Abstract
Prenatal dietary sodium restriction produces profound developmental effects on rat functional taste responses and formation of neural circuits in the brainstem. Converging evidence indicates that the underlying mechanisms for these effects are related to a compromised nutritional state and not to direct stimulus-receptor interactions. We explored whether early malnourishment produces similar functional and structural effects to those seen following dietary sodium restriction by using a protein deficient, sodium replete diet. To determine if early dietary protein-restriction affects the development of the peripheral gustatory system, multi-fiber neurophysiological recordings were made from the chorda tympani nerve and anterograde track tracing of the chorda tympani nerve into the nucleus of the solitary tract (NTS) was accomplished in rats fed a protein-restricted or a control diet (6% and 20%, respectively). The dietary regimens began on embryonic day 7 and continued until rats were used for neurophysiological recordings (postnatal days (P) 35–50) or for chorda tympani terminal field labeling (P40–50). Responses to a concentration series of NaCl, sodium acetate, KCl, and to 0.50 M sucrose, 0.03 M quinine-HCl, and 0.01 N HCl revealed attenuated responses (30–60%) to sodium-specific stimuli in rats fed the 6% protein diet compared with those fed the 20% protein diet. Responses to all other stimuli were similar between groups. Terminal field volumes were nearly twofold larger in protein-restricted rats compared with controls, with the differences located primarily in the dorsal-caudal zone of the terminal field. These results are similar to the results seen previously in rats fed a sodium-restricted diet throughout pre- and postnatal development, suggesting that dietary sodium- and protein-restriction share similar mechanisms in altering gustatory development.
Keywords: nucleus of the solitary tract, gustation, development, prenatal diet, plasticity
Sensory systems adapt to environmental influences by coordinated alterations in structure and function, often referred to as “plastic” changes. Unlike other sensory systems, there is a remarkable amount of environmentally induced plasticity resident in peripheral and brainstem gustatory structures. Some of the most consistent and largest alterations in the gustatory system occur to manipulations of stimuli that also impact other homeostatic systems, especially when the environmental manipulation is instituted during early development.
Perhaps the most dramatic examples of functional and structural plasticity occur in the gustatory system through prenatal dietary manipulations. For example, rats placed on a sodium-restricted diet from embryonic day 3 (E3) through adulthood have a specific and dramatic attenuation of sodium salt taste responses in the chorda tympani nerve, the nerve that innervates taste buds on the anterior tongue (Hill et al., 1986; Hill, 1987; Hill and Przekop, 1988). Oddly, the sodium-restricted diet must be introduced before E10 to produce the functional changes in receptor cells that are due in large part to functional alterations attributed to the epithelial sodium channel (ENaC) on taste receptor cells (Hill et al., 1986; Hill, 1987; Hill and Przekop, 1988; Ye et al., 1993). This early age (E10) is just before the birth of cells in the geniculate ganglia, which contains the soma of the chorda tympani nerve, and just before the tongue first appears (Mistretta, 1972; Altman and Bayer, 1982). These functional changes can be reversed; sodium taste responses in the chorda tympani nerve can return to control levels within 10–15 days after sodium is reintroduced into the diet, even if the replete diet is presented at adulthood (Hill et al., 1986; Hill, 1987; Hill and Przekop, 1988). However, the absorption of sodium is a necessary condition for recovery to occur. Taste stimulation alone fails to restore function (Przekop et al., 1990).
In addition to altered sodium responses, dietary sodium restriction throughout development leads to selective morphological changes in the terminal fields of taste nerves at the first central synaptic relay, in the nucleus of the solitary tract (NTS). In particular, the chorda tympani terminal field is dramatically larger and abnormally shaped in sodium-restricted rats compared with sodium-replete rats (King and Hill, 1991; May and Hill, 2006; Sollars et al., 2006). The fourfold enlargement of the chorda tympani terminal field compared with controls occurs even if dietary sodium restriction is limited to E3–E12 (Krimm and Hill, 1997), reflecting an altered developmental pattern (Mangold and Hill, 2008). Collectively, the results from early dietary sodium-restriction studies show that 1) the diet must be instituted before the peripheral system is established, 2) recovery of salt taste function requires absorption of sodium and can occur even at adulthood, and 3) the developmental program producing altered terminal fields is set before significant peripheral and central gustatory development. These three findings all show that the underlying mechanisms responsible for the neurobiological effects are not simply due to direct stimulation of taste receptors with the appropriate taste stimuli, but are consistent with more global effects that indirectly impact gustatory development. Nutrition may be a key variable in producing such global effects.
Whereas alterations in peripheral taste responses to sodium and the enlarged chorda tympani terminal field in the central gustatory pathway are hallmarks of early dietary sodium restriction, some of the most striking effects of the diet is the animal’s small size and retarded postnatal growth, and the rapid growth rates following dietary recovery (Hill et al., 1986; Hill, 1987; Hill and Przekop, 1988). It is obvious that the diet has widespread effects and is not limited to the gustatory system. Indeed, there are indications that sodium-restricted rats are undernourished (i.e. they are 63% of control body weights at postnatal day 30; P30), and therefore, the consequences attendant to stunted growth may contribute to the lack of gustatory maturation (Hill et al., 1986; Hill, 1987; Hill and Przekop, 1988).
Severe dietary sodium restriction may trigger a cascade of nutritionally related events that impact both somatic growth and gustatory development. For example, there is a clear relationship between sodium and placental transport of nutrients (Novak et al., 1996; Ashworth et al., 2001; McClellan and Novak, 2001; Page et al., 2003; Finch et al., 2004), suggesting that sodium-restriction during early development has a significant effect on pre- and postnatal growth. Furthermore, insulin-like growth factor levels are significantly decreased by malnourishment resulting in smaller fetuses (Fowden, 2003; Gluckman and Pinal, 2003; Gicquel and Le Bouc, 2006), are decreased in sodium-restricted rats (Sollars and Hill, unpublished observations), and impact the functional development of the ENaCs (Blazer-Yost and Cox, 1988), the channel involved in sodium taste transduction (DeSimone and Ferrell, 1985; Avenet and Lindemann, 1988). To test the hypothesis that the functional and structural effects in the gustatory system may be at least partially due to nutritional effects, we sought an experimental dietary manipulation that results in dramatically stunted growth similar to sodium-restricted rats, but has a full complement of dietary sodium and has been widely used in animal models of malnutrition.
A classic and widely used experimental manipulation used to produce malnourishment is through dietary protein restriction (Forbes et al., 1977; Resnick et al., 1979; Resnick and Morgane, 1983; Morgane et al., 1993). Protein is a significant and necessary component involved in neurological development. Briefly, amino acids serve as precursors of enzymes, neurotransmitters, and structural proteins that are necessary for the proper growth of the brain (Morgane et al., 1993; Tonkiss et al., 1993). Therefore, amino acids are not only vital for protein synthesis, but also ensure the proper structural and functional development of the nervous system. Protein restriction also characteristically produces a severely malnourished animal, where body weights are significantly lower than controls (Forbes et al., 1977; Galler and Tonkiss, 1998). Finally, prenatal protein malnutrition produces more severe alterations in neurological development than when protein restriction is limited to postnatal ages (Morgane et al., 1993), similar to what was found for sodium-restricted rats (Krimm and Hill, 1997; Mangold and Hill, 2007).
Due to the similarities in magnitude and timing between sodium and protein restriction in yielding low body weights and a wide array of common neurological consequences, it is possible that a generalized malnourishment effect rather than a specific sodium-restriction effect is responsible for the results seen in sodium-restricted animals. The current study tests the hypothesis that a protein-restricted diet replete in sodium will have similar neurobiological effects on the developing gustatory system compared with the effects resulting from the sodium-restricted diet. We used peripheral functional measurements (chorda tympani nerve recordings) and central morphological measurements (chorda tympani nerve terminal field organization) to begin such comparisons.
EXPERIMENTAL PROCEDURES
All animal procedures were done in accordance with NIH guidelines for humane handling of animals, and the Institutional Animal Care Committee at the University of Virginia approved all protocols. All efforts were made to minimize the number of animals used and their suffering.
Animals
Breeding pairs of Sprague–Dawley rats obtained from Harlan Sprague-Dawley (Dublin, VA, USA) were fed a standard rat chow (20% protein) ad libitum for the first 6 days of breeding. On the seventh day, the breeding pairs were separated, and the dam’s diet was switched to either a 6% protein diet (protein-restricted) or a 20% protein diet (control) (Harlan Teklad, Madison, WI, USA). Dams remained on their specified diet until their offspring were weaned at P21. Pups were then weaned to the same diet previously fed to the dam. The percentage of protein in the diet of restricted and control animals was chosen based on previous research (Morgane et al., 1993; Gressens et al., 1997; Muaku et al., 1997; Kehoe et al., 2001).
All litters (n = 8; four litters/dietary group) were culled to eight pups on P1, with an attempt to retain equal numbers of males and females. Body weights were measured every day until weaning on P21. After weaning, rats were weighed every 5 days until used for neurophysiological recordings or for labeling the chorda tympani terminal nerve.
