Abstract
The twofold cost of sex implies that sexual and asexual reproduction do not coexist easily. Asexual forms tend to outcompete sexuals but may eventually suffer higher extinction rates, creating tension between short- and long-term advantages of different reproductive modes. The ‘short-sightedness’ of asexual reproduction takes a particularly intriguing form in gynogenetic species complexes, in which an asexual species requires sperm from a related sexual host species to trigger embryogenesis. Asexuals are then predicted to outcompete their host, after which neither species can persist. We examine whether spatial structure can explain continued coexistence of the species complex, and assess the evidence based on data on the Amazon molly (Poecilia formosa). A modification of the Levins metapopulation model creates two regions of good prospects for coexistence, connected by a region of poorer patch occupancy levels. In the first case, mate discrimination and/or niche differentiation keep local extinction rates low, and most patches contain both species; the other possibility resembles host–parasite dynamics where parasites frequently drive the host locally extinct. Several dynamical features are counterintuitive and relate to the parasitic nature of interactions in the species complex: for example, high local extinction rates of the asexual species can be beneficial for its own persistence. This creates a link from the evolution of sexual reproduction to that of prudent predation.
Keywords: evolution of sex, metapopulation dynamics, mate choice
1. Introduction
The coexistence of sexual and asexual modes of reproduction is difficult at the best of times. Sexual species suffer from the twofold cost of sex, which implies that they should be quickly outcompeted by asexual forms that use the same resources but can produce eggs at twice the rate (Williams 1975; Maynard Smith 1978; Agrawal 2001, 2006). On the other hand, asexual reproduction appears to be associated with long-term costs such as deleterious mutation accumulation (Muller 1964; Kondrashov 1988; Lynch & Gabriel 1990) and limited ability to adapt to changing environmental conditions (Bell 1982; Waxman & Peck 1999) or higher vulnerability to parasites (reviewed in Hamilton et al. 1990). Asexual lineages are therefore typically fairly short-lived (e.g. Muller 1964; Lynch & Gabriel 1990; Lynch et al. 1993; Crow 1994; Dunbrack et al. 1995; but see ancient asexuals: Judson & Normark (1996)). Indeed, much of the literature on the evolution of sex attempts to weigh the short-term advantage of the twofold benefit of asexual reproduction with the long-term benefits of sex, as well as asking whether sex has significant benefits that act already over shorter time-scales (e.g. Case & Taper 1986; Kondrashov 1988; West et al. 1999; Doncaster et al. 2000; Colegrave 2002; Agrawal 2006).
The ‘short-sightedness’ of asexual reproduction is present in a particularly intriguing and fast-acting form in gynogenetic species (also known as sperm-dependent parthenogenesis, Beukeboom & Vrijenhoek (1998), Schley et al. (2004) and Schlupp (2005)). This is a type of ameiotic unisexual reproduction where offspring are formed parthenogenetically, yet egg development cannot be completed without sperm. Since offspring of such species are all-female, gynogenetic species can only persist in sympatry with closely related bisexual species that offer a continuous supply of donor males. Hence, this mode of reproduction combines certain disadvantages of sexuality with that of asexuality. For example, all-female fish, Amazon mollies, Poecilia formosa, rely on closely related heterospecific males (usually either Poecilia latipinna or Poecilia mexicana; Hubbs & Hubbs 1932) who, in turn, have evolved partial discrimination against Amazon females (which is stronger in regions where Amazons occur, Ryan et al. (1996) and Gabor & Ryan (2001); for additional biological detail, see Heubel et al. (submitted)). The discrimination is clearly not complete (Heubel 2004; Schlupp & Plath 2005; Heubel & Schlupp 2006), however, otherwise the Amazons would have been driven extinct. The short-sightedness of asexuality occurs because, by not suffering the twofold cost of sex, Amazons are quickly expected to outnumber their sexual competitors (Williams 1975; Beukeboom & Vrijenhoek 1998; Schley et al. 2004). However, if this makes it impossible for the sexual species to persist, Amazons obviously lose their access to males and cannot persist either.
The continued coexistence of Amazons and their host species that they only use as sperm donors is therefore a puzzle. Schley et al. (2004) showed that coexistence is indeed impossible unless there are significant differences in the competitive ability of the two species. Coexistence could be enhanced if asexuals have a narrower niche than sexuals (the frozen niche variation hypothesis, Vrijenhoek (1984)). However, this may be a difficult argument to apply for many gynogenetic species complexes. The asexual and sexual species need to be very close relatives for sperm to trigger embryogenesis properly (Hubbs & Hubbs 1932; Schlupp et al. 1998), and speciation in this case is argued to reflect a single hybridization event (Turner 1982; Avise et al. 1991; Schartl et al. 1995) rather than a process of niche differentiation. There is also currently neither evidence of niche differentiation (Heubel 2004) nor differences in reproductive rates in the same environment (Schlupp 2005). Too distinct phenotypes would also make it relatively easy for males to develop preferences for conspecific females (Kawecki 1988; Schmeller et al. 2005) which, once strong enough, could leave asexuals without sufficient sperm to persist (Aspbury & Gabor 2004; Schlupp & Plath 2005; Heubel et al. submitted). While close phylogenetic relatedness is obviously not synonymous with equal competitive ability, it in this case clearly predisposes species to use the same resources, and together with the required physical mating interaction of asexual females and donor males, this makes arguments based on niche differentiation harder than usual to apply.
Elsewhere (Heubel et al. submitted), we examine whether male mate choice or variations in male fertilization efficiency could promote local coexistence. The results suggest that local coexistence is a possibility if males very strongly discriminate against asexual females. However, the most likely outcome is that male mate choice is not sufficient to prevent extinction, yet extinctions with male choice occur in a delayed fashion when compared with no mate choice (Heubel et al. submitted). Here, we will propose and model an alternative explanation for coexistence of sexual and asexual reproduction in gynogenetic species complexes, that can operate even if there are no differences in competitive ability and if mate choice is too weak to guarantee local coexistence. The alternative, also alluded to by Schley et al. (2004), is spatial structure combined with predator–prey (or host–parasite) dynamics. Ecologically, gynogenetic species resemble predators or parasites, in the sense that their reproduction depends on having access to the sexual species that forms the ‘prey’ or ‘host’ species. Indeed, they are called sexual parasites (e.g. Hubbs 1964; Dries 2003; Gumm et al. 2006) or sperm parasites (Beukeboom & Vrijenhoek 1998; Schlupp 2005).
