Abstract
Hendra virus (HeV) is a lethal paramyxovirus which emerged in humans in 1994. Poor understanding of HeV dynamics in Pteropus spp. (flying fox or fruit bat) reservoir hosts has limited our ability to determine factors driving its emergence. We initiated a longitudinal field study of HeV in little red flying foxes (LRFF; Pteropus scapulatus) and examined individual and population risk factors for infection, to determine probable modes of intraspecific transmission. We also investigated whether seasonal changes in host behaviour, physiology and demography affect host–pathogen dynamics. Data showed that pregnant and lactating females had significantly higher risk of infection, which may explain previously observed temporal associations between HeV outbreaks and flying fox birthing periods. Age-specific seroprevalence curves generated from field data imply that HeV is transmitted horizontally via faeces, urine or saliva. Rapidly declining seroprevalence between two field seasons suggests that immunity wanes faster in LRFF than in other flying fox species, and highlights the potentially critical role of this species in interspecific viral persistence. The highest seroprevalence was observed when animals showed evidence of nutritional stress, suggesting that environmental processes that alter flying fox food sources, such as habitat loss and climate change, may increase HeV infection and transmission. These insights into the ecology of HeV in flying fox populations suggest causal links between anthropogenic environmental change and HeV emergence.
Keywords: Hendra virus, Henipavirus, Pteropus scapulatus, zoonotic emerging infectious diseases, flying fox
1. Introduction
The emergence of zoonoses from wildlife is a key threat to global human health (Smolinski et al. 2003) and has important implications for conservation (Cohen 2000; Daszak et al. 2000; Dobson & Foufopoulos 2001). However, elucidating the ecological conditions that contribute to the emergence of these diseases is a major challenge in disease ecology (Plowright et al. 2008). To do this often involves the study of both the behaviour of diseases within hosts and macroecological behaviours of wildlife diseases mediated through large-scale processes, which is a logistical and scientific challenge (Plowright et al. 2008).
Since the mid-1990s, five new zoonotic viral diseases (Hendra virus, Nipah virus, Menangle virus, Australian bat lyssavirus and Melaka virus) have emerged from Australasian Pteropus species bats (Field et al. 2001; Mackenzie et al. 2001, 2003; Chua et al. 2007). These viruses can cause significant morbidity and mortality in humans and domestic animals (Eaton et al. 2006; Chua et al. 2007) and are widely distributed in Pteropus hosts (Field 2005; Epstein et al. 2006). Nevertheless, little is known about their ecology and epidemiology, including how they are transmitted within Pteropus populations or from Pteropus hosts to domestic animals. The nomadic lifestyle and complex social structure of Pteropus bats, as well as the sporadic nature of bat-borne disease outbreaks, has limited large-scale study on these pathogens and limited progress in understanding their dynamics within bats.
Hendra virus (HeV), a paramyxovirus in the genus Henipavirus, was the first of these bat-borne viruses to be recognized. Repeated sporadic spillovers of HeV have occurred in eastern Australia since 1994, resulting in four human cases (two fatal) and more than 30 equine deaths (Murray et al. 1995; Field et al. 2000, 2007; Daszak et al. 2006; Hanna et al. 2006). All human cases have involved spillover of infection from flying foxes to pasture-fed horses, which appear to act as amplifier hosts, facilitating transmission to people (Daszak et al. 2006). HeV does not cause apparent disease in flying fox reservoir hosts (Williamson et al. 1998, 1999). However, the high case fatality rate in humans, and the lack of effective treatment or vaccination, has led to HeV being classified as a biosafety level four (BSL-4) pathogen (Eaton et al. 2006).
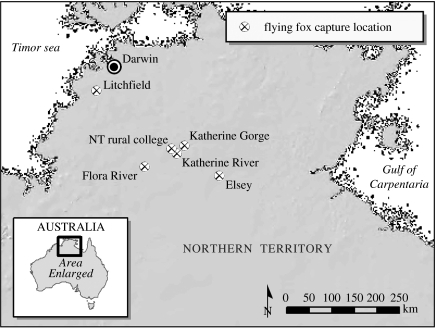
Flying fox populations are highly spatially structured, often consisting of dense aggregations (camps) of tens of thousands to hundreds of thousands of individuals roosting in canopy vegetation during the day, feeding at night. The little red flying fox (LRFF), Pteropus scapulatus, is the only nearly exclusively nectivorous Pteropus spp. and their dependence on this patchily distributed, ephemeral food resource (Palmer & Woinarski 1999; Palmer et al. 2000) drives a nomadic lifestyle, large population sizes and a wide geographical distribution compared with other Australian flying fox species (Hall & Richards 2000; Birt 2004). Recent modelling work (Plowright 2007) suggests that the large number of LRFF populations and their extensive geographical distribution makes them a key reservoir species. As one of only two species that geographically overlap with all known sites of HeV spillover, little red flying foxes may also play an important role in the emergence of HeV into horses. We conducted our longitudinal study of HeV dynamics in LRFF in the northern range of their distribution (figure 1), where they are least migratory due to unfragmented, relatively abundant and diverse flowering vegetation.
Figure 1.
Field sites where LRFF were captured in the Northern Territory of Australia: Katherine Gorge National Park; Northern Territory (NT) Rural College; Flora River Nature Park; Elsey National Park; Litchfield National Park; and Katherine River.
