Abstract
The end-Permian mass extinction, 251 million years (Myr) ago, was the most devastating ecological event of all time, and it was exacerbated by two earlier events at the beginning and end of the Guadalupian, 270 and 260 Myr ago. Ecosystems were destroyed worldwide, communities were restructured and organisms were left struggling to recover. Disaster taxa, such as Lystrosaurus, insinuated themselves into almost every corner of the sparsely populated landscape in the earliest Triassic, and a quick taxonomic recovery apparently occurred on a global scale. However, close study of ecosystem evolution shows that true ecological recovery was slower. After the end-Guadalupian event, faunas began rebuilding complex trophic structures and refilling guilds, but were hit again by the end-Permian event. Taxonomic diversity at the alpha (community) level did not recover to pre-extinction levels; it reached only a low plateau after each pulse and continued low into the Late Triassic. Our data showed that though there was an initial rise in cosmopolitanism after the extinction pulses, large drops subsequently occurred and, counter-intuitively, a surprisingly low level of cosmopolitanism was sustained through the Early and Middle Triassic.
Keywords: alpha, diversity, mass extinction, Permian, Triassic, recovery
1. Introduction
The end-Permian mass extinction was the most dramatic event to impact life on Earth (Erwin 1990, 2006; Benton 2003; Benton & Twitchett 2003). On land and sea, life was nearly extinguished, ecosystems were devastated and many long-lived lineages disappeared. Most studies hitherto have focused on the effects of this extinction on marine life. It has been more difficult to determine the effects on land because rock successions are typically less complete, and only two major sedimentary basins have so far yielded extensive faunas spanning the boundary: the Karoo Basin of South Africa and the South Urals Basin of Russia.
During the last two decades, our understanding of the end-Permian extinction has increased significantly. It is now widely believed that this devastating event was caused by large-scale volcanism in Siberia, which led to major atmospheric changes and the collapse of ecosystems worldwide (Benton 2003). Earlier evidence suggested that this extinction was prolonged through the Late Permian (Erwin 1990; Teichert 1990), but hints of an earlier event, noted first among terrestrial vertebrate faunas (Benton 1985, 1989; King 1991), have been confirmed (Retallack et al. 2006) as the same end-Guadalupian extinction of marine organisms, already noted independently (Jin et al. 1994; Stanley & Yang 1994; Rohde & Muller 2005). During both extinction events, diversity losses were not simply a reflection of increased rates of extinction but also of depressed origination rates (Benton 1998; Bambach et al. 2004).
The terrestrial vertebrate record of the Permian is also confounded by an additional earlier event, or gap, between the Kungurian and Roadian stages, 271 million years (Myr) ago. This marks the turnover between Early Permian pelycosaur-rich faunas to those dominated by therapsids and pareiasaurs and has been termed ‘Olson's gap’ (Lucas & Heckert 2001) or ‘Olson's extinction’ (Sahney & Benton in preparation), depending on whether it is seen as a failure of fossil preservation or a real ecological event. Frustratingly, tetrapod footprint faunas of this time show an even longer hiatus than the skeletal evidence, so that the validity of each interpretation is difficult to verify (Lucas 2004).
The impact of the end-Permian event was devastating. In the sea, the level of species loss was 80–96%, and blastoid echinoderms, tabulate and rugose corals, graptolites, trilobites, eurypterids, acanthodians and placoderms disappeared entirely (Hallam & Wignall 1997). On land, the dominant Glossopteris flora was replaced, eight orders of insects became extinct (Labandeira & Sepkoski 1993) and two-thirds of tetrapod families were lost (Benton 1989). The only tetrapod lineages to survive were procolophonoids, dicynodonts, and presumably therocephalians, cynodonts, and archosauromorphs, and their Triassic recovery was slow (Benton et al. 2004).
The return of diversity after an extinction event can be viewed in terms of taxonomy (Niklas et al. 1983; Labandeira & Sepkoski 1993; Benton 1995), ecology (Bambach 1985; Bambach et al. 2007) or morphology (Foote 1995; Roy & Foote 1997). In this study, we are concerned with the recovery of taxonomic diversity in the context of ecological diversity.
There are several meanings for the term ‘recovery’ after mass extinctions. Past studies have revealed that faunal revival after a devastating ecological event may follow a pattern similar to ecological succession (Sole et al. 2002), and recovery may be considered as the point at which the model is complete and the new ecosystem is stable. On both the scale of modern ecological recovery and recovery from mass extinction, disaster (‘weedy’ or generalist) taxa are known to insinuate themselves into empty guilds, pushing the boundaries of their geographical range and ecospace. Early Triassic terrestrial ecosystems are clearly dominated by a small number of genera, most notably the dicynodont Lystrosaurus, which accounted for approximately 90% of terrestrial vertebrates (Benton 1983). Disaster taxa then experienced rapid turnover in the time immediately following the event, later giving way to more specialized organisms (Benton 2003).
Recovery can also be viewed as a return to pre-extinction conditions, for example the numerical recovery of taxa. Globally, this type of recovery happened quickly after the end-Permian event by the Olenekian (250–245 Myr ago), but then the diversity fell again (figure 1), either as a result of displaced disaster taxa that had filled empty guilds or the devastation caused by another extinction pulse at the end of the Olenekian. In contrast, the ecological recovery of tetrapods and plants was slow, and lost guilds and trophic levels were not readily refilled (Retallack et al. 1996; Benton et al. 2004; Grauvogel-Stamm & Ash 2005).