Body weight data analysis
A repeated measures ANOVA was used to detect differences in body weight from P1–P50, and factorial ANOVAs with post hoc tests were used to detect diet and sex differences. A level of P≤0.05 was regarded as significant in all analyses.
Neurophysiological recordings
Chorda tympani dissection
In order to examine the effects of protein restriction on whole-nerve responses, the chorda tympani nerve was dissected using a method adapted from Hill et al. (1986). At P35–P50, protein-restricted (n = 6) and control rats (n = 6) were anesthetized with urethane (0.9 mg/kg; i.p.) and were placed on a water-circulating heating pad to maintain body temperature. The hypoglossal nerves were transected bilaterally to reduce tongue movements. The animal was then tracheotomized to prevent stimuli from entering the lungs. A non-traumatic head-holder was then used to position the animal (Erickson, 1966). The left zygomatic arch was removed and the left mandible was retracted. The chorda tympani nerve was exposed from overlying tissue by lateral dissection. The nerve was cut near its entrance into the tympanic bulla and desheathed. The chorda tympani nerve was then placed on a platinum electrode with an indifferent electrode in nearby tissues (Hill et al., 1986).
Neurophysiology
Multifiber neural activity from the whole nerve was amplified and monitored with an audio amplifier. For data analysis, the amplified signal was passed through an integrator with a time constant of 0.5 s. The electrical summated activity was displayed and analyzed using Chart5 for Windows (PowerLab, ADInstruments, Colorado Springs, CO, USA). This measure of neural response reflects the summation of single-fiber responses and is an appropriate measure for studying responses from a large population of taste buds (Beidler, 1954).
Stimuli and stimulation procedure
Whole nerve recordings were obtained by stimulating the anterior tongue with concentration series of NaCl, sodium acetate, and KCl. Concentration series of these stimuli were delivered in ascending order (0.05 M, 0.10 M, 0.25 M, and 0.50 M). Additional stimuli included HCl (0.01 N), quinine (0.03 M), and sucrose (0.50 M). All chemicals were dissolved in distilled water and kept at room temperature. Three milliliters of each stimulus at a rate of about 1 ml/s were delivered to the anterior tongue by a syringe and remained on the tongue for 25 s. The tongue was then rinsed with distilled water for at least 1 min. The next stimulus was delivered 35 s after rinsing, thus completing a 2 min cycle for each stimulus. The stability of responses was monitored by comparing 0.50 M NH4Cl responses evoked immediately before and after the concentration series. Responses were considered stable when NH4Cl response magnitudes on each side of the concentration series differed by less than 10%.
Once the recordings for all the stimuli were obtained, a cotton swab soaked in 50 μM amiloride solution was placed on the tongue for 2 min. Amiloride is an ENaC transport blocker that effectively suppresses sodium salt taste responses in adult rats (Heck et al., 1984). A concentration series to NaCl dissolved in 50 μM amiloride solution was then recorded. Rinses consisted of 50 μm amiloride in distilled water. The stability of the responses was also monitored by comparing relative NaCl-amiloride responses to 0.50 M NH4Cl that was also dissolved in the 50 μM amiloride solution.
Data analysis
Responses to each stimulus were measured using Chart5 for Windows (PowerLab, ADInstruments) to calculate an average magnitude for each stimulus response relative to the baseline. Steady-state response magnitudes above spontaneous levels were measured and averaged across 5–15 s following stimulus application. The first 5 s of each stimulus response was excluded from the response magnitude to eliminate responses resulting from tactile stimulation of the tongue and to ensure that responses adapted to a steady state (Hill et al., 1986). The mean response magnitudes to 0.50 M NH4Cl recorded before and after the concentration series were calculated in the same way. The response magnitudes to NH4Cl that bracketed a stimulus series were averaged to give a standardized 0.50 M NH4Cl response. Relative response ratios were calculated by dividing the mean response magnitude of each stimulus by the standardized 0.50 M NH4Cl response.
Independent t-tests were then performed to determine significant differences (P≤0.05) between protein-restricted (n = 6) and control rats (n = 6) for each stimulus.
Chorda tympani terminal field labeling
Anterograde labeling
In order to visualize the chorda tympani nerve terminal field within the NTS, the right chorda tympani nerve which projects exclusively to the ipsilateral NTS was labeled with an anterograde neural tracer. Both groups of rats fed the 6% protein diet (n = 7) and the 20% protein diet (n = 6) were anesthetized with a 100 mg/kg injection of ketamine, i.m. Supplementary injections were given as needed, and body temperature was maintained at 36 °C using a water-circulating heating pad. The rat was then placed in a non-traumatic headholder (Erickson, 1966). A ventral approach was used to expose the chorda tympani nerve within the right tympanic bulla (May and Hill, 2006; Sollars et al., 2006). A longitudinal incision in the ventromedial portion of the neck was made, and the masseter muscle and the posterior belly of the digastricus muscle were retracted. A small hole was made in the tympanic bulla to expose the chorda tympani nerve. The chorda tympani nerve was then cut near the geniculate ganglia and dimethyl sulfoxide (DMSO) was placed on the nerve for approximately 1 min. Once the DMSO had been removed and the area surrounding the nerve was relatively dry, crystals of 3 kD biotinylated dextran amine (BDA; Molecular Probes, Inc., Eugene, OR, USA) were placed on the proximal end of the cut chorda tympani nerve. No attempt was made to deliver the same amount of BDA among rats; rather, the tracer was applied to obtain a gelatinous-like consistency over the cut end of the nerve for all rats. This is the procedure consistently used in previous studies that yielded high fidelity labels in rats of different ages and experimental conditions (Sollars and Hill, 2000; May and Hill, 2006; Mangold and Hill, 2007, 2008). There were no noticeable differences between groups in accessing the chorda tympani nerve and in labeling it with BDA. The incision was then sutured, and the rat remained on the heating pad until it recovered from the anesthetic. Rats were killed approximately 24 h following surgery. Previous work (May and Hill, 2006; Sollars et al., 2006) and pilot experiments showed that this period was optimum in transport of BDA to the NTS. Rats were perfused transcardially with Krebs solution (pH 7.3) containing 0.5% glucose followed by 8% paraformaldehyde (pH 7.0).
Tissue preparation
Brains were removed and postfixed in 4% paraformaldehyde overnight. The cerebellum was removed, and the medulla was blocked and sectioned on a vibratome horizontally at 50 μm throughout the entire NTS. Therefore, the entire rostral–caudal and medial–lateral extent of the terminal fields was visualized (Davis, 1988; Whitehead, 1988; Lasiter et al., 1989). The 50 μm sections of tissue were collected in 0.1 mM phosphate-buffered saline (PBS; pH 7.4). Sections were then incubated for 1 h in 0.2% Triton X in PBS with streptavidin Alexa Fluor 488 (Molecular Probes) at 1:500 to visualize the chorda tympani terminals labeled with BDA. Sections were then rinsed in PBS three times for 5 min per rinse.
Confocal microscopy and data collection
Chorda tympani terminal fields were imaged using an Olympus IX70 microscope and a Fluoview 3 laser confocal microscope system. The argon laser was used to visualize Alexa 488 (absorption: 495 nm; emission: 519 nm). FluoView 3.3 software was used to run the system and visualize the chorda tympani terminal fields (Olympus America Inc., Melville, NY, USA). Each 50 μm section containing terminal field label was mounted between two coverslips with a 2:1 mixture of glycerol and PBS. Optical images were captured every 3 μm throughout the entire extent of the physical section. The total number of optical sections varied as the thickness of the sections varied through vibratome sectioning. Data were obtained only from brains that showed clear and definable labeled chorda tympani axons entering the rostral pole of the NTS (see below). Tissue in which the axons were not robustly labeled was not used in the analyses (approximately 20% for both groups).
Data analysis
Chorda tympani terminal field volume was quantified using Neurolucida software version 4.34 (MicroBrightField, Inc., Colchester, VT, USA). The perimeter of each labeled terminal field was outlined and the area was calculated for each 3 μm optical section. The volume of each 50 μm section was calculated by summing the areas of each optical section and then multiplying by 3 μm, the thickness between optical sections. Total terminal field volume was then calculated by summing the volumes of each 50 μm section. Independent t-tests were performed to determine significant differences (P≤0.05) in total terminal field volume between protein-restricted (n = 7) and control rats (n = 6).
Total NTS volume
Animals
An additional six Sprague–Dawley rats were used to measure the total NTS volume (6% diet, n = 3; 20% diet, n = 3). Rats were deeply anesthetized with 4 mg/kg urethane (ethyl carbamate: Sigma-Aldrich Co., St. Louis, MO, USA; i.p.) and transcardially perfused at approximately P90 with Krebs–Henseleit buffer (pH 7.3) followed by 8% paraformaldehyde (pH 7.2).