There are important differences to ‘ordinary’ parasitism. Most importantly, sperm parasites do not directly harm the fitness of sperm donors in the way parasites often weaken or kill each individual they infest. However, the situation remains harmless only if neither resources nor sperm availability limits reproduction, which would predict that both Amazons and the host species can achieve their full reproductive potential. This harmlessness cannot be expected to last long over ecological time-scales (Kiester et al. 1981) for two reasons. Firstly, asexual females compete for the same (or similar, Schley et al. (2004)) resources as their sexual sister species and they reproduce faster, thus continually outcompeting sexuals. This alone could lead to extinction of the sexuals (Peck et al. 1999). Secondly, the decline may also be hastened because sperm limitation adds to the damage inflicted on the host. Sperm limitation is caused by an ever-increasing proportion of asexuals inevitably diminishing the ratio of males to females (the latter comprising both sexual and asexual females).
In gynogenesis, as well as in the related context of gynodioecy where populations consist of females and hermaphrodites, these processes can result in population collapse due to sperm (or pollen) shortage (gynogenesis: Kiester et al. 1981, Schley et al. 2004; gynodioecy: Stewart-Cox et al. 2005). In the context of gynodioecy, it has already been shown that spatial structure can help maintain the coexistence of females and hermaphrodites (Stewart-Cox et al. 2005). This result generalizes to a pattern commonly found in predator–prey systems: local coexistence is difficult or impossible to achieve, but spatial structure can dramatically improve the prospects of coexistence for predators and their prey in a metapopulation with local extinctions and colonizations of new patches (e.g. Holyoak & Lawler 1996; Ellner et al. 2001; Bonsall et al. 2002). The possibility that this could apply in the context of gynogenesis too was already raised by Kiester et al. (1981), but this verbal assertion has never been examined mathematically.
2. Material and methods
A model by Schley et al. (2004) showed that asexual gynogenetic species could outcompete sexuals to extinction when they occupied the same ecological niche. However, if such extinctions took place within the context of a metapopulation, it is possible that colonization of patches could sustain the detrimental asexual behaviour. Here, we focus on the dynamics of patch colonization and extinction. A model of within-patch dynamics (Heubel et al. submitted), shows that patches that are invaded by asexuals are often expected to go extinct after a number of generations; this number varies depending on precise assumptions of male fertilization efficiency and to what extent males discriminate against heterospecific matings, but it often falls between 10 and 20 generations. Based on these results, here we simply assume that patches that contain the sperm parasite will have a significantly higher extinction rate than patches inhabited by sexuals only (also, the latter extinction rate may equal zero).
As our framework, we use the Levins metapopulation model (Levins 1969) where the environment consists of a large number of equally sized patches. The model is not spatially explicit. Instead, we are interested in deriving the proportion of patches that are occupied at any given time. In the classic metapopulation setting (Levins 1969), there is only one species that colonizes empty patches at a rate c, and extinction occurs in occupied patches at a rate e. This leads to the equation dp/dt=cp(1−p)−ep for the proportion of occupied patches, p. The solution of dp/dt=0 yields the equilibrium =1−e/c in addition to the trivial equilibrium =0. It is clear that if e>c, the population cannot persist (Levins 1969).
In our two-species case with asexual and sexual fish, we will have three types of patches. In addition to empty patches and patches that are occupied by sexuals only (proportion p), there are also patches that contain both sexuals and asexuals (proportion q). As a simplification, we ignore the possibility that a patch contains asexuals only: as asexuals cannot reproduce on their own, such populations will not persist for a significant amount of time. For the same reason, we assume that the extinction of sexuals in a patch will always cause the immediate extinction of asexuals too.
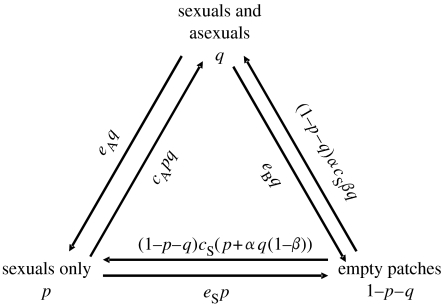
To derive the sexual–asexual dynamics, the parameters c and e in the Levins metapopulation model will be replaced by a list of different colonization and extinction possibilities (see table 1 for notation). We assume that patches in which the sexual species resides alone can go extinct ‘on their own’ (rate eS), but the extinction rate is heightened to eB in patches that contain both species. This reflects the fact that coexistence within a single patch is difficult (Kiester et al. 1981; Schley et al. 2004), which we elsewhere show to be true even with male mate choice (Heubel et al. submitted). The rate eB refers to both species going extinct, due to our assumption that the extinction of asexuals must immediately follow that of the sexuals. Finally, it is also possible that asexuals go extinct on their own, such that the sexual species still survives in the patch (rate eA).
Table 1.
Summary of notation used.
| symbol | description |
|---|---|
| p | proportion of S patches, i.e. containing the sexual species only |
| q | proportion of B patches, i.e. containing both species |
| 1−p−q | proportion of E patches, i.e. empty patches |
| cS | rate at which sexuals, that originate from S patches, colonize empty (E) patches |
| cA | rate at which asexuals (from B patches) colonize sexual (S) patches |
| α | proportion of sexual colonization rate cS that is still maintained if the departure patch contains asexuals |
| β | proportion of colonizations of empty (E) patches which include both sexuals and asexuals, to form patches with both (B) |
| eS | extinction rate of sexual (S) patches |
| eB | extinction rate of patches containing both species (B) |
| eA | rate at which patches containing both species become sexual-only patches due to extinction of the asexuals |
We next define the rules of colonization. It is conceivable that some types of dispersal, such as flooding events, easily lead to both species colonizing new patches together, but it is also likely that some sexual populations become established without their asexual parasites. The asexual species obviously cannot establish new populations alone. Finally, it is possible that the colonization rate of the sexual species depends on whether dispersal occurs from a patch where sexuals exist on their own, or together with their sperm parasites. These assumptions can be captured using the following parameters: the colonization rate of sexuals into empty patches is cS if sexuals exist on their own, but this is changed to αcS when they share a patch with asexuals. If α<1, the presence of asexuals in some way hampers the colonization ability of sexuals, for example, by diminishing the number of sexuals in a patch. When sexuals and asexuals share a patch, a fraction β of sexual colonization events is accompanied by asexuals. Finally, we assume that asexuals can colonize patches in which sexuals alone have resided; this rate of invasion by asexuals is denoted cA.
