Abstract
Akt is a phospholipid-binding protein and the downstream effector of the phosphoinositide 3-kinase (PI3K) pathway. Akt has three isoforms: Akt1, Akt2, and Akt3. All of these isoforms are expressed in rod photoreceptor cells, but the individual functions of each isoform are not known. In this study we found that light induces the activation of Akt1. The membrane binding of Akt1 to rod outer segments (ROS) is insulin receptor (IR)/PI3K-dependent as demonstrated by reduced binding of Akt1 to ROS membranes of photoreceptor-specific IR knockout mice. Membrane binding of Akt1 is mediated through its Pleckstrin homology (PH) domain. To determine whether binding of the PH domain of Akt1 to photoreceptor membranes is regulated by light, various green fluorescent protein (GFP)/Akt1-PH domain fusion proteins were expressed in rod photoreceptors of transgenic Xenopus laevis under the control of the Xenopus opsin promoter. The R25C mutant PH domain of Akt1, which does not bind phosphoinositides, failed to associate with plasma membranes in a light-dependent manner. This study suggests that light-dependent generation of phosphoinositides regulates the activation and membrane binding of Akt1 in vivo. Our results also suggest that actin cytoskeletal organization may be regulated through light-dependent generation of phosphoinositides.
Keywords: Retina, Protein kinase B, Akt isoforms, Phosphoinositides, Membrane binding, Pleckstrin homology domain, Cell survival
INTRODUCTION
The serine/threonine protein kinase B (PKB)/Akt is a key mediator of signal transduction processes downstream of phosphoinositide 3-kinase (PI-3 kinase). PI 3-kinase is the key enzyme catalyzing the transfer of phosphate from ATP to the D-3 position of the inositol ring of membrane-localized phosphoinositides, thereby generating 3′-phosphorylated phosphoinositides, such as phosphotidylinositol 3,4,5-trisphosphate [PtdIns (3,4,5)P3, PIP3] and phosphotidylinositol 3,4-bisphosphate [PtdIns (3,4) P2], upon growth factor stimulation. The production of PIP3 and PI(3, 4)P2 recruits Akt from the cytoplasm to the plasma membrane through the PH domain of Akt (Andjelkovic et al. 1997;Meier et al. 1997;Wijkander et al. 1997;Zhang and Vik 1997;Sable et al. 1998;Currie et al. 1999). The relocalization of Akt to the plasma membrane brings Akt in proximity to regulatory kinases, such as 3-phosphoinositide-dependent protein kinase 1 (PDK1), which phosphorylate and activate Akt. Akt has also been observed to translocate to the nucleus after its activation in some cells (Carvalho et al. 2000;Bijur and Jope 2003;Testa and Bellacosa 1997;Borgatti et al. 2000).
The PKB/Akt family consists of three members, PKBα/Akt1, PKBβ/Akt2 and PKBγ/Akt3, which are products of three separate genes located on distinct chromosomes. The isoforms share a high degree of structural and sequence conservation through evolution (Masure et al. 1999;Staal 1987;Brodbeck et al. 1999;Jones et al. 1991a;Jones et al. 1991b;Nakatani et al. 1999). Akt1 and Akt2 are ubiquitously expressed (Coffer and Woodgett 1991), whereas Akt3 is found primarily in brain with little expression in heart, kidney, and placenta (Masure et al. 1999). Considering the complexity of biological outcomes elicited by PKB/Akt activity in a variety of cells and tissues, as well as its involvement in the etiology of various human diseases, a detailed understanding of the potential isoform-specific functions of PKB/Akt family members is needed for a comprehensive understanding of this important kinase family.
Growth factor-induced activation of Akt has been shown in transformed retinal neurons (Barber et al. 2001) and mouse models of retinal degeneration (Samardzija et al. 2006). In situ hybridizations have shown that all three Akt messenger RNAs are present in different layers of the retina (Reiter et al. 2003). We have previously reported that only Akt2 is essential for stress-induced photoreceptor survival and maintenance, although all three Akt isoforms are present in photoreceptor cells (Li et al. 2007). PKB/Akt is a downstream effector of the PI3K pathway, and we have previously reported that PI3K activity in the retina is regulated through the light-induced tyrosine phosphorylation of the retinal insulin receptor (IR) (Rajala et al. 2002;Rajala et al. 2007).
In the current study, we examined the activation of individual Akt isoforms in response to light adaptation. We found that Akt1 was the only isoform in the murine retina activated by exposure to room light. We show in this study that Akt1 is regulated through IR/PI3K-dependent pathway as tissue-specific deletion of IR in photoreceptor cells resulted in reduced p85 and Akt1 recruitment to rod outer segment membranes. We used photoreceptor-specific GFP-Akt1 transgenic Xenopus laevis to confirm the membrane binding of Akt1 in the retina. Confocal microscopy of transgenic retinas revealed that the membrane binding of the GFP-PH domain of Akt1 is light dependent. The R25C mutant PH domain of Akt1, which does not bind phosphoinositides, failed to associate with the membranes in a light-dependent manner. Our study demonstrates for the first time that light-dependent generation of phosphoinositides regulates the activation of Akt1.
EXPERIMENTAL PROCEDURES
Materials
Polyclonal anti-IRβ antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-p85 antibody was obtained from Upstate Biotechnology (Lake Placid, NY). Anti-Akt, anti-Akt1, anti-Akt2, anti-Akt3, anti-pAkt (S473) antibodies, and PI3K inhibitor LY294002 were obtained from Cell Signaling (Danvers, MA). Monoclonal anti-arrestin antibody was a kind gift from Dr. Paul Hargrave (University of Florida, Gainesville). Anti-opsin (RD14) antibody was a kind gift from Dr. Robert Molday (University of British Columbia, Vancouver, Canada). [γ32P]ATP was obtained from Perkin Elmer (Waltham, MA). Actin antibody was obtained from Affinity BioReagents (Golden, CO). D-myo-Phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2) and PIP-Strips were obtained from Echelon Research laboratories, Inc (Salt Lake City, UT). Human insulin R (rDNA origin) was obtained from Eli Lilly & Company (Indianapolis, IN). All other reagents were of analytical grade and from Sigma (St. Louis, MO).
Animals
All animal work was performed in strict accordance with the Association for Research in Vision and Ophthalmology statement on the “Use of Animals in Ophthalmic and Vision Research.” All protocols were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center and the Dean McGee Eye Institute. A breeding colony of albino Sprague-Dawley (SD) rats was maintained in our vivarium in cyclic light (5 lux; 12 h on/12 h off). Experiments were carried out on both male and female rats (150–200 g). Photoreceptor specific conditional insulin receptor knockout mice (Rajala et al. 2008) were born in 60-lux cyclic light (12h on/off) in the animal facility and maintained under these lighting conditions until they were used in an experiment.
Retinal Organ Cultures
Six to eight week-old SD rats that were born and raised in dim cyclic light (5 lux; 12 h on/12 h off) were euthanized and the eyes were enucleated. The retinas were incubated at 37°C in Dulbecco’s modified Eagle’s medium (Invitrogen, CA) in the presence or the absence of 1 μM insulin for 5–30 min. At the indicated times, the retinas were either processed for biochemistry or the eye cups rinsed in phosphate-buffered saline and then fixed in 4% paraformaldehyde (PFA). The eye cups were then processed for frozen sections as described in the methods under the tissue preparation and immunocytochemistry section.
PI 3-Kinase Assay
Enzyme assays were carried out as previously described (Kaplan et al. 1987). Briefly, assays were performed directly on anti-IRβ immunoprecipitates (Ruderman et al. 1990) in 50 μl of reaction mixture containing 0.2 mg/ml PI-4,5-P2, 50 μM ATP, 10 μCi [γ32P] ATP, 5 mM MgCl2, and 10 mM HEPES buffer (pH 7.5). The reactions were carried out for 30 min at room temperature and stopped by the addition of 100 μl of 1 N HCl followed by 200 μl of chloroform/methanol (1/1, v/v). Lipids were extracted and resolved on oxalate-coated TLC plates (silica gel 60) with a solvent system of 2-propanol/2 M acetic acid (65/35, v/v). The plates were coated in 1% (w/v) potassium oxalate in 50% (v/v) methanol and then baked in an oven at 100 °C for 1 h prior to use. TLC plates were exposed to X-ray film overnight at −70 °C and radioactive lipids were scraped and quantified by liquid scintillation counting.
Preparation of Rat and Mouse Rod Outer Segments
Rod outer segments (ROS) were prepared from retinas using a discontinuous sucrose gradient centrifugation as previously described (Rajala et al. 2007;Rajala et al. 2002). Retinas were homogenized in ice-cold 47% sucrose solution containing 100 mM NaCl, 1 mM EDTA, and 10 mM Tris-HCl (pH 7.4) (buffer A). Retinal homogenates were transferred to either 15 ml (rat) or 4 ml (mouse) centrifuge tubes and sequentially overlaid with 42%, 37%, and 32% sucrose dissolved in buffer A. The gradients were spun at 82,000 × g for 90 min at 4°C. The 32/37% interfacial sucrose band containing ROS membranes was harvested and diluted with 10 mM Tris-HCl (pH 7.4) containing 100 mM NaCl and 1 mM EDTA and centrifuged at 27,000 × g for 30 min. The ROS pellets were resuspended in 10 mM Tris-HCl (pH 7.4) containing 100 mM NaCl and 1 mM EDTA and stored at −20°C. All protein concentrations were determined by the BCA reagent following the manufacturer’s instructions (PIERCE, Rockford, IL).
Immunoprecipitation
ROS membranes were solubilized for 30 min at 4°C in a lysis buffer (1% Nonidet P-40, 20 mM HEPES (pH 7.4), and 2 mM EDTA) containing phosphatase inhibitors (100 mM NaF, 10 mM Na4P2O7, 1 mM NaVO3, and 1 mM molybdate) and protease inhibitors (10 μM leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF). Insoluble material was removed by centrifugation at 17,000 × g for 20 min at 4°C. Either ROS or retina lysates were precleared by incubation with 40 sl of protein A-Sepharose for 1 h at 4°C with mixing. The supernatant was incubated with anti-IRβ, anti pAkt (S473), anti-Akt1, anti-Akt2 and anti-Akt3 antibodies overnight at 4°C and subsequently with 40 μl of protein A-Sepharose for 1 h at 4°C. Following centrifugation at 14,000 rpm for 1 min, immune complexes were washed three times with wash buffer [50 mM HEPES (pH 7.4) containing 118 mM NaCl, 100 mM NaF, 2 mM NaVO3, 0.1% (w/v) SDS, and 1% (v/v) Triton X-100]. Immunoprecipitates were subjected to immunoblot analysis with anti-phospho-IR (1:1000), anti-Akt (1:1000), anti-Akt1 (1:1000), anti-Akt2 (1:1000), or anti-Akt3 (1:1000) antibodies.