Tissue preparation and confocal microscope transmitted light imaging
The procedure has been described previously (Mangold and Hill, 2007). Briefly, brains were removed and post-fixed in 8% paraformaldehyde overnight. The medulla was blocked and sectioned in the horizontal plane on a vibratome at 50 μm and collected and prepared for imaging as described above. Sections were imaged on the scanning confocal microscope using the transmitted light function with the 488 nm argon laser. The NTS is relatively translucent within the brainstem and appears similar to phase-contrast images when imaged with the transmitted light.
Data collection
The NTS volume was measured using Neurolucida computer software (version 4.34, MicroBrightField). To calculate volume, the measurements from all the sections were summed and multiplied by the section thickness (50 μm).
Statistical analysis
Total NTS volumes were compared between rats fed the 6% and the 20% protein diet using a Mann-Whitney nonparametric test. A statistical result with an α level of P≤0.05 is reported as significant.
RESULTS
Survival and body weights
All rats born to mothers placed on the 6% and 20% protein diets survived to the age at which they were used for neurophysiological recordings or chorda tympani terminal field labeling.
A repeated measures ANOVA revealed a significant overall effect for Age (F(26, 728) = 1977.2) and significant interactions of Age×Diet (F(26, 728) = 989.2), Age×Sex (F(26, 728) = 28.8), and Age×Diet×Sex (F(26, 728) = 24.8) (Ps<0.0001). Post hoc tests showed that male rats fed the 6% protein diet weighed significantly more than female rats fed the 6% protein diet from birth through P16 (P>0.05). This is due primarily to the small standard errors for each group and not large differences in body weights (Fig. 1). In contrast, male and female rats fed the 20% diet did not differ for body weights until P25 and after, where males weighed significantly more than females (P<0.001; Fig. 1). Finally, male and female rats fed the 20% protein diet weighed significantly more than their 6% protein diet counterparts from birth through P50 (P<0.0001; Fig. 1). For example, body weights of male and female rats fed the 6% protein diet were approximately 40% of control body weights by P10. Body weight differences between groups continued to diverge throughout the remainder of development (P<0.001; Fig. 1), so that by P50, male and female rats on the 6% protein diet were only 17% and 21% of the weight seen in rats fed the 20% protein diet, respectively (Fig. 1).
Fig. 1.
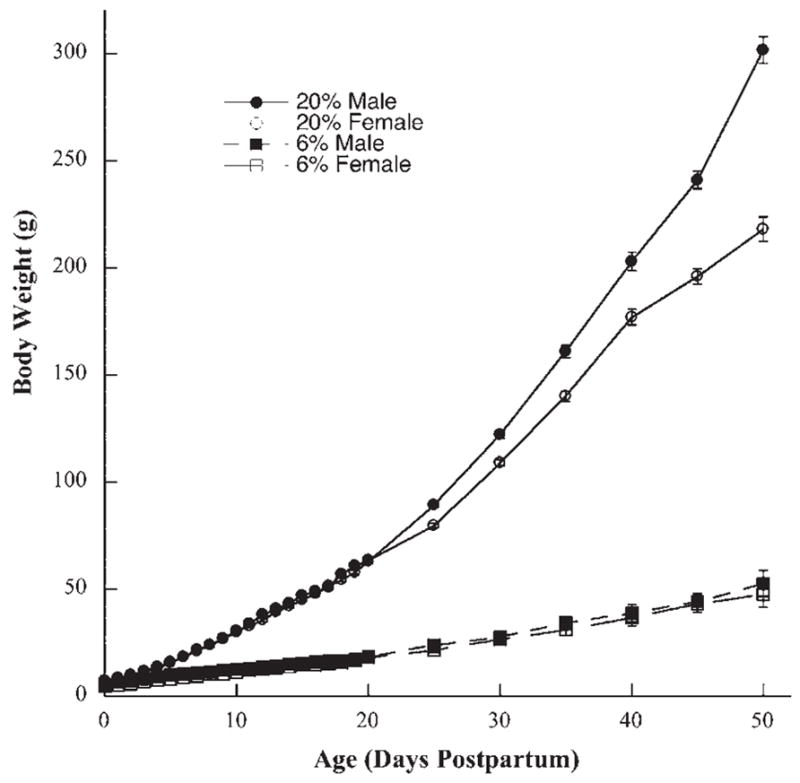
Mean (±S.E.M.) body weights for male (solid symbols) and female (open symbols) rats raised on a 20% protein diet (circles) and rats raised on a 6% protein diet (squares) from birth (0 days) to 50 days postnatal. Mean body weights for rats fed the 6% protein diet were significantly less than rats fed the 20% protein diet beginning at birth and extending through P50.
Multi-fiber neurophysiological responses
Protein restriction maintained throughout development had a significant and selective effect on peripheral taste responses. Responses to all concentrations of sodium salt stimuli (NaCl and sodium acetate) were significantly smaller in rats fed 6% protein throughout development compared with those fed a 20% protein diet (P<0.05) (Figs. 2 and 3). More profound differences in relative responses between groups were observed as the concentration of the sodium-specific stimulus increased (Figs. 2 and 3). For example, responses to 0.5 M NaCl and 0.5 M sodium acetate in 6% protein fed rats were 59% and 50% of that in 20% protein fed rats, respectively (Figs. 2 and 3). The attenuated responses to sodium-specific stimuli in protein-restricted rats were exclusive. Responses to non-sodium containing compounds including KCl, HCl (0.01 N), quinine (0.03 M), and sucrose (0.50 M) displayed no group differences at any concentration (P>0.05; Fig. 4).
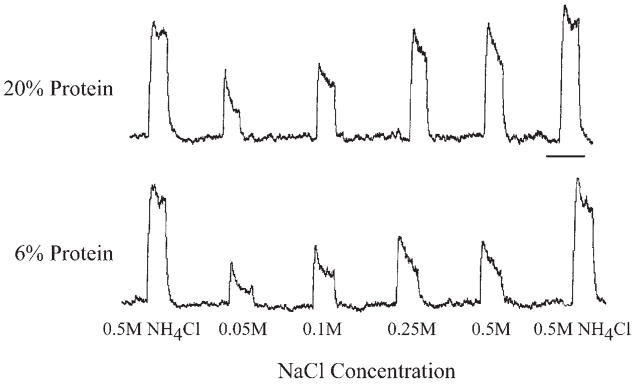
Fig. 2.
Integrated responses from the chorda tympani nerve to a concentration series of NaCl and to 0.5 M NH4Cl in a rat raised on a 20% protein diet (20% protein) and in a rat raised on a 6% protein diet (6% protein). Scale bar=1 min.
Fig. 3.
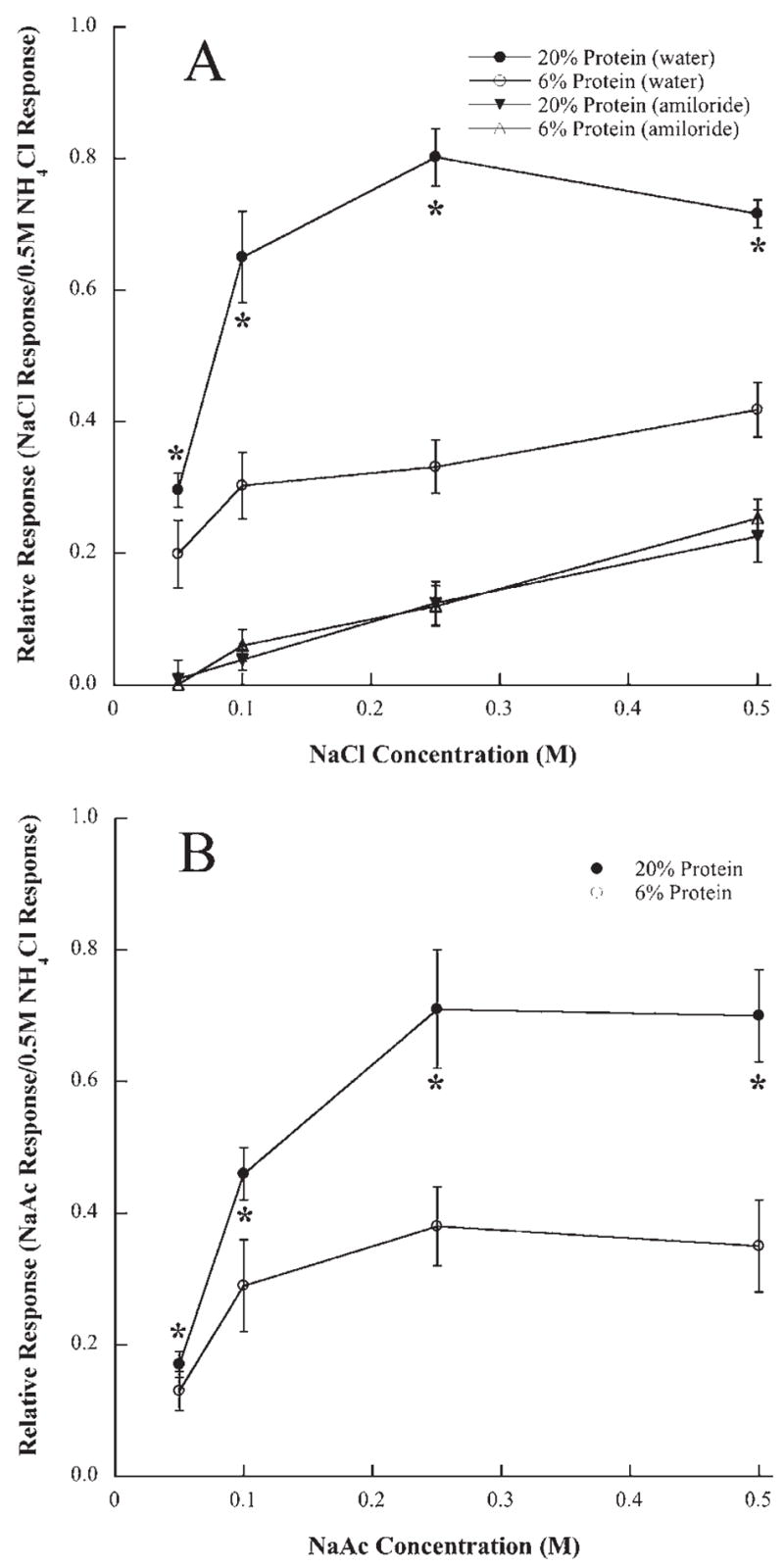
(A) Mean (±S.E.M.) responses of the chorda tympani nerve to a concentration series of NaCl before (circles) and after (triangles) lingual application of amiloride in rats fed a 20% (solid symbols) or a 6% protein (open symbols) diet during development. (B) Mean (± S.E.M.) responses of the chorda tympani nerve to a concentration series of sodium acetate (NaAc) in rats fed a 20% or a 6% protein diet during development. Asterisks denote responses from rats fed the 20% protein diet were significantly higher than those in rats fed the 6% diet (P<0.05).