The rates of extinction and colonization are summarized in figure 1. Taking into account the fraction of patches p and q that provide relevant populations for the colonization and extinction events, we obtain the dynamics,
| (2.1) |
In other words, sexual patches are created through sexuals colonizing empty patches (the frequency of which is 1−p−q), and these sexuals may originate from sexual patches (proportion p, rate cS) or from patches containing both species (proportion q, rate α(1−β)cS). Sexual-only patches can also be created by the asexual species going extinct and leaving sexuals alone in the given patch (proportion q, rate eA). Sexual patches (proportion p) can be destroyed in two ways: they may cease to be sexual-only patches through being invaded by asexuals (at a rate cAq) or they may go extinct by themselves (rate eS). A patch that contains both species (proportion q) can be created by asexuals that colonize a sexual patch or by both species colonizing an empty patch. Such patches cease to exist when both species are driven extinct (rate eB) or if asexuals go extinct (rate eA).
Figure 1.
Model assumptions. See table 1 for details of the notation used, and main text for details.
3. Results
Solving for dp/dt=0 and dq/dt=0 yields the proportion of patches with sexuals only (p), the proportion with both (q) and the total proportion of patches that contain the sexual species (p+q). The trivial equilibrium p=q=0 is always a solution of equation (2.1). Another equilibrium describes the absence of asexuals q=0, and in this case the model unsurprisingly reduces to the classical metapopulation model with sexuals only present, p=1−eS/cS and q=0. This solution obviously requires cS>eS for it to be biologically relevant (Levins 1969). There are also two other solutions, of which one describes biologically relevant p and q values between 0 and 1 (i.e. the coexistence solution). In the case of β>0, the expressions for this equilibrium are very unwieldy. Hence we treat the case β=0 separately and explore the general case β≥0 numerically.
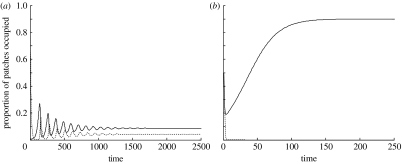
The main question is whether coexistence is possible even if asexual sperm parasites outcompete sexuals and cause their own demise. The local impact of the parasitic asexual fish is strong when the ratio eB/eS is high (i.e. asexuality significantly hastens extinction). An example of the dynamics (figure 2) shows that coexistence is certainly possible despite high eB/eS ratios, even if colonization events by sexuals are always followed by the sperm parasite too (β=1). In figure 2a, the presence of the asexual fish increases the local extinction rate by 25-fold when compared with the background extinction rate of sexuals, eS, and the dynamics show damping cyclicity towards a stable (albeit low) proportion of occupied patches. This prediction remains qualitatively unchanged and also quantitatively very similar (not shown) if the relative importance of asexuals causing extinction is increased, up to the point where they are the only cause of extinction (this is achieved by setting eS=0). However, if the relative harm of the presence of asexuals is increased in an alternative way, by increasing eB while keeping eS unchanged (figure 2b), asexuals will go extinct. The system then equilibrates at the classical one-species Levins equilibrium, with much improved occupancy prospects for the sexual fish species (figure 2b: occupancy proportion for sexual fish is 1−eS/cS=0.9).
Figure 2.
(a,b) Two example dynamics of the system. The proportion of patches occupied by sexuals only (p) is represented by the solid line, while the dashed line represents the proportion of patches colonized by both sexuals and asexuals (q). In (a) there is stable coexistence between sexuals and asexuals (extinction rate for patches containing both sexuals and asexuals eB=0.25), while in (b) the asexuals are driven extinct, and only patches containing the sexual species remain (extinction rate for patches containing both sexuals and asexuals is very high, eB=2.0). Other parameters used in the figure are eA=0.01, eS=0.01, α=1, β=1, cS=0.1 and cA=2.
While figure 2 shows that coexistence is possible in principle, it is important to study the generality of any comparisons. Figure 2 was derived using β=1. Before proceeding to consider a large set of parameter values, we will consider the special case β=0, i.e. we assume that patches do not get colonized simultaneously by both sexuals and asexuals. This case yields interesting analytical insight because the equilibria that potentially describe coexistence simplify greatly. Setting dp/dt=dq/dt=0 in equation (2.1) and simplifying, one obtains candidate equilibria,
| (3.1) |
where and .
Necessary conditions for coexistence are 0≤<1 and 0<+<1. Any solution with coexistence must have <1, and from equation (3.1) it is clear that this implies cA≥eA+eB. This in turn guarantees that B>0, which allows us to reject one-candidate equilibrium for coexistence: we can deduce that , and , regardless of the sign of A. Therefore, we can sum up that coexistence in the case of β=0 requires cA>eA+eB, and the equilibrium is described by
| (3.2) |
The analogy between the conditions cA>eA+eB and cS>eS is clear. The former is required for coexistence, i.e. the parasitic asexual species must be able to persist. The latter describes the condition allowing the sexual species to persist alone. In both cases, a species must be able to colonize suitable patches fast enough to compensate for all of its local extinctions.
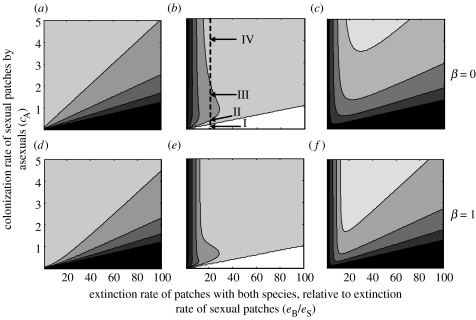
While the solutions are more complicated in the case β≥0, the numerical solutions turn out to depend relatively little on β. Figure 3 shows coexistence conditions and the total proportions of occupied patches , and for the two extremes β=0 and 1, for varying values cA and eB/eS. When cA>eA+eB, coexistence is possible. It is intriguing to note that this condition neither requires any specific relationship between cS and cA, nor is it influenced by the value of α (although once conditions for coexistence are filled, the equilibrium frequencies reached will depend on these parameters). In other words, coexistence is possible when asexuals are either better or poorer colonizers than their sexual hosts.
