Abstract
This paper reports on a unique preservation of soft tissues in the ventrolateral region of the plant-eating dinosaur Psittacosaurus from the Jehol biota of China. The preservation is of a deep cross section through the dermis, which includes multiple layers of collagenous fibres in excess of 25, among the highest recorded in vertebrates, with a further 15 more layers (poorly preserved) estimated for the entire height of the section. Also, for the first time in a dinosaur two fibre layers parallel to the skin surface are preserved deep within the dermis at the base of the cross section. These fibre layers comprise regularly disposed fibres arranged in left- and right-handed geodesic helices, matching the pattern at the surface and reasonably inferred for the entire section. As noted from the studies on modern-day animals, this fibre structure plays a critical part in the stresses and strains the skin may be subjected to and is ideally suited to providing support and protection. Psittacosaurus gives a remarkable, unprecedented understanding of the dinosaur skin.
Keywords: Psittacosaurus, dermal cross section, helical fibres, multiple layers
1. Introduction
The structures and mechanical properties of the skin of interest to the present study concern the dermis. As a connective tissue, the dermis may be said to consist of a fibrillar framework, enmeshed in, and partially united with, an ‘amorphous’ ground substance of mucopolysaccharides (glycosaminoglycans). The three-dimensional framework may vary in complexity in vertebrates but is always composed of collagen fibres. While this dermal architecture participates variably in several other functions in different species, it appears to carry out widely in vertebrates one particular function of providing the biomechanically important role of protection and/or support for the enclosed body mass (Moss 1972).
A number of studies in vertebrates in the past 30 years have shown exactly how the skin provides both a rigid framework to support the body contents and a flexible covering to allow changes in shape associated with mobility and locomotion (Wainwright et al. 1978; Alexander 1987; Pabst 1996; Lingham-Soliar 2005a,b and references therein). Rather inextensible dermal collagen fibres are central to these functions. Application of this knowledge has been possible in only one fossil vertebrate group, to the author's knowledge, the ichthyosaur (Lingham-Soliar & Reif 1998; Lingham-Soliar 1999, 2001; Lingham-Soliar & Plodowski 2007), largely owing to the excellence of soft tissue preservations from the Posidonia Shale of Baden Württemberg (Toarcian), southern Germany. On the other hand, dinosaur integumental preservations were rather sparse, that is, until the explosion of finds from the Lower Cretaceous Yixian Formation in Liaoning Province, China in the mid-1990s.
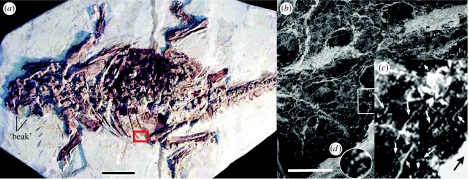
Psittacosaurus (from the Greek for parrot lizard) was a bipedal, plant-eating, ceratopsian dinosaur (about the size of a gazelle; figure 1) occupying a phylogenetic position far removed from theropod dinosaurs and bird origins (Feduccia et al. 2005 and references therein). It is understood from the preservation of different parts of the body in another specimen that Psittacosaurus was covered by epidermal scales (Mayr et al. 2002). Erosion of the scales in many areas in that specimen has exposed the underlying dermis and its fibres (figure 1b). In the present specimen, the epidermal scales are further eroded and the dermal fibres even more widely exposed, in particular, in the thoracic rib area (Feduccia et al. 2005). Here, however, seen for the first time in a dinosaur, a cross section of the dermis cut at right angles to the surface and, also for the first time, tangential sections, i.e. sections cut on a plane parallel to the skin surface, deep within the dermis. This extraordinary preservation opens a window of understanding into the deep structure of dinosaur skin that parallels in many ways similar investigations of large extant vertebrates in recent years.
Figure 1.
The dinosaur Psittacosaurus. (a) Specimen MV 53 (Nanjing Museum of Geology and Palaeontology, China). (b) Specimen SMF R 4970 (Forschungsinstitut Senckenberg, Germany). The figure shows the detail of the skin preservation of the left shoulder; plate-like scales are separated by numerous small polygonal and tubercle-like scales (photo courtesy of Prof. Gerhard Plodowski). (c) Demarcated area in (b); shows eroded epidermal scales and underlying fibres of the dermis. (d) Detail of beaded fibre. Scale bars, (a) 5 cm and (b) 1 cm.
