Abstract
Animals at advanced ages exhibit a reduction in central leptin sensitivity. However, changes in growth, metabolism, and obesity risk occur much earlier in life, particularly during the transition from youth to middle age. To determine when initial decreases in central leptin sensitivity occur, leptin-dependent suppression of food intake was tested in 8-, 12-, and 20-wk-old male, chow-fed Sprague Dawley rats. Intracerebroventricular leptin injection (3 μg) suppressed 24-h food intake in 8- and 12-wk-old rats (P < 0.05) but not 20-wk-old rats. To identify potential cellular mediators of this resistance, we focused on protein tyrosine phosphatase 1B (PTP1B), a recently described inhibitor of leptin signaling. PTP1B protein levels, as determined by Western blot, were significantly higher in mediobasal hypothalamic punches collected from 20-wk-old rats, compared with 8-wk-old rats (P < 0.05). When 20-wk-old rats were fasted for 24 h, levels of hypothalamic PTP1B decreased (P < 0.05), coincident with a restoration of leptin sensitivity. To directly test whether inhibition of PTP1B restores leptin sensitivity, 20-wk-old chow-fed rats were pretreated with a pharmacological PTP1B inhibitor 1 h before leptin, and 24-h food intake was recorded. As expected, leptin alone produced a small but nonsignificant reduction in food intake. However, pretreatment with the PTP1B inhibitor resulted in a marked improvement in leptin-dependent suppression of food intake (P < 0.05). These data are consistent with the hypothesis that increases in PTP1B contribute to hypothalamic leptin resistance as rats transition into middle age.
LEPTIN ACTS ON key neuronal circuits within the brain to reduce food intake, limit body adiposity, and enhance peripheral glucose metabolism. The absence of leptin leads to obesity, indicating that leptin plays an essential role in the maintenance of energy homeostasis. However, leptin deficiency is rarely the basis for obesity, and instead most obese individuals exhibit elevated circulating leptin levels and are less responsive to exogenously administered leptin (1, 2), consistent with a state of leptin resistance. Leptin resistance is widely associated with obesity and diabetes, but the cellular and physiological mechanisms underlying leptin resistance are poorly defined.
Consumption of highly palatable, calorie-dense diets is associated with the development of leptin resistance and obesity, and models of diet-induced obesity have been used to explore the mechanisms underlying leptin resistance (3-6). However, leptin resistance also develops with age independent of diet, and multiple studies have used aging as a model to identify mechanisms of leptin resistance (7-12). Such studies frequently focus on animals at advanced ages, such as rats older than 1 or even 2 yr of age (11-13), despite the fact that increases in the predisposition to obesity occur much earlier in life, particularly during the transition from youth to middle age (14, 15). Therefore, focusing on the cellular mechanisms underlying the development of leptin resistance within this earlier time frame may provide unique insight into the initial events contributing to the development of leptin resistance.
Protein tyrosine phosphatase 1B (PTP1B) is an intracellular phosphatase implicated in the inhibition of leptin signaling. PTP1B dephosphorylates Janus kinase 2 (Jak2), the initial kinase in the leptin receptor signal transduction pathway (16–18), and in cell culture PTP1B expression is inversely proportional to leptin sensitivity (16, 17, 19). Mice with targeted deletion of PTP1B in all tissues resist dietary obesity and exhibit improved sensitivity to leptin (16, 19–21), and this resistance to diet-induced obesity is recapitulated by loss of PTP1B selectively within neurons (22). The present study focuses on the early development of leptin resistance that occurs during maturation. This work indicates that hypothalamic PTP1B levels increase coincident with the initial development of leptin resistance and that inhibition of hypothalamic PTP1B restores leptin sensitivity.
Materials and Methods
Animals
All procedures were performed in accordance with National Institutes of Health guidelines for the care and use of animals and were approved by the Animal Care and Use Committee of Pennington Biomedical Research Center. Male Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN; Charles River Laboratories, Wilmington, MA) were housed singly and maintained on a 12-h light,12-h dark cycle with ad libitum access to standard rat chow and water unless otherwise noted. For third ventricular [intracerebroventricular (icv)] cannulation, rats were anesthetized and placed into a stereotaxic device. Using aseptic techniques, the skull was exposed, head screws inserted, and a 22G stainless steel cannula implanted at coordinates −2.2 from bregma and −7.5 from dura (23). After anchoring the cannula and sealing all skull openings with dental acrylic, the incision was sutured and a 28G obdurator placed into the cannula. After surgery animals were treated with analgesics and allowed to recover at least 1 wk before further study.
Experiment 1: effect of age and fasting on hypothalamic leptin action
Eight, 12-, and 20-wk-old male (n = 5– 8/group) Sprague Dawley rats bearing third ventricular cannula were used to test the effects of age on leptin-dependent inhibition of food intake. Rats were fed standard rodent chow ad libitum and randomly assigned to receive icv injection of either leptin (3 μg dissolved in 2 μl PBS) or vehicle, approximately 2 h before lights off. Food was removed 4 h before lights off and returned 30 min before lights off, and 24 h food intake was recorded. To test the effects of fasting on leptin inhibition of food intake, 20-wk-old rats were fasted for 24 h, and after the fast leptin (3 μg icv) or vehicle was injected and 24 h food intake determined as above. To test the effects of leptin on hypothalamic signal transducer and activator of transcription 3 (Stat3) phosphorylation, 8- or 20-wk-old rats were treated with vehicle or leptin (1 and 3 μg) as above. Twenty minutes after leptin injection, rats were rapidly decapitated and brains blocked and frozen on crushed dry ice for subsequent protein extraction and Western blot.
Experiment 2: chronic and acute regulation of hypothalamic PTP1B protein levels by age and fasting
Chow-fed Sprague Dawley rats at either 8 or 20 wk of age (5/group) were killed and brains, epididymal and retroperitoneal fat pad weights, and serum were collected at the time the animals were killed. Serum was used for analysis of leptin and insulin levels via RIA (Linco Research, St. Charles, MO) according to manufacturer’s instructions. Brains were blocked and frozen on crushed dry ice for subsequent protein extraction and Western blot. In similar fashion, a separate group of mature (20 wk old) rats were fasted or fed for 24 h and subsequently killed and brains collected as above for analysis of hypothalamic PTP1B.