(a) Hypotheses
In this paper, we test the following hypotheses on the transmission and ecology of HeV within flying foxes. Experimental infection suggests that HeV can be transmitted transplacentally (Williamson et al. 1999). However, we hypothesize that horizontal transmission is the primary mode of transmission in wild populations, for two reasons. First, HeV has been detected in urine, rectal swabs and throat swabs of experimentally infected animals (K. Halpin 2003–2004, unpublished data), and, second, the highly social nature of flying fox aggregations makes horizontal transmission via these secretions very likely. We hypothesize that pregnancy plays an important epidemiological role, because pregnancy is associated with increased susceptibility to disease in other mammalian systems (Sheldon & Verhulst 1996) and most HeV spillover events coincide with peak flying fox birthing seasons (Field et al. 2001, 2007). Lastly, we hypothesize that seasonal increase in contact rates, and the immunosuppressive effects of testosterone associated with mating, may drive temporal fluctuations in HeV dynamics and the risk of spillover to horses and humans. To test these hypotheses, we constructed age-specific seroprevalence curves to examine temporal patterns of exposure. We also sampled flying foxes during each stage of the highly seasonal reproductive cycle (mating, birthing, lactation and weaning) and examined the relative risk of exposure in relation to sex and reproductive status in individuals, as well as seasonal population-level seroprevalence patterns.
2. Material and methods
(a) Source of the data
A total of 664 LRFF were sampled over five field seasons (November 2004 to March 2006) in the Northern Territory of Australia (figure 1; table 1). Flying fox campsite locations shifted between seasons as the distribution of ephemeral nectar resources changed, requiring sampling at multiple locations within the study area. Sampling periods were chosen to coincide with mating (spring), late pregnancy/early birthing (autumn) and late lactation/early weaning (winter). Sampling was not possible in summer, due to the inaccessibility of sites during the wet season, or in winter 2006, when no LRFF could be located in the study area.
Table 1.
Description of risk factors for Hendra virus serostatus included in univariate analysis. (Data were collected from 664 LRFF sampled over five field seasons between November 2004 and March 2006.)
| variable | description | no. of animals |
|---|---|---|
| sex | ||
| male | 321 | |
| female | 343 | |
| age | ||
| 0–1 month | a pup that is dependent on its mother, usually up to the age of three months; as we were sampling during the peak birthing time, these animals were all less than a month old | 8 |
| 3 months | juveniles sampled in July; the pup is too large to be carried by the mother, but has not yet reached full size; mandibular third molars have not fully erupted; permanent dentition still fragile with slight pinkish tone (appears almost transparent in places) | 50 |
| 6 months | juveniles sampled in November; the pup is independent, but has not yet reached full size; all teeth have erupted and have reached their full length; teeth show no signs of wear (cusps are sharp), but slight (diet dependent) lateral staining is possible | 64 |
| pre-breeding | an animal that is full size but not sexually mature; from 1 to 2 years; in females, nipples are small and surrounded by fur; males have underdeveloped testes regardless of the season; dentition likely to show signs of staining on lateral surfaces (diet dependent), cusps of premolar teeth are becoming slightly oblong, but there is no wear or staining on canine teeth | 198 |
| mature | a full-sized sexually mature animal (potentially breeding); from 2 years to at least 16 years old; mature males have well developed testes (particularly in the mating season); in females that have bred, nipples are enlarged, irrespective of whether they have lactated recently; also, lactating and post-lactating females have less fur around the nipple; teeth are mostly heavily stained from all sides, often showing signs of discoloration and decay, but in various stages of wear | 344 |
| season | ||
| spring 2004 | November 2004, Elsey National Park | 170 |
| autumn 2005 | April 2005, Flora Nature Park | 103 |
| winter 2005 | July 2005, Flora Nature Park (n=69) and Northern Territory Rural College (n=116) | 185 |
| spring 2005 | November 2005, Elsey National Park (n=93) and Katherine River (n=87) | 180 |
| autumn 2006 | March 2006, Katherine Gorge National Park (n=18) and Litchfield National Park (n=8) | 26 |
| weight | body weight (g) | 664 |
| forearm | length from the styloid process of the third metacarpal to the olecranon process of the ulna (mm) when the wing is folded; forearm length does not increase once the animal reaches maturity (approx. 2 years) | 664 |
| weight/forearm ratio | body weight (g) divided by forearm length (mm) gives an indication of body condition by taking into account body size | 664 |
| lactating | enlarged nipples expressing milk | 22 |
| pup | dependent pup attached to its mother | 8 |
| pregnant | pregnancy detected via palpation | 34 |
(b) Capture and sampling methods
Flying foxes were caught in mist-nets at ‘fly in’, the two hours before dawn when animals return to roosting sites after foraging. Mist-nets were raised between either ground-mounted masts, masts placed at either end of a small outboard-powered boat, or hoisted between ropes in the tree canopy. Each animal was anaesthetized via mask induction of isoflurane as described by Jonsson et al. (2004), or with an intramuscular injection of ketamine (6–7 mg kg−1) and medetomidine (60–70 μg kg−1) followed by reversal with atipamezole (300–350 μg kg−1).
Sex, reproductive status, forearm length, weight and age were recorded for individual animals. Criteria for classification into age classes are described in detail in table 1. An approximate age for mature flying foxes was determined by tooth wear. We aged a subset of individuals (n=163) by the analysis of annual cementum growth layers in an extracted first left mandibular premolar as described by Divljan et al. (2006).
Blood was collected by venepuncture from the propatagial (leading edge wing membrane) vein, and in accordance with the Australian Bird and Bat Banding Scheme, each individual was fitted with an individually numbered thumb band so that recaptured individuals could be identified. After full recovery from anaesthesia, flying foxes were released at the capture site.
Population estimates were not possible due to thickly vegetated terrain, lack of topographic variation and ‘fly outs’ of large numbers of animals that occurred over a 360° radius. Roughly, we estimated camp sizes to be in the hundreds of thousands of individuals, except for autumn 2006, when only a few thousand individuals could be located.