2. Material and methods
(a) Preserved soft tissue of Psittacosaurus sp.
Psittacosaurus sp. (not Psittacosaurus lujiatunensis, see Averianov et al. 2006) specimen MV53 came from the Lower Cretaceous Yixian Formation in Liaoning Province, China and is part of the Jehol biota. It is housed in the Palaeontological Museum, Nanjing (Nanjing Institute of Geology and Palaeontology).
The cross section of preserved soft tissue, directly adjacent to the surface integumental fibres1 (Feduccia et al. 2005), lies on the ventrolateral part of the body near the last three thoracic ribs and anterior to the left femur (figure 1a, red rectangle). The fine details of the preserved tissue in Psittacosaurus MV53 are probably a consequence of rapid burial and mineralization (Briggs 2003). However, preservation of integumental structures in Liaoning dinosaurs, e.g. Sinosauropteryx (Chen et al. 1998; Currie & Chen 2001; Lingham-Soliar et al. 2007) and Sinornithosaurus (Xu et al. 2001; Feduccia et al. 2005), barring the thick tail structures in the German Psittacosaurus, has been described as ‘blurry or foggy’ (Fucheng et al. 2006, p. 401). The ‘blurry’ preservation may be attributed to brittleness that frequently occurs with pyritized soft tissue (Leng & Yang 2003). Hence, finer details down to the collagen fibril level (approx. 50–100 nm thickness), which ordinarily might benefit from scanning electron microscope (SEM) analysis, may be difficult in pyritized fossil preservations of the Jehol biota and the cost of searching in terms of damaging valuable and limited material (Allison 1988) would be too high to justify. Furthermore, conventional light microscopy used here has proved successful in studies on dermal collagen fibre and fibre bundle organization in modern-day animals such as sharks (Motta 1977; Lingham-Soliar 2005a,b) and dolphins (Pabst 1996).
(b) Decomposing dermal tissue of Carcharodon carcharias
Partially decomposing dermal tissue of the white shark C. carcharias (left for 4 weeks at room temperature, approx. 25–30°C, and allowed to dehydrate for an additional 72 hours in a desiccator) was used for SEM analyses along with fresh tissue of the animal.
3. Results and descriptions
(a) Description of the integumental structures of Psittacosaurus
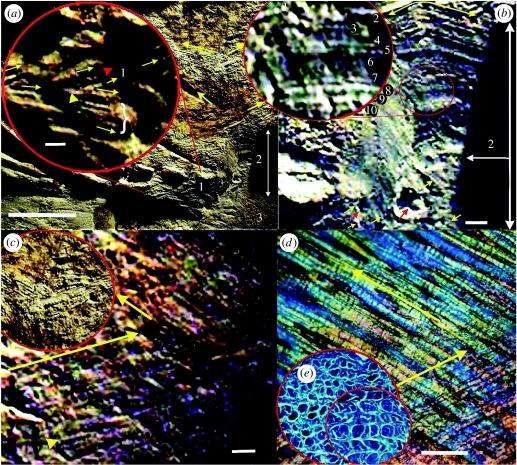
The entire cross section lies adjacent to surface integumental structures and extends from a height of approximately 10–15 mm (figure 2a,b: areas 1 and 2, respectively) and width of approximately 35–40 mm. Fairly well-preserved fibres were described on the surface of the fossil (see figs. 12 and 13 in Feduccia et al. (2005) and figure 2c inset, in this paper). The vertical face in area 1 is more fractured than in area 2. As a consequence of uneven fracturing, cross sections of the fibres are apparent in opposing directions (figure 2a (inset): yellow and red arrowheads). This is suggested with caution because fibre orientations can only be reliably assessed from indisputable tangential views (discussed below). The best preserved fibre layer in area 1 (figure 2a (inset): yellow arrowhead) shows remarkable clarity despite the problems already mentioned concerning Liaoning fossils.