Figure 3.
Contour plots showing the proportion of patches occupied by (a,d) sexuals only, (b,e) asexual parasites and sexuals together (both) and (c,f) the total proportion of occupied patches (all fish). The dashed line in (b), and the points indicated (I–IV), represents a cross section detailing what happens when the colonization rate of asexuals increases. Shading indicates proportions from white to black as follows: 0 (white), less than 0.2, 0.4, 0.6, 0.8 and less than 1 (black). In (a–c), Amazons and sexuals cannot colonize patches together (β=0), while in (d–f) they can (β=1). Other parameters used in the figures are eA=0.01, eS=0.01, α=0.5 and cS=0.3.
The consequences of each parameter combination for the dynamics of sexual and asexual species are intriguing. In figure 3, darkest areas in figure 3a,d show where the sexual hosts persist best, in the sense of high equilibrium frequency of sexual-only patches. The darkest areas in figure 3c,f, on the other hand, show where the sexual hosts persist best measured as the total number of patches that contain sexuals, either alone or together with asexual sperm parasites. Both measures indicate, unsurprisingly, that the sexual host benefits when the sperm parasite is not a good colonizer (dark areas are associated with low values of cA). Alternatively, the sexual host also persists at high frequencies if the ratio eB/eS is very low (solutions near the y-axis in rightmost graphs of figure 3). In this case, almost all patches contain both the sexual and the asexual species and extinctions are rare, as are sexual-only patches.
Somewhat less intuitively, both measures also indicate that the sexual host species benefits, in the sense of reaching high occupancy levels, if the sperm parasite causes local extinction very rapidly (dark areas are associated with high values of eB/eS). This is because a parasite that overexploits its host rapidly has itself trouble persisting. The overall frequency of parasite-infested patches remains low (or zero) everywhere towards the right end of figure 3b,e, and the sexual species consequently persists at a level close to its single-species Levins equilibrium.
A more detailed look at the effect of cA, the colonizing ability of the sperm parasite, on the dynamics reveals that good ability to find new host patches (high cA) is not always beneficial to the persistence of the sperm parasite either. If sperm parasites often cause extinction of all individuals in a patch (high eB/eS), then the values of cA that lead to the highest prevalence of the asexual species are intermediate ones (figure 3b,e), not the highest ones. We can see this by looking at the dashed cross section in figure 3b: the point represented by I shows that asexual species first inhabit only few patches, and the proportion of occupancy first increases with their colonization rate (between points II and III along the cross section). However, if the colonization rate is increased even further, they can act to aid their own demise, as shown by point IV of the cross section, where their occupancy fraction is lower than at point II or III (figure 3b).
Altogether, our results also confirm that ‘parasitism’ by gynogenesis is the correct term to apply, as the invasion of the asexual species decreases the total abundance of the host species, sometimes dramatically (figure 3: in the absence of the sperm parasite, all sexual solutions would lie in the darkest region of highest abundance). This result applies whether or not sexual colonizations are accompanied by asexuals (compare β=0 with β=1 in figure 3), and also regardless of the values of α and eA. The effect of α is straightforward: if the ability of sexuals to colonize new patches is clearly hampered when they are accompanied by asexuals (i.e. α is low), the frequency of patches containing both species is reduced, while the proportions of sexual-only patches remain largely unaffected (not shown).
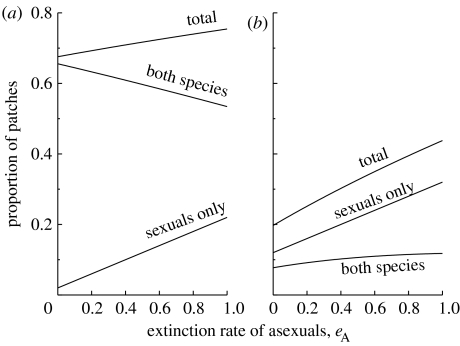
The effect of extinctions in which only asexuals disappear from a patch, leaving sexuals intact (eA), is intriguing. The sexual species, unsurprisingly, becomes more abundant if eA is high, which is in accordance with the idea that the asexual species is a parasite. Yet high asexual-only extinction rates can, under some conditions, be beneficial for the asexual species too, in the sense that high local extinction rates can increase the equilibrium frequency of patches that the asexual species is able to occupy (figure 4). This occurs if sperm parasites are very harmful, such that the local coexistence prospects of sexuals and asexuals are always poor (i.e. short-lived). The parasitic species then tends to overexploit its resource, and preventing it from doing so (high eA reverts some of the asexual-infested patches back to a sexual-only state) then leads to higher overall resource abundance and, consequently, a larger equilibrium frequency of patches with asexual sperm parasites (figure 4b).
Figure 4.
The proportions of patches containing the sexual species only, both asexual and sexual fish, and the total of occupied patches of either kind, as a function of the rate eA at which asexual fish go extinct in patches leaving the sexual population intact. In (a), asexual parasites cause within-patch extinction of both asexual and sexual species at a much lower rate (eB=0.1) than in (b) where eB=0.6. In (b), the high value of eB depresses the overall amount of the resource (total frequency of non-empty patches) that the asexual species depends on. This depression is less severe when eA is high, explaining the paradox that in (b), the frequency of patches with the asexual species present is higher when the asexual species suffers from a higher extinction rate eA.
4. Discussion
Our results show how spatial dynamics can be essential for explaining the continued coexistence of systems with an asexual species continually outcompeting its host. We elsewhere show that even if the host species adapts so that its males discriminate against mating with the sperm parasite, coexistence is not a self-evident outcome of competition. On the other hand, factors such as mate choice or specific values of fertilization efficiency can substantially prolong the time to extinction should it occur (Heubel et al. submitted). The results of the current paper show that these time-scales matter. If individuals of the sexual host species have time to colonize new patches while their local patch is following its transient dynamics towards extinction, the system can reach an equilibrium where coexistence is globally possible despite local extinctions.