(c) Serologic tests
Serum neutralization tests (SNTs), the reference standard for HeV (Daniels et al. 2001), were performed on serum or plasma samples from each bat in the Biosecurity level 4 laboratory at the Australian Animal Health Laboratory in Geelong, Australia. Neutralization of HeV by the sample at a dilution of 1 : 5 or greater was regarded as a positive test based on titres generated by experimentally infected flying foxes (Williamson et al. 1998; Halpin et al., unpublished data). Samples that were unable to neutralize HeV at any dilution were regarded as negative, while samples that were toxic to the cell layer were regarded as unknown. The samples were retested using a Luminex assay (Bossart et al. 2007) to ensure that laboratory error was not a cause of the patterns observed. As HeV does not cause disease-induced mortality in flying foxes (Williamson et al. 1998, 1999), and induces long-lived detectable immunity in Pteropus conspicillatus and Pteropus poliocephalus (spectacled and black flying foxes; Field 2005; H. Field 1998–2001, unpublished data), we assumed that seroprevalence measures cumulative past exposure to HeV.
(d) Statistical approach
All variables (described in table 1) were first screened using a univariate analysis and a chi-square test to check for statistically significant associations with serological status. Data were then analysed using logistic regression and chi-square statistics in R (R Development Core Team 2006). Variables were selected for inclusion in the multivariate model based on the likelihood ratio test using forward and backward methods. Continuous variables were categorized and checked graphically to verify the linearity of the log odds. Potential confounding variables were identified based on a change of greater than 10% in the odds ratio of other variables after adding the potential confounder to the model, previous knowledge and biological reasoning. Adjusted odds ratios and 95% CIs were used to assess the strength of association between risk factors and HeV serostatus.
3. Results
Of the 664 flying foxes sampled over the five field seasons, we obtained 601 SNT results with an overall seroprevalence of 23.62%. Based on univariate analyses, season, age, forearm length, weight, pregnancy, lactation and sex were significantly (p<0.05) associated with serological status (table 2). No individual flying foxes were recaptured.
Table 2.
Univariate analysis of risk factors for Hendra virus serostatus in LRFF: proportion seropositive, odds ratios with 95% CIs and chi-square p-values for variables included in the analysis.
| variable | category | proportion seropositive | odds ratio | 95% CI | chi-square p-value |
|---|---|---|---|---|---|
| sex and reproductive status | non-pregnant female | 0.16 | 1.00 | ||
| pregnant female | 0.83 | 25.47 | 9.23–70.22 | <0.001 | |
| lactating female | 0.52 | 5.6 | 2.24–14.01 | <0.001 | |
| male | 0.23 | 1.55 | 1.02–2.38 | 0.041 | |
| age | 0–1 month | 1.00 | 3×108 | 0–inf | 0.971 |
| 3 months | 0.14 | 9.47 | 1.1–81.79 | 0.041 | |
| 6 months | 0.02 | 1.00 | |||
| pre-breeding | 0.12 | 8.46 | 1.12–64.17 | 0.039 | |
| adult | 0.35 | 32.62 | 4.46–238.54 | <0.001 | |
| season | spring 2004 | 0.31 | 1.00 | ||
| autumn 2005 | 0.47 | 1.66 | 0.97–2.83 | 0.066 | |
| winter 2005 | 0.16 | 0.41 | 0.24–0.71 | 0.002 | |
| spring 2005 | 0.06 | 0.14 | 0.07–0.28 | <0.001 | |
| autumn 2006 | 0.76 | 6.99 | 2.63–18.62 | <0.001 | |
| forearm | forearm length (mm) | 1.09 | 1.06–1.12 | <0.001 | |
| weight | body weight (g) | 1.01 | 1.00–1.01 | <0.001 | |
| weight/forearm ratioa | body weight (g)/forearm length (mm) | 1.75 | 0.80–3.83 | 0.163 |
Excluding pregnant females.
(a) Stratified analysis
(i) Age and size
Apparent maternal immunity in pups waned over the first six months of life, after which seroprevalence increased with age, creating a typical concave-shaped curve (figure 2a). Seroprevalence increased with forearm length (figure 2b), a trend that was significant after we accounted for age (p<0.001). The analysis of extracted teeth demonstrated that seroprevalence continues to increase with age within the mature class; however, most teeth were removed during winter 2005 and spring 2005 when there were very few seropositive animals, hence the strength of association between age and serostatus is dampened in this subset of data. Figure 2c illustrates the age distribution of animals from which teeth were removed for analysis.
Figure 2.
Patterns of HeV seroprevalence in relation to age and size. (a) Age-specific HeV seroprevalence as determined by criteria in table 1: animals less than one month old were all seropositive (n=8), reflecting the serostatus of their mothers, which were also all seropositive. Error bars represent 95% binomial CIs. (b) Relationship between forearm length (for animals with forearm length≥124 mm) and HeV seroprevalence. The risk of HeV infection increases with body size. Animals with a forearm length of above 130 mm were all mature animals. Error bars represent 95% binomial CIs. A, 125–130; B, 131–135; C, 136–140; D, 141–145; E, 146–150; F, 151–155; G, 156–160. (c) Age distribution of animals from which teeth were analysed.
(ii) Sex and reproductive status
Seroprevalence in males and females differed significantly only during the 2005 birthing season (autumn), when the seroprevalence in pregnant and lactating females was more than four times the seroprevalence in adult males (p<0.0001) and non-reproductive females (p<0.0001; figure 3a). In autumn 2006, no females were pregnant or lactating, presumably due to poor body conditions and nutritional stress observed, yet both males and females of all age groups had a high seroprevalence, equivalent to that of the reproductive females in the 2005 birthing season.