Figure 2.
Integumental fibres. (a) Left posterolateral side of the body shows a vertical fracture, i.e. a cross section, in the fossil (extending from a variable height of approx. 10–15 mm and width 35–40 mm) areas 1 and 2. The surface of the fossil shows fibres extending in opposing directions (described in detail in Feduccia et al. 2005); the vertical section, area 1 (inset), is more fractured than area 2, as a consequence the cross section shows fibres in two slightly different planes (yellow arrows) and the fibre bundles are clearly visible (yellow arrowhead). (b) Cross section of numerous, compacted layers of structural fibres. A few fibres extending out of the cross section (yellow arrows) are also noted. Inset (from approximate circled area): photographed at a slightly different angle to the main picture; despite representing the poorest of the layers preserved, the fibre layers are clearly evident (10, numbered) excluding few missing layers. (c) Opposing layers of fibres (area 3 in (a)) in tangential view, i.e. parallel to the surface fibres, exposed at the base of the section; arrows show respective fibre angles; inset, also in tangential view, shows detail of the surface fibres from (a) oriented in opposing directions. (d,e) Dermal fibres in the white shark C. carcharias. (d) Tangential view of fibre bundles (left-handed); owing to the thinness of the histological section only a trace of the right-handed fibres are present (bottom right). (e) Cross section of fibre bundles from skin from the body of the shark. Scale bars, (a) 20 mm, (b) 1.5 mm and (c,d) 1 mm.
Area 2 represents a cross section of the fibre layers. Preserved layers are clearly visible, numerous and tightly stacked (figure 2b), comparable to those of sharks (Lingham-Soliar 2005a,b; see also figure 2e). Remarkably, an excess of 25 fibre layers are present in the upper two-thirds of the section, ignoring gaps arising from degradation that would clearly have represented further layers (figure 2b, demarcated by white horizontal arrow). In figure 2b (inset), despite the blurry or foggy preservation mentioned above (a problem not exclusive to Liaoning material but also associated with material from Kazakhstan and Russia), it is possible to discern with little trouble 10 fibre layers (numbered 1–10). This part of the cross section despite being more blurry than many other layers (above the horizontal white arrow) is on the other hand notable for fewer gaps between the layers, i.e. 10 layers preserved out of a possible 13 (the missing layers either completely eroded or showing minimal traces). The ‘puffiness’ and ‘whitening’ of the fibres in places, particularly towards the bottom of the inset, are not uncommon in pyritic, and even occasionally phosphatic, degradation (e.g. ichthyosaur dermal fibres; see fig. 9 in Lingham-Soliar 2001). Fibres in the lower one-third of the section (below the horizontal white arrow) are poorly preserved (see below for possible causes). It seems reasonable to estimate a further 15 more layers including the two well-preserved layers exposed on the tangential plane at the base of the section (figure 2c, area 3 in (a)).
Figure 3.
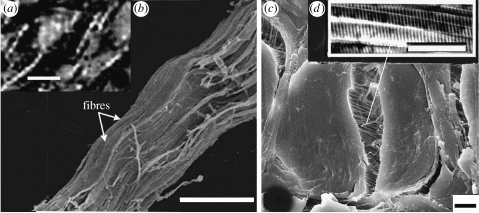
SEM of collagen fibre bundles from the white shark C. carcharias. (a) Degraded and dehydrated collagen fibre bundles showing beaded structure. (b) High resolution of (a): shows fibres within the collagen fibre bundle; the waviness indicates loss of tension (see text). The waves transfer to the fibre bundle and ultimately create the beaded structure. (c) Fresh collagen fibre bundle in cross section. The fibre bundle is fractured down the middle, showing fibres still retaining original tension. (d) Component fibrils from the fibre showing 67 nm axial banding. Scale bars, (a) 500 μm, (b) 50 μm, (c) 10 μm and (d) 1 μm.