SDS-PAGE and Western Blotting
Proteins were resolved by 10% SDS-PAGE and transferred onto nitrocellulose membranes. The blots were washed two times for 10 min with TTBS (20 mM Tris-HCl (pH 7.4), 100 mM NaCl, and 0.1% Tween 20) and blocked with either 5% bovine serum albumin (BSA) or nonfat dry milk powder (Bio-Rad) in TTBS for 1 h at room temperature (RT). Blots were then incubated with anti-IRβ (1:1000), anti-Actin (1:1000), anti-p85 subunit of PI3K (1:4000), anti-arrestin (1;1000) and anti-opsin (1:10,000), anti-Akt1 (1:1000), anti-Akt2 (1:1000), anti-Akt3 (1:1000), anti-Akt (1:1000), or anti-pAkt (S473) (1:1000) antibodies overnight at 4°C. Following primary antibody incubations, immunoblots were incubated with horseradish peroxidase (HRP)-linked secondary antibodies (either anti-rabbit or anti-mouse) and developed by SuperSignal Western Blotting Kits (PIERCE, Rockford, IL) according to the manufacturer’s instructions. Immunoblots were performed using Kodak Image Station 4000R (Eastman Kodak Co.) in the linear range of detection.
Site-Directed Mutagenesis
Site-directed mutagenesis (SDM) was performed by using Quick-Change SDM kit (Stratagene Inc, LaJolla, CA) with a PTC100 programmable thermal controller (MJ Research, Inc, Watertown, Mass MA). The reaction mixture contained SDM buffer (200 mM Tris-HCl (pH8.8), 100 mM KCl, 100 mM NH4SO4, 1% Triton X-100, 1 mg/ml nuclease-free BSA) 1 mM deoxynucleotide mix (dATP, dCTP, dTTP, and dGTP), 50 ng of TOPO TA Cloning vector (Invitrogen, CA) containing Akt1-AH domain, and 125 ng of sense and antisense primers with mutations in a total volume of 50 μl. To this mixture, 2.5 units of Pfu DNA polymerase were added. The mutant primers are shown in Table 1. The extension parameters of SDM were as follows: initial denaturation at 95°C for 30 sec, follow by 16 cycles at 95°C for 30 sec, 55°C for 1 min and 68°C for 12 min (2 min/kb). The reaction was placed on ice for 2 min, and then 10 units of DPN1 restriction enzyme were added. The mixture was incubated at 37ºC for 60 min. Transformation was carried out using 1 μl of the reaction, and the sequence of each clone was verified by DNA sequencing.
Table 1.
Primers used in this study
| Forward: Akt1 transgenic Xenopus Xho I | CTC GAG CCA CCA TGA ACG ACG TAG CCA TT |
| Reverse: Akt1 transgenic Xenopus BamHI | GGA TCC ATG GCT GTG CCA CTG GCT GAG TAG |
| Forward: Akt1-AH transgenic Xenopus XhoI | CTC GAG CCA CCA TGA ACG ACG TAG CCA TT |
| Reverse: Akt1-AH transgenic Xenopus BamHI | GGA TCC ATC ATG GTC ACA CGG TGC TTG |
| Forward: Akt1-AH R25C transgenic Xenopus | GGA TCC ATG GCT GTG CCA CTG GCT GAG TAG |
| Reverse: Akt1-AH R25C transgenic Xenopus | GGA TCC ATC ATG GTC ACA CGG TGC TTG |
| Forward: AH R25C site-directed mutagenesis | CCT GGC GGC CAT GCT ACT TCC TCC TCA AG |
| Reverse: AH R25C site-directed mutagenesis | CTT GAG GAG GAA GTA GCA TGG CCG CCA GG |
| Forward: GST-4T1-AH/and AH R25C BamHI | AGG GAT CCA CCA TGA ACG ACG TAG CCA TTG T |
| Reverse: GST-4T1-AH/and AH R25C SalI | CGT GTC GAC ATC ATG GTC ACA CGG TGC TTG |
| Forward: GST-4T1-Akt1BamHI | AGG GAT CCA CCA TGA ACG ACG TAG CCA TTG T |
| Reverse: GST-4T1-Akt1 SalI | GTC GAC TCA GGC TGT GCC ACT GGC TGA GTA G |
| Forward: GST-4T1-Akt2 BamHI | GGA TCC ATG AAT GAG GTAT CTG TC |
| Reverse: GST-4T1-Akt2 SalI | GTC GAC TCA CTC TCG GAT GCT GGC |
| Forward: GST-4T1-Akt3 BamHI | GGATCC ATG AGC GAT GTT ACC ATT G |
| Reverse: GST-4T1-Akt3 SalI | GTC GAC TTA TTC CCG TCC GCT TGC AG |
Construction of GST Fusion Proteins and Pull-Down Experiments
We constructed GST fusion proteins for Akt1, Akt2, Akt3 and AH domain of Akt1. The cDNA encoding the Akt1, Akt2 and Akt3 were amplified by PCR of reverse transcribed retinal RNA using primers designed on the basis of either rat or mouse brain. The full-length Akt isoforms and AH domain of Akt1 were cloned into a pGEX-4T-1 (Pharmacia, Piscataway, NJ) GST fusion vector. The sequence of each clone was verified by DNA sequencing. The expression vectors were transformed into BL21 (DE3) competent cells and the transformed cells were incubated with IPTG (1 mM). Expressed fusion proteins were purified through GST-Sepharose 4B matrix. All inductions yielded proteins of the expected size as judged by Coomassie staining or immunoblot blot analysis.
Protein-Lipid Overlay Assay
Membrane arrays (PIP-Strips) spotted with 100 pmol of phospholipids were purchased from Echelon Research Laboratories (Salt Lake City, UT) and used for protein-lipid overlay assays by following the manufacturer’s instructions. Briefly, membranes were blocked with 3% (w/v) fatty acid-free BSA (Sigma) in TBST [10 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.1% (w/v) Tween 20] for 1 h at RT. Blocked membranes were incubated with 0.5 μg/mL GST/GST fusion proteins (wild type and mutant (R25C) AH domain of Akt1, Akt1, Akt2, Akt3 and Grip) overnight at 4°C with gentle agitation. They were then washed three times with TBST plus 3% fatty acid-free BSA. The membranes were subjected to immuno blot analysis using anti-GST antibody to detect the bound GST fusion proteins.
Generation of DNA Constructs
FANS-xop1.3-hrGFP-N1 vector was kindly provided by David Papermaster (University of Connecticut Health Center, Farmington, Connecticut). All X. laevis expression constructs were based on peGFP-C1 (BD Biosciences Clontech, Palo Alto, CA), which was modified to contain the proximal X. laevis opsin promoter (XOP1.3GFP-C1) as described previously (Tam et al. 2000). The N1 vectors have multiple cloning sites (MCS) just after the promoter followed by the GFP coding region, which has two in-frame stop codons at the end. The Stratagene HrGFP cDNA, which is 2 times brighter and more soluble than EGFP was cloned into the GFP site. The rat Akt1 and Akt1-AH (PH domain containing the linker sequence) DNA sequences were amplified by RT-PCR from rat retina cDNA with a Kozak start site added at the N-terminus. Sense and antisense primers are shown in Table 1. XhoI and BamHI (New England Biolabs, Beverly, MA) were the restriction enzymes used. The primers were designed so to be inserted in frame with GFP. The final constructs were verified by DNA sequencing. The resulting plasmids encoded a GFP which was preceded by full-length Akt (fAkt1), Akt1-AH, or Akt1-AH R25C. The ligated plasmid was cloned into bacteria (XL1 Blue Escherichia coli; Stratagene, La Jolla, CA) and the plasmids were purified with QIAprep spin miniprep kit (Qiagen, Valencia, CA). Expression vectors were linearized by digestion with ApaLI and purified using the GeneClean kit (Q-BIOgene, Carlsbad, CA). Purified DNA was used to generate transgenic Xenopus as described in the following section.
Generation of Transgenic Xenopus laevis Embryos
Adult Xenopus laevis frogs were obtained from Xenopus I (Dexter, MI) and maintained in aquaria for at least 2 weeks in a 12-hour light-dark cycle at 20°C. A modification of the protocol of Kroll and Amaya (Kroll and Amaya 1996) was used for generating transgenic Xenopus laevis frogs. This research adhered to the ARVO Statement for the “Use of Animals in Ophthalmic and Vision Research” and the Declaration of Helsinki and Principles of Laboratory Animal Care of the National Institute of Health.
Xenopus sperm nuclei were prepared by macerating testes removed from adult male Xenopus laevis that were killed by immersion in 2% tricaine methylsulfonate (MS 222; Sigma-Aldrich, St. Louis, MO) for 20 minutes. Nuclei were isolated from sperm, as described previously (Kroll and Amaya 1996), and then cryoprotected and stored at −80°C until time of use. The oocyte high-speed cytoplasmic extract was prepared according to previously published methods (Kroll and Amaya 1996;Murray 1991;Wiechmann et al. 2003). Oocytes were retrieved from the frogs 18 h after HCG injections and were dejellied with 2% cysteine, rinsed in 1x MMR, and placed in 0.4x MMR and 6% Ficoll (Sigma-Aldrich) for immediate use in transgenesis. ApaLI was used to linearize fragments containing the XOP-GFP construct or the XOP-GFP-Akt1 constructs. These constructs were purified and injected into each oocyte as described (Wiechmann et al. 2003). Normally cleaving embryos were selected at the four- to eight-cell stage and incubated in 0.4x MMR/6% Ficoll with 50 sag/mL gentamicin (Sigma-Aldrich) at 18°C. Tadpoles were transferred to 22°C at approximately stage 20. At approximately stage 45, tadpoles were transferred to a solution of 0.2 M Na2HPO4 and 50 g/L of a salt mixture (Instant Ocean; Aquarium Systems, Mentor, OH) at 22°C. Staging of the Xenopus embryos was performed according the methods of Nieuwkoop and Faber (Nieuwkoop and Farber J (eds) 1956).
Transgenic tadpoles were split into two groups: one group (control) was dark-adapted overnight and then immediately placed in fixative, and the other group was dark-adapted overnight, transferred to transparent plastic Petri dish (Fisher Pittsburgh, PA, USA) and light-adapted for 30 min at 1400 lux. Light levels were measured using an International Light IL1700 radiometer. Following light adaptation, all of the tadpoles were transferred as a group to fixative within 30 seconds. Tadpoles used for these experiments ranged from 2 to 4 weeks old (Stages 47–54). For most experiments, light adaptation was initiated at 9 AM.
Transgenic adult males were mated with wild-type females to produce F1 offspring, which were used for light/dark-adaptation experiments. After 2–3 weeks, F1 tadpoles were visually screened for green fluorescence and segregated into strong, weak, or no transgene expression.