Experiment 3: effects of pharmacological PTP1B inhibition on food intake and leptin sensitivity
Twenty-week-old male Sprague Dawley rats bearing third ventricular cannula were treated with a pharmacological PTP1B inhibitor (0.3 nmol icv) or vehicle, followed 1 h later by leptin (3 μg icv) or vehicle, to generate four treatment groups (n = 8 –10/group): Veh-Veh, Veh-Leptin, PTP1Bi-Veh, and PTP1Bi-Leptin. Specific pharmacological properties for this cell-permeable inhibitor have been previously described by Xie et al. (24) (termed compound II), including the inhibitor’s effect to acutely reduce PTP1B activity and enhance insulin signaling in vitro. Leptin injections occurred approximately 2 h before lights off, and food was removed 4 h before lights off, returned 30 min before lights off, and 4- and 24-h food intake was recorded.
To determine whether higher doses of the inhibitor alone would exert direct effects on food intake, a separate group of 20-wk-old rats were treated with vehicle or 1 or 3 nmol of PTP1B inhibitor icv approximately 2 h before lights off, and 4- and 24-h food intake was recorded as above. These doses are 3- and 10-fold higher than those used to block the effects of exogenously administered leptin.
Experiment 4: effects of pharmacological Jak2 inhibition on leptin and melanotan II (MTII)-dependent inhibition of food intake
Male Sprague Dawley rats (16 wk old) were surgically implanted with indwelling third ventricular cannula as above. Because 16-wk-old rats may exhibit some defects in leptin action, these rats were fasted for 24 h to ensure a robust response to leptin. After the fast, rats were treated with a single injection of the Jak2 inhibitor AG490 (0.1 nmol icv) or vehicle (5% dimethylsulfoxide in PBS), followed 1 h later by leptin (3 μg icv) or PBS vehicle to generate four treatment groups (n = 6 – 8/group): Veh-Veh, AG490-Veh, Veh-Leptin, and AG490-Leptin. Leptin injections occurred approximately 2 h before lights off, food was returned 30 min before lights off, and 4- and 24-h food intake was recorded. In similar fashion, a separate group of rats were fasted for 24 h and subsequently treated with the Jak2 inhibitor AG490 (0.1 nmol icv) or vehicle, followed 1 h later by MTII (0.3 nmol icv) or vehicle to generate four treatment groups (n = 5– 6/group): Veh-Veh, AG490-Veh, Veh-MTII, and AG490-MTII. Leptin/MTII injections occurred approximately 2 h before lights off, food was returned 30 min before lights off, and 4- and 24-h food intake was recorded.
Protein extraction and Western blot
Frozen brain blocks containing hypothalamus were placed on a cryostat and trimmed on each side to reproducible anatomical points just before and after the hypothalamic arcuate nucleus (∼3 mm thick). A single punch was made over each arcuate nucleus (two punches per section) using a 0.8-mm-diameter punch. Punches were made to slightly overlap each other along the midline (over the third ventricle). Whereas punches contain principally arcuate nucleus, we cannot rule out the inclusion of other brain areas, and for this reason refer to them as punches of mediobasal hypothalamus. Specifically, it is likely that portions of the ventromedial nucleus were also included in the punch. In addition, some residual parts of the superchiasmatic nucleus and premammillary areas from the rostral and caudal parts of the section, respectively, may also be included. Punches were placed into lysis buffer [1% Triton X-100, 100 mmol/liter Tris (pH 7.4), 100 mmol/liter sodium pyrophosphate, 100 mmol/liter sodium fluoride, 10 mmol/liter EDTA, 10 mmol/liter sodium vanadate, 2 mmol/liter phenylmethylsulfonyl fluoride, and 0.1 g/liter aprotinin] and snap frozen using liquid nitrogen. Subsequently the extracts were centrifuged at 100,000 × g at 4 C for 45 min to remove insoluble material. Fifty micrograms of total protein were electrophoretically separated on an SDS-PAGE gel and transferred to a nitrocellulose membrane. Blots were blocked in 5% nonfat dry milk or 1% BSA and probed with a commercially available antibodies for PTP1B antibody (1:1000; Upstate Biotechnology, Lake Placid, NY), phospho-Stat3 (1:5000; Cell Signaling, Danvers, MA), or Stat3 (1:5000; Cell Signaling). Positive signal was imaged using a chemiluminescent substrate (enhanced chemiluminescence reagent; Amersham Biosciences, Piscataway, NJ) and exposure to autoradiograph film, and afterward the blot was stripped and reprobed for levels of β-actin.
Statistical analysis
Data were analyzed using the SAS software package (version 8; SAS Institute, Cary, NC) using a two-tailed t test or ANOVA using the general linear model (general linear model) procedure. When experiment wide tests were significant, post hoc comparisons were made using the least squares-mean statement with the PDIFF option and thus represent least significant differences tests for preplanned comparisons. In some studies animal numbers were spread across two independent replicates, with replicate included in the statistical model. For Western blot analysis, levels of PTP1B were normalized to β-actin and phospho-Stat3 to total Stat3 and a two-tailed t test used to test significant differences between treatments. All data are expressed as mean ± sem, with a probability value of less than 0.05 considered statistically significant.
Results
Experiment 1: effect of age and fasting on hypothalamic leptin action
To determine the age of initial onset of leptin resistance in maturing rats, the ability of centrally administered leptin (3 μg icv) to suppress 24-h food intake was determined in 8-, 12-, and 20-wk-old chow-fed Sprague Dawley rats. Third ventricular administration of leptin significantly suppressed food intake in 8- and 12-wk-old rats (P < 0.05; Fig. 1), compared with vehicle (2 μl PBS). However, icv leptin did not alter food intake in 20-wk-old rats (Fig. 1). To determine whether fasting could restore leptin action in these mature, leptin-resistant rats, a separate group of 20-wk-old rats was fasted for 24 h and the effect of icv leptin administration on 24-h food intake determined. As expected, fasting increased subsequent 24-h intake vs. nonfasted controls (Fig. 1). Treatment with leptin significantly suppressed this fasting-induced increase in food intake (P < 0.05, Fig. 1), but the reduction was only to levels of food intake of vehicle-treated, nonfasted controls.
Fig. 1.

Changes in leptin sensitivity with age and fasting. Male chow-fed Sprague Dawley rats at 8, 12, and 20 wk of age (five to eight rats/group) were treated with a single icv injection of leptin (3 μg), and 24-h food intake was recorded. In addition, a separate group of 20-wk-old rats was fasted for 24 h, injected with leptin after the fast, and 24-h food intake recorded. Leptin treatment significantly suppressed food intake in 8- and 12-wk-old rats (*, P < 0.05) but had no effect on food intake in 20-wk-old rats. However, when 20-wk-old rats were fasted for 24 h, leptin blocked fasting-induced increases in food intake (*, P < 0.05).