Area 3 (figure 2a,c), at the base of the preserved cross section, shows two fibre layers exposed deep within the dermis along the tangential plane. The fibres are present as parallel arrays, with one layer in left-handed orientation and the other in right-handed orientation, at approximately 40° to the animal's long axis. The orientations closely match those on the surface of the animal shown here (figures 2a (arrows) and 3 (insets)), as well as at other locations in the same specimen in Feduccia et al. (2005), suggesting a uniformity of the fibre orientations.
(b) Decomposing dermal tissue of C. carcharias
The decomposing dermis of C. carcharias under low-power SEM (approx. 10×) shows a beaded structure of the collagen fibres (figure 3a) that is strikingly similar to that of Psittacosaurus (figures 1c,d and 2c) and other fossilized animals (Lingham-Soliar 1999, 2001; Feduccia et al. 2005; Lingham-Soliar & Plodowski 2007). At high-power SEM, the fibres comprising the bundles are found to form regular waves (figure 3b, arrows); this shows the onset of dehydration of the dermal tissue. More severe dehydration would increase the magnitude of the waves of the fibres to give the pronounced beaded aberrations of fibre bundles and ultimately result in their distortion and eventual breakup during decay. A similar beaded phenomenon is noted in the histological preparations of fibrous tissue for polarized light microscopy, which requires dehydration to aid birefringence (figure 2d). In contrast, high-power SEM of fibre bundles and fibres in fresh tissue shows that they are straight (figure 3c), reflecting their original tension.
4. Discussion
Collagenous tissue, except in the arthropods, provides the majority of the passive structural elements such as skin (dermis), cartilage, tendon and bone in most animals (Wainwright et al. 1976). The versatility of collagen in vertebrates, often governed by function rather than by taxonomic affiliations, provides properties of both pliancy and rigidity. The dermal tissue of sharks, with a special reference to collagen, is used here for ease of experimentation and availability. Indeed, it is shown in Feduccia et al. (2005) that complex fibre architectures occur in the dermis of a range of living reptiles including the archosaur, Crocodilus niloticus.
(a) Dermal fibre structure of Psittacosaurus and comparisons with modern animals
To date, all integumental structures described in dinosaurs, whether interpreted as ‘protofeathers’ or structural fibres, occur on the surface of the animal or on adjacent substrate. The present findings with respect to the dermis of Psittacosaurus represent a significant development in so far as it is the first cross-sectional exposure to reveal a complex architecture of structural fibres in the dermis of a dinosaur.
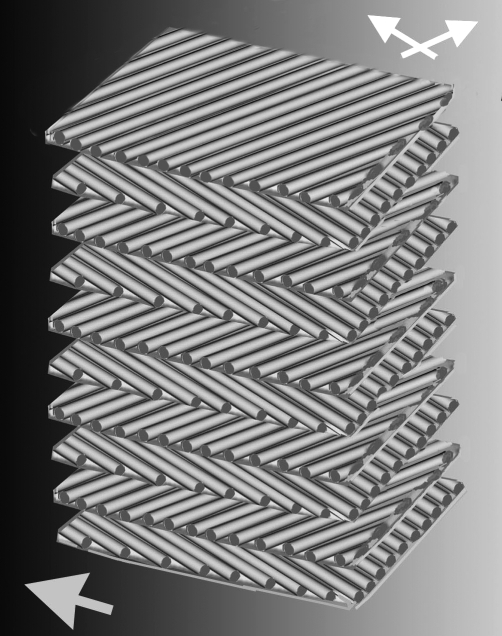
An understanding of the three-dimensional helical structure of dermal collagen in living animals has been achieved by serial cross and tangential sections of the dermis, as well as by confocal laser scanning microscopy. Hence, the type of information gained would ordinarily be beyond the scope of fossilized soft tissue. However, the unique preservation of integumental structures in Psittacosaurus MV53, emanating from freak combinations of circumstances that exposed the skin not only in the tangential plane at the surface and base but also in the cross section, provides a depth of information from a single section that virtually matches that of living animals in the following essential details: (i) the cross section reveals multiple fibre layers through the height of the dermis, (ii) the tangential sections show a highly ordered array of bands of tensile fibres, (iii) the tangential sections at the surface and base of the section show a geodesic arrangement of the fibres oriented in the left- and right-handed directions in alternate layers that probably represented the entire structure in this region of the dermis, and (iv) tangential preservations at the surface and the base show that the fibre angles are relatively low, approximately 35–40° (discussed below).