Figure 3.
HeV seroprevalence as a function of reproductive status and season. (a) Seroprevalence across reproductive classes in autumn 2005 (preg, pregnant; lact, lactating; not preg/lact, not pregnant or lactating). (b) Overall seroprevalence across seasons (Spr04, spring 2004; Aut05, autumn 2005; Win05, winter 2005; Spr05, spring 2005; Aut06, autumn 2006). Error bars represent 95% binomial CIs.
(iii) Season and location within seasons
Seroprevalence differed significantly between seasons (table 2; figure 3b), although the patterns were not annually predictable. Overall seroprevalence in spring 2004 (mating), autumn 2005 (birthing) and autumn 2006 (birthing season but animals not reproducing) was more than twice as high as seroprevalence in winter 2005 (weaning) and spring 2005 (mating). Sampling location within a season was not a significant risk factor for serostatus (table 2): there were no significant differences between the Flora River and the Northern Territory Rural College in winter 2005 (p=0.91), the Katherine River and the Elsey National Park in spring 2005 (p=0.10), or the Litchfield National Park and the Katherine Gorge in autumn 2006 (p=0.85). Repeated analyses using luminex test results revealed the same patterns in the data.
(b) Logistic regression analysis
Age, season and sex/reproductive status were included in the main effects model (table 3). In the final model, mature flying foxes, pregnant and lactating females and flying foxes caught in autumn 2006 had a significantly higher risk of being seropositive (p<0.05), while flying foxes caught in winter and spring 2005 had a significantly lower risk of being seropositive (p<0.01). Notably, when adjusted for sex and age, the odds of being seropositive in autumn 2006 were 14–42 times higher than the odds of being seropositive in any other season. Lactating females were sampled during the birthing season, when seroprevalence was high, and during winter, when seroprevalence was low. In contrast, pregnant females were only sampled during birthing; therefore, lactation was associated with a lower relative risk than pregnancy. Forearm length, weight and weight/forearm ratio did not significantly improve the fit of the model after adjusting for other covariates (based on the likelihood ratio test), and were therefore excluded. Two-way interactions among the main effects could not be assessed due to the lack of data in some cells.
Table 3.
Logistic regression model of risk factors for Hendra virus serostatus in LRFF: coefficients, chi-square p-values and odds ratios with 95% CIs for variables included in the model.
| variable | category | coefficient estimate | chi-square p-value | odds ratio | 95% CI |
|---|---|---|---|---|---|
| sex and reproductive status | non-pregnant female | reference | |||
| pregnant female | 2.74 | <0.001 | 15.48 | 4.41–54.35 | |
| lactating female | 1.38 | 0.010 | 3.96 | 1.4–11.24 | |
| male | 0.02 | 0.932 | 1.02 | 0.61–1.71 | |
| age | age 0 | 19.33 | 0.972 | 2×108 | 0–inf |
| 3 months (weaning) | 2.19 | 0.060 | 8.91 | 0.91–87.17 | |
| 6 months | reference | ||||
| pre-breeding | 1.65 | 0.120 | 5.19 | 0.65–41.38 | |
| mature | 2.65 | 0.011 | 14.14 | 1.86–107.75 | |
| season | spring 2004 | reference | |||
| autumn 2005 | −0.70 | 0.075 | 0.49 | 0.23–1.07 | |
| winter 2005 | −0.97 | 0.007 | 0.38 | 0.19–0.76 | |
| spring 2005 | −1.85 | <0.001 | 0.16 | 0.07–0.33 | |
| autumn 2006 | 1.90 | <0.001 | 6.67 | 2.43–18.31 | |
4. Discussion
Our analysis provides empirical evidence that nutritional status and reproduction are important drivers of HeV seroprevalence. These results have implications for the management and control of HeV outbreaks, because they indicate that seasonality and environmental stresses may lead to epidemics of HeV in flying foxes. Age-specific seroprevalence curves indicate that horizontal transmission is the principal form of intraspecific transmission, suggesting that contaminated faeces, urine or saliva may be responsible for spillover to other species. Waning acquired immunity in LRFF is suggested, and it could be an important driver of within-host dynamics in this system, as well as in the persistence of HeV within interspecific flying fox communities.
When experimentally infected with HeV, flying foxes develop viraemia and excrete virus in urine, faeces and saliva for approximately one week (Halpin et al., unpublished data). In their typically dense, three-dimensional aggregations, flying foxes excrete urine and faeces throughout the day, and it is not uncommon to see a fine mist of urine in the camp. Under these conditions, particularly given regular grooming behaviour, horizontal transmission is very likely. The concave curve in figure 2a suggests waning maternal immunity followed by cumulative risk of exposure with age and is consistent with horizontal transmission. The finding that maternal antibody status declines over the first six months of life (descending portion of curve in figure 2a) corresponds with the findings from Field (2005), in which maternal antibodies in captive-born P. conspicillatus and P. poliocephalus declined over seven months.
Vertical transmission has been documented in flying foxes on two occasions: in parenterally inoculated pregnant P. poliocephalus (Williamson et al. 1999; but not in oronasally inoculated pregnant Pteropus alecto, Halpin et al., unpublished data) and in two injured free-living flying foxes (P. alecto and P. poliocephalus) in temporary captivity (Halpin et al. 2000). The lack of antibody status in juvenile flying foxes after maternal immunity has waned, along with the concave age-specific seroprevalence curve, suggests that if vertical transmission occurs in LRFF, it is not the predominant form of transmission.