Fig. 4.
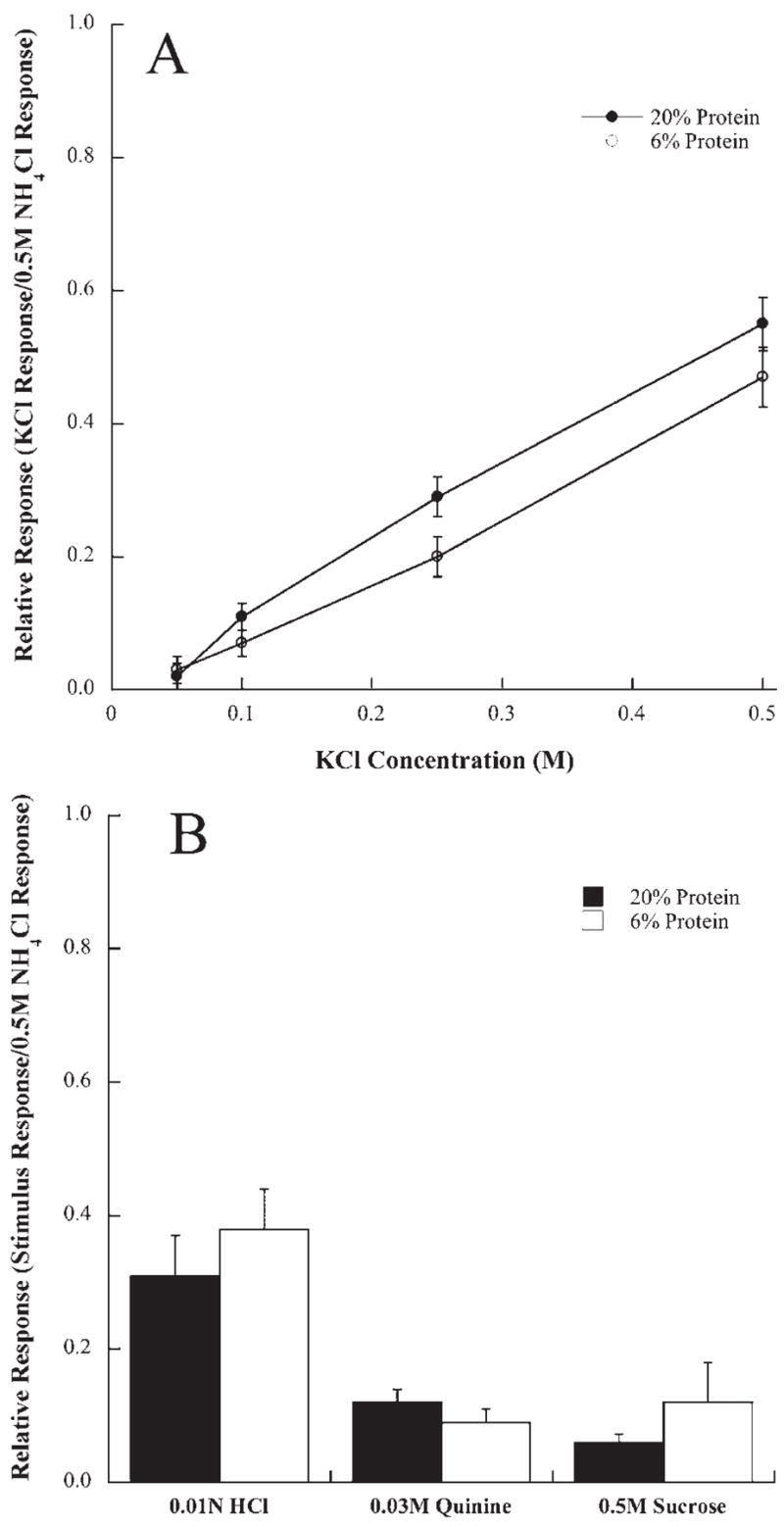
(A) Mean (±S.E.M.) responses of the chorda tympani nerve to a concentration series of KCl in rats fed a 20% (solid circles) or a 6% protein diet (open circles) during development. (B) Mean responses (±S.E.M.) to 0.01 N HCl, 0.03 M quinine HCl, and 0.5 M sucrose in rats fed a 20% protein (solid bars) or a 6% protein diet (open bars).
The group-related differences in relative responses to sodium salt stimuli disappeared following the lingual application of amiloride. That is, amiloride suppressed NaCl responses to the same magnitude between dietary groups. Amiloride suppressed all responses to NaCl by as much as 55–60% in 20% protein fed rats and by 45–50% in 6% protein fed rats (Fig. 3A). The absolute response suppression of chorda tympani responses by amiloride was greater for higher concentrations of NaCl. No significant differences between groups existed after amiloride treatment (P>0.05; Fig. 3A).
Chorda tympani terminal field volumes
Total chorda tympani terminal field volumes
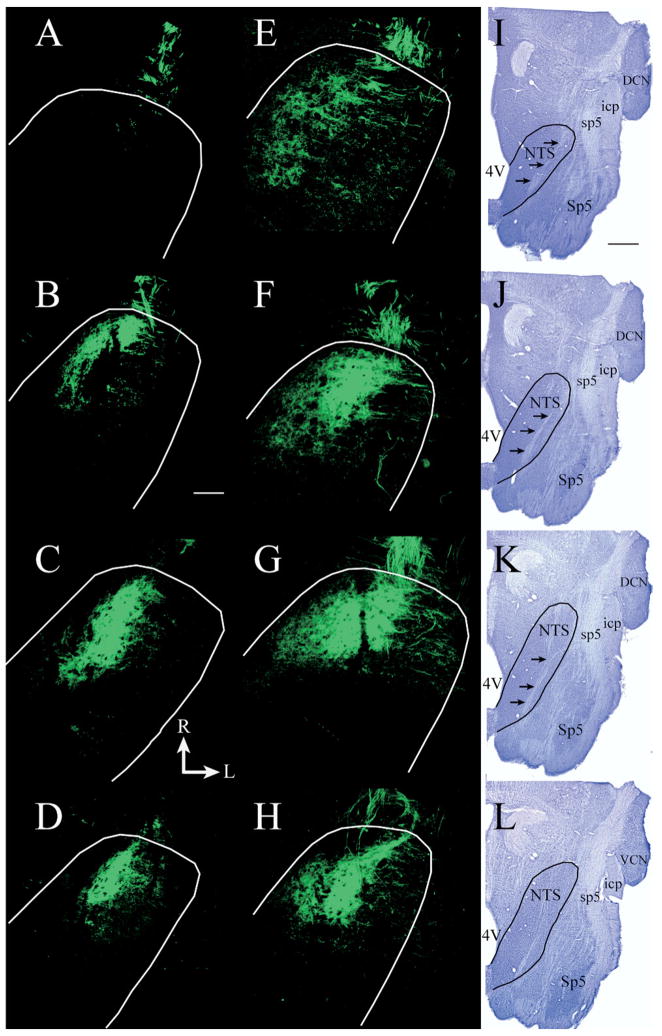
Total terminal field volumes were measured throughout the entire dorsal–ventral extent of the NTS. Only labels that were robust and distinct were included in the data (Fig. 5). Fig. 6 shows that the total mean chorda tympani nerve terminal field volume (±S.E.M.) was about twice as large in rats fed the 6% protein diet (29.0×106 μm3±5.1) than in those fed the 20% protein diet (16.0×106 μm3±3.5; P=0.04). Overall, chorda tympani nerve terminal fields in protein-deprived rats extended more caudally and laterally, particularly in the dorsal zone, compared with controls (Fig. 5).