The fibre structures from the tangential and cross sections of the skin of Psittacosaurus may be closely compared with those from the dermis of a modern-day analogue, C. carcharias (figure 2d,e: tangential and cross sections, respectively), even to the extent of the beaded nature of the fibre bundles (cf. figure 2c,d). There seems to be little doubt that the distinctive three-dimensional dermal architecture of fibres of Psittacosaurus (figure 4) is identifiable with that of many vertebrates thus far studied, such as sharks (Motta 1977; Lingham-Soliar 2005a,b), dolphins (e.g. Pabst 1996), modern reptiles (Feduccia et al. 2005) and the extinct ichthyosaurs (e.g. Lingham-Soliar et al. 2007). We may reasonably assume, as they were for all the above groups, that the structural fibres were collagen, the predominant fibre of the dermis (Moss 1972). Recently, the author was able to confirm that the dermal fibrils in C. carcharias show the repeat 67 nm axial banding or d-bands characteristic of collagen at the ultrastructural level (figure 3d; see Kemp (2005) for collagen fibril d-bands in shark ceratotrichia).
Figure 4.
A schematic three-dimensional view of the dermal fibre architecture in Psittacosaurus MV53 showing fibre layers and orientations, based on cross and tangential sections in the present study. Large arrow shows long axis of animal.
Palaeontology as a science depends on comparisons with modern-day animals, which may permit investigative methods that are precluded by fossil materials alone. One aspect of study that undoubtedly benefits from experiments on modern-day animals concerns animal decay processes prior to fossilization: ‘The most appropriate basis for interpreting the morphology preserved in a soft-bodied fossil is not a pristine carcass, but one that has been subjected to some decay… observations of morphological changes during decay can provide important insights, even when the fossil belongs to an extinct group’ (Briggs 2003, p. 277). To this end, the experimentation on the dehydration of shark dermal tissue (cf. experiments on a decomposing dolphin in Lingham-Soliar (2003)), which includes SEM analysis that is difficult on fossilized integument for reasons mentioned above, provides an important insight into the interpretation of fossilized collagen fibre bundles, with particular reference here to the beaded nature of fibre bundles (figure 2c) frequently observed in fossilized soft tissue (not to be confused with the d-bands of collagen fibrils).
(b) Biomechanical implications of the dermal fibre architecture of Psittacosaurus
The helical fibre structure in the dermis of vertebrates and its behaviour under load are much the same as the trellis web of a beam or truss (Gordon 1978) and play a critical part in the stresses and strains the skin may be subjected to (discussed below). In a classic study, Clark & Cowey (1958) demonstrated how change of shape is controlled in nemertean and turbellarian worms by inextensible fibres in the epidermis arranged in alternate left- and right-handed geodesic helices. In helical fibres that are free from the skeleton, fibre angle plays a critical role with respect to strain due to bending of the body: at high strains (greater stiffness) the fibre angles are greater (approx. 60–70°) than at low strains (greater flexibility) where fibre angles are relatively small (approx. 20–50°; Alexander 1987). The skin of an animal is usually under tension even when it is not being deformed by some movement (Wainwright et al. 1976). However, the skin is by no means isotrophic or uniform over the animal's surface. For instance, in the living animal, low or high modulus regions must reflect major differences in the mechanical function of the skin in different areas of the body. In Psittacosaurus, the preferred directions and extensions of the fibres at low angles (figure 4) would reflect the mechanical function in the ventrolateral and ventral regions of the body where there would be stress variations connected with expansion of the stomach and gut during feeding. Indeed, tensile tests on the skin of sharks have shown the effectiveness of helical fibres reorienting towards such varying stress axes (Naresh et al. 1997). The mechanical function may be significantly different, for example, along the animal's back and would be reflected by higher fibre angles in a state of tension (see Lingham-Soliar (2005a,b) for stress and fibre angle variations in the body of C. carcharias).