Tissue Preparation and Immunocytochemistry
Whole-head preparations of transgenic and normal tadpoles were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and transferred to 30% sucrose in 0.1 M phosphate buffer for 16 to 20 hours at 4°C. Sagittal, 12-sm sections were cut on a cryostat microtome and collected on glass slides. To analyze the tissue distribution of GFP expression, sections were labeled with 0.0005% 4′, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). Sections were rinsed in PBS (pH 7.4) and coverslips were mounted on the slides with mounting matrix (Cytoseal 60; Stephens Scientific, Kalamazoo, MI).
For immunocytochemical localization of phosphorylated Akt (pAkt 473) in the retina, Xenopus were anesthetized with MS-222 and whole eyes were fixed by immersion in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Cryostat sections were rinsed in PBS and then incubated in buffer [5% normal goat serum (Sigma-Aldrich) 5% BSA, 0.2% Triton X-100, and 0.004% sodium azide in PBS] for 1 h at RT. In control experiments, the pAkt antibody was incubated for 2 h at 4°C with its blocking peptides before being applied to the section. Sections were then incubated with the pAkt (S473) antibody (1:100) in blocking buffer overnight at 4°C. Sections were rinsed with PBS and incubated in a mixture of Alexa Fluor 568 (red) conjugated to goat anti-rabbit (1:1000, Molecular Probes, Eugene, OR) and 0.0005% DAPI nuclear stain for 1 h at RT. Sections were rinsed in PBS and coverslips were mounted with mounting medium (VECTASHIELD with DAPI, Vector laboratories Inc, Burlingame, CA). GFP-labeled sections were viewed under a laser-scanning confocal microscope (Olympus IX81-FV500, NY). Samples were excited with 405, 488, and 546 nm wavelength lasers (Olympus, Melville, NY). At least five transgenic animals were examined by confocal microscopy for each construct. Due to the wide variety of expression levels, no combination of settings of iris, gain, and laser power was found that would provide suitable visualization of all transgenic animals. Therefore, only animals with strong Akt1-GFP expression were used in experiments. Laser settings were chosen such that the presence of autofluorescence could not be detected in wild-type retinas, and the GFP signal was not saturated. These low gain settings were rigorously fixed for each image.
RESULTS
Activation of IR by insulin in retinal organ cultures
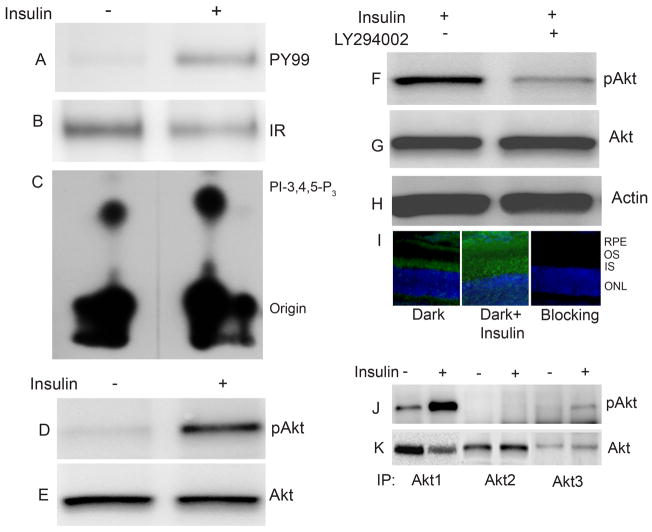
Addition of insulin to the DMEM incubation medium resulted in activation of the IR, measured by the immunoprecipitation of the IR with anti-IRβ antibody (Fig. 1B), followed by immunoblot analysis with anti-PY99 antibody (Fig. 1A). The results indicate the phosphorylation of IR in response to insulin ex vivo.
Figure 1.
Insulin induced activation of IR, PI3K and Akt activation ex vivo. Retinas in organ culture were stimulated with or without 1μM insulin and incubated for 5 min at 37 °C. Insulin stimulated or unstimulated retinal proteins were subjected to immunoprecipitation with anti-Irβ followed by either immunoblot analysis with anti-PY99 antibody (A). The blot was stripped and reprobed with anti-IRβ antibody to ensure equal amount of protein in each lane (B). IRβ immunoprecipitates from insulin stimulated or unstimulated retinal proteins were directly measured for IR associated PI3K activity using PI-4,5-P2 and [γ32P] ATP as substrates (C). Rat retinas were dissected and incubated in DMEM medium with or without 1 μM insulin. After incubation, the retinas were lysed and 25 μg of protein was subjected immunoblot analysis with anti-pAkt (Ser 473) antibody (D). The blot was stripped and reprobed with anti-Akt antibody to ensure equal amount of protein in each lane (E). Rat retinas were pre-incubated in DMEM medium with or without the PI3K inhibitor LY294002 (50 μM) for 60 min prior to insulin treatment for 5 min at 37 °C. Twenty five micrograms of retina proteins were subjected to immuno blot analysis with anti-phospho-Akt (Ser 473) (F), total Akt (G) and actin (H) antibodies. Rat eye cups were dissected from dark-adapted rats and incubated in DMEM medium with or without 1 μM insulin 5 min at 37 °C. The retinas were fixed and stained for pAkt (S473) antibody (I), and the nuclei were stained with DAPI. Insulin stimulated and unstimulated retinas were lysed and subjected to immunoprecipitation with anti-Akt1, anti-Akt2 and anti-Akt3 antibodies followed by immuno blot analysis with anti-pAkt antibody (J). The blot was stripped and reprobed with anti-Akt antibody to ensure equal amount of protein in each immunoprecipitate (K).
Insulin-induced activation of PI3K in retinal organ cultures
To test the effect of insulin on PI3K activity, insulin stimulated or unstimulated retinas in organ culture were lysed and immunoprecipitated with anti-IRβ antibody followed by measurement of the PI3K activity associated with anti-IRβ immunoprecipitates. Higher PI3K activities were observed in the anti-IRβ immunoprecipitates of insulin stimulated retinas (Fig. 1C). These results suggest that insulin induced the activation of PI3K through tyrosine phosphorylated IR ex vivo.
Insulin induced activation of Akt
Retinas in culture were stimulated with or without insulin and the retinal proteins were subjected immunoblot analysis with anti-pAkt antibody (Fig. 1D). The blot was stripped and reprobed with anti-Akt antibody to ensure equal amount of protein in each lane (Fig. 1E). The results indicate the activation of Akt in response to insulin ex vivo.
PI3K-dependent activation of Akt
To determine whether insulin induced activation of Akt is regulated through PI3K activation, we incubated retinas in organ culture in the presence and absence of PI3K inhibitor LY294002 for 30 min followed by treatment with or without insulin. Retinal proteins were subjected to immunoblot analysis with anti-pAkt antibody (Fig. 1F). The blots were stripped and reprobed with anti-Akt (Fig. 1G) and anti-actin (Fig. 1H) antibodies to ensure equal amount of protein in each lane. The phosphorylation of Akt was reduced when the retinas were incubated with PI3K inhibitor LY294002 prior to the addition of insulin (Fig. 1F), although total Akt levels were similar in both conditions (Fig. 1G). These results suggest that insulin-stimulated Akt activation is mediated through PI3K. To substantiate the biochemical data, we carried out immunohistochemistry to examine the insulin-induced activation of Akt in retinal sections prepared from eye cups that were stimulated with insulin in organ cultures. Dark-adapted rat retinal eye cups were stimulated with or without insulin in culture for 5 min. The retinas were embedded in OCT compound (Sakura, Tokyo, Japan) and 12-μm thick cryo-sections were cut and stained for pAkt immunoreactivity. The results indicated only weak pAkt immunoreactivity in the inner segment in untreated retinas. However, upon addition of insulin, a significant activation of pAkt was observed in the inner segment, outer segment and retinal pigment epithelial cell layers (Fig. 1I). These results further suggest the activation of Akt in response to insulin ex vivo.
Insulin-induced activation of Akt isoforms
There are three Akt isoforms, all of which are expressed in photoreceptors as demonstrated with the advent of single rod cell PCR (Reiter et al. 2003). To determine which isoform is activated in response to insulin, we immunoprecipitated the retinal lysates prepared from insulin stimulated and unstimulated retinas in ex vivo cultures with anti-Akt1, anti-Akt2 and anti-Akt3 antibodies (Fig. 1K). The immune complexes were subjected to immunoblot analysis with pAkt antibody. The results indicate that insulin activates Akt1 and Akt3 isoforms, but not Akt2 (Fig. 1J). Insulin-induced Akt1 activation was much higher compared to Akt3. These results suggest that insulin specifically activates the Akt1 and also induces a weak activation of Akt3 isoforms.
Light-activation of the retinal Akt in vivo
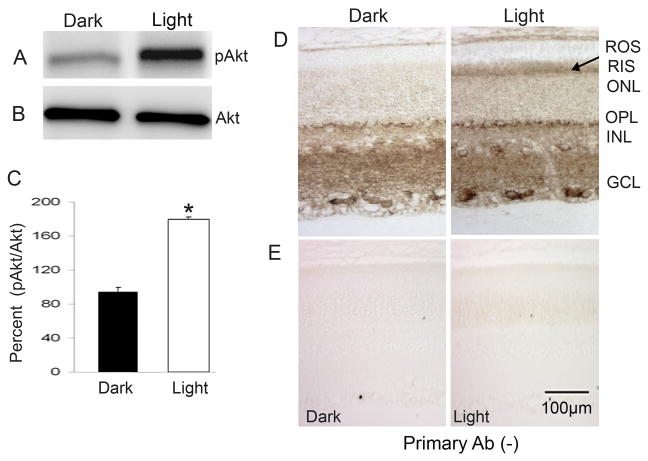
We previously reported that light causes increased tyrosine phosphorylation of the IR in vivo, which leads to the downstream activation of the PI3K in rod photoreceptor cells (Rajala et al. 2002). More recently, we demonstrated that IR activation is mediated through the G-protein-coupled receptor rhodopsin (Rajala et al. 2007). Akt is a downstream effector molecule of IR/PI3K pathway, and we sought to determine whether light also induces the activation of Akt. To determine the light-dependent activation of Akt, we examined pAkt immunoreactivity in retinal lysates from dark- and light-adapted rats. The results indicate an increased level of Akt phosphorylation in light-adapted compared to dark-adapted retinas (Fig. 2A). The blots were stripped and reprobed with total Akt to ensure equal amounts of Akt in both lysates (Fig. 2B). Densitometric analysis of pAkt immunoblot was performed in the linear range of detection and absolute values were then normalized to total Akt (Fig. 2C). These results suggest that activation of Akt is light-induced in vivo. To determine which retinal cells responded to light-induced Akt activation, dark- and light-adapted rat retinal sections stained for pAkt showed significantly increased pAkt immunoreactivity in the rod inner segment layer (Fig. 2D). These results suggest that physiological light induces the activation of Akt in rod photoreceptor cells.
Figure 2.