Our results show two fairly distinct parameter regions where coexistence is possible, in the sense that most patches are occupied at equilibrium. In one of them, the parasitic asexual species is abundant but causes little damage (i.e. it does not markedly increase the local extinction rate), while in the other, the presence of the sperm parasite is relatively detrimental to the sexual species and causes extinctions fast, but these extinctions also help to keep the frequency of parasitic invasion low from the point of view of the host. Either case allows the sexual host to persist at a frequency that is not significantly reduced when compared with existing alone in the habitat network. These two regions are connected by a region where persistence is much hindered by parasitism, and the overall frequency of occupied patches remains low in a network of suitable habitat patches (indicated by the pale area between the two darker slopes in figure 3).
Which of these regions might be most relevant in natural populations? In the case of Amazon mollies, the first alternative requires that asexuals do not cause much population-level damage, i.e. do not often cause local extinction despite the assumed ability of asexuals to outcompete sexually reproducing species (Williams 1975; Agrawal 2001, 2006). Sperm limitation that hampers asexual reproduction more than that of sexuals (due to male mate choice, Ryan et al. (1996) and Gabor & Ryan (2001); or differential sperm availability, Aspbury & Gabor (2004) and Schlupp & Plath (2005)) or competitive asymmetries (Schley et al. 2004) could push a gynogenetic species complex towards this solution and thus explain coexistence. This alternative predicts that most local habitat patches contain both species. On the other hand, our model also shows that a gynogenetic system can also conceivably persist without any mechanisms that favour local coexistence. In such a case, there is a balance of local extinctions and recolonizations, and this can lead to long-term persistence as a metapopulation with frequent local extinction. This requires that both species are reasonably good colonizers. In this case, the balance of sexual-only and jointly occupied patches depends on exact parameter values, but in general there should be a sizeable proportion of sexual-only patches.
Which alternative is supported by field studies? In the case of mollies, in regions of sexual–asexual sympatry, most local patches contain both sexuals and asexuals (Hubbs 1964; Darnell & Abramoff 1968; Balsano et al. 1981; Schlupp et al. 2002; Heubel 2004). There certainly is evidence for some of the mechanisms aiding local coexistence, e.g. males from both an allopatric and a sympatric population produce more sperm when in the presence of a conspecific female than in the presence of a heterospecific asexual female (Aspbury & Gabor 2004). Furthermore, P. mexicana males transfer more sperm to their conspecific sexual females than to Amazons (Schlupp & Plath 2005).
However, whether such mechanisms are sufficient to enhance local coexistence (keep eB low) requires estimating how often, and over what time-scale, the invasion of asexuals is kept at bay or leads to local extinction. So far, data are limited, but they show that local extinctions definitely occur: in a field study, monitoring population dynamics of six mixed P. latipinna/P. formosa populations over a period of 3 years (approx. six to nine generations) at least six independent local extinctions occurred (Heubel 2004; K. Heubel & I. Schlupp 2001–2004, unpublished data), and they always followed a similar pattern: the proportion of asexuals increases heavily, often until patches become purely Amazon-occupied before collapsing. At several locations in central Texas, Amazons have colonized formerly asexual-free patches, e.g. San Marcos River in the 1950s (Drewry et al. 1958; Hubbs 1964), and during the last decade at San Marcos Springs (Schlupp et al. 2002), Comal River (Heubel 2004) and San Antonio River (Edwards 2001). To sum up, currently it would be premature to attempt to evaluate the precise values of coexistence parameters (figure 3) for the molly gynogenetic complex, yet we find support both for the importance of mechanisms that aid local coexistence and for the role of extinction–colonization dynamics. One should also note that transient dynamics (figure 2) can bring asexual sperm parasites perilously close to extinction over spatial scales that are larger than one patch. With added stochasticity, sizeable areas could occasionally become free of sperm parasites before recolonization eventually occurs.
In general, the ecological dynamics of sperm-dependent asexual–sexual species complexes are similar to host–parasite models. Furthermore, some of our results have good analogues in studies of virulence evolution in microparasites. If there was no trade-off between virulence and transmission, pathogens should evolve to be very benign (Boots et al. 2004; Read & Keeling 2006). However, efficient transmission is often difficult to achieve without harming the host. This explains the evolution of virulent strains, sometimes up to the point that hosts tend to become locally extinct (Boots & Sasaki 2003; de Castro & Bolker 2005). Many models agree that local depletion of susceptible hosts can select for somewhat lower virulence, and details of spatial structure have a strong influence on the solutions (Read & Keeling 2006; Kamo et al. 2007, and references therein). Likewise, in predator–prey systems, one can predict competition between genotypes that follow a strategy of ‘prudent’ predation (Slobodkin 1974; van Baalen & Sabelis 1995) and those who adopt ‘rapacious’ strategies that rapidly overexploit local populations and subsequently have to rely on dispersal to find new hosts to exploit. In a wholly asexual context, the evolutionary outcome of this competition has been shown to depend strongly on dispersal patterns (Kerr et al. 2006); in other words, colonization ability.
Ours is a population dynamic model, and as such does not consider that rates of reproduction, extinction or colonization may evolve. Nevertheless, some of our population dynamics consequences of the traits of the asexual species could prove very intriguing in this context. The parasitic nature of the asexual species predicts several counterintuitive results where the parasite persists less well if it spreads ‘too’ efficiently. If asexual forms locally outcompete sexuals very quickly, then asexuals lose and sexuals win in terms of overall abundance in the habitat. It follows that a ‘too high’ colonization rate of asexual individuals can lead to decreasing population-level performance (occupancy fraction) of the asexual species. Also, if asexual species often causes local extinction of its host, then asexuals persist the better, the higher their own extinction rate (figure 4). Theoretical work on dispersal (e.g. Heino & Hanski 2001; Bowler & Benton 2005; Kokko & López-Sepulcre 2006) as well as models of parasite reproduction and virulence (e.g. Kamo et al. 2007), both suggest dynamic feedback between selective pressures on a life-history trait (dispersal or reproduction) and the trait's dynamic, population-wide consequences. Our results suggest that gynogenetic species complexes could provide illuminating examples for the study of this feedback. Because some of the predicted population-level consequences appear counterintuitive, they might allow us to test whether the predicted tension between individual- and population-level benefits of dispersal (Kokko & López-Sepulcre 2006) matters in dispersal evolution.