HeV seroprevalence showed a scaling relationship with increasing forearm length, even after we controlled for age (figure 2b). This phenomenon suggests the possibility that risk of disease exposure increases with host body size, since forearm length, which does not increase in mature animals, serves as a proxy for adult body size. One explanation for this pattern is that size is associated with dominance rank and that dominant animals have higher contact rates. Behavioural studies of flying foxes have documented hierarchies where dominant males control larger harems of females during mating (Markus 2002), and dominant individuals defend feeding sites against the intrusion of other individuals (Birt 2004).
The fourfold higher seroprevalence in pregnant and lactating females in autumn 2005, compared with seroprevalence in males and pre-breeding females, indicates that pregnancy and lactation are risk factors for viral infection. Increased susceptibility to infectious disease during pregnancy and lactation has been demonstrated in other mammalian systems (Lloyd 1983; Alexander & Stimson 1988; Festa-Bianchet 1989; Sheldon & Verhulst 1996; Nelson 2004; Cattadori et al. 2005; Trevejo et al. 2005) due to hormonal suppression of immunity (Alexander & Stimson 1988) and the allocation of resources to reproduction at the expense of immune defence (Sheldon & Verhulst 1996; Nelson 2004). The modified behaviour of pregnant and lactating flying foxes may also promote exposure.
This is the first study to show an association between birthing and HeV infection in a flying fox population, and supports the hypothesis that HeV spillover is linked to flying fox reproduction. A temporal association between the peak birthing season in flying foxes and some disease events in horses (Young et al. 1997; Field et al. 2000, 2007; Halpin et al. 2000), and evidence of vertical transmission (Williamson et al. 1999; Halpin et al. 2000), has led to speculation that pasture contaminated with aborted foetuses or birthing fluids was the source of infection for horses (Young et al. 1997; Field et al. 2000; Halpin et al. 2000). Our data demonstrate that HeV risk is high in all reproducing females, including lactating and near-term pregnant animals (animals that have not aborted their foetus), suggesting that increased numbers of infected animals, as opposed to simply abortion events, could drive the higher force of infection to spillover hosts during the reproductive season.
In the autumn 2006 birthing period, no females were pregnant or lactating. Coincident with this period was a shortage of blossom and nectar, presumably due to high rainfall and a prolonged wet season. Low body weights, poor body conditions and observations of extremely abnormal diurnal feeding indicate that the population was nutritionally stressed, which probably accounts for the non-reproductive state of adult females at this time. Despite the lack of reproduction, all demographic groups exhibited approximately 80% seroprevalence, 14–42 times higher than the odds of seropositivity in any other season after adjusting for sex, reproductive status and age (table 3). The elevated seroprevalence in a nutritionally stressed population could be explained by increased viral susceptibility due to substantial energetic costs of immune responses (Gulland 1992; Sheldon & Verhulst 1996; Nelson 2004). Furthermore, nutritional and environmental stresses can change behaviour, for example causing animals to crowd around restricted food resources or to share food with other species. Our data (figure 4) demonstrate that body condition and seroprevalence were related in all field seasons: seroprevalence increased when body condition decreased, with the exception of males during the two mating seasons.
Figure 4.
Relationship between seroprevalence and body condition (weight/forearm ratio). Points represent mean seroprevalence per field season as a function of mean body weight/forearm ratio for adult non-pregnant females (squares and solid line) and adult males (diamonds and dashed line). Dividing body weight by forearm measurement accounts for body size and therefore gives a crude measurement of body condition. 1, spring 2004; 2, autumn 2005; 3, winter 2005; 4, spring 2005; 5, autumn 2006.
We hypothesized that mating would be associated with increased risk of viral transmission, because it is a time of intense interaction (Markus 2002) and the immunosuppressive effects of testosterone have been shown to increase disease risk (Zuk & McKean 1996). In the spring 2004 mating season, there was a high population seroprevalence (figure 3b), particularly in adult males. However, during the spring 2005 mating season, we observed low seroprevalence in all animals, yet mating was observed, and age distributions and population size were comparable with those of spring 2004. A plausible explanation for our results from spring 2005 is that HeV infection was not present in the population at that time. This interpretation is supported by modelling efforts of HeV infection dynamics in the other flying fox species, which suggest that HeV does not persist endemically within spatially discrete local populations, but persists globally via metapopulation dynamics (Plowright 2007).
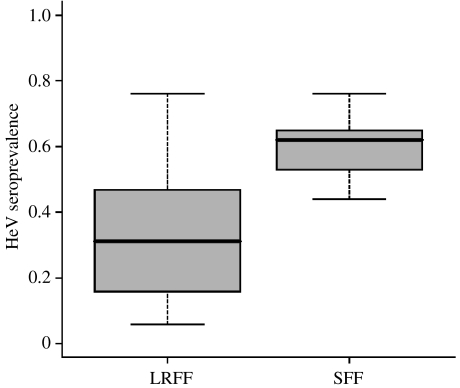
Although the absence of infection provides a plausible explanation for the low HeV seroprevalence in LRFF in spring 2005, it is an improbable explanation for the marked decline in seroprevalence we observed between autumn 2005 and winter/spring 2005 (figure 3b). It may be that, unlike detectable immunity in other flying fox species, detectable immunity in LRFF wanes over short time scales. IgG to HeV in P. conspicillatus and P. poliocephalus is detectable for years-to-a-lifetime (Field 2005; H. Field, unpublished data), resulting in relatively stable seroprevalence (figure 5) over time (Field 2005).
Figure 5.
Variance in HeV seroprevalence over five field seasons in LRFF compared with variance in HeV seroprevalence over five field seasons in a Northern Queensland population of spectacled flying foxes (A. C. Breed 2005–2006, unpublished data).