Light activation of Akt in vivo. Thirty micrograms of dark- and light-adapted rat retina lysate was subjected to immuno blot analysis with pAkt antibody (A). The blot was stripped and reprobed with anti-Akt (B) antibody. Densitometric analysis of pAkt immunoblots was performed in the linear range of detection and absolute values were then normalized to total Akt (C). Data are mean ± SD, (n=4). The dark-adapted control was set as 100 percent. *p<0.001. Immunocytochemical analysis of pAkt in dark- and light-adapted rat retinas. Paraffin-fixed sections of dark- and light-adapted (30 min) rat retinas were stained for pAkt (D). The pAkt signal was developed with 3′,3′-diaminobenzidine (Dako) as chromogen (Tanito et al. 2007). The arrow indicates the light-induced activation of Akt in the rod inner segment. Antibody staining was blocked with omitting primary antibody (E). ROS, rod outer segments; RIS, rod inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer
Specificity of light-induced activation of Akt in vivo
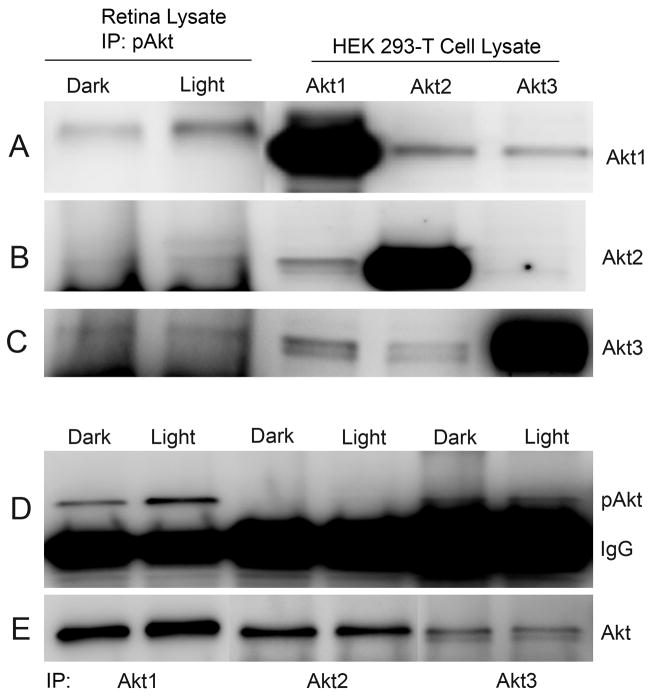
To identify which Akt isoform is activated in response to normal physiological light stimulation, we immunoprecipitated pAkt from dark- and light-adapted retinas and carried out immunoblot analysis, probing with anti-Akt1, anti-Akt2, and anti-Akt3 antibodies. The results indicate an increased amount of Akt1 was recovered from light-adapted rat retina lysates immunoprecipitated with the pAkt antibody (Fig. 3A). A weak recovery of Akt2 from light-adapted rat retina lysates was also observed with pAkt antibody (Fig. 3B), and there was no difference in the recovery of Akt3 between dark- and light-adapted retinas (Fig. 3C). We observed a mobility shift of Akt1 in the immunoprecipitates and this could be due to the phosphorylation state of the protein. The results suggest that Akt1 may be the major light-activatable isoform in the inner segment. To confirm the specificity of Akt isoform specific antibodies, we expressed Akt1, Akt2, and Akt3 in HEK-293T cells and subjected lysates to immunoblot analysis with anti-Akt1, anti-Akt2, and anti-Akt3 antibodies. The results indicate no cross reactivity between the individual isoforms, which further attests to the selectivity of the antibodies towards each isoform (Fig. 3A–C). To further confirm that Akt1 is activated in response to light stimulation, we immunoprecipitated the isoforms with anti-Akt1, anti-Akt2, and anti-Akt3 antibodies, followed by immunoblot analysis with anti-pAkt antibody. The results indicate increased pAkt immunoreactivity in light-adapted retina lysates that were immunoprecipitated with anti-Akt1 antibody (Fig. 3D) and show that light induced the activation of Akt1 in rod photoreceptors. The blot was stripped and reprobed with anti-Akt1, anti-Akt2, and anti-Akt3 antibodies to ensure equal amount of protein in each lane (Fig. 3E). Collectively these experiments clearly suggest that Akt1 is the major light-activatable isoform in the rod photoreceptors.
Figure 3.
Light-dependent activation of Akt1 in vivo. Retina lysates from dark- and light-adapted rats were immunoprecipitated with pAkt antibody followed by immuno blot analysis with anti-Akt1 (A), anti-Akt2 (B) and anti-Akt3 (C) antibodies. Five micrograms of recombinant Akt1 (A), Akt2 (B), and Akt3 (C) expressed in HEK-293T cells were subjected to immuno blot analysis with anti-Akt1, anti-Akt2 and anti-Akt3 antibodies. Retina lysates from dark- and light-adapted rats were immunoprecipitated with anti-Akt1, anti-Akt2 and anti-Akt3 antibodies followed by immunoblot analysis with anti-pAkt antibody (D). The blot was stripped and reprobed with anti-Akt antibody to ensure equal amount of protein in each immunoprecipitate (E).
In vivo Akt1 binding to ROS is IR/PI3K-dependent
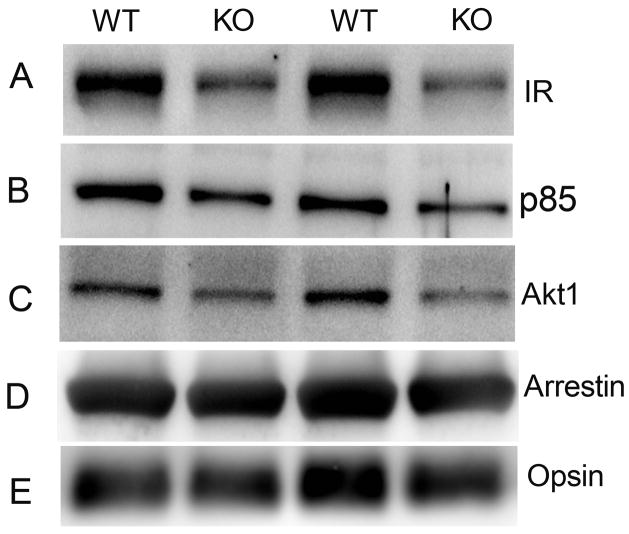
Experiments described in Figures 2 and 3 do not confirm whether the light-dependent activation of Akt1 is IR/PI3K-dependent. To address this question, we used the Cre/lox system to specifically inactivate the IR gene in rod photoreceptors. This was achieved by mating rod-expressing Cre mice and floxed IR mice to generate conditional IR knockout mice (Rajala et al. 2008). For this study, we used the 0.2-kb mouse opsin promoter-controlled Cre recombinase mouse line (Le et al. 2006). We recently reported the reduced binding of p85 subunit of PI3K and total Akt to ROS membranes prepared from IR knockout mouse retinas (Rajala et al. 2008). To support our current study that Akt1 membrane binding is IR/PI3K-dependent, we have done similar experiments using different mice born of different mothers and of different generations. To confirm the conditional deletion of IR, we examined the levels of IR expression and also the binding of p85 subunit of PI3K as a positive readout by immunoblot analysis with anti-IRβ (Fig. 4A) and anti-p85 (Fig. 4B) antibodies. The results indicate reduced levels of IR resulted in reduced binding of p85 to ROS membranes prepared from IR knockout mouse retinas compared to wild type. These results support our previously reported findings (Rajala et al. 2008). However, in the present study, it was necessary to again demonstrate the level of IR and p85 binding since different lines of conditional IR knockout mice can give different levels of recombination (different levels of IR deletion) and to ensure that these animals were true conditional IR knockouts.
Figure 4.
Binding of Akt1 to ROS in photoreceptor-specific IR knockout mice. Five micrograms of protein from two independent ROS preparations from wild-type and IR knockout mouse retinas (5 mice from each group) were subjected to immuno blot analysis with anti-IRβ (A), anti-p85 (B), anti-Akt1 (C), anti-arrestin (D) and anti-opsin (E) antibodies.
The IR-depleted ROS membranes were examined for the binding of different Akt isoforms. ROS membranes from wild type and conditional IR knockout mice were analyzed by using immunoblot analysis with anti-Akt1, anti-Akt2, and anti-Akt3 antibodies. The levels of Akt1 in ROS from IR knockout mouse line were less than that of wild-type control mice (Fig. 4C). No binding of Akt2 and Akt3 to ROS was observed in either wild type or IR knockout mouse retinas (data not shown). These results suggest that Akt1 binding to ROS is IR/PI3K-dependent in vivo. The photoreceptor specific proteins arrestin (Fig. 4D) and opsin (Fig. 4E) were used as loading control for ROS membranes. Collectively, these results clearly suggest that decreased IR expression in ROS from conditional IR knockout mice leads to reduced recruitment of p85 and Akt1 to ROS membranes. This reduced recruitment may be due to decreased activation of Akt1.
PH Domain Mediates Akt Interaction with Phosphoinositides
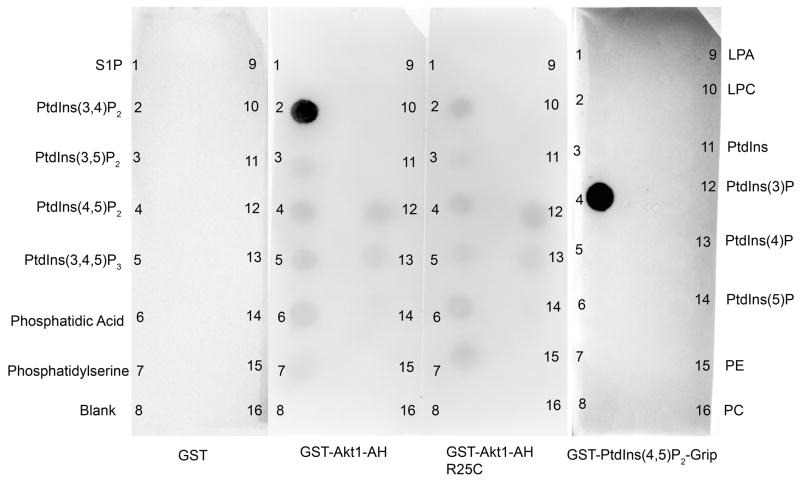
To evaluate the binding specificity of Akt PH domain to various phospholipids, we employed a protein-lipid overlay assay using membranes that had been spotted with equal amounts (100 pmol) of different phospholipids. Purified GST fusion proteins containing the AH domain of Akt1 (PH domain containing the linker domain (Fig. 7A), a mutant AH domain of Akt1 (R25C) which fails to interact with phosphoinositides, Akt1, Akt2, Akt3, PIP2 Grip (PLC-d1 PH) which binds to PtdIns (4,5) P2 (positive control supplied by the Echelon), or GST alone were incubated with the membrane strips as described under Experimental Procedures. After washing, the bound GST fusion proteins were detected by immunoblot analysis with anti-GST antibody. The GST control did not bind to any of the phospholipids (Fig. 5) while GST-AH domain of Akt1 (Fig. 5), GST-Akt1, GST-Akt2 and GST-Akt3 bound only to PtdIns (3,4) P2 (data not shown). The mutant AH domain of Akt1 (R25C) failed to bind to PtdIns (3,4) P2 (Fig. 5). The positive control PIP2 Grip (PLC-d1 PH domain) binds only to PtdIns (4,5) P2 (Fig. 5) further attesting to the specificity of binding. These results indicate that Akt isoforms preferentially interact with D3-phosphoinositides via their PH domains. These results further confirm that Akt is a phospholipid binding protein.
