10.1111/j.1558-5646.1999.tb05381.x. doi:10.2307/2640727 [DOI] [PubMed] [Google Scholar]
- Veen T, Borge T, Griffith S.C, Saetre G.-P, Bures S, Gustafsson L, Sheldon B.C. Hybridization and adaptive mate choice in flycatchers. Nature. 2001;411:45–50. doi: 10.1038/35075000. doi:10.1038/35075000 [DOI] [PubMed] [Google Scholar]
- Wade M.J, Patterson H, Chang N.W, Johnson N.A. Postcopulatory, prezygotic isolation in flour beetles. Heredity. 1994;72:163–167. doi: 10.1038/hdy.1994.23. [DOI] [PubMed] [Google Scholar]
- West-Eberhard M.J. Sexual selection, social competition, and speciation. Q. Rev. Biol. 1983;58:155–183. [Google Scholar]
- Westneat D.F, Stewart I.R.K. Extra-pair paternity in birds: causes, correlates, and conflict. Annu. Rev. Ecol. Evol. Syst. 2003;34:365–396. doi:10.1146/annurev.ecolsys.34.011802.132439 [Google Scholar]
- Wiernasz D.C, Kingsolver J.G. Wing melanin patterns mediates species recognition in Pieris occidentalis. Anim. Behav. 1992;43:89–94. doi:10.1016/S0003-3472(05)80074-0 [Google Scholar]
- Wiley C, Bengtson J.M, Svedin N, Qvarnström A. Hybridization cost of delayed maturation of secondary sexual traits in the collared flycatcher. Evolution. 2005;59:2311–2316. [PubMed] [Google Scholar]
- Wiley C, Fogelberg N, Sæther S.A, Veen T, Svedin N, Vogel Kehlenbeck J, Qvarnström A. Direct benefits and costs for hybridizing Ficedula flycatchers. J. Evol. Biol. 2007;20:854–864. doi: 10.1111/j.1420-9101.2007.01316.x. doi:10.1111/j.1420-9101.2007.01316.x [DOI] [PubMed] [Google Scholar]