Waning immunity would significantly enhance the persistence of HeV in LRFF, conferring on them a critical role in the maintenance of HeV across Pteropus spp. Given that LRFF resolve as the most basal lineage of Pteropus bats (O'Brien 2005), and are genetically distant (Bastian et al. 2002; O'Brien 2005), and ecologically and morphologically distinct (Hall & Richards 2000; Birt 2004), from other Australian flying fox species, waning immunity may reflect a more mature host–pathogen relationship in this species.
In our study, flying fox campsites moved in relation to ephemeral patches of blossom and nectar; therefore, we sampled in multiple locations. In our analysis, we assumed that individuals sampled at different locations were part of a single population and that the patterns observed were not due to sampling of different populations. We based this assumption on: (i) almost identical seroprevalence among locations within seasons, suggesting regional synchrony of viral dynamics, (ii) satellite and radio telemetry studies which indicated considerable movement of individuals among roost sites within the study area (R. K. Plowright, C. Smith, C. Palmer, A. Divljan & H. Field 2005, unpublished data; C. Palmer, C. Smith, A. Divljan & R. K. Plowright 2005, unpublished data), and (iii) the rapid decline in seroprevalence observed between autumn and winter 2005, which was probably not due to sampling of different populations, as we sampled at the same location consecutively during those two seasons. Furthermore, we are confident that at least the lactating females in autumn and winter 2005 represent a single population, because lactating females typically do not move during pup dependency (Birt 2004) and we observed the same pattern of declining seroprevalence in these animals. We therefore conclude that disparate local populations may actually constitute one regional population for viral dynamics, that sampling different populations did not confound our results, and that the overall rapid decline in seroprevalence observed between seasons is not due to sampling of different populations.
In summary, our research identified a pattern of horizontal transmission, probably through urine, faeces or saliva, with increasing age significantly increasing risk of infection through direct contact. Excreta- or saliva-contaminated pastures, or discarded fruit, are probable routes of viral transmission to horses, and our data suggest that risk of transmission increases during flying fox reproductive periods and in times of nutritional stress. Our conclusion that LRFF immune dynamics seem to differ from dynamics in the three other Australian Pteropus species may be critical for understanding viral dynamics in all Pteropus spp.
Increased infection risk in reproductively and nutritionally stressed animals may have important ramifications for the dynamics of henipaviruses in Pteropus species. First, it suggests that there is probably an element of seasonality to infection dynamics that may be useful in spillover prevention and control. Second, anthropogenic habitat loss, habitat alteration, roost disturbance, urbanization and persecution by hunters (Fujita & Tuttle 1991; Mickleburg et al. 1992; Duncan et al. 1999), all of which are known to alter food availability and cause nutritional stress in Pteropus populations (Hall & Richards 2000; P. Eby 1998–2007, unpublished data), may drive HeV spillover events. Finally, nectar and fruit availability are dependent on climatic conditions (Law et al. 2000), and thus flying foxes—particularly the highly nectar-dependent LRFF—may be greatly affected by climate change. Our data suggest that such environmental stresses lead directly to changes in henipavirus infection and excretion dynamics. Future work to test the hypothesis that nutritional stress, driven by climate change and other anthropogenic alterations to the environment, is an important driver of disease emergence for HeV, and perhaps other emerging bat viruses, is urgently needed.
Acknowledgments
Charles Darwin University Animal Ethics Committee gave permission for work on P. scapulatus (project reference: A04033). Permits were granted by the Parks and Wildlife Commission of the Northern Territory (permit number: 18597).
We thank the Northern Territory Government's Department of Natural Resources, Environment and the Arts, particularly Katherine region staff and John Woinarski from Biodiversity Conservation Division, Darwin, for their great support during the project. We also thank Greer Meehan, Rhys Fogarty, John Burke, Justin Perry, Rhonda Scoccinarro, Tracey Blackney, Sam Veloz, Liz Chamberlin, Adam Porter, Damian Milne, Dave Fuller, Carol de Jong, Kerryn Parry-Jones, Marion Cook, Peter Cook, Chris Kinard, David Hooper, Andy Peckham, Amanda McLaughlin, Sam Wushusen and Katharine Bossart for their assistance with the field and laboratory work; Les Hall, Patrina Birt, Peggy Eby, Kim Halpin, Juliet Pulliam, Patrick Foley, Phil Kass, Andrew Breed, Alex Hyatt, Deborah Middleton, Andy Dobson, Jon Epstein, Lin-Fa Wang, Bryan Eaton, Andrew Cunningham and Kevin Olival for their insightful conversations about LRFF and HeV; Rosie Woodroffe, Bruno Chomel, Leslie Bienen, Paul Cross, John Winnie Jr and Sara Gregory for their comments on the manuscript.
This work was supported in part by an NIH/NSF ‘Ecology of Infectious Diseases’ award from the John E. Fogarty International Center R01-TW05869, by core funding to the Consortium for Conservation Medicine from the V. Kann Rasmussen Foundation and is published in collaboration with the Australian Biosecurity Cooperative Research Center for Emerging Infectious Diseases (AB-CRC). R.K.P. was supported by the V. Kann Rasmussen Foundation, the Australian–American Fulbright Commission, the Foundation for Young Australians and grants from the UC Davis Graduate Group in Ecology.