Spatial structure can influence competition between sexuals and asexuals in several different ways. For example, Salathé et al. (2006) have argued that spatial structure will diminish the twofold cost of sexuals, because if dispersal and competition are local, then sexual individuals will mostly compete with sexuals and asexuals with asexuals. This diminishes the relative reproductive advantage of the latter and accelerates mutation accumulation due to small local population size (see also Peck et al. 1999). Our results bear more resemblance to those of Stewart-Cox et al. (2005), who in a different context (gynodioecious populations of flowering plants) show how exploitative types may cause local extinction. Here, the helping hand of spatial structure is once again closely related to results in predator–prey and host–parasite theories. Whether or not parasites evolve to be so damaging that they cause local extinctions of their host species (Boots & Sasaki 2003; de Castro & Bolker 2005), a spatially structured environment provides means of global coexistence as it allows a sufficient number of hosts to escape parasitism (Holyoak & Lawler 1996; Ellner et al. 2001; Bonsall et al. 2002). This mechanism may be essential in explaining the maintenance of gynogenetic organisms.
Whether spatial structure is a sufficient explanation for coexistence is an intriguing topic for further study. It may also act together with locally stabilizing factors such as some degree of niche differentiation (Balsano et al. 1981; Schley et al. 2004). Differences in susceptibility to parasites is a possibility, as asexual forms should lack the genetic means to find novel solutions to pathogens or other forms of novel environments. However, a study testing for differences in parasite loads between P. formosa and its sexual host P. latipinna found none (Tobler & Schlupp 2005). There is more evidence for another stabilizing mechanism, male mate choice (Ryan et al. 1996; Gabor & Ryan 2001; Aspbury & Gabor 2004; Schlupp & Plath 2005; Gumm et al. 2006). While male choice may have to reach unrealistically strong levels to wholly prevent local extinctions, a weak male tendency to prefer conspecific females can slow down extinction rates considerably (Heubel et al. submitted). Together with the current results this means much improved chances of coexistence. Such models may also prove important in explaining a wider range of behaviours that may be detrimental to population-wide reproductive output. There is no general guarantee that evolution steers populations away from extinction (Boots & Sasaki 2003; Rankin & López-Sepulcre 2005; Dercole et al. 2006; Rankin et al. 2007), but if extinctions first occur on a local scale then sufficient recolonizing ability can aid persistence.
Acknowledgments
We thank three anonymous reviewers for constructive comments. Funding was provided by the Academy of Finland. D.J.R. is currently funded by SNSF grant 3100A0-105626 (to M. Taborsky).
References
- Agrawal A.F. Sexual selection and the maintenance of sexual reproduction. Nature. 2001;411:692–695. doi: 10.1038/35079590. doi:10.1038/35079590 [DOI] [PubMed] [Google Scholar]
- Agrawal A.F. Evolution of sex: why do organisms shuffle their genotypes? Curr. Biol. 2006;16:R696–R704. doi: 10.1016/j.cub.2006.07.063. doi:10.1016/j.cub.2006.07.063 [DOI] [PubMed] [Google Scholar]
- Aspbury A.S, Gabor C.R. Discriminating males alter sperm production between species. Proc. Natl Acad. Sci. USA. 2004;101:15 970–15 973. doi: 10.1073/pnas.0405653101. doi:10.1073/pnas.0405653101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avise J.C, Trexler J, Travis J, Nelson W.S. Poecilia mexicana is the recent female parent of the unisexual fish Poecilia formosa. Evolution. 1991;45:1530–1533. doi: 10.1111/j.1558-5646.1991.tb02657.x. doi:10.2307/2409901 [DOI] [PubMed] [Google Scholar]
- Balsano J.S, Kucharski K, Randle E.J, Rasch E.M, Monaco P.J. Reduction of competition between bisexual and unisexual females of Poecilia in northeastern Mexico. Environ. Biol. Fishes. 1981;6:39–48. doi:10.1007/BF00001798 [Google Scholar]
- Bell G. Croom Helm; London, UK: 1982. The masterpiece of nature: the evolution and genetics of sexuality. [Google Scholar]
- Beukeboom L.W, Vrijenhoek R.C. Evolutionary genetics and ecology of sperm-dependent parthenogenesis. J. Evol. Biol. 1998;11:755–782. doi:10.1007/s000360050117 [Google Scholar]
- Bonsall M.B, French D.R, Hassell M.P. Metapopulation structures affect persistence of predator–prey interactions. J. Anim. Ecol. 2002;71:1075–1084. doi:10.1046/j.1365-2656.2002.00670.x [Google Scholar]
- Boots M, Sasaki A. Parasite evolution and extinctions. Ecol. Lett. 2003;6:176–182. doi:10.1046/j.1461-0248.2003.00426.x [Google Scholar]
- Boots M, Hudson P.J, Sasaki A. Large shifts in pathogen virulence relate to host population structure. Science. 2004;303:842–844. doi: 10.1126/science.1088542. doi:10.1126/science.1088542 [DOI] [PubMed] [Google Scholar]
- Bowler D.E, Benton T.G. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 2005;80:205–225. doi: 10.1017/s1464793104006645. doi:10.1017/S1464793104006645 [DOI] [PubMed] [Google Scholar]
- Case T.J, Taper M.L. On the coexistence and coevolution of asexual and sexual competitors. Evolution. 1986;40:366–387. doi: 10.1111/j.1558-5646.1986.tb00478.x. doi:10.2307/2408816 [DOI] [PubMed] [Google Scholar]