Fig. 5.
Fluorescent photomicrographs of horizontal sections through the NTS showing the CT terminal field in a rat fed a 20% protein diet (A–D) and in a rat fed a 6% protein diet (E–H) rat. The dorsal-most sections of the fields are shown in A and E, more ventrally located sections from the dorsal zone are shown in B and F, sections from the intermediate zone are shown in C and G, and sections from the ventral zone are shown in D and H. The perimeters of the NTS are shown in white. The CT terminal field in the dorsal zone of the rat fed the 6% protein diet (E, F) is larger than in the rat fed the 20% protein diet (A, B). The appearance of terminal fields in the intermediate (C, G) and in the ventral (D, H) zones is similar for rats fed the 20% and the 6% protein diet. (I–L) Nissl-stained tissue from a separate rat fed the 6% protein diet, illustrating the shape of the NTS and other brainstem structures at the respective dorsal to ventral level to the fluorescent images in the same row. In I–L, the NTS is outlined in black and black arrows point to the solitary tract. R, rostral; L, lateral; M, medial; C, caudal. Scale bar=200 μm in B and refers to A–H. Scale bar=1 mm in I and refers to I–L. 4V, Fourth ventricle; DCN, dorsal cochlear nucleus; icp, inferior cerebellar peduncle; Sp5, spinal trigeminal nucleus; sp5, spinal trigeminal tract; VCN, ventral cochlear nucleus.
Fig. 6.
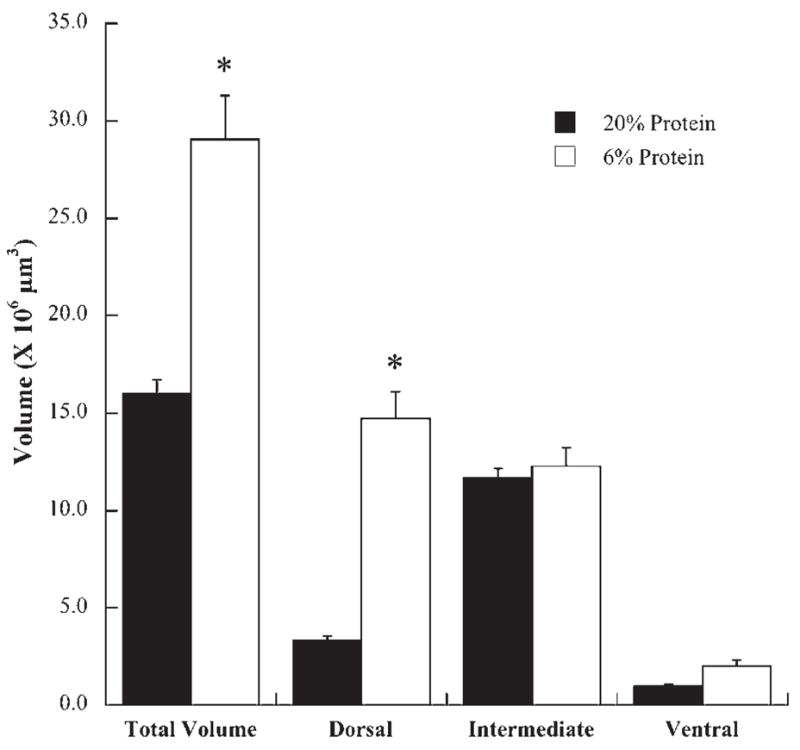
Mean (±S.E.M.) total chorda tympani terminal field volume and terminal field volumes in the dorsal, intermediate, and ventral zones in rats fed a 20% (solid bars) or a 6% protein diet (open bars). Asterisks denote significantly greater than the mean for rats fed the 20% diet.
Zonal distribution of the terminal field
Consistent with previous sodium-restriction studies of the chorda tympani terminal fields (King and Hill, 1991; Krimm and Hill, 1997), the NTS was divided into dorsal, intermediate, and ventral zones to examine the effects of dietary protein-restriction on terminal field volumes. The dorsal zone contained sections in which the solitary tract was most visible and included sections in which the fourth ventricle occupied the largest medial–lateral extent (Fig. 5I, J). The dorsal zone was further characterized by the spinal trigeminal tract extending to approximately the rostral-most extent of the NTS and by the lack of the hypoglossal nucleus (Fig. 5I, J). In controls, the dorsal zone was usually contained within dorsal-most 150–200 μm (i.e. three to four sections; Fig. 5A, B). The intermediate zone (100 μm) was characterized by the decrease in the fourth ventricle volume compared with the dorsal zone, by the dorsal extent of the hypoglossal nucleus, by the extension of the spinal trigeminal tract rostrally beyond the inferior cerebellar peduncle, and by the presence of the dorsal extent of the facial nucleus (Fig. 5K). The chorda tympani terminal field in the intermediate zone appeared more oval in shape (Fig. 5C, G). The ventral zone occupied the final 100–150 μm of tissue (Fig. 5D, H), and was characterized by an expanded hypoglossal nucleus and facial nucleus compared with the intermediate sections (Fig. 5L). This zone was located by the much smaller and more compact chorda tympani terminal field (Fig. 5D, H).
The terms “dorsal,” “intermediate,” and “ventral” zones used here are consistent with earlier reports (King and Hill, 1991; Krimm and Hill, 1997; Sollars et al., 2006) and are used as descriptors of the serial horizontal sections. The orientation of the NTS within the brainstem is such that the caudal portion of the NTS is dorsal to the ventral-most portion. That is, the NTS is inclined from rostral to caudal. Therefore, the “dorsal” zone more accurately represents the dorsal–caudal portion of the field in the NTS and the intermediate and ventral sections represent a more ventral–rostral portion of the terminal field in the NTS.
Chorda tympani terminal field volume by zone
The differences in total terminal field volumes between controls and protein-restricted rats were largely accounted for in the dorsal zone (Figs. 5 and 6) (control: 3.3×106 μm3±0.5; protein-restricted: 14.7×106 μm3±3.2; P=0.007). The dorsal zone in protein-restricted animals extended more caudally and laterally extended than in controls (Fig. 5A, B, E, F). Chorda tympani nerve terminal field volumes in the intermediate zone (Figs. 5C, G and 6) (control: 11.7×106 μm3±1.0; protein-restricted: 12.3×106 αm3±2.2; P = 0.8) and in the ventral zone (control: 1.0×106 μm3±0.2; protein-restricted: 2.0×106 μm3×0.7; P=0.22; Fig. 6D, H) were not different.
NTS volume
Analysis of the NTS volume revealed that adult rats fed the 6% protein diet throughout development had volumes approximately 19% smaller than in adult rats fed the 20% diet (6%: 10.4×108 μm3±0.03; 20%: 12.8×108 μm3±0.02). Therefore, the enlarged chorda tympani nerve terminal field in rats fed the 6% protein diet was contained within an NTS that was significantly smaller (P<0.05) than in rats fed the 20% diet.
DISCUSSION
Dietary protein restriction throughout pre- and postnatal development had major effects on body weights, chorda tympani whole nerve responses, and chorda tympani terminal field volumes in the NTS. Rats fed a protein-restricted diet (6% protein) early in gestation throughout adulthood had significantly lower body weights beginning on P10 compared with rats fed a protein-replete diet (control; 20% protein). The differences in body weight continued into adulthood, indicating a malnourished and severely growth-retarded state. The protein-restricted rats also had selective and highly attenuated peripheral taste responses to sodium-specific stimuli. The application of amiloride eliminated the differences in response magnitudes between control and restricted rats, suggesting that early protein dietary restriction altered functional ENaC development. Group-related differences were also seen in the chorda tympani nerve terminal field volumes in the NTS. The protein-restricted group had total chorda tympani nerve terminal field volumes that were nearly twice as large as controls, with the group-related effects predominantly in the dorsal zone of the chorda tympani terminal field. Moreover, the enlarged terminal field was contained within a relatively smaller NTS, thereby exaggerating the diet-related terminal field volume differences.
Comparisons with previous studies
The results reported here are strikingly similar to previous studies that used dietary sodium-restriction rather than protein-restriction to alter gustatory development (Hill et al., 1986; Hill, 1987; Hill and Przekop, 1988). In both sodium-restricted and protein-restricted rats, whole nerve responses to sodium salt stimuli were lower for all concentrations of NaCl stimuli (Fig. 7). In both types of dietary restricted rats, the response magnitudes to NaCl increased by 60–80% and reached a maximal response when the stimulus concentration of NaCl was increased from 0.05 M to 0.10 M. (Fig. 7). By contrast, the response magnitudes in control rats increased by 60–115% as the stimulus concentration increased from 0.1 M to 0.25 M NaCl (Fig. 7). Therefore, both sodium- and protein-restricted diets produced lowered taste responses to sodium-specific stimuli. These differences in response magnitude compared with controls were not seen with non-sodium stimuli.
