Abstract
Variation in the monoamine-oxidase-A gene has been associated with volumetric changes in corticolimbic regions with differences in their response to relevant emotional tasks. Here we show no changes in baseline regional brain metabolism as a function of genotype indicating that, unchallenged, corticolimbic activity is not modulated by the MAOA genotype.
Keywords: MAO A, FDG, baseline
1. Introduction
Monoamine oxidase A (MAO-A) degrades neurotransmitters, including serotonin, dopamine and norepinephrine implicated in regulating mood and behavior (Shih & Thompson, 1999). A functional polymorphism in the MAO-A gene promoter is described as 4-tandem-repeats (high MAO-A activity) in 60% and 3-repeats (low MAO-A activity) in 40% in healthy men (Sabol et al., 1998). Studies in humans (Foley et al., 2004) and primates (Newman et al., 2005) support a link between the low MAO-A genotype and susceptibility to antisocial behavior in the face of childhood maltreatment. Functional MRI studies found that healthy subjects with the low MAO-A genotype have mostly reduced volume (Meyer-Lindenberg et al., 2006) and changed cortical activity to cognitive and emotional tasks as compared to the high genotype (Fan et al., 2003; Passamonti et al., 2006; Meyer-Lindenberg et al., 2006).
It is not known, however, whether brain function without a task, at resting baseline would differ as a function of the MAO-A genotype. As compared to fMRI, glucose utilization measured by PET is a quantifiable and absolute measure of brain activity. Using the aggregation of brain activity over time without a specific challenge yet at an alert state, may provide an informative gestalt of baseline neuronal activity. We hypothesized that brain function without any task, therefore without a specific challenge to expose susceptibility, will not show genotype modulated differences in brain metabolism.
2. Method
Thirty-eight male subjects (32±6 years of age) participated in this PET study following a thorough physical examination and interview by a neurologist to verify healthy status. All 38 control subjects were fasting four hours before this PET study. Non-smoking status was ascertained by self-report and verified by breath CO test. Two PET scans were obtained 2 hours apart at rest: a [11C]clorgyline [reported elsewhere (Fowler et al., 2007)] followed by a 18FDG scan to measure glucose metabolism both done at rest.
All subjects provided cheek swab samples containing their DNA which was analyzed for MAO-A (Freeman et al., 2003). Polymerase chain reactions (PCRs) were performed as described previously (Sabol et al., 1998). The PCR products were analyzed on an Applied Biosystems 3100 Genetic analyzer resulting in 12 subjects (32%) having the 3-repeat allele (low) and 26 (68%) having the 4-repeat allele (high), distributions that parallel previous studies (Meyer-Lindenberg et al, 2006). Note that we did not have subjects with 3.5 or 5 repeat alleles in this sample possibly due to the relative rarity of these alleles in the population (less than 2%). There were no significant differences between the two genotype groups on age, education, right hand dominance (Oldfield, 1971), socioeconomic status (Hollingshead, 1975), verbal (Wilkinson, 1993) and non-verbal (Wechsler, 1999) measures of intelligence, and on self-reported depression (Beck et al., 1996) (all P > 0.62). Subjects were fully informed and provided written consent in accordance with the local Institutional Review Board.
The 18FDG scans were acquired on a whole body PET scanner (Siemen’s HR+ 4.5×4.5×4.8 mm at center of field-of-view) in 3D mode providing 63 contiguous planes of 2.4mm each. To stabilize the head, an individually molded head-holder was made for each subject. Subjects were kept supine with their eyes open in a quiet room with a nurse monitoring to prevent sleep and maintain awake state. Catheters were placed in the antecubital vein for radiotracer injection and the radial artery for blood sampling. A transmission scan was obtained with a 68Ge rotating rod source before the emission scan to correct for attenuation before the radiotracer injection.18FDG (Hamacher et al., 1986) (3–5 mCi) was injected. Serial blood samples were taken from time of 18FDG injection through 55 minutes. Emission data were attenuation-corrected and reconstructed using filtered back projection and metabolic images were computed (Reivich et al., 1985).