The role of collagen fibres is not merely to stiffen the tissue at high strains or enable mobility. Numerous layers of fibres also contribute very much to its toughness and high work of fracture. A thick, highly reinforced dermis in dinosaurs probably evolved as a means of mechanical protection against predation as, for example, the highly thickened dermis, with an excess of 40 fibre layers, of the nurse shark, Ginglymostoma cirratum (Motta 1977). A generalization of the primary functional role of the dermis in the protection and/or support for the enclosed body mass may be extended to most vertebrates (Moss 1972) with the possible exception of birds, wherein the dermis plays a unique role with respect to feather attachments (Homberger & de Silva 2003). Furthermore, given that a ground plan of the smallest scales in the ventral area of the body of dinosaurs (Czerkas 1997) would provide limited protection, which indeed might apply to the smooth tubercles (Gohlich & Chiappe 2006) that covered most of the body in small predatory dinosaurs, a highly thickened dermis incorporating a deep, complex architecture of structural fibres is considered not only essential but also functionally appropriate.
How would the dermis of a theropod dinosaur compare structurally with that of Psittacosaurus? Notwithstanding probable differences in thickness, it is reasonable to think that there would not have been fundamental differences in structure between the dermis of a theropod and that of a non-theropod dinosaur in so far as protection and support are concerned (Moss 1972). The dermis of a theropod dinosaur, even allowing for a thickness of just one-quarter of that of Psittacosaurus, would still accommodate numerous layers of collagen fibres and consequently it would not be surprising to find more and more fossils with preserved fibres (not to mention from frills, cartilage, tendon and bones). Indeed, this view is supported by observations of several such fibre layers in Sinosauropteryx (Lingham-Soliar et al. 2007; figure 4).
(c) Possible causes of the fractured skin of Psittacosaurus
An excavation near the base of the cross section and another smaller excavation alongside apparently represent the tips of tooth impressions, the smaller probably made by a slightly shorter tooth (figure 2b, red arrows). Both impressions, despite a difference in size, show apparent radiating stress fractures emanating from the tooth tips that are strikingly similar in shape. The fracture could have been caused by a predator/scavenger's bite. The concave shape of the fracture (figure 2b, double-headed arrow) would also match the convex outward tooth face of a predator or scavenger. Furthermore, trauma around the possible tooth tips, inferred from fibres in the vicinity emerging as it were out of the section and a general raggedness (figure 2b, yellow arrows) as well as from the exposure of fibre layers on the tangential plane at the base of the section (figure 2c), is suggestive of the ‘cut and pull’ feeding technique—the tear at the base extending along the plane of least resistance to expose a ‘clean’ tangential section. Although circumstantial, the arguments presented here are plausible and may give a hint of the life and death struggles of extinct animals (Rothschild & Martin 1993; Lingham-Soliar 2004). An alternative, though less probable explanation, is that the exposure may be the consequence of a serendipitous fracture of the fossil by natural forces.
Acknowledgments
The author is grateful to Drs Pei-ji Chen, Weiguo Sun, Qun Yang and Lao Sha (Nanjing Institute of Geology and Palaeontology) and to Prof. W.J. Hillenius (College of Charleston) and Prof. John Ruben (Oregon State University) for making available material on Psittacosaurus MV53 and advice. The author also thanks Prof. Gerhard Plodowski for material on Psittacosaurus SMF R 4970.
Endnote
Note that as with most studies in which dermal fibre architectures have been investigated (e.g. sharks and dolphins in Motta (1977) and Pabst (1996), respectively), reference to the term ‘collagen fibre’ is one of convenience because, strictly speaking, observations at the light microscopic level show fibre bundles (approx. 50–250 μm across) rather than fibres (approx. 4–20 μm across).