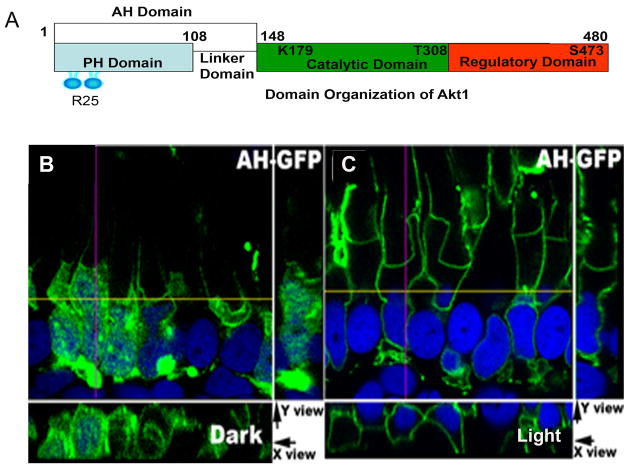
Figure 7.
Membrane binding of AH-Akt1 domain. Domain organization of Akt1 (1–480 amino acids) shows the N-terminal PH domain followed by linker domain (AH domain, 1–148 amino acids), Arginine 25 is the key residue involved in the interaction with the phosphoinositides, catalytic domain containing the ATP binding site (K179) and threonine 308 phosphorylation site followed by regulatory subunit containing serine 473 phosphorylation site (A). An AH-Akt1-GFP construct was used to generate the transgenic Xenopus (AH-GFP). Tadpoles were dark-adapted overnight (B) or dark adapted overnight and then light-adapted for 30 min (C). Experiments were done using both primary injection and the F1 offspring, at least 5 tadpoles for each group, and representative images are shown.
Figure 5.
Phospholipid binding specificities of AH domain of Akt1. Five micrograms of purified proteins of GST or GST-AH-Akt1 or GST-AH-Akt1 (R25C) or GST-Grip (PLC-d1 PH domain) were incubated with nitrocellulose strips that had been spotted with various phospholipids. The bound proteins were detected by immunoblot analysis using anti-GST antibody. 1) S1P, spingosine 1 phosphate; 2) PtdIns(3,4)P2, phosphatidylinositol 3,4-diphosphate; 3) PtdIns(3,5)P2, phosphatidylinositol 3,5-diphosphate; 4) PtdIns(4,5)P2, phosphatidylinositol 4,5-diphosphate; 5) PtdIns(3,4,5)P2, phosphatidylinositol 3,4,5-triphosphate; 6) PA, phosphatidic acid; 7) PS phosphatidylserine; 8) blank; 9) LPA, lysophosphatidic acid; 10) LPC, lysophosphatidylcholine; 11) PtdIns, phosphatidylinositol; 12) PtdIns(3)P, phosphatidylinositol 3-phosphate; 13) PtdIns(4)P, phosphatidylinositol 4-phosphate; 14) PtdIns(5)P, phosphatidylinositol 5-phosphate; 15) PE phosphatidylethanolamine; 16) PC, phosphatidylcholine.
GFP distribution in both dark and light conditions in a transgenic Xenopus model
Low expression levels of Akt isoforms (Rajala, unpublished results) precluded the detection of any possible light-induced changes in the Akt subcellular compartmentalization in rat retina. Therefore, studying the Akt membrane binding is difficult under these conditions and a better approach was to use frog technology to study protein targeting in photoreceptors (Tam et al. 2000;Luo et al. 2004;Peterson et al. 2003). Generating transgenic mouse lines with Akt is time-consuming; however, a transgenic frog approach would be ideal to test the protein targeting in a shorter time. Therefore, a transgenic Xenopus model was used to study the membrane binding and activation of Akt1, using a full-length Akt1-GFP (fAkt1-GFP) fusion construct. To make sure that GFP alone does not translocate in response to light, a GFP construct driven by a Xenopus rod-specific opsin promoter was used to generate transgenic frogs (Tam et al. 2000). After each injection, 30–50 tadpoles were positive out of the thousands of eggs injected. GFP fluorescence was first detected between post-fertilization day (pfd) 5–7, and the tadpoles were used for experiments at pfd 15 (stage 47/48). GFP-positive transgenic tadpoles were dark-adapted overnight and half were exposed to normal light for 30 minutes, and both were then analyzed for GFP fluorescence. The GFP expression was exclusively cytoplasmic and no significant membrane binding of GPF was detected in the light-adapted retains compared with dark-adapted retinas (data not shown).
Light-stimulated activation and membrane binding of Akt1 in a transgenic Xenopus model
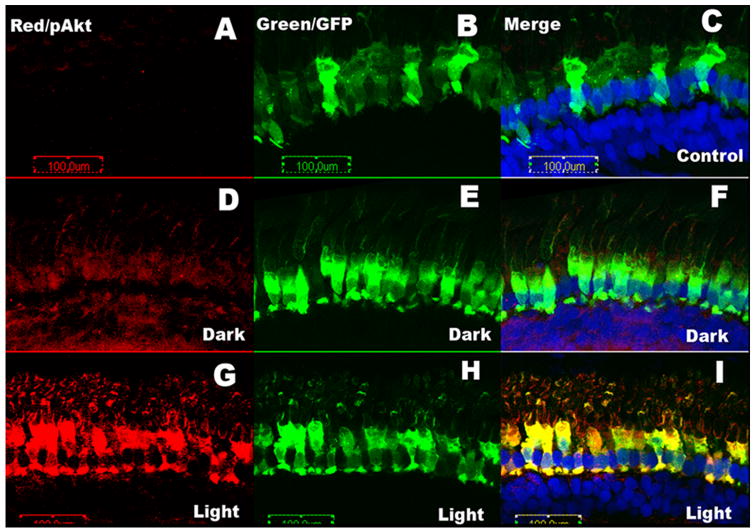
After determining that GFP alone does not change in its subcellular compartmentalization in response to light, the transgenic Xenopus model was used to study the membrane binding and activation of Akt1 using a fAkt1-GFP fusion construct. fAkt1 was inserted to the C-terminus of GFP and expression of the Akt1-GFP fusion proteins was driven by a Xenopus rod-specific opsin promoter (Tam et al. 2000). The tadpoles were used for experiments at pfd 15 (stage 47/48). GFP-positive transgenic tadpoles were dark-adapted overnight; half were exposed to normal light for 30 min, after which both groups were analyzed for GFP fluorescence. Tadpoles from both the primary injection and F1 offspring were used, and all showed similar patterns of fAkt1-GFP expression. No morphological differences between wild type and fAkt1 transgenic tadpoles were detected. To study the activation of Akt, pAkt antibody was used on the dark- and light-adapted Xenopus retinal sections. Control experiments were carried out with GFP expressed tadpoles. We failed to observe the pAkt signal in control GFP under dark- and light-adapted conditions (Fig. 6A). The pAkt immunofluorescence (red) was higher in the retinas from light-adapted tadpoles (Figure 6G and I) than in the retinas from the dark-adapted tadpoles (Fig. 6D and F). The GFP (green) was used to locate the exogenous Akt1 (Fig. 6B, E, H), while the red indicated where pAkt signal localized (Figure 6A, D and G). A very strong colocalization was observed in the light-adapted rods expressing fAkt1-GFP (Fig. 6I) compared to dark-adapted rods (Fig. 6F). We noticed weak pAkt immunoreactivity in dark-adapted retinas (Fig. 6D) compared to control retinas (Fig. 6A), suggesting a weak activation of exogenously expressed fAkt. No significant membrane binding of the Akt1 was detected in the light-adapted retinas compared with the dark-adapted retinas.
Figure 6.
Membrane binding and activation of Akt1 after light stimulation in transgenic Xenopus. After dark adaptation overnight, half of the animals were maintained in the dark, or exposed to light for 30 min. pAkt was immunolocalized (red channel) in dark-adapted tadpoles or light-adapted tadpoles. Nuclei were stained with a blue nuclear dye (DAPI). pAkt was co-localized with the Akt1-GFP fusion protein in photoreceptors in both the dark-and light-adapted groups. More intense staining of pAkt was observed in the light-adapted group.
Light-stimulated membrane binding of AH domain of Akt1 in transgenic Xenopus
Although we did not detect any membrane binding of the fAkt1-GFP fusion protein, our earlier immunoblot analyses of rodent retinas and immunohistochemistry of retinas from both rodents (Fig. 2 and 3) and fAkt1 transgenic Xenopus (Fig. 6) support the concept of Akt1 activation by light. We believe that the reason that we were not able to detect the membrane binding of fAkt1 after light stimulation is due to the dynamic process of Akt binding and release from the plasma membrane. Because the activation of Akt is responsible for its conformation change and subsequent release from the membrane, we generated transgenic Xenopus with Akt1 AH-GFP fusion protein, which consists of the PH domain and a linker domain but not the catalytic and regulatory domains (Fig. 7A). The reason we used AH domain instead of PH domain is because of the similarity of Akt isoform PH domains. Using PH domains alone cannot distinguish the specific isoforms. Therefore, the AH domain, which is composed of the PH domain plus the linker domain between the PH domain and the catalytic domain was used (Fig. 7A). The resulting transgenic AH-Akt1 Xenopus only reflected changes in Akt1, but not Akt2 or Akt3. We observed that in dark-adapted animals, the AH-GFP was present in the cytosol as well as on plasma membranes (Fig. 7B). However, in the light-adapted animals, the AH-GFP was localized mainly to plasma and nuclear membranes (Fig. 7C). These results suggest that phosphoinositides are generated in rod membranes in a light-dependent manner and that the AH domain of Akt1 binds to these membranes.