References
- Alexander J, Stimson W.H. Sex-hormones and the course of parasitic infection. Parasitol. Today. 1988;4:189–193. doi:10.1016/0169-4758(88)90077-4 [Google Scholar]
- Bastian S.T, Jr, Tanaka K, Anunciado R.V.P, Natural N.G, Sumalde A.C, Namikawa T. Evolutionary relationships of flying foxes (Genus Pteropus) in The Philippines inferred from DNA sequences of cytochrome b gene. Biochem. Genet. 2002;40:101–116. doi: 10.1023/a:1015161305843. doi:10.1023/A:1015161305843 [DOI] [PubMed] [Google Scholar]
- Birt, P. 2004 Mutualistic interactions between the nectar-feeding little red flying fox Pteropus scapulatus (Chiroptera: Pteropodidae) and flowering eucalypts (Myrtaceae): habitat utilisation and pollination. PhD thesis, University of Queensland, Brisbane, Australia.
- Bossart K.N, McEachern J.A, Hickey A.C, Choudhry V, Dimitrov D.S, Eaton B.T, Wang L.F. Neutralization assays for differential henipavirus serology using Bio-Plex protein array systems. J. Virol. Methods. 2007;142:29–40. doi: 10.1016/j.jviromet.2007.01.003. doi:10.1016/j.jviromet.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Cattadori I.M, Boag B, Bjornstad O.N, Cornell S.J, Hudson P.J. Peak shift and epidemiology in a seasonal host-nematode system. Proc. R. Soc. B. 2005;272:1163–1169. doi: 10.1098/rspb.2004.3050. doi:10.1098/rspb.2004.3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua K.B, et al. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc. Natl Acad. Sci. USA. 2007;104:11 424–11 429. doi: 10.1073/pnas.0701372104. doi:10.1073/pnas.0701372104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.L. Changing patterns of infectious disease. Nature. 2000;406:762–767. doi: 10.1038/35021206. doi:10.1038/35021206 [DOI] [PubMed] [Google Scholar]
- Daniels P, Ksiazek T, Eaton B.T. Laboratory diagnosis of Nipah and Hendra virus infections. Microbes Infect. 2001;3:289–295. doi: 10.1016/s1286-4579(01)01382-x. doi:10.1016/S1286-4579(01)01382-X [DOI] [PubMed] [Google Scholar]
- Daszak P, Cunningham A.A, Hyatt A.D. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. doi:10.1126/science.287.5452.443 [DOI] [PubMed] [Google Scholar]
- Daszak P, et al. The emergence of Nipah and Hendra virus: pathogen dynamics across a wildlife–livestock–human continuum. In: Collinge S, et al., editors. Disease ecology: community structure and pathogen dynamics. Oxford University Press; Oxford, UK: 2006. pp. 186–201. [Google Scholar]
- Divljan A, Parry-Jones K, Wardle G.M. Age determination in the grey-headed flying fox. J. Wildl. Manage. 2006;70:607–611. doi:10.2193/0022-541X(2006)70[607:ADITGF]2.0.CO;2 [Google Scholar]
- Dobson A, Foufopoulos J. Emerging infectious pathogens of wildlife. Phil. Trans. R. Soc. B. 2001;356:1001–1012. doi: 10.1098/rstb.2001.0900. doi:10.1098/rstb.2000.0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A, Baker G.B, Montgomery N. Environment Australia; Canberra, Australia: 1999. The action plan for Australian bats. [Google Scholar]
- Eaton B.T, Broder C.C, Middleton D, Wang L.F. Hendra and Nipah viruses: different and dangerous. Nat. Rev. Microbiol. 2006;4:23–35. doi: 10.1038/nrmicro1323. doi:10.1038/nrmicro1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J.H, Field H.E, Luby S, Pulliam J.R, Daszak P. Nipah virus: impact, origins, and causes of emergence. Curr. Infect. Dis. Rep. 2006;8:59–65. doi: 10.1007/s11908-006-0036-2. doi:10.1007/s11908-006-0036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa-Bianchet M. Individual differences, parasites, and the costs of reproduction for bighorn ewes (Ovis canadensis) J. Anim. Ecol. 1989;58:785–795. doi:10.2307/5124 [Google Scholar]
- Field, H. 2005 The ecology of Hendra virus and Australian bat lyssavirus. PhD thesis, The University of Queensland, Brisbane.
- Field H, Barrat P.C, Hughes R.J, Shield J, Sullivan N.D. A fatal case of Hendra virus infection in a horse in north Queensland—clinical and epidemiological features. Aust. Vet. J. 2000;78:279–280. doi: 10.1111/j.1751-0813.2000.tb11758.x. [DOI] [PubMed] [Google Scholar]
- Field H, Young P, Yob J.M, Mills J, Hall L, Mackenzie J. The natural history of Hendra and Nipah viruses. Microbes Infect. 2001;3:307–314. doi: 10.1016/s1286-4579(01)01384-3. doi:10.1016/S1286-4579(01)01384-3 [DOI] [PubMed] [Google Scholar]
- Field H.E, Breed A.C, Shield J, Hedlefs R.M, Pittard K, Pott B, Summers P.M. Epidemiological perspectives on Hendra virus infection in horses and flying foxes. Aust. Vet. J. 2007;85:268–270. doi: 10.1111/j.1751-0813.2007.00170.x. doi:10.1111/j.1751-0813.2007.00170.x [DOI] [PubMed] [Google Scholar]
- Fujita M.S, Tuttle M.D. Flying foxes (Chiroptera: Pteropodidae): threatened animals of key ecological and economic importance. Conserv. Biol. 1991;5:455–463. doi:10.1111/j.1523-1739.1991.tb00352.x [Google Scholar]
- Gulland F.M. The role of nematode parasites in Soay sheep (Ovis aries L.) mortality during a population crash. Parasitology. 1992;105(Pt 3):493–503. doi: 10.1017/s0031182000074679. [DOI] [PubMed] [Google Scholar]