Fig. 7.
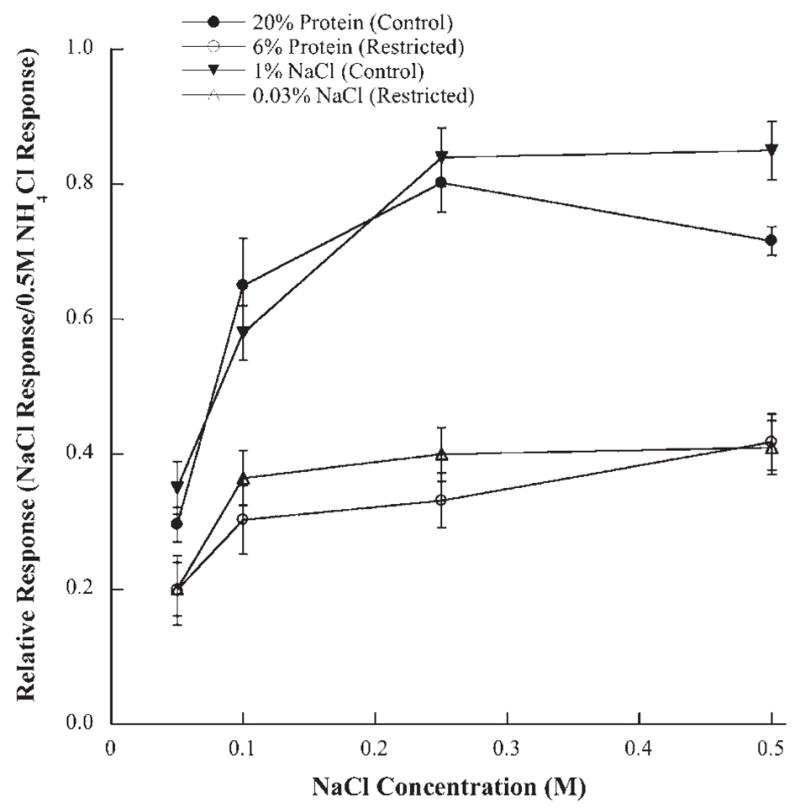
Mean (±S.E.M.) chorda tympani responses to a concentration series of NaCl from rats restricted of protein (6% protein; open circles) or of NaCl (0.03% NaCl; open triangles) compared with their respective control groups (20% protein; solid circles: 1% NaCl; solid triangles). All of the mean responses from the control groups are significantly higher than those from the respective dietary restriction group (P < 0.05).
Earlier studies demonstrated that decreased afferent activity in sodium-restricted rats led to decreased pruning with age and expanded gustatory terminal fields in the NTS (Sollars et al., 2006; Mangold and Hill, 2008). In the current study, protein-restricted rats also had expanded chorda tympani terminal fields in the NTS as well as decreased peripheral neural responses. The chorda tympani terminal field volumes of protein-restricted rats were similar to the volumes seen in sodium-restricted rats (Fig. 6). Consistent also with sodium-restriction studies (King and Hill, 1991; Krimm and Hill, 1997; May and Hill, 2006; Sollars et al., 2006), the dorsal zone of the chorda tympani terminal field was particularly affected by early dietary protein restriction (Fig. 6). The intermediate and ventral zones remained unchanged compared with controls in both protein- and sodium-restricted animals (Fig. 6). Therefore, protein-restriction, like sodium-restriction, produced expanded chorda tympani terminal fields that may have resulted from decreased activity-dependent pruning in the NTS (Sollars et al., 2006). Taken together, there are remarkable similarities in peripheral functional response alterations and terminal field organization between sodium- and protein-restricted rats. Such similarities may relate to a common nutritional-dependent pathway(s).
General malnutrition is responsible for altering gustatory development
Malnutrition is operationally defined as a diet deficient in one or more nutrients (Morgane et al., 1993, 2002). We chose to examine the effects of protein restriction as a means to institute malnutrition because of the significant amount of work relating how dietary protein levels influence somatic growth (Kanarek et al., 1986), the development of dendritic morphology (Benitez-Bribiesca et al., 1999; Cordero et al., 2003), neurogenesis (King et al., 2002, 2004), sensory development (Puthuraya et al., 1980; Sjostrom et al., 1984, 1985, 1987a,b; Almli et al., 1985; Conradi et al., 1985, 1989, 1990; Sjostrom and Conradi, 1987, 1989; Conradi and Sjostrom, 1989; Vilela et al., 2005), as well as influence a variety of behaviors and underlying neural circuits (Cintra et al., 1997; Morgane et al., 2002; Mokler et al., 2003). Clearly, other dietary manipulations may yield similar (or different) results than found here because insufficient amounts of a single nutrient can significantly alter nervous system development. Indeed, it is possible that prenatal restriction of other dietary constituents such as fats, carbohydrates, and essential minerals could lead to the effects seen here; however, it is clear from this study that early protein restriction leads to malformations that represent delayed or arrested nervous system maturation in the gustatory system as noted in other systems (Morgane et al., 1993). Since an absence of either protein or sodium leads to similar alterations in gustatory development, the strikingly similar effects resulting from two distinctly different types of dietary restriction suggest that a common pathway involving malnutrition is responsible for the alterations in gustatory development and function. If malnutrition in general is responsible for altering gustatory development, both protein and sodium-restriction may operate under a similar mechanism(s) that causes these developmental changes. Indeed, there are clear sodium-dependent transport systems related to glucose and amino acid transfer across the placenta (McClellan and Novak, 2001). Therefore, decreased maternal sodium levels in sodium-restricted rats could produce malnutrition effects similar to that produced through protein restriction.
Potentially, maternal dietary restriction may cause the reduction of certain circulating factors like hormones and growth factors that are essential for proper fetal development (Hill and Przekop, 1988). The absence of these circulating factors may compromise ENaC function in taste buds (Hill and Przekop, 1988). A non-functional channel would lead to lowered peripheral responses. Although the candidate circulating factors and pathways are numerous, there are some that are especially attractive because of their regulatory roles on somatic growth and channel function. Specifically, insulin-like growth factor 1 (IGF1) is a key factor in mediating fetal growth (Baker et al., 1993; Liu et al., 1993; Gicquel and Le Bouc, 2006), is decreased in sodium-restricted rats (Sollars and Hill, personal communication), and plays a major role in amino acid transport and in regulation of ENaCs (Blazer-Yost and Cox, 1988; Blazer-Yost et al., 1989). The potential alterations in IGF1 may help explain the specificity of the functional changes in the chorda tympani nerve. Namely, an exclusive down-regulation of the transduction channel used for sodium taste through decreased IGF1 levels would help explain why amiloride reduced the responses in control rats to that seen in protein- or sodium-restricted rats and why the non-sodium stimuli were not affected by dietary manipulations. We emphasize here that only responses from the chorda tympani nerve were examined; therefore, it is possible that other taste modalities (e.g. sugar responses) could be affected in other taste receptor populations (e.g. circumvallate taste buds). Moreover, while IGF1 may provide an attractive scenario, it is possible that other factors play a role in producing the diet-related functional changes seen in peripheral gustatory structures.
For central morphological changes, the dampened afferent activity resulting from lack of ENaC function in protein- and sodium-restricted rats may prevent the proper activity-dependent pruning of the gustatory terminal fields in the developing NTS and have a major effect on circuit formation (Sollars et al., 2006; Mangold and Hill, 2008; May et al., 2008). Activity-dependent changes in central morphology are consistently seen in other developing sensory systems (Hubel and Wiesel, 1970; Catalano and Shatz, 1998; Crowley and Katz, 2002).
Finally, the timing of the dietary insult is a more crucial factor than the type of insult for both dietary sodium and protein restriction, again pointing to a common pathway. For example, dietary sodium restriction must occur on or before E8 to cause changes in peripheral responses to sodium-specific stimuli (Hill and Przekop, 1988), and prenatal sodium-restriction limited to E3–E12 sufficiently and permanently alters the development of the chorda tympani terminal field (Krimm and Hill, 1997; Mangold and Hill, 2007, 2008). Likewise, protein restriction limited to prenatal periods increases tonic inhibition in the hippocampus (Chang et al., 2003); decreases the numerical density of hippocampal CA3 excitatory synapses (Granados-Rojas et al., 2004); and produces smaller hypothalami and a suppressed corticosterone response to acute and chronic isolation stress (Kehoe et al., 2001). The timing of the dietary insult in all of these instances must occur during an early critical period to have a large-scale effect: a time when the nervous system experiences exuberant growth and is susceptible to insult (Morgane et al., 1993, 2002).
Protein-restriction as an effective health-related model
The striking and similar effects caused by protein- and sodium-restriction suggest that malnutrition in general is the primary vehicle for altering gustatory development. Since protein-restriction is likely to be a more relevant and widespread concern in human nutrition than sodium-restriction, the current data may be especially important in providing new insights into the effects of early protein-malnutrition on sensory and brainstem development.