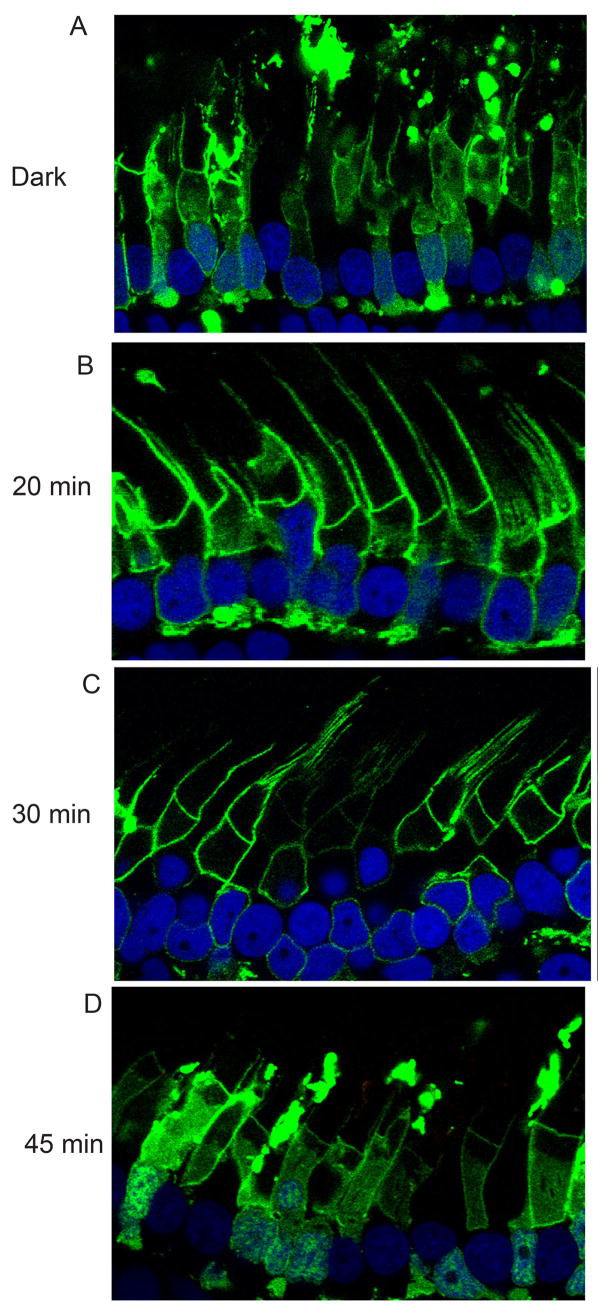
To determine the time-dependent membrane binding of the AH domain of Akt1-GFP in transgenic Xenopus, we examined the membrane binding of the AH domain fusion in darkness and at 20, 30, and 45 min after light-adaptation. We observed that in the dark-adapted animals, the AH-GFP was present in the cytosol as well as on plasma membranes (Fig. 8A). In the light-adapted animals, after 20 min, more AH-GFP was localized to plasma membranes and less to the cytosol (Fig. 8B) and at 30 min, the majority of AH-GFP was the plasma membrane-localized (Fig. 8C). Forty-five minutes after light-adaptation, the AH-GFP was mainly localized in the cytosol (Fig. 8D). These experiments suggest that the change in the AH-GFP localization may be due the change in the phosphoinositide concentration in the plasma membrane.
Figure 8.
Time-dependent membrane binding of AH-Akt1 domain. An AH-Akt1-GFP construct was used to generate the transgenic Xenopus (AH-GFP). Tadpoles were dark-adapted overnight (A) or dark adapted overnight and then light-adapted for 20 (B), 30 (C) and 45 (D) min. Experiments were done using both primary injection and the F1 offspring, at least 5 tadpoles for each group, and representative images are shown.
Light-dependent generation of phosphoinositides regulate the membrane binding of AH domain of Akt1 in transgenic Xenopus
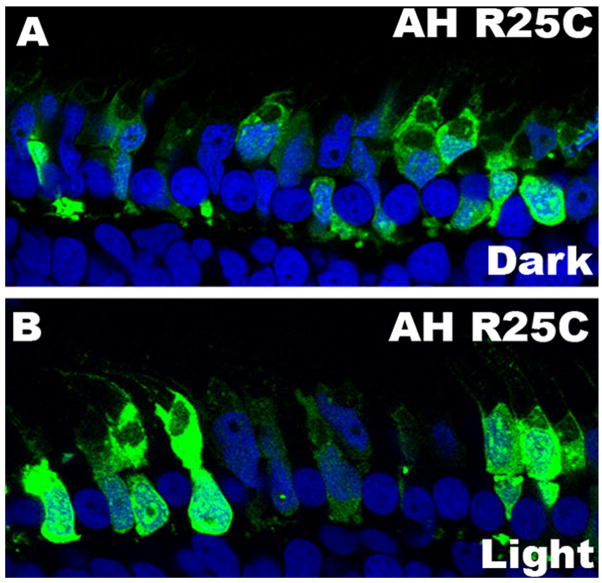
The PH domain of the Akt family is well known for its binding to phospholipids (Watton and Downward 1999;Frech et al. 1997). Indeed, the high affinity of the PH domain of Akt to the lipid made it such a valuable tool that the researchers have been using the Akt PH-GFP to localize PtdIns (3,4) P2 and PtdIns (3,4,5)P3, instead of the lipid antibodies, in both cell culture system and transgenic mouse models (Nishio et al. 2007;Gassama-Diagne et al. 2006;Varnai et al. 2005). To determine whether the Akt1 AH domain membrane binding is dependent on the light-dependent generation of phosphoinositides in the transgenic Xenopus model, we generated transgenic Xenopus with a mutant Akt1 AH domain, the R25C mutant. The AH R25C mutant introduces a cysteine to replace the arginine at position 25 and causes a loss of affinity to PtdIns (3,4)P2 or PtdIns (3,4,5)P3 (Franke et al. 1995;Thomas et al. 2002). We observed that the fluorescence distribution pattern of the Akt1 AH R25C-GFP transgenic tadpoles was different from that of the AH-GFP tadpoles. In the Akt1 AH R25C tadpoles, no binding to the membrane was seen (Fig. 9). We also did not find any difference between the dark and light condition in the distribution of the AH R25C-GFP in photoreceptors (Fig.9A and B). These results suggest that the membrane interaction of the Akt1 AH domain is dependent on the light-induced generation of phosphoinositides.
Figure 9.
Membrane binding of AH R25C-Akt1 domain. An AH R25C-Akt1-GFP construct was used to generate the transgenic Xenopus (AH-GFP). Tadpoles were dark-adapted overnight (A) or dark adapted overnight and then light-adapted for 30 min (B). Protein localization was visualized using the endogenous fluorescence of GFP. Experiments were done using at least 5 tadpoles for each group, and representative images are shown.
Light-dependent generation of phosphoinositides reorganizes actin cytoskeleton
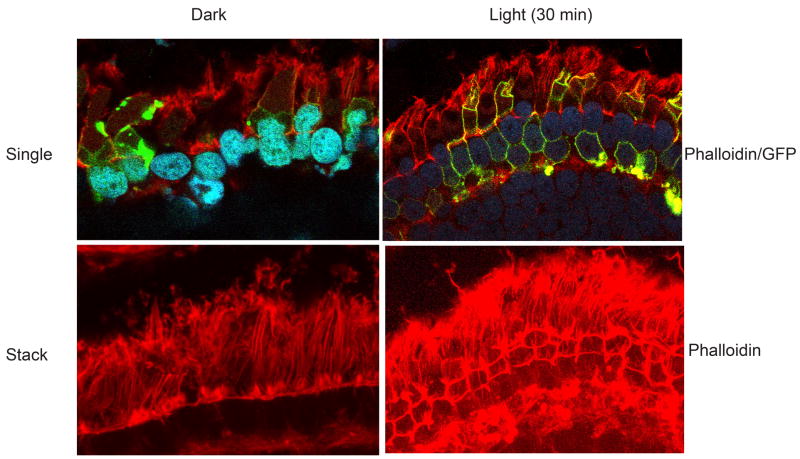
Phosphoinositdies are known to reorganize the actin cytoskeleton (Takenawa and Itoh 2001). To determine whether the light-dependent generated phosphoinositides regulate the actin cytoskeleton, we stained the dark- and light-adapted transgenic tadpole retinal sections with rhodamine phalloidin. Our results indicate that the GFP-Akt1-AH domain co localizes with the actin cytoskeleton under light-adapted conditions (Fig. 10). In dark-adapted retinas the actin appears to be stress fiber phenotype and in response to light we could observe the reorganization of the actin cytoskeleton (Fig. 10). These results suggest that light generated PI3K products facilitate the actin cytoskeletal reorganization.
Figure 10.
Light-dependent generation of phosphoinositides reorganize actin cytoskeleton. Tadpoles were dark-adapted overnight or dark adapted overnight and then light-adapted for 30 min. Eye tissues were fixed and the sections were stained with rhodamine phalloidin (red) and visualized the AH-Akt1 domain using the endogenous fluorescence of GFP. Representative single (phalloidin/GFP) and stack (Phalloidin) images are shown.
DISCUSSION
Growth factor-induced activation of Akt has been shown in transformed retinal neurons (Barber et al. 2001). In this study we made a novel finding that Akt1, but not Akt2 or Akt3 was activated by normal room light. These findings were further confirmed by using a transgenic Xenopus model of the full-length Akt1 and the AH domain of the Akt1. The transgenic frogs with the AH domain of Akt1 showed the translocation from cytosol to plasma membrane upon light activation. Our studies also suggest that the membrane binding of Akt1 is dependent on the light-induced generation of phosphoinositides.
In the retina, Reiter et al (Reiter et al. 2003) showed that only Akt1 responded to insulin, suggesting that IR activation leads to the activation of Akt1. In this study, we also observed the Akt1 activation in response to insulin ex vivo. Furthermore, the insulin-induced activation of Akt was completely blocked by a PI3K inhibitor, suggesting that the activation of Akt is dependent on the activation of PI3K, the downstream effector of the IR (Reiter et al. 2003;Rajala et al. 2004). Similar to insulin, light also induces the activation of IR which results in the activation of PI3K (Rajala et al. 2002;Rajala et al. 2007) and Akt1. The in vivo membrane binding of Akt1 is IR/PI3K-dependent as photoreceptor specific IR knockout retinas exhibited reduced binding of Akt1 to ROS membranes. Further, ex vivo retinal organ cultures in the presence of LY294002 failed to activate Akt in response to light (data not shown). These experiments clearly suggest that light activation and membrane binding of Akt1 is IR/PI3K-dependent.