To more directly assess leptin signaling within the hypothalamus, the effects of age on leptin-induced Stat3 phosphorylation was tested. As with food intake, icv leptin treatment significantly increased Stat3 phosphorylation in mediobasal hypothalamus from 8-wk-old rats (P < 0.05; Fig. 2) but had no effect in 20-wk-old rats.
Fig. 2.
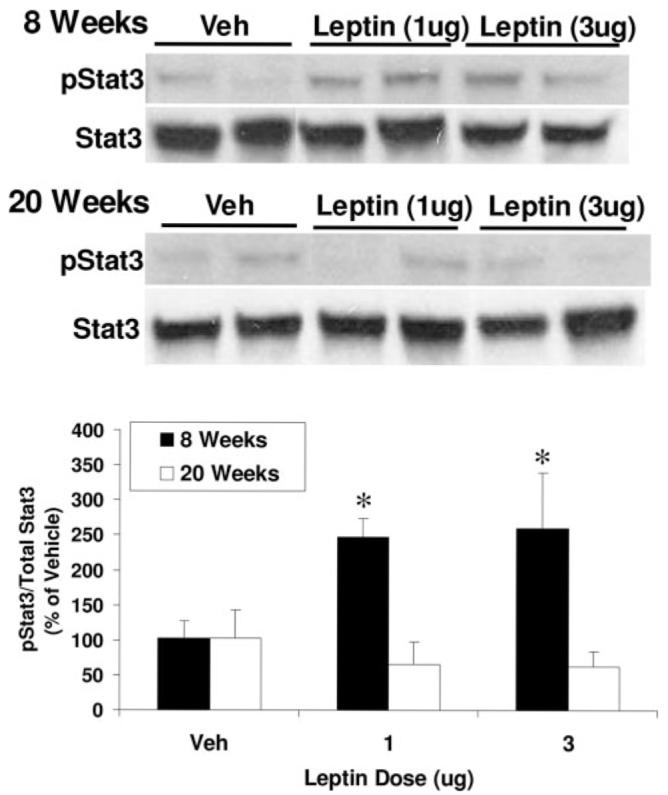
Effects of age on leptin activation of Stat3 within the medio-basal hypothalamus. Eight and 20-wk-old Sprague Dawley rats were treated icv with vehicle (Veh) or 1 or 3 μg leptin. Twenty minutes after leptin administration, rats were rapidly decapitated and mediobasal hypothalamus collected for analysis of Stat3 phosphorylation via Western blot. Intracerebroventricular leptin induced a significant increase in Stat3 phosphorylation in 8-wk-old rats at both doses tested (P < 0.05) but had no effect on Stat3 phosphorylation 20-wk-old rats.
Experiment 2: chronic and acute regulation of hypothalamic PTP1B protein levels by age and fasting
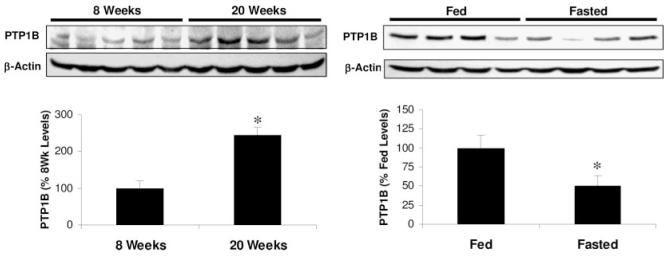
To explore the basis for this leptin resistance, hypothalamic expression of PTPB was examined in 8- and 20-wk-old chow-fed Sprague Dawley rats. Western blot analysis of mediobasal hypothalamic punches revealed increased PTP1B in 20-wk-old rats, compared with 8-wk-old animals (P < 0.05, Fig. 3). Carcass analysis of 8- and 20-wk-old rats indicated that 20-wk-old rats weighed more and had significantly larger epididymal and retroperitoneal fat pads than 8-wk-old rats, even if expressed per unit body weight (Table 1). Similarly, circulating levels of leptin and insulin assessed in trunk blood taken at the time the animals were killed were higher in 20-wk-old rats, compared with 8-wk-old rats (P < 0.05; Table 1).
Fig. 3.
Effects of age and fasting on hypothalamic PTP1B protein expression. Levels of PTP1B protein were determined on small punches of mediobasal hypothalamus collected from 8- and 20-wk-old Sprague Dawley rats by Western blot (five rats/group). Blots were probed with a specific PTP1B antibody, and levels are expressed relative to β-actin content. The 20-wk-old rats exhibited significantly greater levels of PTP1B protein, compared with 8-wk-old rats (P < 0.05). A separate group of 20-wk-old rats were fed or fasted for 24 h and levels of PTP1B protein in mediobasal hypothalamic punches determined via Western blot. Fasting decreased hypothalamic levels of PTP1B in these mature rats (P < 0.05).
TABLE 1.
Comparison of body weight and composition between 8- and 20-wk-old rats
| 8 wk | 20 wk | |
|---|---|---|
| Body weight (g) | 268.1 ± 6.3 | 471.3 ± 4.88a |
| Epididymal weight (g) | 1.91 ± 0.04 | 5.3 ± 0.45a |
| Epididymal weight (%BW) | 0.71 ± 0.04 | 1.12 ± 0.09a |
| Retroperitoneal weight (g) | 0.67 ± 0.1 | 3.3 ± 0.5a |
| Retroperitoneal weight (%BW) | 0.25 ± 0.04 | 0.70±.11a |
| Leptin (ng/ml) | 1.79 ± 0.23 | 2.84 ± 040a |
| Insulin (ng/ml) | 1.64 ± 0.39 | 3.04 ± 0.48a |
Sprague Dawley rats fed standard chow ad libitum were killed at 8 and 20 wk of age (five rats per age) for measures of body weight, retroperitoneal and epididymal fat pad weights, and serum leptin and insulin levels. Fat pad weights are presented as raw weights and as a percentage per gram of body weight (%BW). Data are presented as mean ± SEM.
P < 0.05, 8 vs. 20 wk.