Acknowledgments
This work was supported by NIH grant DC-00407.
Abbreviations
- BDA
biotinylated dextran amine
- DMSO
dimethyl sulfoxide
- E
embryonic day
- ENaC
epithelial sodium channel
- IGF1
insulin-like growth factor 1
- NTS
nucleus of the solitary tract
- P
postnatal day
- PBS
phosphate-buffered saline
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- Almli CR, Henault MA, Velozo CA, Morgane PJ. Ontogeny of electrical activity of main olfactory bulb in freely-moving normal and malnourished rats. Brain Res. 1985;350:1–11. doi: 10.1016/0165-3806(85)90245-7. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer S. Development of the cranial nerve ganglia and related nuclei in the rat. Adv Anat Embryol Cell Biol. 1982;74:1–90. doi: 10.1007/978-3-642-68479-1. [DOI] [PubMed] [Google Scholar]
- Ashworth CJ, Finch AM, Page KR, Nwagwu MO, McArdle HJ. Causes and consequences of fetal growth retardation in pigs. Reprod Suppl. 2001;58:233–246. [PubMed] [Google Scholar]
- Avenet P, Lindemann B. Amiloride-blockable sodium currents in isolated taste receptor cells. J Membr Biol. 1988;105:245–255. doi: 10.1007/BF01871001. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Beidler LM. A theory of taste stimulation. J Gen Physiol. 1954;38:133–139. doi: 10.1085/jgp.38.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Bribiesca L, De la Rosa-Alvarez I, Mansilla-Olivares A. Dendritic spine pathology in infants with severe protein-calorie malnutrition. Pediatrics. 1999;104:e21. doi: 10.1542/peds.104.2.e21. [DOI] [PubMed] [Google Scholar]
- Blazer-Yost BL, Cox M. Insulin-like growth factor 1 stimulates renal epithelial Na+ transport. Am J Physiol. 1988;255:C413–C417. doi: 10.1152/ajpcell.1988.255.3.C413. [DOI] [PubMed] [Google Scholar]
- Blazer-Yost BL, Cox M, Furlanetto R. Insulin and IGF I receptor-mediated Na+ transport in toad urinary bladders. Am J Physiol. 1989;257:C612–C620. doi: 10.1152/ajpcell.1989.257.4.C612. [DOI] [PubMed] [Google Scholar]
- Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- Chang YM, Galler JR, Luebke JI. Prenatal protein malnutrition results in increased frequency of miniature inhibitory postsynaptic currents in rat CA3 interneurons. Nutr Neurosci. 2003;6:263–267. doi: 10.1080/1028415031000151549. [DOI] [PubMed] [Google Scholar]
- Cintra L, Granados L, Aguilar A, Kemper T, DeBassio W, Galler J, Morgane P, Duran P, Diaz-Cintra S. Effects of prenatal protein malnutrition on mossy fibers of the hippocampal formation in rats of four age groups. Hippocampus. 1997;7:184–191. doi: 10.1002/(SICI)1098-1063(1997)7:2<184::AID-HIPO5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Conradi NG, Sjostrom A. Functional development of the visual system in normal and protein-deprived rats. VII. Lamination of oxidative enzyme activity in the visual cortex during post-natal development. Acta Physiol Scand. 1989;136:589–596. doi: 10.1111/j.1748-1716.1989.tb08706.x. [DOI] [PubMed] [Google Scholar]
- Conradi NG, Sjostrom A, Gustafsson B, Wigstrom H. Decreased nerve conduction velocity in optic nerve following early post-natal low-dose lead exposure. Acta Physiol Scand. 1990;140:515–519. doi: 10.1111/j.1748-1716.1990.tb09028.x. [DOI] [PubMed] [Google Scholar]
- Conradi NG, Sjostrom A, Karlsson B, Sourander P. Functional development of the visual system in normal and protein deprived rats. II. Morphometric and biochemical studies on adult optic nerve. Acta Physiol Scand. 1985;125:277–283. doi: 10.1111/j.1748-1716.1985.tb07716.x. [DOI] [PubMed] [Google Scholar]
- Conradi NG, Sjostrom A, Rydenhag B. Functional development of the visual system in normal and protein-deprived rats. VIII. Post-natal development of optic nerve axons. Acta Physiol Scand. 1989;136:597–603. doi: 10.1111/j.1748-1716.1989.tb08707.x. [DOI] [PubMed] [Google Scholar]
- Cordero ME, Valenzuela CY, Rodriguez A, Aboitiz F. Dendritic morphology and orientation of pyramidal cells of the neocortex in two groups of early postnatal undernourished-rehabilitated rats. Brain Res Dev Brain Res. 2003;142:37–45. doi: 10.1016/s0165-3806(03)00013-0. [DOI] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Ocular dominance development revisited. Curr Opin Neurobiol. 2002;12:104–109. doi: 10.1016/s0959-4388(02)00297-0. [DOI] [PubMed] [Google Scholar]
- Davis BJ. Computer-generated rotation analyses reveal a key three-dimensional feature of the nucleus of the solitary tract. Brain Res Bull. 1988;20:545–548. doi: 10.1016/0361-9230(88)90213-4. [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Ferrell F. Analysis of amiloride inhibition of chorda tympani taste response of rat to NaCl. Am J Physiol. 1985;249:R52–R61. doi: 10.1152/ajpregu.1985.249.1.R52. [DOI] [PubMed] [Google Scholar]
- Erickson R. Nontraumatic headholders for mammals. Physiol Behav. 1966;1:97–98. [Google Scholar]
- Finch AM, Yang LG, Nwagwu MO, Page KR, McArdle HJ, Ashworth CJ. Placental transport of leucine in a porcine model of low birth weight. Reproduction. 2004;128:229–235. doi: 10.1530/rep.1.00193. [DOI] [PubMed] [Google Scholar]
- Forbes WB, Tracy C, Resnick O, Morgane PJ. Effects of maternal dietary protein restriction on growth of the brain and body in the rat. Brain Res Bull. 1977;2:131–135. doi: 10.1016/0361-9230(77)90009-0. [DOI] [PubMed] [Google Scholar]
- Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta. 2003;24:803–812. doi: 10.1016/s0143-4004(03)00080-8. [DOI] [PubMed] [Google Scholar]
- Galler JR, Tonkiss J. The effects of prenatal protein malnutrition and cocaine on the development of the rat. Ann N Y Acad Sci. 1998;846:29–39. [PubMed] [Google Scholar]
- Gicquel C, Le Bouc Y. Hormonal regulation of fetal growth. Horm Res. 2006;65 (Suppl 3):28–33. doi: 10.1159/000091503. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Pinal CS. Regulation of fetal growth by the somatotrophic axis. J Nutr. 2003;133:1741S–1746S. doi: 10.1093/jn/133.5.1741S. [DOI] [PubMed] [Google Scholar]
- Granados-Rojas L, Aguilar A, Diaz-Cintra S. The mossy fiber system of the hippocampal formation is decreased by chronic and postnatal but not by prenatal protein malnutrition in rats. Nutr Neurosci. 2004;7:301–308. doi: 10.1080/10284150400017306. [DOI] [PubMed] [Google Scholar]
- Gressens P, Muaku SM, Besse L, Nsegbe E, Gallego J, Delpech B, Gaultier C, Evrard P, Ketelslegers JM, Maiter D. Maternal protein restriction early in rat pregnancy alters brain development in the progeny. Brain Res Dev Brain Res. 1997;103:21–35. doi: 10.1016/s0165-3806(97)00109-0. [DOI] [PubMed] [Google Scholar]
- Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- Hill DL. Susceptibility of the developing rat gustatory system to the physiological effects of dietary sodium deprivation. J Physiol (Lond) 1987;393:413–424. doi: 10.1113/jphysiol.1987.sp016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DL, Mistretta CM, Bradley RM. Effects of dietary NaCl deprivation during early development on behavioral and neurophysiological taste responses. Behav Neurosci. 1986;100:390–398. doi: 10.1037//0735-7044.100.3.390. [DOI] [PubMed] [Google Scholar]
- Hill DL, Przekop PR., Jr Influences of dietary sodium on functional taste receptor development: a sensitive period. Science. 1988;241:1826–1828. doi: 10.1126/science.3175625. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanarek RB, Schoenfeld PM, Morgane PJ. Maternal malnutrition in the rat: effects on food intake and body weight. Physiol Behav. 1986;38:509–515. doi: 10.1016/0031-9384(86)90418-x. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Mallinson K, Bronzino J, McCormick CM. Effects of prenatal protein malnutrition and neonatal stress on CNS responsiveness. Brain Res Dev Brain Res. 2001;132:23–31. doi: 10.1016/s0165-3806(01)00292-9. [DOI] [PubMed] [Google Scholar]
- King CT, Hill DL. Dietary sodium chloride deprivation throughout development selectively influences the terminal field organization of gustatory afferent fibers projecting to the rat nucleus of the solitary tract. J Comp Neurol. 1991;303:159–169. doi: 10.1002/cne.903030114. [DOI] [PubMed] [Google Scholar]