References
- Alexander R.McN. Bending of cylindrical animals with helical fibres in their skin or cuticle. J. Theor. Biol. 1987;124:97–110. doi:10.1016/S0022-5193(87)80255-2 [Google Scholar]
- Allison P.A. Phosphatized soft bodied squids from the Jurassic Oxford Clay. Lethaia. 1988;21:403–410. doi:10.1111/j.1502-3931.1988.tb01769.x [Google Scholar]
- Averianov A.O, Voronkevich A.V, Leshchinskiy S.V, Fayngertz A.V. A ceratopsian dinosaur Psittacosaurus sibiricus from the Early Cretaceous of West Siberia, Russia and its phylogenetic relationships. J. Syst. Paleontol. 2006;4:359–395. doi:10.1017/S1477201906001933 [Google Scholar]
- Briggs D.E.G. The role of decay and mineralization in the preservation of soft-bodied fossils. Annu. Rev. Earth Planet. Sci. 2003;31:275–301. doi:10.1146/annurev.earth.31.100901.144746 [Google Scholar]
- Chen P.-J, Dong Z.M, Zheng S.N. An exceptionally well preserved theropod dinosaur from the Yixian formation of China. Nature. 1998;391:147–152. doi:10.1038/34356 [Google Scholar]
- Clark R.B, Cowey J.B. Factors controlling the change of shape of certain nemertean and turbellarian worms. J. Exp. Biol. 1958;35:731–748. [Google Scholar]
- Currie P.J, Chen P.-J. Anatomy of Sinosauropteryx prima from Liaoning, northeastern China. Can. J. Earth Sci. 2001;38:1705–1727. doi:10.1139/cjes-38-12-1705 [Google Scholar]
- Czerkas S.A. Skin. In: Currie P.J, Padian K, editors. Encyclopedia of dinosaurs. Academic Press; San Diego, CA: 1997. pp. 669–675. [Google Scholar]
- Feduccia A, Lingham-Soliar T, Hinchcliffe J.R. Do feathered dinosaurs exist? Testing the hypothesis on neontological and paleontological evidence. J. Morphol. 2005;266:125–166. doi: 10.1002/jmor.10382. doi:10.1002/jmor.10382 [DOI] [PubMed] [Google Scholar]
- Fucheng Z, Zhou Z, Dyke G. Feathers and ‘feather-like’ integumentary structures in Liaoning birds and dinosaurs. Geol. J. 2006;41:395–404. doi:10.1002/gj.1057 [Google Scholar]
- Gohlich U.B, Chiappe L.M. A new carnivorous dinosaur from the Late Jurassic Solnhofen archipelago. Nature. 2006;440:329–332. doi: 10.1038/nature04579. doi:10.1038/nature04579 [DOI] [PubMed] [Google Scholar]
- Gordon J.E. Penguin; Harmondsworth, UK: 1978. Structures. [Google Scholar]
- Homberger D.G, de Silva K.N. The role of mechanical forces on the patterning of the avian feather-bearing skin: a biomechanical analysis of the integumentary musculature in birds. J. Exp. Zool. B (Mol. Dev. Evol.) 2003;298:123–139. doi: 10.1002/jez.b.30. doi:10.1002/jez.b.30 [DOI] [PubMed] [Google Scholar]
- Kemp N.E. Banding pattern and fibrillogenesis of ceratotrichia in shark fins. J. Morphol. 2005;154:187–203. doi: 10.1002/jmor.1051540202. doi:10.1002/jmor.1051540202 [DOI] [PubMed] [Google Scholar]
- Leng Q, Yang H. Pyrite framboids associated with the Mesozoic Jehol biota in northeastern China: implications for microenvironment during early fossilization. Prog. Nat. Sci. 2003;13:206–212. [Google Scholar]
- Lingham-Soliar T. Rare soft tissue preservation showing fibrous structures in an ichthyosaur from the Lower Lias (Jurassic) of England. Proc. R. Soc. B. 1999;266:2367–2373. doi:10.1098/rspb.1999.0933 [Google Scholar]
- Lingham-Soliar T. The ichthyosaur integument: skin fibers, a means for a strong, flexible and smooth skin. Lethaia. 2001;34:287–302. doi:10.1080/002411601753293042 [Google Scholar]