- Colegrave N. Sex releases the speed limit on evolution. Nature. 2002;420:664–666. doi: 10.1038/nature01191. doi:10.1038/nature01191 [DOI] [PubMed] [Google Scholar]
- Crow J.F. Advantages of sexual reproduction. Dev. Genet. 1994;15:205–213. doi: 10.1002/dvg.1020150303. doi:10.1002/dvg.1020150303 [DOI] [PubMed] [Google Scholar]
- Darnell R.M, Abramoff P. Distribution of the gynogenetic fish, Poecilia formosa, with remarks on the evolution of the species. Copeia. 1968;1968:354–361. doi:10.2307/1441764 [Google Scholar]
- de Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecol. Lett. 2005;8:117–126. doi:10.1111/j.1461-0248.2004.00693.x [Google Scholar]
- Dercole F, Ferrière R, Gragnani A, Rinaldi S. Coevolution of slow–fast populations: evolutionary sliding, evolutionary pseudo-equilibria and complex red queen dynamics. Proc. R. Soc. B. 2006;273:983–990. doi: 10.1098/rspb.2005.3398. doi:10.1098/rspb.2005.3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncaster C.P, Pound G.E, Cox S.J. The ecological cost of sex. Nature. 2000;404:281–285. doi: 10.1038/35005078. doi:10.1038/35005078 [DOI] [PubMed] [Google Scholar]
- Drewry G.E, Delco E.A, Hubbs C. Occurence of the Amazon molly Mollienesia formosa at San Marcos, Texas. Tex. J. Sci. 1958;10:489–490. [Google Scholar]
- Dries L.A. Peering through the looking glass at a sexual parasite: are Amazon mollies red queens? Evolution. 2003;57:1387–1396. doi: 10.1111/j.0014-3820.2003.tb00346.x. doi:10.1111/j.0014-3820.2003.tb00346.x [DOI] [PubMed] [Google Scholar]
- Dunbrack R.L, Coffin C, Howe R. The cost of males and the paradox of sex: an experimental investigation of the short-term competitive advantages of evolution in sexual populations. Proc. R. Soc. B. 1995;262:45–49. doi:10.1098/rspb.1995.0174 [Google Scholar]
- Edwards R.J. New additions and persistence of the introduced fishes of the upper San Antonio River, Bexar County, Texas. Tex. J. Sci. 2001;53:3–12. [Google Scholar]
- Ellner S.P, et al. Habitat structure and population persistence in an experimental community. Nature. 2001;412:538–543. doi: 10.1038/35087580. doi:10.1038/35087580 [DOI] [PubMed] [Google Scholar]
- Gabor C.R, Ryan M.J. Geographical variation in reproductive character displacement in mate choice by male sailfin mollies. Proc. R. Soc. B. 2001;268:1063–1070. doi: 10.1098/rspb.2001.1626. doi:10.1098/rspb.2001.1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumm J.M, Gonzalez R, Aspbury A.S, Gabor C.R. Do I know you? Species recognition operates within and between the sexes in a unisexual–bisexual species complex of mollies. Ethology. 2006;112:448–457. doi:10.1111/j.1439-0310.2005.01175.x [Google Scholar]
- Hamilton W.D, Axelrod R, Tanese R. Sexual reproduction as an adaptation to resist parasites. Proc. Natl Acad. Sci. USA. 1990;87:3566–3573. doi: 10.1073/pnas.87.9.3566. doi:10.1073/pnas.87.9.3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino M, Hanski I. Evolution of migration rate in a spatially realistic metapopulation model. Am. Nat. 2001;157:495–511. doi: 10.1086/319927. doi:10.1086/319927 [DOI] [PubMed] [Google Scholar]
- Heubel, K. U. 2004 Population ecology and sexual preferences in the mating complex of the unisexual Amazon molly Poecilia formosa PhD thesis, University of Hamburg, Hamburg.
- Heubel K.U, Schlupp I. Turbidity affects association behaviour in male Poecilia latipinna. J. Fish Biol. 2006;68:555–568. doi:10.1111/j.0022-1112.2006.00941.x [Google Scholar]
- Heubel, K. U., Rankin, D. J. & Kokko, H. Submitted. Population consequences of male mate choice and fertilization efficiency in a gynogenetic species complex.
- Holyoak M, Lawler S.P. Persistence of an extinction–prone predator–prey interaction through metapopulation dynamics. Ecology. 1996;77:1867–1879. doi:10.2307/2265790 [Google Scholar]
- Hubbs C. Interactions between bisexual fish species and its gynogenetic sexual parasite. Tex. Mem. Mus. Bull. 1964;8:1–72. [Google Scholar]
- Hubbs C.L, Hubbs L.C. Apparent parthenogenesis in nature in a form of fish of hybrid origin. Science. 1932;76:628–630. doi: 10.1126/science.76.1983.628. doi:10.1126/science.76.1983.628 [DOI] [PubMed] [Google Scholar]
- Judson O.P, Normark B.B. Ancient asexual scandals. Trends Ecol. Evol. 1996;11:41–46. doi: 10.1016/0169-5347(96)81040-8. doi:10.1016/0169-5347(96)81040-8 [DOI] [PubMed] [Google Scholar]
- Kamo M, Sasaki A, Boots M. The role of trade-off shapes in the evolution of parasites in spatial host populations: an approximate analytical approach. J. Theor. Biol. 2007;244:588–596. doi: 10.1016/j.jtbi.2006.08.013. doi:10.1016/j.jtbi.2006.08.013 [DOI] [PubMed] [Google Scholar]
- Kawecki T.J. Unisexual/bisexual breeding complexes in Poeciliidae: why do males copulate with unisexual females? Evolution. 1988;42:1018–1023. doi: 10.1111/j.1558-5646.1988.tb02520.x. doi:10.2307/2408917 [DOI] [PubMed] [Google Scholar]
- Kerr B, Neuhauser C, Bohannan B.J.M, Dean A.M. Local migration promotes competitive restraint in a host–pathogen ‘tragedy of the commons’. Nature. 2006;442:75–78. doi: 10.1038/nature04864. doi:10.1038/nature04864 [DOI] [PubMed] [Google Scholar]
- Kiester A.R, Nagylaki T, Shaffer B. Population dynamics of species with gynogenetic sibling species. Theor. Popul. Biol. 1981;19:358–369. doi:10.1016/0040-5809(81)90026-5 [Google Scholar]
- Kokko H, López-Sepulcre A. From individual dispersal to species ranges: perspectives for a changing world. Science. 2006;313:789–791. doi: 10.1126/science.1128566. doi:10.1126/science.1128566 [DOI] [PubMed] [Google Scholar]