- King RS, DeBassio WA, Kemper TL, Rosene DL, Tonkiss J, Galler JR, Blatt GJ. Effects of prenatal protein malnutrition and acute postnatal stress on granule cell genesis in the fascia dentata of neonatal and juvenile rats. Brain Res Dev Brain Res. 2004;150:9–15. doi: 10.1016/j.devbrainres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- King RS, Kemper TL, DeBassio WA, Ramzan M, Blatt GJ, Rosene DL, Galler JR. Birthdates and number of neurons in the serotonergic raphe nuclei in the rat with prenatal protein malnutrition. Nutr Neurosci. 2002;5:391–397. doi: 10.1080/1028415021000055934. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Hill DL. Early prenatal critical period for chorda tympani nerve terminal field development. J Comp Neurol. 1997;378:254–264. [PubMed] [Google Scholar]
- Lasiter PS, Wong DM, Kachele DL. Postnatal development of the rostral solitary nucleus in rat: dendritic morphology and mitochondrial enzyme activity. Brain Res Bull. 1989;22:313–321. doi: 10.1016/0361-9230(89)90059-2. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (IGF1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Mangold JE, Hill DL. Extensive reorganization of primary afferent projections into the gustatory brainstem induced by feeding a sodium-restricted diet during development: less is more. J Neurosci. 2007;27:4650–4662. doi: 10.1523/JNEUROSCI.4518-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold JE, Hill DL. Postnatal reorganization of primary afferent terminal fields in the rat gustatory brainstem is determined by prenatal dietary history. J Comp Neurol. 2008;509:594–607. doi: 10.1002/cne.21760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May OL, Erisir A, Hill DL. Modifications of gustatory nerve synapses onto nucleus of the solitary tract neurons induced by dietary sodium-restriction during development. J Comp Neurol. 2008;508:529–541. doi: 10.1002/cne.21708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May OL, Hill DL. Gustatory terminal field organization and developmental plasticity in the nucleus of the solitary tract revealed through triple-fluorescence labeling. J Comp Neurol. 2006;497:658–669. doi: 10.1002/cne.21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan R, Novak D. Fetal nutrition: how we become what we are. J Pediatr Gastroenterol Nutr. 2001;33:233–244. doi: 10.1097/00005176-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Mistretta CM. Topographical and histological study of the developing rat tongue, palate and taste buds. In: Bosma JF, editor. Third symposium on oral sensation and perception. Springfield, IL: Thomas; 1972. pp. 163–187. [Google Scholar]
- Mokler DJ, Galler JR, Morgane PJ. Modulation of 5-HT release in the hippocampus of 30-day-old rats exposed in utero to protein malnutrition. Brain Res Dev Brain Res. 2003;142:203–208. doi: 10.1016/s0165-3806(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Austin-LaFrance R, Bronzino J, Tonkiss J, Diaz-Cintra S, Cintra L, Kemper T, Galler JR. Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev. 1993;17:91–128. doi: 10.1016/s0149-7634(05)80234-9. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev. 2002;26:471–483. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Muaku SM, Thissen JP, Gerard G, Ketelslegers JM, Maiter D. Postnatal catch-up growth induced by growth hormone and insulin-like growth factor-I in rats with intrauterine growth retardation caused by maternal protein malnutrition. Pediatr Res. 1997;42:370–377. doi: 10.1203/00006450-199709000-00019. [DOI] [PubMed] [Google Scholar]
- Novak DA, Beveridge MJ, Malandro M, Seo J. Ontogeny of amino acid transport system A in rat placenta. Placenta. 1996;17:643–651. doi: 10.1016/s0143-4004(96)80083-x. [DOI] [PubMed] [Google Scholar]
- Page KR, Ashworth CJ, McArdle HJ, Finch AM, Nwagwu MO. Sodium transport across the chorioallantoic membrane of porcine placenta involves the epithelial sodium channel (ENaC) J Physiol. 2003;547:849–857. doi: 10.1113/jphysiol.2002.031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przekop P, Jr, Mook DG, Hill DL. Functional recovery of the gustatory system after sodium deprivation during development: how much sodium and where. Am J Physiol. 1990;259:R786–R791. doi: 10.1152/ajpregu.1990.259.4.R786. [DOI] [PubMed] [Google Scholar]
- Puthuraya KP, Nayar U, Deo MG, Manchanda SK. Effects of undernutrition on the visually evoked responses in rats during development. Dev Neurosci. 1980;3:162–173. doi: 10.1159/000112389. [DOI] [PubMed] [Google Scholar]
- Resnick O, Miller M, Forbes W, Hall R, Kemper T, Bronzino J, Morgane PJ. Developmental protein malnutrition: influences on the central nervous system of the rat. Neurosci Biobehav Rev. 1979;3:233–246. doi: 10.1016/0149-7634(79)90011-3. [DOI] [PubMed] [Google Scholar]
- Resnick O, Morgane PJ. Animal models for small-for-gestational-age (SGA) neonates and infants-at-risk (IAR) Brain Res. 1983;312:221–225. doi: 10.1016/0165-3806(83)90138-4. [DOI] [PubMed] [Google Scholar]
- Sjostrom A, Conradi NG. Functional development of the visual system in normal and protein-deprived rats. VI. Evoked responses in adult rats, protein deprived in early life and nutritionally rehabilitated from weaning. Acta Physiol Scand. 1987;130:713–721. doi: 10.1111/j.1748-1716.1987.tb08196.x. [DOI] [PubMed] [Google Scholar]
- Sjostrom A, Conradi NG. Functional development of the visual system in normal and protein-deprived rats. IX. Visual evoked response in young rats. Acta Physiol Scand. 1989;136:605–609. doi: 10.1111/j.1748-1716.1989.tb08708.x. [DOI] [PubMed] [Google Scholar]
- Sjostrom A, Conradi NG, Andersson SA. Functional development of the visual system in normal and protein deprived rats. I. Persistent changes in light-induced cortical evoked response. Acta Physiol Scand. 1984;120:585–594. doi: 10.1111/j.1748-1716.1984.tb07424.x. [DOI] [PubMed] [Google Scholar]
- Sjostrom A, Conradi NG, Andersson SA. Functional development of the visual system in normal and protein-deprived rats. IV. Latencies in the specific visual pathway of adult rats. Acta Physiol Scand. 1987a;130:695–703. doi: 10.1111/j.1748-1716.1987.tb08194.x. [DOI] [PubMed] [Google Scholar]
- Sjostrom A, Conradi NG, Andersson SA. Functional development of the visual system in normal and protein-deprived rats. V. Specific cortical response and repetitive stimulation in adult rats. Acta Physiol Scand. 1987b;130:705–711. doi: 10.1111/j.1748-1716.1987.tb08195.x. [DOI] [PubMed] [Google Scholar]
- Sjostrom A, Conradi NG, Gustafsson B, Wigstrom H. Functional development of the visual system in normal and protein deprived rats. III: Recordings from adult optic nerve in vitro. Acta Physiol Scand. 1985;125:353–358. doi: 10.1111/j.1748-1716.1985.tb07729.x. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Hill DL. Lack of functional and morphological susceptibility of the greater superficial petrosal nerve to developmental dietary sodium restriction. Chem Senses. 2000;25:719–727. doi: 10.1093/chemse/25.6.719. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Walker BR, Thaw AK, Hill DL. Age-related decrease of the chorda tympani nerve terminal field in the nucleus of the solitary tract is prevented by dietary sodium restriction during development. Neuroscience. 2006;137:1229–1236. doi: 10.1016/j.neuroscience.2005.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkiss J, Galler J, Morgane PJ, Bronzino JD, Austin-LaFrance RJ. Prenatal protein malnutrition and postnatal brain function. Ann N Y Acad Sci. 1993;678:215–227. doi: 10.1111/j.1749-6632.1993.tb26124.x. [DOI] [PubMed] [Google Scholar]
- Vilela MC, Mendonca JE, Bittencourt H, Lapa RM, Alessio ML, Costa MS, Guedes RC, Silva VL, Andrade da Costa BL. Differential vulnerability of the rat retina, suprachiasmatic nucleus and intergeniculate leaflet to malnutrition induced during brain development. Brain Res Bull. 2005;64:395–408. doi: 10.1016/j.brainresbull.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Whitehead MC. Neuronal architecture of the nucleus of the solitary tract in the hamster. J Comp Neurol. 1988;276:547–572. doi: 10.1002/cne.902760409. [DOI] [PubMed] [Google Scholar]
- Ye Q, Stewart RE, Heck GL, Hill DL, DeSimone JA. Dietary Na+-restriction prevents development of functional Na+ channels in taste cell apical membranes: proof by in vivo membrane voltage perturbation. J Neurophysiol. 1993;70:1713–1716. doi: 10.1152/jn.1993.70.4.1713. [DOI] [PubMed] [Google Scholar]