To determine whether changes in hypothalamic PTP1B might contribute to fasting-induced increases in leptin sensitivity in 20-wk-old rats, the effect of a 24-h fast on PTP1B levels within the mediobasal hypothalamus was determined. As expected, fasting significantly reduced circulating levels of leptin from 2.35 ± 0.30 to 1.48 ± 0.23 ng/ml (P = 0.03) and also reduced insulin levels from 2.43 ± 0.22 to 1.26 ± 0.21 ng/ml (P < 0.01). Fasting also decreased hypothalamic PTP1B expression by approximately 50% (P < 0.05; Fig. 3). These data indicate that PTP1B levels are increased in leptin-resistant rats and that fasting reduces hypothalamic PTP1B and increases leptin sensitivity.
Experiment 3: acute pharmacological inhibition of PTP1B restores leptin sensitivity in mature, leptin-resistant rats
To test whether the acute inhibition of hypothalamic PTP1B signaling improves leptin sensitivity, 20-wk-old rats were pretreated with a highly selective, cell-permeable inhibitor of PTP1B (24) 1 h before icv injection of leptin (3 μg) or vehicle. As expected, leptin produced a small, nonsignificant decrease in food intake in the absence of the inhibitor (Fig. 4). However, pretreatment with the PTP1B inhibitor, at a dose (0.3 nmol), which had no effect alone, enhanced the anorectic effect of leptin at 4 and 24 h (P < 0.05; Fig. 4). To test whether higher doses of the PTP1B inhibitor might enhance sensitivity to endogenous leptin in leptin-resistant rats, the response to icv administration of 1 or 3 nmol of the PTP1B inhibitor was tested. Increasing the PTP1B inhibitor dose by 3- and 10-fold resulted in a dose-dependent decrease in food intake at both 4 and 24 h (P < 0.05; Fig. 5). Collectively, these data support the conclusion that increased PTP1B contributes to leptin resistance in 20-wk-old rats.
Fig. 4.
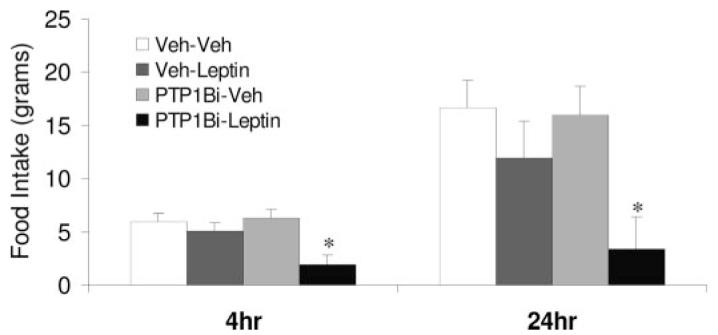
Pharmacological inhibition of PTP1B improves leptin sensitivity in mature, leptin-resistant rats. Twenty-week-old rats bearing third cerebroventricular cannula (eight to 10 rats/group) were injected with a selective, cell-permeable PTP1B inhibitor (0.3 nmol icv) previously described by Xie et al. (24). One hour after the inhibitor injection, rats were treated with leptin (3 μg icv) or vehicle, and 4- and 24-h food intake was recorded. Neither leptin nor the inhibitor alone significantly affected food intake in these 20-wk-old rats. However, pretreatment with the PTP1B inhibitor markedly improved leptin-dependent suppression of food intake (*, P < 0.05).
Fig. 5.
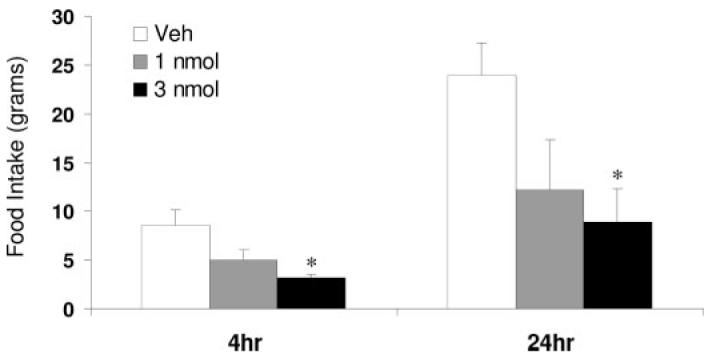
Pharmacological PTP1B inhibition reduces food intake. Twenty-week-old rats bearing icv cannula were treated with higher doses of the PTP1B inhibitor (1 and 3 nmol, icv) than used to enhance sensitivity to exogenous leptin. Intracerebroventricular administration of the PTP1B inhibitor at these higher doses resulted in a dose-dependent inhibition of food intake (P < 0.05) at both 4 and 24 h, consistent with an increase in sensitivity to endogenous leptin.
Experiment 4: Jak2 inhibition blocks leptin-dependent suppression of food intake
PTP1B inhibits leptin signaling by dephosphorylating Jak2. To determine whether inhibition of Jak2 is sufficient to explain PTP1B-induced leptin resistance, 16-wk-old Sprague Dawley rats were fasted for 24 h to maximize leptin sensitivity and then received a single injection of either the Jak2 inhibitor AG490 (1 nmol icv) or vehicle followed 1 h later by a single icv injection of leptin (3 μg). As expected for 24-h fasted animals, leptin significantly suppressed 4- and 24-h food intake (P < 0.05, Fig. 6). However, pretreatment with the Jak2 inhibitor AG490 blocked leptin’s ability to suppress food intake, indicating that loss of Jak2 signaling is sufficient to induce leptin resistance.
Fig. 6.