- Hall L, Richards G. Australian natural history series. University of New South Wales Press Ltd; Sydney, Australia: 2000. Flying foxes: fruit and blossom bats of Australia. [Google Scholar]
- Halpin K, Young P.L, Field H.E, Mackenzie J.S. Isolation of Hendra virus from Pteropid bats: a natural reservoir of Hendra virus. J. Gen. Virol. 2000;81:1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- Hanna J.N, McBride W.J, Brookes D.L, Shield J, Taylor C.T, Smith I.L, Craig S.B, Smith G.A. Hendra virus infection in a veterinarian. Med. J. Aust. 2006;185:562–564. doi: 10.5694/j.1326-5377.2006.tb00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson N.N, Johnston S.D, Field H, de Jong C, Smith C. Field anaesthesia of three Australian species of flying fox. Vet. Rec. 2004;154:664. doi: 10.1136/vr.154.21.664. [DOI] [PubMed] [Google Scholar]
- Law B, Mackowski C, Schoer L, Tweedie T. Flowering phenology of myrtaceous trees and their relation to climatic, environmental and disturbance variables in northern New South Wales. Austral Ecol. 2000;25:160–178. doi:10.1046/j.1442-9993.2000.01009.x [Google Scholar]
- Lloyd S.S. Immunosuppression during pregnancy and lactation. Ir. Vet. J. 1983;37:64–70. [Google Scholar]
- Mackenzie J.S, et al. Emerging viral diseases of Southeast Asia and the Western Pacific. Emerg. Infect. Dis. 2001;7:497–504. doi: 10.3201/eid0707.017703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J.S, Field H.E, Guyatt K.J. Managing emerging diseases borne by fruit bats (flying foxes), with particular reference to henipaviruses and Australian bat lyssavirus. J. Appl. Microbiol. 2003;94:59s–69s. doi: 10.1046/j.1365-2672.94.s1.7.x. doi:10.1046/j.1365-2672.94.s1.7.x [DOI] [PubMed] [Google Scholar]
- Markus N. Behaviour of the black flying fox Pteropus alecto: 2. Territoriality and courtship. Acta Chiropt. 2002;4:153–166. [Google Scholar]
- Mickleburg S, Hutson A, Racey P. International Union for the Conservation of Nature and Natural Resources (IUCN); Gland, Switzerland: 1992. Old world fruit bats: an action plan for their conservation. [Google Scholar]
- Murray K, et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. doi:10.1126/science.7701348 [DOI] [PubMed] [Google Scholar]
- Nelson R.J. Seasonal immune function and sickness responses. Trends Immunol. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. doi:10.1016/j.it.2004.02.001 [DOI] [PubMed] [Google Scholar]
- O'Brien, J. 2005 Phylogeography and conservation genetics of the fruit bat genus Pteropus (Megachiroptera) in the western Indian Ocean. PhD thesis, National University of Ireland, Dublin.
- Palmer C, Woinarski J.C.Z. Seasonal roosts and foraging movements of the black flying fox (Pteropus alecto) in the Northern Territory: resource tracking in a landscape mosaic. Wildl. Res. 1999;26:823–838. doi:10.1071/WR97106 [Google Scholar]
- Palmer C, Price O, Bach C. Foraging ecology of the black flying fox (Pteropus alecto) in the seasonal tropics of the Northern Territory Australia. Wildl. Res. 2000;27:169–178. doi:10.1071/WR97126 [Google Scholar]
- Plowright, R. K. 2007 The ecology and epidemiology of Hendra virus in flying foxes (Pteropus spp.). PhD thesis, University of California, Davis.
- Plowright R.K, Sokolow S.H, Gorman M.E, Daszak P, Foley J.E. Causal inference in disease ecology: investigating ecological drivers of disease emergence. Front. Ecol. Environ. 2008;6 doi:10.1890/070086 [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2006. R: a language and environment for statistical computing. [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. doi:10.1016/0169-5347(96)10039-2 [DOI] [PubMed] [Google Scholar]
- Smolinski M.S, Hamburg M.A, Lederberg J. The National Academies Press; Washington, DC: 2003. Microbial threats to health: emergence, detection, and response. [PubMed] [Google Scholar]
- Trevejo R.T, Barr M.C, Robinson R.A. Important emerging bacterial zoonotic infections affecting the immunocompromised. Vet. Res. 2005;36:493–506. doi: 10.1051/vetres:2005011. doi:10.1051/vetres:2005011 [DOI] [PubMed] [Google Scholar]
- Williamson M.M, Hooper P.T, Selleck P.W, Gleeson L.J, Daniels P.W, Westbury H.A, Murray P.K. Transmission studies of Hendra virus (equine morbillivirus) in fruit bats, horses and cats. Aust. Vet. J. 1998;76:813–818. doi: 10.1111/j.1751-0813.1998.tb12335.x. [DOI] [PubMed] [Google Scholar]
- Williamson M.M, Hooper P.T, Selleck P.W, Westbury H.A, Slocombe R.F. Experimental Hendra virus infection in pregnant guinea-pigs and fruit bats (Pteropus poliocephalus) J. Comp. Pathol. 1999;122:201–207. doi: 10.1053/jcpa.1999.0364. doi:10.1053/jcpa.1999.0364 [DOI] [PubMed] [Google Scholar]
- Young, P., Halpin, K., Field, H. & Mackenzie, J. 1997 Finding the wildlife reservoir of equine morbillivirus. In Recent advances in microbiology, vol. 5 (ed. V. Asche), pp. 1–12. Melbourne, Australia: Australian Society of Microbiology, Inc.
- Zuk M, McKean K.A. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 1996;26:1009–1023. doi:10.1016/S0020-7519(96)00086-0 [PubMed] [Google Scholar]