The binding of Akt to the plasma membrane upon growth factor stimulation has been well studied (Andjelkovic et al. 1997;Meier et al. 1997;Wijkander et al. 1997;Zhang and Vik 1997;Sable et al. 1998;Currie et al. 1999). In this study we have not directly measured the light induced generation of phosphoinositidies in vivo and it is very difficult to measure the endogenous levels of 3′-phosphoinositides from photoreceptor membranes. Although it may be straightforward to directly measure membrane bound 3′-phosphoinositides from cell lines in culture, we are dealing with a complex tissue containing at least 7 types of retinal cells. As a result, it is not trivial to establish which cell type may generate phosphoinositides in vivo. With the exception of the rod outer segment, we cannot isolate a pure membrane fraction from the entire photoreceptor cell. As a result, we decided to express fluorescently-tagged phosphoinositide probes in photoreceptors, in vivo, to both study the interaction of Akt with photoreceptor membranes and to gain insight into the phosphoinositide species generated in response to light exposure. Fluorescently-tagged protein domains (e.g., PH domains) as phosphoinositide sensors have been used by a number of independent laboratories (Halet 2005;Lorenzo et al. 2005;Dormann et al. 2004;Pattni et al. 2001;Rusten and Stenmark 2006) and we applied this approach to an in vivo model to demonstrate the light-dependent generation of 3′-phosphoinositides specifically in photoreceptor cells using the Akt1-AH domain as the cellular probe. The light-dependent binding of this probe coupled with its in vitro binding affinity for 3′-phosphoinositides strongly suggest that PtdIns(3,4)P2 is generated in photoreceptor membranes in response to light exposure. Identification and quantitation of these phosphoinositide species in response to light in vivo would further advance our understanding of the role of individual phosphoinositides in the regulation of specific phospholipid-binding proteins.
In the present study, we showed that the AH domain translocated to the plasma membrane after light stimulation. This membrane binding was lipid dependent as the AH R25C-GFP lost its ability to bind to the plasma membrane independent of light status. Arginine 25 had been shown to be essential for Akt binding to lipid both by biochemical and structural studies (Franke et al. 1995;Thomas et al. 2002). We did not observe the membrane binding phenomenon in the fAkt1 transgenic tadpoles, for which there may be several explanations. First, limitations of the technique may be inadequate to detect the subtle changes of Akt1. Second, Akt1 may move to the membrane, become activated, and then leave the membrane, which had been shown in various studies (Downward 1998). Consistent with these studies, we also found the activation of Akt1 in the cytosol of transgenic Xenopus photoreceptors. Therefore, after phosphorylation of Akt1 by the PDK1 at the membrane, a conformational change occurs (Milburn et al. 2003), and Akt1 detaches from the membrane, leaving less Akt1 on the membrane at the time of observation. In contrast, the AH domain without the catalytic domain will not release from the plasma membrane. Our detection of a difference with the AH domain could be due to its lack of a catalytic domain. Further, our time course experiments suggest that dissociation of AH-GFP from the membranes is due to a decrease in the concentration of phosphoinositides in the membranes. Our studies with the AH domain and the mutant AH domain of Akt1 and the lipid overlay assays clearly suggest the light-dependent generation of phosphoinositides, probably PtdIns (3,4)P2 in ROS membranes through the action of PI3K. Regardless of the activation of Akt, the AH domain of Akt could be used as a probe to detect the light-dependent generation of phosphoinositides. In the retina all three Akt isoforms are expressed (Reiter et al. 2003;Li et al. 2007), but only the Akt1 activation is light-dependent. This differential activation could be explained based on the recently identified isoform specific phosphatases, PHLPP1 and PHLPP2 (Brognard et al. 2007). Both Akt isoform specific phosphatases are expressed in the retina (Rajala, unpublished observation). Further studies are required to understand the role of PHLPP1 and PHLPP2 on isoform specific regulation of Akt in the retina.
PI3K-generated phosphoinositides are involved in the membrane targeting of rhodopsin as well as the disc morphogenesis in mammalian photoreceptors (Chuang et al. 2007). In retina, deletion of two phosphoinositide binding proteins, Akt2 (Li et al. 2007) and IRS-2 (Yi et al. 2005) results in photoreceptor degeneration. Drosophila arrestin has a phosphoinositide lipid binding domain that binds PtdIns (3,4,5)P3 and controls the movement of arrestin (Lee et al. 2003). Myosin II binds to the PH domain of Akt (Tanaka et al. 1999), which raises interesting possibilities, since myosin VIIA gene is mutated in Usher syndrome 1B patients (Weil et al. 1995). Modulation of phototransduction gain by changes in phosphoinositide levels have also been reported (He et al. 2004). All these studies underscore the importance of phosphoinositides in photoreceptor structure and function.
Phosphoinositides are the key molecules for regulation of actin cytoskeletal organization and membrane traffic from plasma membrane (Takenawa and Itoh 2001). It is interesting to note in this study that light-dependent generated phosphoinositides appears to regulate the actin cytoskeleton. Consistent with this observation, we also observed reduced ROS associated actin (presumably F-actin) in IR knockout mouse retinas compared to wild type mouse retinas (data not shown). These observations along with our current findings suggest that light-induced activation of IR/PI3K may regulate the actin cytoskeletal organization. Dysfunction of the actin cytoskeleton is a key event in the pathogenesis of diabetic nephropathy (Conway et al. 2004), diabetic neuropathy (McLean 1997;McLean et al. 1995) and diabetic cardiomyopathy (Olson et al. 1998;Fein et al. 1984). The light-induced activation of IR/PI3K-generated phosphoinositides in the retina might play an important role in the control of diabetic retinopathy.
Activation of Akt has been shown to have roles in cell growth (Downward 1998), cell survival (Lawlor and Alessi 2001), glucose metabolism (Whiteman et al. 2002;Brazil and Hemmings 2001) and cell polarity (Yoshimura et al. 2006;Gartner et al. 2006;Arimura and Kaibuchi 2005;Menager et al. 2004) in a variety of cell types. The function of the light-dependent activation of Akt1 in the retina is not known. In retinal photoreceptor cells, Akt1 activity is driven by light, which indicates that it may have some functions involved in the light-induced, rod-specific activities such as photoreceptor outer segment disk shedding (Basinger et al. 1976), biogenesis of new ROS membranes (Hollyfield and Rayborn 1979), light adaptation, or assisting the movement of other proteins (Muresan et al. 1993). Moreover, since Akt has been shown to be involved in neuronal cell polarity and the growth of axons, the light-driven activation of Akt1 may be involved in the maintenance the polarity of photoreceptors, which are highly polarized cells. Akt1 may also regulate axon formation or connection to transduce the light signal from the peripheral to central nervous system and participate in the formation of vision. These speculations require further studies involving knockout mice to understand the light-dependent activation of Akt1 in photoreceptor functions.
Acknowledgments
The authors are grateful to Shannon Brazeal and Mindy Pidek (University of Oklahoma Health Science Center) for generating and maintaining the transgenic Xenopus Laevis colonies used in this study. We thank Dr. Michael Elliott for the critical review of the manuscript. We thank Dr. Morris Birnbaum (University of Pennsylvania) for his generous gift of mammalian expression constructs of Akt. We also thank Dr. David Papermaster (University of Connecticut) for providing Xenopus opsin constructs. The authors also thank Dr. Masaki Tanito for his help in the immunohistochemistry. This study was supported by grants from the National Institutes of Health (EY016507, EY00871, and NEI Core grant EY12190); Research to Prevent Blindness, Inc.
ABBREVIATIONS
- PKB
protein kinase B
- PI3K
phosphoinositide 3-kinase
- IR
insulin receptor
- ROS
rod outer segments
- PDK1
phosphoinositide-dependent protein kinase 1
- SDM
site-directed mutagenesis
- PH
pleckstrin homology
- GFP
green fluorescent protein
References
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Arimura N, Kaibuchi K. Key regulators in neuronal polarity. Neuron. 2005;48:881–884. doi: 10.1016/j.neuron.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem. 2001;276:32814–32821. doi: 10.1074/jbc.M104738200. [DOI] [PubMed] [Google Scholar]
- Basinger S, Hoffman R, Matthes M. Photoreceptor shedding is initiated by light in the frog retina. Science. 1976;194:1074–1076. doi: 10.1126/science.1086510. [DOI] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgatti P, Martelli AM, Bellacosa A, Casto R, Massari L, Capitani S, Neri LM. Translocation of Akt/PKB to the nucleus of osteoblast-like MC3T3-E1 cells exposed to proliferative growth factors. FEBS Lett. 2000;477:27–32. doi: 10.1016/s0014-5793(00)01758-0. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Brodbeck D, Cron P, Hemmings BA. A human protein kinase Bgamma with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain. J Biol Chem. 1999;274:9133–9136. doi: 10.1074/jbc.274.14.9133. [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Carvalho E, Eliasson B, Wesslau C, Smith U. Impaired phosphorylation and insulin-stimulated translocation to the plasma membrane of protein kinase B/Akt in adipocytes from Type II diabetic subjects. Diabetologia. 2000;43:1107–1115. doi: 10.1007/s001250051501. [DOI] [PubMed] [Google Scholar]
- Chuang JZ, Zhao Y, Sung CH. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell. 2007;130:535–547. doi: 10.1016/j.cell.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffer PJ, Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- Conway BR, Maxwell AP, Savage DA, Patterson CC, Doran PP, Murphy M, Brady HR, Fogarty DG. Association between variation in the actin-binding gene caldesmon and diabetic nephropathy in type 1 diabetes. Diabetes. 2004;53:1162–1165. doi: 10.2337/diabetes.53.4.1162. [DOI] [PubMed] [Google Scholar]
- Currie RA, Walker KS, Gray A, Deak M, Casamayor A, Downes CP, Cohen P, Alessi DR, Lucocq J. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J. 1999;337(Pt 3):575–583. [PMC free article] [PubMed] [Google Scholar]
- Dormann D, Weijer G, Dowler S, Weijer CJ. In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J Cell Sci. 2004;117:6497–6509. doi: 10.1242/jcs.01579. [DOI] [PubMed] [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- Fein FS, Malhotra A, Miller-Green B, Scheuer J, Sonnenblick EH. Diabetic cardiomyopathy in rats: mechanical and biochemical response to different insulin doses. Am J Physiol. 1984;247:H817–H823. doi: 10.1152/ajpheart.1984.247.5.H817. [DOI] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Frech M, Andjelkovic M, Ingley E, Reddy KK, Falck JR, Hemmings BA. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272:8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- Gartner A, Huang X, Hall A. Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3beta) independently of Akt/PKB serine phosphorylation. J Cell Sci. 2006;119:3927–3934. doi: 10.1242/jcs.03159. [DOI] [PubMed] [Google Scholar]
- Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol. 2006;8:963–970. doi: 10.1038/ncb1461. [DOI] [PubMed] [Google Scholar]
- Halet G. Imaging phosphoinositide dynamics using GFP-tagged protein domains. Biol Cell. 2005;97:501–518. doi: 10.1042/BC20040080. [DOI] [PubMed] [Google Scholar]
- He F, Mao M, Wensel TG. Enhancement of phototransduction g protein-effector interactions by phosphoinositides. J Biol Chem. 2004;279:8986–8990. doi: 10.1074/jbc.M311488200. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG, Rayborn ME. Photoreceptor outer segment development: light and dark regulate the rate of membrane addition and loss. Invest Ophthalmol Vis Sci. 1979;18:117–132. [PubMed] [Google Scholar]
- Jones PF, Jakubowicz T, Hemmings BA. Molecular cloning of a second form of rac protein kinase. Cell Regul. 1991a;2:1001–1009. doi: 10.1091/mbc.2.12.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A. 1991b;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Whitman M, Schaffhausen B, Pallas DC, White M, Cantley L, Roberts TM. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987;50:1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- Le YZ, Zheng L, Zheng W, Ash JD, Agbaga MP, Zhu M, Anderson RE. Mouse opsin promoter-directed Cre recombinase expression in transgenic mice. Mol Vis. 2006;12:389–398. [PubMed] [Google Scholar]
- Lee SJ, Xu H, Kang LW, Amzel LM, Montell C. Light adaptation through phosphoinositide-regulated translocation of Drosophila visual arrestin. Neuron. 2003;39:121–132. doi: 10.1016/s0896-6273(03)00390-8. [DOI] [PubMed] [Google Scholar]
- Li G, Anderson RE, Tomita H, Adler R, Liu X, Zack DJ, Rajala RV. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J Neurosci. 2007;27:203–211. doi: 10.1523/JNEUROSCI.0445-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Urbe S, Clague MJ. Analysis of phosphoinositide binding domain properties within the myotubularin-related protein MTMR3. J Cell Sci. 2005;118:2005–2012. doi: 10.1242/jcs.02325. [DOI] [PubMed] [Google Scholar]
- Luo W, Marsh-Armstrong N, Rattner A, Nathans J. An outer segment localization signal at the C terminus of the photoreceptor-specific retinol dehydrogenase. J Neurosci. 2004;24:2623–2632. doi: 10.1523/JNEUROSCI.5302-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masure S, Haefner B, Wesselink JJ, Hoefnagel E, Mortier E, Verhasselt P, Tuytelaars A, Gordon R, Richardson A. Molecular cloning, expression and characterization of the human serine/threonine kinase Akt-3. Eur J Biochem. 1999;265:353–360. doi: 10.1046/j.1432-1327.1999.00774.x. [DOI] [PubMed] [Google Scholar]
- McLean WG. The role of axonal cytoskeleton in diabetic neuropathy. Neurochem Res. 1997;22:951–956. doi: 10.1023/a:1022466624223. [DOI] [PubMed] [Google Scholar]
- McLean WG, Roberts RE, Mullins FH. Post-translational modifications of microtubule- and growth-associated proteins in nerve regeneration and neuropathy. Biochem Soc Trans. 1995;23:76–80. doi: 10.1042/bst0230076. [DOI] [PubMed] [Google Scholar]
- Meier R, Alessi DR, Cron P, Andjelkovic M, Hemmings BA. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J Biol Chem. 1997;272:30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- Menager C, Arimura N, Fukata Y, Kaibuchi K. PIP3 is involved in neuronal polarization and axon formation. J Neurochem. 2004;89:109–118. doi: 10.1046/j.1471-4159.2004.02302.x. [DOI] [PubMed] [Google Scholar]
- Milburn CC, Deak M, Kelly SM, Price NC, Alessi DR, van Aalten DM. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem J. 2003;375:531–538. doi: 10.1042/BJ20031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan V, Joshi HC, Besharse JC. Gamma-tubulin in differentiated cell types: localization in the vicinity of basal bodies in retinal photoreceptors and ciliated epithelia. J Cell Sci. 1993;104(Pt 4):1229–1237. doi: 10.1242/jcs.104.4.1229. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Nakatani K, Sakaue H, Thompson DA, Weigel RJ, Roth RA. Identification of a human Akt3 (protein kinase B gamma) which contains the regulatory serine phosphorylation site. Biochem Biophys Res Commun. 1999;257:906–910. doi: 10.1006/bbrc.1999.0559. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P, Farber J, editors. Normal Tables of Xenopus laevis. North Holland Publishing; Amsterdam: 1956. [Google Scholar]
- Nishio M, Watanabe K, Sasaki J, Taya C, Takasuga S, Iizuka R, Balla T, Yamazaki M, Watanabe H, Itoh R, Kuroda S, Horie Y, Forster I, Mak TW, Yonekawa H, Penninger JM, Kanaho Y, Suzuki A, Sasaki T. Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nat Cell Biol. 2007;9:36–44. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- Olson TM, Michels VV, Thibodeau SN, Tai YS, Keating MT. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science. 1998;280:750–752. doi: 10.1126/science.280.5364.750. [DOI] [PubMed] [Google Scholar]
- Pattni K, Jepson M, Stenmark H, Banting G. A PtdIns(3)P-specific probe cycles on and off host cell membranes during Salmonella invasion of mammalian cells. Curr Biol. 2001;11:1636–1642. doi: 10.1016/s0960-9822(01)00486-9. [DOI] [PubMed] [Google Scholar]
- Peterson JJ, Tam BM, Moritz OL, Shelamer CL, Dugger DR, McDowell JH, Hargrave PA, Papermaster DS, Smith WC. Arrestin migrates in photoreceptors in response to light: a study of arrestin localization using an arrestin-GFP fusion protein in transgenic frogs. Exp Eye Res. 2003;76:553–563. doi: 10.1016/s0014-4835(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Rajala A, Anderson RE, Ma JX, Lem J, Al Ubaidi MR, Rajala RV. G-protein-coupled Receptor Rhodopsin Regulates the Phosphorylation of Retinal Insulin Receptor. J Biol Chem. 2007;282:9865–9873. doi: 10.1074/jbc.M608845200. [DOI] [PubMed] [Google Scholar]
- Rajala A, Tanito M, Le YZ, Kahn CR, Rajala RV. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J Biol Chem. 2008;283:19781–19792. doi: 10.1074/jbc.M802374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala RV, McClellan ME, Ash JD, Anderson RE. In vivo regulation of phosphoinositide 3-kinase in retina through light-induced tyrosine phosphorylation of the insulin receptor beta-subunit. J Biol Chem. 2002;277:43319–43326. doi: 10.1074/jbc.M206355200. [DOI] [PubMed] [Google Scholar]
- Rajala RV, McClellan ME, Chan MD, Tsiokas L, Anderson RE. Interaction of the Retinal Insulin Receptor beta-Subunit with the P85 Subunit of Phosphoinositide 3-Kinase. Biochemistry. 2004;43:5637–5650. doi: 10.1021/bi035913v. [DOI] [PubMed] [Google Scholar]
- Reiter CE, Sandirasegarane L, Wolpert EB, Klinger M, Simpson IA, Barber AJ, Antonetti DA, Kester M, Gardner TW. Characterization of insulin signaling in rat retina in vivo and ex vivo. Am J Physiol Endocrinol Metab. 2003;285:E763–E774. doi: 10.1152/ajpendo.00507.2002. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Kapeller R, White MF, Cantley LC. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci U S A. 1990;87:1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusten TE, Stenmark H. Analyzing phosphoinositides and their interacting proteins. Nat Methods. 2006;3:251–258. doi: 10.1038/nmeth867. [DOI] [PubMed] [Google Scholar]
- Sable CL, Filippa N, Filloux C, Hemmings BA, Van Obberghen E. Involvement of the pleckstrin homology domain in the insulin-stimulated activation of protein kinase B. J Biol Chem. 1998;273:29600–29606. doi: 10.1074/jbc.273.45.29600. [DOI] [PubMed] [Google Scholar]
- Samardzija M, Wenzel A, Aufenberg S, Thiersch M, Reme C, Grimm C. Differential role of Jak-STAT signaling in retinal degenerations. FASEB J. 2006;20:2411–2413. doi: 10.1096/fj.06-5895fje. [DOI] [PubMed] [Google Scholar]
- Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T, Itoh T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim Biophys Acta. 2001;1533:190–206. doi: 10.1016/s1388-1981(01)00165-2. [DOI] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Hurd LB, Papermaster DS. Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J Cell Biol. 2000;151:1369–1380. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Konishi H, Touhara K, Sakane F, Hirata M, Ono Y, Kikkawa U. Identification of myosin II as a binding protein to the PH domain of protein kinase B. Biochem Biophys Res Commun. 1999;255:169–174. doi: 10.1006/bbrc.1999.0162. [DOI] [PubMed] [Google Scholar]
- Tanito M, Kaidzu S, Anderson RE. Delayed loss of cone and remaining rod photoreceptor cells due to impairment of choroidal circulation after acute light exposure in rats. Invest Ophthalmol Vis Sci. 2007;48:1864–1872. doi: 10.1167/iovs.06-1065. [DOI] [PubMed] [Google Scholar]
- Testa JR, Bellacosa A. Membrane translocation and activation of the Akt kinase in growth factor-stimulated hematopoietic cells. Leuk Res. 1997;21:1027–1031. doi: 10.1016/s0145-2126(97)00093-3. [DOI] [PubMed] [Google Scholar]
- Thomas CC, Deak M, Alessi DR, van Aalten DM. High-resolution structure of the pleckstrin homology domain of protein kinase b/akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr Biol. 2002;12:1256–1262. doi: 10.1016/s0960-9822(02)00972-7. [DOI] [PubMed] [Google Scholar]
- Varnai P, Bondeva T, Tamas P, Toth B, Buday L, Hunyady L, Balla T. Selective cellular effects of overexpressed pleckstrin-homology domains that recognize PtdIns(3,4,5)P3 suggest their interaction with protein binding partners. J Cell Sci. 2005;118:4879–4888. doi: 10.1242/jcs.02606. [DOI] [PubMed] [Google Scholar]
- Watton SJ, Downward J. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr Biol. 1999;9:433–436. doi: 10.1016/s0960-9822(99)80192-4. [DOI] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Vrieze MJ, Dighe R, Hu Y. Direct modulation of rod photoreceptor responsiveness through a Mel(1c) melatonin receptor in transgenic Xenopus laevis retina. Invest Ophthalmol Vis Sci. 2003;44:4522–4531. doi: 10.1167/iovs.03-0329. [DOI] [PubMed] [Google Scholar]
- Wijkander J, Holst LS, Rahn T, Resjo S, Castan I, Manganiello V, Belfrage P, Degerman E. Regulation of protein kinase B in rat adipocytes by insulin, vanadate, and peroxovanadate. Membrane translocation in response to peroxovanadate. J Biol Chem. 1997;272:21520–21526. doi: 10.1074/jbc.272.34.21520. [DOI] [PubMed] [Google Scholar]
- Yi X, Schubert M, Peachey NS, Suzuma K, Burks DJ, Kushner JA, Suzuma I, Cahill C, Flint CL, Dow MA, Leshan RL, King GL, White MF. Insulin receptor substrate 2 is essential for maturation and survival of photoreceptor cells. J Neurosci. 2005;25:1240–1248. doi: 10.1523/JNEUROSCI.3664-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Arimura N, Kaibuchi K. Signaling networks in neuronal polarization. J Neurosci. 2006;26:10626–10630. doi: 10.1523/JNEUROSCI.3824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Vik TA. Growth factor stimulation of hematopoietic cells leads to membrane translocation of AKT1 protein kinase. Leuk Res. 1997;21:849–856. doi: 10.1016/s0145-2126(97)00055-6. [DOI] [PubMed] [Google Scholar]