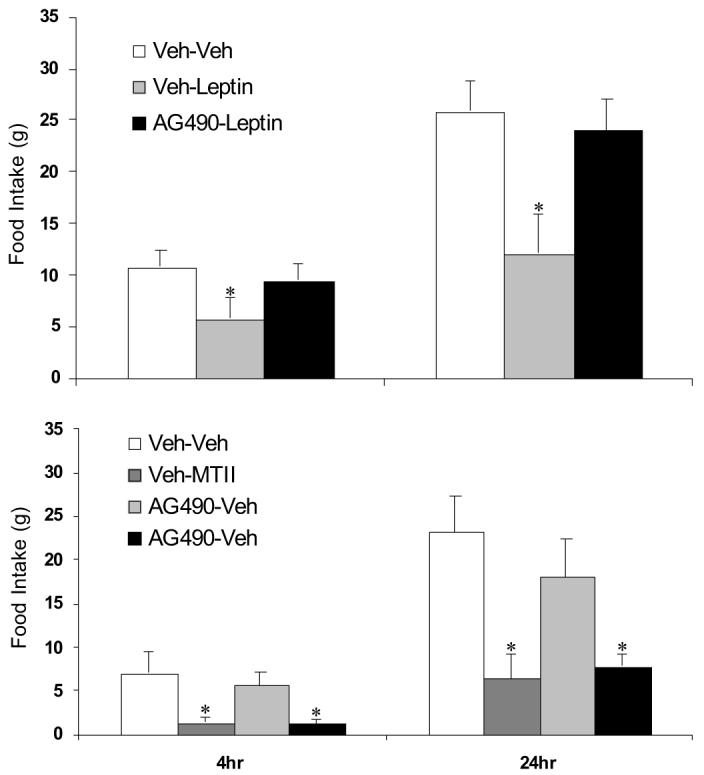
Effects of a Jak2 inhibitor on regulation of food intake by leptin and MTII. Sprague Dawley rats (16 wk old, six to eight/group) bearing third cerebroventricular cannula were fasted for 24 h and subsequently pretreated with a cell-permeable Jak2 inhibitor (AG490, 1 nmol) or vehicle 1 h before treatment with leptin (3 μg, icv) or vehicle, and 4 and 24 h food intake was measured. Leptin alone significantly suppressed 24-h food intake (*, P < 0.05); however, pretreatment with the Jak2 inhibitor AG490 completely blocked leptin’s ability to suppress food intake. A separate group of Sprague Dawley rats were fasted for 24 h and pretreated as above with AG490 (1 nmol icv) or vehicle 1 h before treatment with the melanocortin agonist MTII (0.3 nmol icv) or vehicle. MTII significantly reduced 4 and 24-h food intake (P < 0.05), but AG490 pretreatment had no effect on MTII-dependent suppression of food intake.
To rule out a nonspecific effect of AG490 on food intake, we tested the ability of the melanocortin receptor agonist MTII to reduce food consumption in animals pretreated with AG490. As above, rats bearing third ventricular cannula were fasted for 24 h and treated with either AG490 (1 nmol icv) or vehicle, followed 1 h later by MTII (0.3 nmol icv). MTII alone suppressed food intake at both 4 and 24 h (P < 0.05; Fig. 6), and this effect was not altered by pretreatment with AG490. These findings indicate that pretreatment with a Jak2 inhibitor blocks leptin’s anorectic effect at a site upstream of melanocortin receptors.
Discussion
Leptin resistance is a common characteristic of obesity, but the underlying mechanism remains a subject of active investigation. The current work focuses on an early-onset model of age-associated leptin resistance. These data indicate that PTP1B levels within the mediobasal hypothalamus increase during maturation and that pharmacological or physiological reductions in PTP1B improve leptin sensitivity in leptin-resistant animals. Taken together, these observations suggest that PTP1B contributes to the initial decline in leptin responsiveness with age.
A large body of evidence provides compelling support for age-associated increases weight gain and concomitant reductions in peripheral and central leptin sensitivity (7-12, 25). Although age-induced leptin resistance is therefore well accepted, much of the work in this area has focused on rats and mice at advanced ages (i.e. greater than 1 or 2 yr of age). Given that increased fat deposition and relative adiposity typically occur much earlier, we focused on an earlier age range with the goals of identifying when leptin resistance first becomes evident and whether increased hypothalamic PTP1B plays an important role. Published work implicates PTP1B as a cellular mediator of leptin resistance. PTP1B dephosphorylates Jak2, the initial tyrosine kinase mediating intracellular leptin signaling (16–18), and work in cell culture documents an inverse relationship between PTP1B expression and leptin sensitivity (16, 17, 19). Mouse genetic models indicate that deletion of PTP1B in all tissues enhances basal leptin sensitivity and conveys resistance to diet-induced obesity (16, 17, 19), whereas loss of PTP1B exclusively within neurons largely recapitulates the phenotype of the global knockout (22). These data indicate that PTP1B is well placed to regulate hypothalamic leptin sensitivity, but little work has focused specifically on changes in hypothalamic PTP1B as a mechanism for regulating leptin sensitivity with age.
The responsiveness of rats to a single icv injection of leptin was tested at 8, 12, and 20 wk of age to identify when initial decreases in leptin sensitivity occur. Reductions in the anorectic effect of leptin were first detected in rats at 20 wk of age. Similarly, defects in leptin-mediated induction of Stat3 phosphorylation within the mediobasal hypothalamus were also detected in 20-wk-old rats. These observations are significant, considering that the age range from 8 to 20 wk reflects a transition from youth to middle age in male rats and is associated with changes in tissue accretion, metabolism, and peripheral insulin sensitivity (26). This time period is also marked by differences in the sensitivity to caloric density, with older rats being more susceptible to the development of diet-induced obesity, compared with younger rats (27). Because the current study used only a single dose of leptin, it is unclear whether 20-wk-old rats are absolutely unresponsive to leptin, or whether they would remain responsive to higher doses. Because previous studies clearly document reduced leptin sensitivity in much older rats, we focused on defining a single dose that would produce divergent effects in young vs mature rats. Considering that leptin responsiveness has been detected in 5- to 6-month-old animals in separate studies using either higher doses or longer treatment regimens (13, 28), we suggest that 20-wk-old rats are not absolutely leptin resistant. However, the current work clearly documents that 20-wk-old rats are less responsive to the chosen dose of leptin when compared with younger animals.
In the current study, 20-wk-old rats were resistant to icv leptin injection, indicative of defective leptin signaling within the brain (9, 12). The hypothalamic arcuate nucleus is a key brain area mediating leptin action, and the arcuate nucleus also contains significant levels of PTP1B (16). To test whether PTP1B represents a local cellular mediator of this leptin resistance, levels of PTP1B were compared in medio-basal hypothalamus (which contains arcuate nucleus) from 8- and 20-wk-old rats. Twenty-week-old rats exhibited an increase in PTP1B, compared with leptin sensitive, 8-wk-old rats, indicating that increases of PTP1B were temporally associated with defects in leptin action within the mediobasal hypothalamus. Whereas these observations do not establish cause and effect, they are consistent with our hypothesis that changes in PTP1B are an important mechanism for regulating leptin sensitivity.