- Lingham-Soliar T. The dinosaurian origin of feathers: perspectives from dolphin (Cetacea) collagen fibers. Naturwissenschaften. 2003;90:563–567. doi: 10.1007/s00114-003-0483-7. doi:10.1007/s00114-003-0483-7 [DOI] [PubMed] [Google Scholar]
- Lingham-Soliar T. Palaeopathology and injury in the extinct mosasaurs (Lepidosauromorpha, Squamata) and implications for modern reptiles. Lethaia. 2004;37:255–262. doi:10.1080/00241160410006519 [Google Scholar]
- Lingham-Soliar T. Dorsal fin in the white shark Carcharodon carcharias: a dynamic stabilizer for fast swimming. J. Morphol. 2005a;263:1–11. doi: 10.1002/jmor.10207. doi:10.1002/jmor.10207 [DOI] [PubMed] [Google Scholar]
- Lingham-Soliar T. Caudal fin in the white shark, Carcharodon carcharias (Lamnidae): a dynamic propeller for fast, efficient swimming. J. Morphol. 2005b;264:233–252. doi: 10.1002/jmor.10328. doi:10.1002/jmor.10328 [DOI] [PubMed] [Google Scholar]
- Lingham-Soliar T, Plodowski G. Taphonomic evidence for high-speed adapted fins in thunniform ichthyosaurs. Naturwissenschaften. 2007;94:65–70. doi: 10.1007/s00114-006-0160-8. doi:10.1007/s00114-006-0160-8 [DOI] [PubMed] [Google Scholar]
- Lingham-Soliar T, Reif W.-E. Taphonomic evidence for fast tuna-like swimming in Jurassic and Cretaceous ichthyosaurs. Neues Jahrb. Geol. Palaontol. Abh. 1998;207:171–183. [Google Scholar]
- Lingham-Soliar, T., Feduccia, A. & Wang, X. 2007 A new Chinese specimen indicates that ‘protofeathers’ in the Early Cretaceous theropod dinosaur Sinosauropteryx are degraded collagen fibres. Proc. R. Soc. B274, 1823–1829. (doi:10.1098/rspb.2007.0352) [DOI] [PMC free article] [PubMed]
- Mayr G, Peters D.S, Plodowski G, Vogel O. Bristle-like integumentary structures at the tail of the horned dinosaur Psittacosaurus. Naturwissenschaften. 2002;89:361–365. doi: 10.1007/s00114-002-0339-6. doi:10.1007/s00114-002-0339-6 [DOI] [PubMed] [Google Scholar]
- Moss M. The vertebrate dermis and the integumental skeleton. Am. Zool. 1972;12:27–34. [Google Scholar]
- Motta P.J. Anatomy and functional morphology of dermal collagen fibers in sharks. Copeia. 1977;1977:454–464. doi:10.2307/1443263 [Google Scholar]
- Naresh M.D, Arumugam V, Sanjeevi R. Mechanical behaviour of shark skin. J. Biosci. 1997;22:431–437. doi:10.1007/BF02703189 [Google Scholar]
- Pabst D.A. Morphology of the subdermal connective sheath of dolphins: a new fiber-wound, thin-walled, pressurized cylinder model for swimming vertebrates. J. Zool. Lond. 1996;238:35–52. [Google Scholar]
- Rothschild B.M, Martin L.D. CRC Press; Boca Raton, FL: 1993. Paleopathology. Disease in the fossil record. [Google Scholar]
- Wainwright S.A, Biggs W.D, Currey J.D, Gosline J.M. Edward Arnold; London, UK: 1976. Mechanical design in organisms. [Google Scholar]
- Wainwright S.A, Vosburgh F, Hebrank J.H. Shark skin: function in locomotion. Science. 1978;202:747–749. doi: 10.1126/science.202.4369.747. doi:10.1126/science.202.4369.747 [DOI] [PubMed] [Google Scholar]
- Xu X, Zhou Z, Prum R.O. Branched integumental structures in Sinornithosaurus and the origin of birds. Nature. 2001;410:200–204. doi: 10.1038/35065589. doi:10.1038/35065589 [DOI] [PubMed] [Google Scholar]