Data were analyzed using Statistical Parametric Mapping (SPM) (Friston et al., 1995) on the “absolute” and the “relative” (images normalized to the mean metabolic activity of all voxels within the brain) metabolic images. The relative scaling corrects for these individual differences by accounting for differences in global metabolism. For this purpose the metabolic measures were spatially normalized using a 2×2×2 mm3 voxel size and the template provided in the SPM 99 package and subsequently smoothed with a 16 mm isotropic Gaussian kernel. Two separate independent-samples t-tests were performed to compare the absolute and the relative images obtained from the participants during rest.
Manual regions of interest (ROI) were also drawn on the metabolic images of each subject. For ROI placement we re-sliced the metabolic images along the AC-PC line and summed the 63 planes in groups of 2 obtaining 23 planes of 4.76mm thickness with 11 planes above and 11 planes below the thalamus. We applied Talairach and Tournoux (1988) template while manually adjusting the position of the ROI for each individual. Values were computed using the weighted average from the different planes for the regions in Figure 1. Each of these ROI was identified in at least two contiguous slices. To obtain a global metabolic value for each individual we chose 10–12 of these planes (depending on the size of the individual brain) typically choosing 6–7 planes above, and 4–5 planes below the thalamus using a program that thresholded at 20% of the maximum metabolic rate for the plane (Volkow et al., 2006).
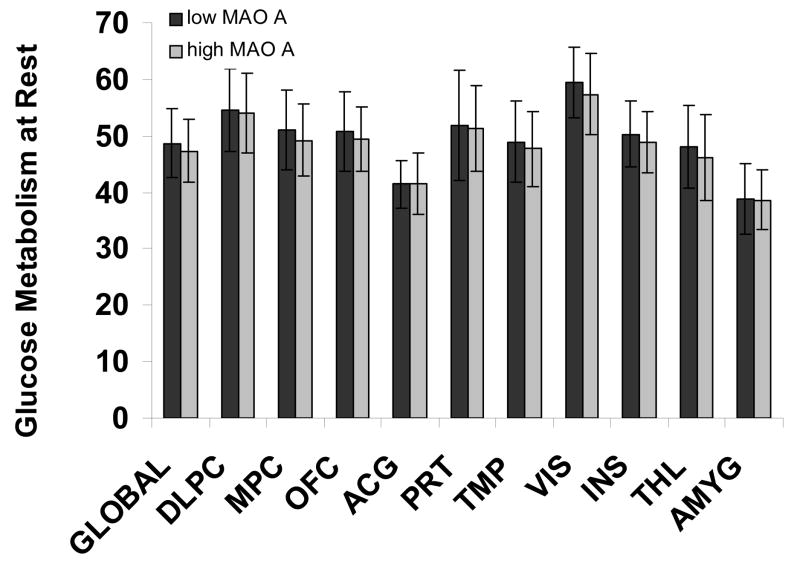
Fig. 1.
Absolute Glucose Metabolism at Rest in the low and high MAO-A Genotype Groups. From manually drawn bilateral (averaged) ROI in dorsolateral prefrontal cortex (DLPC), medial prefrontal cortex (MPC), lateral orbitofrontal cortex (OFC), anterior cingulate gyrus (ACG), parietal cortex (PRT), medial and lateral temporal cortices (TMP), the visual cortex (VIS), insula (INS), thalamus (THL) and amygdala (AMYG). Error bar represent standard deviation.
Whole-brain SPM significance was set at P<0.05, cluster-level corrected, though uncorrected thresholds were also inspected. The ROI from manual drawings are reported at the threshold level of P<0.05, Bonferroni corrected for regions implicated in the MAO A genotype (Buckholtz et al., 2007).
3. Results
Using whole brain analyses in SPM, there were no significant differences in absolute or relative baseline metabolism between the genotyped groups. Reducing the significance threshold to P<0.05, uncorrected, there were still no differences in absolute or relative metabolism. The ROI results confirmed the non significant SPM findings (all P>0.30) (Figure 1).
4. Discussion
Baseline metabolism, a marker of resting brain function (Volkow et al., 2006) did not differ as a function of the MAO-A genotype. Posthoc analysis revealed that the sample size in this study would yield a power of 80% for the t-test to detect differences at effect size 1 (P<0.05, two-tailed). In our study the pooled SDs was about 20% of the population mean. This indicates that the effect of MAO-A genotype on brain metabolism at rest is smaller than the variability in brain metabolism at rest in adult subjects. Failure to see a difference could reflect the need to challenge with a relevant task to detect the differences in activation patterns as a function of genotype. Given volumetric differences in amygdala and cingulate that were observed as a function of genotype (Meyer-Lindenberg et al., 2006) one would expect differences in metabolism unless the lack of challenge created a ceiling effect in these healthy subjects.