To more directly test the role of PTP1B in leptin resistance, the effect of local inhibition of hypothalamic PTP1B was tested in 20-wk-old, leptin-resistant rats. This was accomplished by pretreating animals with a potent, cell-permeable, and highly selective inhibitor of PTP1B (24), which has been used in multiple published studies to define the role played by PTP1B in insulin-mediated signaling and glucose uptake, as well as other cellular processes including integrin and caveolin function (24, 29–32). Acute administration of this PTP1B inhibitor before leptin treatment markedly improved the anorectic effects of leptin in 20-wk-old, leptin-resistant rats, indicating that the elevated PTP1B levels in the hypothalamus of 20-wk-old rats contribute to their resistance to icv leptin administration. Because pharmacological inhibitors can exert nonspecific effects, it is possible that the above observation is due to an effect of the inhibitor independent of leptin signaling. However, it should be pointed out that the dose of the inhibitor used had no effect on food intake alone. This observation suggests that is it unlikely that the inhibitor was directly influencing feeding behavior independently from leptin. The current observations with this highly selective inhibitor are also fully consistent with the improvement in leptin sensitivity observed in PTP1B-deficient mice (22).
In the above study, a low dose of a pharmacological PTP1B inhibitor enhanced leptin sensitivity in leptin-resistant rats. Considering that these rats also exhibit elevated endogenous leptin, it seems likely that increased hypothalamic PTP1B would promote resistance to this endogenous leptin. To test whether loss of hypothalamic PTP1B would enhance sensitivity to endogenous leptin, higher doses of the PTP1B inhibitor were administered icv, and the effect on food intake was assessed. Increasing the dose of the PTP1B inhibitor by 3- and 10-fold resulted in a dose-dependent decrease in food intake. Although it is possible that effects on additional signaling pathways (i.e. insulin) may contribute to the decreases in food intake observed with high doses of the inhibitor, this observation is most consistent with a model in which inhibition of hypothalamic PTP1B increases sensitivity to endogenous leptin, resulting in a decrease in food intake. These observations, together with those described above, are also consistent with previous observations in PTP1B-deficient mice (16, 19–21) and particularly with very recent work in mice bearing neuron-specific PTP1B deletion (22). In these mice, loss of PTP1B selectively within neurons recapitulates the enhancement of leptin sensitivity and resistance to diet-induced obesity observed in the constitutive knockouts, indicating PTP1B acts primarily within the brain to promote diet-induced leptin resistance. The current data suggest that PTP1B also represents a mechanism for leptin resistance with age and indicate that local changes in hypothalamic PTP1B can acutely affect central leptin sensitivity.
Fasting activates a series of physiological responses designed to protect against excessive weight loss and restore body adiposity once the nutrient challenge ends. Leptin plays a key role in this response to fasting (33), and fasted animals are particularly responsive to leptin, suggesting that fasting may enhance leptin sensitivity. In the current study, 20-wk-old rats responded to a 24-h fast by increasing food intake when food was restored. Treatment with leptin effectively blocked this fasting-induced hyperphagia, even though the animals were leptin resistant in the fed state. These observations indicated that fasting can improve leptin sensitivity and that defects in central leptin action are not permanent but can instead be acutely reversed. Because fasting improves leptin sensitivity in formerly leptin-resistant rats, the effect of fasting on hypothalamic PTP1B was also tested. The fasting-induced restoration of leptin responsiveness in 20-wk-old rats was accompanied by a reduction in hypothalamic PTP1B. These observations are consistent with a model in which physiological or pharmacological alterations in hypothalamic PTP1B affect leptin sensitivity.
A large body of work exists documenting at link between PTP1B and Jak2, the initial kinase in the leptin receptor signaling cascade (16–19, 34, 35). This work documents that Jak2 is a substrate for PTP1B, with PTP1B acting to dephosphorylate and thus inhibit Jak2 signaling. Loss of Jak2 therefore provides the cellular mechanism for PTP1B-induced leptin resistance. If so, then loss of Jak2 signaling in leptin-sensitive animals would therefore be predicted to recapitulate the leptin resistance observed in animals expressing elevated PTP1B. Our observations support this hypothesis, in that pretreatment with the Jak2 inhibitor AG490 (36, 37) blocked leptin-dependent suppression of food intake in leptin-sensitive, 24-h-fasted rats. The effect of AG490 was also specific to leptin because this inhibitor did not attenuate the ability of the melanocortin agonist MTII to suppress food intake. MTII is a well-described inhibitor of feeding that acts via central melanocortin receptors (38), which are seven-transmembrane receptors proposed to signal via pathways independent from Jak2.
Taken together, the above observations not only indicate that Jak2 signaling is necessary for leptin action, but they also support a model in which PTP1B-dependent inhibition of Jak2 is sufficient to mediate the leptin resistance observed in 20-wk-old rats. Because Jak2 represents the initial kinase mediating all aspects of downstream leptin signaling, loss of Jak2 would be predicted to attenuate leptin-dependent regulation of multiple signaling pathways, including both Stat3 and phosphatidylinositol 3-kinase. The current manuscript documents a temporal association between increased PTP1B and reduced leptin activation of hypothalamic Stat3 in 20-wk-old rats, but it does not definitively demonstrate that the increase in PTP1B is causal. Future studies are therefore necessary to determine whether PTP1B, by inhibiting Jak2, simultaneously impairs leptin activation of multiple signaling pathways within leptin-sensitive neurons.
In the present study, hypothalamic PTP1B expression was sensitive to both chronic (age) and acute (fasting) regulation, but the physiological and cellular mechanisms mediating these changes are unclear. Studies in peripheral tissues or cultured cells indicate that insulin resistance is associated with changes in PTP1B within muscle, fat, or liver and that insulin may act to increase PTP1B (39 – 44). In the current study, older rats exhibited higher levels of hypothalamic PTP1B and also increased circulating insulin and leptin levels. Similarly, fasting acted to concurrently reduce these end points. Thus, it is possible that changes in insulin or leptin contributed to alterations in hypothalamic PTP1B. However, an alternative interpretation is that increases in PTP1B induce leptin and insulin resistance, leading to increases in their circulating levels. Further work is therefore necessary to determine the mechanisms underlying changes in hypothalamic PTP1B and whether leptin or insulin directly contribute to these changes.