- Kondrashov A.S. Deleterious mutations and the evolution of sexual reproduction. Nature. 1988;336:435–440. doi: 10.1038/336435a0. doi:10.1038/336435a0 [DOI] [PubMed] [Google Scholar]
- Levins R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull. Entomol. Soc. Am. 1969;71:237–240. [Google Scholar]
- Lynch M, Gabriel W. Mutation load and the survival of small populations. Evolution. 1990;44:1725–1737. doi: 10.1111/j.1558-5646.1990.tb05244.x. doi:10.2307/2409502 [DOI] [PubMed] [Google Scholar]
- Lynch M, Burger R, Butcher D, Gabriel W. The mutational meltdown in asexual populations. J. Hered. 1993;84:339–344. doi: 10.1093/oxfordjournals.jhered.a111354. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Cambridge University Press; Cambridge, UK: 1978. The evolution of sex. [Google Scholar]
- Muller H.J. The relation of recombination to mutational advance. Mutat. Res. 1964;1:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- Peck J.R, Yearsley J, Barreau G. The maintenance of sexual reproduction in a structured population. Proc. R. Soc. B. 1999;266:1857–1863. doi:10.1098/rspb.1999.0857 [Google Scholar]
- Rankin D.J, López-Sepulcre A. Can adaptation lead to extinction? Oikos. 2005;111:616–619. doi:10.1111/j.1600-0706.2005.14541.x [Google Scholar]
- Rankin D.J, Bargum K, Kokko H. The tragedy of the commons in evolutionary biology. Trends Ecol. Evol. 2007;22:643–651. doi: 10.1016/j.tree.2007.07.009. doi:10.1016/j.tree.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Read J.M, Keeling M.J. Disease evolution across a range of spatio-temporal scales. Theor. Popul. Biol. 2006;70:201–213. doi: 10.1016/j.tpb.2006.04.006. doi:10.1016/j.tpb.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Ryan M.J, Dries L.A, Batra P, Hillis D.M. Male mate preferences in a gynogenetic species complex of Amazon mollies. Anim. Behav. 1996;52:1225–1236. doi:10.1006/anbe.1996.0270 [Google Scholar]
- Salathé M, Salathé R, Schmid-Hempel P, Bonhoeffer S. Mutation accumulation in space and the maintenance of sexual reproduction. Ecol. Lett. 2006;9:941–946. doi: 10.1111/j.1461-0248.2006.00942.x. doi:10.1111/j.1461-0248.2006.00942.x [DOI] [PubMed] [Google Scholar]
- Schartl M, Wilde B, Schlupp I, Parzefall J. Evolutionary origin of a parthenoform, the Amazon Molly Poecilia formosa, on the basis of a molecular genealogy. Evolution. 1995;49:827–835. doi: 10.1111/j.1558-5646.1995.tb02319.x. doi:10.2307/2410406 [DOI] [PubMed] [Google Scholar]
- Schley D, Doncaster C.P, Sluckin T. Population models of sperm-dependent parthenogenesis. J. Theor. Biol. 2004;229:559–572. doi: 10.1016/j.jtbi.2004.04.031. doi:10.1016/j.jtbi.2004.04.031 [DOI] [PubMed] [Google Scholar]
- Schlupp I. The evolutionary ecology of gynogenesis. Annu. Rev. Ecol. Evol. Syst. 2005;36:399–417. doi:10.1146/annurev.ecolsys.36.102003.152629 [Google Scholar]
- Schlupp I, Plath M. Male mate choice and sperm allocation in a sexual/asexual mating complex of Poecilia (Poeciliidae, Teleostei) Biol. Lett. 2005;1:169–171. doi: 10.1098/rsbl.2005.0306. doi:10.1098/rsbl.2005.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlupp I, Nanda I, Döbler M, Lamatsch D.K, Epplen J.T, Parzefall J, Schmid M, Schartl M. Dispensable and indispensable genes in an ameiotic fish, the Amazon molly Poecilia formosa. Cytogenet. Cell Genet. 1998;80:193–198. doi: 10.1159/000014979. doi:10.1159/000014979 [DOI] [PubMed] [Google Scholar]
- Schlupp I, Parzefall J, Schartl M. Biogeography of the unisexual Amazon molly, Poecilia formosa. J. Biogeogr. 2002;29:1–6. doi:10.1046/j.1365-2699.2002.00651.x [Google Scholar]
- Schmeller D.S, O'Hara R, Kokko H. Male adaptive stupidity: male mating pattern in hybridogenetic frogs. Evol. Ecol. Res. 2005;7:1039–1050. [Google Scholar]
- Slobodkin L.B. Prudent predation does not require group selection. Am. Nat. 1974;108:665–678. doi:10.1086/282942 [Google Scholar]
- Stewart-Cox J.A, Britton N.F, Mogie M. Space mediates coexistence of females and hermaphrodites. Bull. Math. Biol. 2005;67:1273–1302. doi: 10.1016/j.bulm.2005.02.003. doi:10.1016/j.bulm.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Tobler M, Schlupp I. Parasites in sexual and asexual mollies (Poecilia, Poeciliidae, Teleostei): a case for the red queen? Biol. Lett. 2005;1:166–168. doi: 10.1098/rsbl.2005.0305. doi:10.1098/rsbl.2005.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B.J. The evolutionary genetics of a unisexual fish, Poecilia formosa. In: Barigozzi C, editor. Mechanisms of speciation. Alan R. Liss; New York, NY: 1982. pp. 265–305. [PubMed] [Google Scholar]
- van Baalen M, Sabelis M.W. The milker–killer dilemma in spatially structured predator–prey interactions. Oikos. 1995;74:391–400. doi:10.2307/3545984 [Google Scholar]
- Vrijenhoek R.C. Ecological differentiation among clones: the frozen niche variation model. In: Wohrmann K, Loeschcke V, editors. Population biology and evolution. Springer; Berlin, Germany: 1984. pp. 217–231. [Google Scholar]
- Waxman D, Peck J.R. Sex and adaptation in a changing environment. Genetics. 1999;153:1041–1053. doi: 10.1093/genetics/153.2.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S.A, Lively C.M, Read A.F. A pluralist approach to sex and recombination. J. Evol. Biol. 1999;12:1003–1012. doi:10.1046/j.1420-9101.1999.00119.x [Google Scholar]
- Williams G.C. Princeton University Press; Princeton, NJ: 1975. Sex and evolution. [Google Scholar]