This finding mirrors our negative results in the same sample with [11C]clorgyline, a radiotracer with specificity for brain MAO-A (Fowler et al., 2007). It appears that the MAO-A genotype has a modulatory effect on neuronal maturation in utero, and is relevant in childhood only through sensitivity to environmental insult (Caspi et al., 2002) and in adulthood through reactivity to emotional stimuli in fMRI studies (Meyer-Lindenberg, et al., 2006). However, fMRI experiments cannot measure brain function at absolute baseline making it difficult to distinguish differences due to the task challenge from those pertaining to general brain function at baseline (Canli et al., 2005). Although Independent Component Analysis (ICA) can detect baseline fluctuations and map regions that have similar time-varying responses ICA does not provide absolute measures in fMRI. Since we measure a substantial period of time when the brain is not harnessed task demands we call this absolute baseline. Here we show that at resting baseline, the MAO-A genotype does not impart a significant effect on glucose metabolism in brain.
This finding corroborates our hypothesis that in the context of externalizing behavior phenotypes (such as antisocial behavior), significant associations between genotype and behavior emerge primarily in response to a challenge serving to perturb the individual beyond their baseline.
Acknowledgments
This work was carried out at Brookhaven National Laboratory under contract DE-AC-298CH10886 with the U.S. Department of Energy and supported by its Office of Biological and Environmental Research and by NIH-NIDA (K05DA020001), NIH CGRC (MO1RR10710) and by the National Association for Research on Schizophrenia and Depression (NARSAD). We thank the PET team for advice and assistance in different aspects of these studies. We are also grateful to the subjects who volunteered for these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, Egan MF, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Molecular Psychiatry. 2007 doi: 10.1038/sj.mp.4002020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proceedings of the National Academy of Science U S A. 2005;102:12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proceedings of the National Academy of Science U S A. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Archives of General Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Alia-Klein N, Kriplani A, Logan J, Williams B, Zhu W, Craig IW, Telang F, Goldstein R, Volkow ND, Vaska P, Wang GJ. Evidence That Brain MAO-A Activity Does Not Correspond to MAO-A Genotype in Healthy Male Subjects. Biological Psychiatry. 2006;62:355–358. doi: 10.1016/j.biopsych.2006.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA obtained from buccal swabs recruited by mail: An evaluation of the effects of storage on long- term stability and suitability for multiplex PCR genotyping. Behavioral Genetics. 2003;33:67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2:166–72. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Hamacher K, Coenen HH, Stocklin G. Efficient stereospecific synthesis of no carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. Journal Nuclear Medicine. 1986;27:235–238. [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social Class and Mental Illness: A Community Study. New York: John Wiley & Sons; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana BR, Hariri A, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings National Academy of Science U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biological Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Handedness Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fera F, Magariello A, Cerasa A, Gioia MC, Muglia M, Nicoletti G, Gallo O, Provinciali L, Quattrone A. Monoamine oxidase-a genetic variations influence brain activity associated with inhibitory control: new insight into the neural correlates of impulsivity. Biological Psychiatry. 2006;59:334–340. doi: 10.1016/j.biopsych.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Reivich M, Alavi A, Wolf A, Fowler J, Russell J, Arnett C, MacGregor RR, Shiue CY, Atkins H, Anand A. Glucose metabolic rate kinetic model parameter determination in humans: the lumped constants and rate constants for [18F]fluorodeoxyglucose and [11C]deoxyglucose. Journal Cerebral Blood Flow Metabolism. 1985;5:179–192. doi: 10.1038/jcbfm.1985.24. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Human Genetics. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Shih JC, Thompson RF. Monoamine oxidase in neuropsychiatry and behavior. American Journal Human Genetics. 1999;65:593–598. doi: 10.1086/302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Plannar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Volkow ND, Wang GJ, Franceschi D, Fowler JS, Thanos PP, Maynard L, Gatley SJ, Wong C, Veech RL, Kunos G, Kai Li T. Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage. 2006;29:295–301. doi: 10.1016/j.neuroimage.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wilkinson G. The Wide-Range Achievement Test 3- Administration Manual. Wilmington, DE: Wide Range Inc; 1993. [Google Scholar]