In addition to changes in leptin sensitivity, aging is also associated with changes in body adiposity and obesity risk. We propose that changes in leptin sensitivity contribute to aging associated changes in body adiposity and that increases in PTP1B provide a cellular mechanism for reduced leptin sensitivity. If leptin resistance is a cause of obesity, then changes in leptin sensitivity or hypothalamic PTP1B should occur before significant changes in body adiposity. In support of this hypothesis, previously published work indicates that leptin resistance develops with age, even when energy intake is restricted and animals exhibit no change in body adiposity (7, 11). However, it should be noted that this work was conducted in much older animals and that conflicting data do exist (12). In the current study, 20-wk-old rats had relatively larger retroperitoneal and epididymal fat pads and higher circulating leptin levels, indicative of increased body adiposity. Previously published observations indicate that rats continue to gain body adipose mass and increase serum leptin levels well into their second year of life (26, 45, 46). Therefore, the 20-wk-old rats in the current study are in the early stages of body adipose accretion and would be expected to continue to gain adipose mass with age. Nonetheless, because a disassociation between the increase in adiposity and hypothalamic PTP1B was not detected, the current work is inconclusive regarding whether changes in leptin sensitivity or hypothalamic PTP1B precede increases in body adiposity, and further work will be necessary to conclusively test whether leptin resistance is causal to the initial changes in body adiposity with age.
Alterations in leptin sensitivity are associated with obesity in many models. The current work provides evidence for an aging-associated leptin resistance that occurs much earlier than previously described. This resistance develops independent of dietary manipulation but occurs as animals are transitioning from youth into middle age, an age range that is itself associated with changes in the predisposition to obesity. These data also implicate increases in hypothalamic PTP1B as a cellular mechanism for this aging-induced leptin resistance. Hypothalamic PTP1B levels were elevated within mature, leptin-resistant rats, and pharmacological or physiological (fasting) reductions in hypothalamic PTP1B enhanced leptin sensitivity. The results also indicate that a PTP1B-dependent inhibition of Jak2 signaling is sufficient to explain the development of leptin resistance. In summary, this work provides a potential cellular model for leptin-resistance and suggests that pharmacological inhibition of PTP1B may provide a means to improve leptin sensitivity.
Acknowledgments
We gratefully acknowledge the excellent technical support of Amy Whittington.
This work was supported by the Pennington Medical Foundation (to C.D.M.), American Diabetes Association (to T.W.G.), and National Institutes of Health Grants NS051570 (to C.D.M.), DK074772 (to T.W.G.), and DK068447 (to Z.-Y.Z.).
Abbreviations
- icv
Intracerebroventricular
- Jak2
Janus kinase 2
- MTII
melanotan II
- PTP1B
protein tyrosine phosphatase 1B
- Stat3
signal transducer and activator of transcription 3
Footnotes
Disclosure Statement: C.D.M., C.L.W., Z.W., S.-Y.L., Z.-Y.Z., and T.W.G. have nothing to declare. D.S.L. has equity interests in Onset Therapeutics and is an inventor on U.S. application serial no. 10-490-836. W.T.C. consults for and received lecture fees from Amylin, Lilly, and Pfizer.
References
- 1.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol. 2004;286:R143–R150. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- 2.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, doseescalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 3.Prpic V, Watson PM, Frampton IC, Sabol MA, Jezek GE, Gettys TW. Differential mechanisms and development of leptin resistance in A/J versus C57BL/6J mice during diet-induced obesity. Endocrinology. 2003;144:1155–1163. doi: 10.1210/en.2002-220835. [DOI] [PubMed] [Google Scholar]
- 4.Scarpace PJ, Matheny M, Tumer N, Cheng KY, Zhang Y. Leptin resistance exacerbates diet-induced obesity and is associated with diminished maximal leptin signalling capacity in rats. Diabetologia. 2005;48:1075–1083. doi: 10.1007/s00125-005-1763-x. [DOI] [PubMed] [Google Scholar]
- 5.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 6.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson L. Middle-aged C57BL/6 mice have impaired responses to leptin that are not improved by calorie restriction. Am J Physiol Endocrinol Metab. 2002;282:E786–E793. doi: 10.1152/ajpendo.00495.2001. [DOI] [PubMed] [Google Scholar]
- 8.Ma XH, Muzumdar R, Yang XM, Gabriely I, Berger R, Barzilai N. Aging is associated with resistance to effects of leptin on fat distribution and insulin action. J Gerontol A Biol Sci Med Sci. 2002;57:B225–B231. doi: 10.1093/gerona/57.6.b225. [DOI] [PubMed] [Google Scholar]
- 9.Scarpace PJ, Tumer N. Peripheral and hypothalamic leptin resistance with age-related obesity. Physiol Behav. 2001;74:721–727. doi: 10.1016/s0031-9384(01)00616-3. [DOI] [PubMed] [Google Scholar]
- 10.Scarpace PJ, Matheny M, Shek EW. Impaired leptin signal transduction with age-related obesity. Neuropharmacology. 2000;39:1872–1879. doi: 10.1016/s0028-3908(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 11.Gabriely I, Ma XH, Yang XM, Rossetti L, Barzilai N. Leptin resistance during aging is independent of fat mass. Diabetes. 2002;51:1016–1021. doi: 10.2337/diabetes.51.4.1016. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Galaz C, Fernandez-Agullo T, Perez C, Peralta S, Arribas C, Andres A, Carrascosa JM, Ros M. Long-term food restriction prevents ageing-associated central leptin resistance in Wistar rats. Diabetologia. 2002;45:997–1003. doi: 10.1007/s00125-002-0851-4. [DOI] [PubMed] [Google Scholar]
- 13.Scarpace PJ, Matheny M, Moore RL, Tumer N. Impaired leptin responsiveness in aged rats. Diabetes. 2000;49:431–435. doi: 10.2337/diabetes.49.3.431. [DOI] [PubMed] [Google Scholar]
- 14.Mizuno T, Shu IW, Makimura H, Mobbs C. Obesity over the life course. Sci Aging Knowledge Environ. 2004;2004:re4. doi: 10.1126/sageke.2004.24.re4. [DOI] [PubMed] [Google Scholar]
- 15.Sclafani A, Gorman AN. Effects of age, sex, and prior body weight on the development of dietary obesity in adult rats. Physiol Behav. 1977;18:1021–1026. doi: 10.1016/0031-9384(77)90006-3. [DOI] [PubMed] [Google Scholar]
- 16.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 17.Kaszubska W, Falls HD, Schaefer VG, Haasch D, Frost L, Hessler P, Kroeger PE, White DW, Jirousek MR, Trevillyan JM. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol. 2002;195:109–118. doi: 10.1016/s0303-7207(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 18.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 19.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 20.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 21.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 23.Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab. 2005;289:E1051–E1057. doi: 10.1152/ajpendo.00094.2005. [DOI] [PubMed] [Google Scholar]
- 24.Xie L, Lee SY, Andersen JN, Waters S, Shen K, Guo XL, Moller NP, Olefsky JM, Lawrence DS, Zhang ZY. Cellular effects of small molecule PTP1B inhibitors on insulin signaling. Biochemistry. 2003;42:12792–12804. doi: 10.1021/bi035238p. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Scarpace PJ. Circumventing central leptin resistance: lessons from central leptin and POMC gene delivery. Peptides. 2006;27:350–364. doi: 10.1016/j.peptides.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 26.Barzilai N, Rossetti L. Relationship between changes in body composition and insulin responsiveness in models of the aging rat. Am J Physiol. 1995;269:E591–E597. doi: 10.1152/ajpendo.1995.269.3.E591. [DOI] [PubMed] [Google Scholar]
- 27.Sclafani A, Springer D. Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes. Physiol Behav. 1976;17:461–471. doi: 10.1016/0031-9384(76)90109-8. [DOI] [PubMed] [Google Scholar]
- 28.Shek EW, Scarpace PJ. Resistance to the anorexic and thermogenic effects of centrally administrated leptin in obese aged rats. Regul Pept. 2000;92:65–71. doi: 10.1016/s0167-0115(00)00151-8. [DOI] [PubMed] [Google Scholar]
- 29.Lee H, Xie L, Luo Y, Lee SY, Lawrence DS, Wang XB, Sotgia F, Lisanti MP, Zhang ZY. Identification of phosphocaveolin-1 as a novel protein tyrosine phosphatase 1B substrate. Biochemistry. 2006;45:234–240. doi: 10.1021/bi051560j. [DOI] [PubMed] [Google Scholar]
- 30.Liang F, Lee SY, Liang J, Lawrence DS, Zhang ZY. The role of protein-tyrosine phosphatase 1B in integrin signaling. J Biol Chem. 2005;280:24857–24863. doi: 10.1074/jbc.M502780200. [DOI] [PubMed] [Google Scholar]
- 31.Shen K, Keng YF, Wu L, Guo XL, Lawrence DS, Zhang ZY. Acquisition of a specific and potent PTP1B inhibitor from a novel combinatorial library and screening procedure. J Biol Chem. 2001;276:47311–47319. doi: 10.1074/jbc.M106568200. [DOI] [PubMed] [Google Scholar]
- 32.Sun JP, Fedorov AA, Lee SY, Guo XL, Shen K, Lawrence DS, Almo SC, Zhang ZY. Crystal structure of PTP1B complexed with a potent and selective bidentate inhibitor. J Biol Chem. 2003;278:12406–12414. doi: 10.1074/jbc.M212491200. [DOI] [PubMed] [Google Scholar]
- 33.Ahima RS. Leptin and the neuroendocrinology of fasting. Front Horm Res. 2000;26:42–56. doi: 10.1159/000061014. [DOI] [PubMed] [Google Scholar]
- 34.Lund IK, Hansen JA, Andersen HS, Moller NP, Billestrup N. Mechanism of protein tyrosine phosphatase 1B-mediated inhibition of leptin signalling. J Mol Endocrinol. 2005;34:339–351. doi: 10.1677/jme.1.01694. [DOI] [PubMed] [Google Scholar]
- 35.Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 36.Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145:4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- 37.Mehebik N, Jaubert AM, Sabourault D, Giudicelli Y, Ribiere C. Leptin-induced nitric oxide production in white adipocytes is mediated through PKA and MAP kinase activation. Am J Physiol Cell Physiol. 2005;289:C379–C387. doi: 10.1152/ajpcell.00320.2004. [DOI] [PubMed] [Google Scholar]
- 38.Irani BG, Haskell-Luevano C. Feeding effects of melanocortin ligands—a historical perspective. Peptides. 2005;26:1788–1799. doi: 10.1016/j.peptides.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad F, Considine RV, Bauer TL, Ohannesian JP, Marco CC, Goldstein BJ. Improved sensitivity to insulin in obese subjects following weight loss is accompanied by reduced protein-tyrosine phosphatases in adipose tissue. Metabolism. 1997;46:1140–1145. doi: 10.1016/s0026-0495(97)90206-7. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad F, Azevedo JL, Cortright R, Dohm GL, Goldstein BJ. Alterations in skeletal muscle protein-tyrosine phosphatase activity and expression in insulin-resistant human obesity and diabetes. J Clin Invest. 1997;100:449–458. doi: 10.1172/JCI119552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusari J, Kenner KA, Suh KI, Hill DE, Henry RR. Skeletal muscle protein tyrosine phosphatase activity and tyrosine phosphatase 1B protein content are associated with insulin action and resistance. J Clin Invest. 1994;93:1156–1162. doi: 10.1172/JCI117068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenner KA, Hill DE, Olefsky JM, Kusari J. Regulation of protein tyrosine phosphatases by insulin and insulin-like growth factor I. J Biol Chem. 1993;268:25455–25462. [PubMed] [Google Scholar]
- 43.Lam NT, Lewis JT, Cheung AT, Luk CT, Tse J, Wang J, Bryer-Ash M, Kolls JK, Kieffer TJ. Leptin increases hepatic insulin sensitivity and protein tyrosine phosphatase 1B expression. Mol Endocrinol. 2004;18:1333–1345. doi: 10.1210/me.2002-0193. [DOI] [PubMed] [Google Scholar]
- 44.Gum RJ, Gaede LL, Koterski SL, Heindel M, Clampit JE, Zinker BA, Trevillyan JM, Ulrich RG, Jirousek MR, Rondinone CM. Reduction of protein tyrosine phosphatase 1B increases insulin-dependent signaling in ob/ob mice. Diabetes. 2003;52:21–28. doi: 10.2337/diabetes.52.1.21. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Matheny M, Nicolson M, Tumer N, Scarpace PJ. Leptin gene expression increases with age independent of increasing adiposity in rats. Diabetes. 1997;46:2035–2039. doi: 10.2337/diab.46.12.2035. [DOI] [PubMed] [Google Scholar]
- 46.Schemmel R, Hu D, Mickelsen O, Romsos DR. Dietary obesity in rats: influence on carbohydrate metabolism. J Nutr. 1982;112:223–230. doi: 10.1093/jn/112.2.223. [DOI] [PubMed] [Google Scholar]