Figure 1.
Global diversity (dashed line) and mean alpha diversity (solid line) of Permo-Triassic tetrapod families. Extinctions are labelled as 1, Olson's extinction; 2, end-Guadalupian extinction; and 3, end-Permian extinction. Geological stages (Gradstein & Ogg 2004) are as follows: Ar, Artinskian; K, Kungurian; R, Roadian; W, Wordian; Ca, Capitanian; Wu, Wuchiapingian; Ch, Changhsingian; I, Induan; O, Olenekian; An, Anisian; L, Ladinian; Cr, Carnian.
The third interpretation defines recovery as the point when an ecological equilibrium is reached, even if this balance is unlike that of the pre-extinction fauna. Though faunal turnovers occurred continually before and after the end-Permian event, it is difficult to say when the ecosystem reached stability, i.e. a time of high biodiversity, low turnover, resistance to invaders and a complex trophic structure (Sole et al. 2002). Immediately after the end-Permian event, amphibians made a relatively quick recovery, specifically the families Capitosauridae and Trematosauridae, which filled the role of semi-aquatic predators. Among reptiles, therapsids filled disaster taxa roles but their dominance gave way to archosauromorphs.
In this paper, we present the first community-scale analysis of tetrapod recovery from the end-Permian extinction and the preceding Guadalupian extinction events.
2. Material and methods
Diversity is most commonly assessed by tallying the number of taxa on a global scale (Benton 1985, 1995, 1998; Padian & Clemens 1985); such studies provide a synoptic overview but are subject to the vagaries of sampling. This is especially true in the fossil record where patterns of global diversity often reflect patterns of fossiliferous rock availability (Raup 1972; Peters & Foote 2001; Smith 2001; Smith & McGowan 2005). In this study, we use an alternative approach by sampling individual fossil communities (Alroy et al. 2001). By examining well-preserved and well-studied faunas, the taxonomic and ecological recovery of communities after the Permo-Triassic extinction event can be examined more accurately, and the problems of geological bias between time bins are largely avoided.
Data were collected for 69 tetrapod communities from the Artinskian to the end of the Carnian and stored in a relational database management system, the alpha diversity database (ADD). We surveyed all fossil tetrapod findings from the Permian and Triassic and chose only those localities/formations with a high quality of preservation, a completeness of community and a thorough collection and publication history. The study is therefore global and we made no geographical restrictions. Inevitably, however, we could not find suitably substantial faunas from every time bin from every continent: the Early Permian sample is dominated by North American localities, the Middle and Late Permian and Early Triassic time bins by sections in Russia (Pechora Basin, Moscow Basin, South Urals Basin) and southern Africa (Karoo Basin, Ruhuhu Valley), with additional sites from Greenland, Australia, Madagascar, Antarctica and Germany. Sampling in the Middle and Late Triassic is broader, with localities from Western Europe, Russia, South America, North America, Africa, Madagascar, India and China.
The time scale employed was that of Gradstein & Ogg (2004) and abbreviations for time units were taken from Benton (1993). Sedimentary environment, climate and biome were refined based on the discussion with H. Falcon-Lang (2006, personal communication).
The classification of orders and families was taken from Benton (1993, 2005). Ambiguous taxonomic information was included in the database but was tagged, so that the analyses could be run with and without the unclear data. The structure allowed for the inclusion of taxa identified as nomina dubia as well as specimens labelled ?, aff., cf. or otherwise indeterminate or unidentified. Stratigraphical ranges and geographical distributions of families were taken from Benton (1993) and updated using Carroll et al. (1998), and Schoch & Milner (2000). Ecomorphs based on size and diets were taken from Benton (1996).
3. Extinction and recovery in the Middle to Late Permian
Three distinct extinction pulses were responsible for the mass extinction of tetrapods in the Permian and Triassic: Olson's extinction; the end-Guadalupian event; and the end-Permian event. Olson's extinction, in the Early Guadalupian (Roadian, Wordian), reveals an extended period of low diversity when worldwide two-thirds of terrestrial vertebrate life was lost. Global diversity rose dramatically in the Capitanian, probably the result of disaster taxa filling empty guilds, only to fall again when the end-Guadalupian event caused a diversity drop in the Wuchiapingian. Globally, terrestrial vertebrates recovered the high familial diversity of the Artinskian (39 non-singleton families found worldwide) in the Changhsingian, the last stage of the Permian (figure 1), only to be devastated again by the end-Permian event.
The communities in this study are globally dispersed and despite the differences in latitude and climate, they are fairly well constrained in their diversity. Community diversity falls dramatically during Olson's extinction and diverse Artinskian faunas, which featured large amniote predators and herbivores such as Dimetrodon and Diadectes, and a variety of semi-aquatic tetrapods, including Eryops, Archeria and Ophiacodon, disappear. The mean number of families in Early Guadalupian communities is reduced to a paltry 13% of the Artinskian high. A detailed look at the Russian Permian sequence confirms that the diversity drop was severe; only two families crossed the boundary from the Wordian to the Capitanian, but recovery began immediately with the origination of five families in the latter stage (Benton et al. 2004). Globally, alpha diversity does not fully recover; it slowly reaches a plateau (approx. 57% of Artinskian diversity) by the end of the Permian (figure 1). The pattern of diversification is similar at all taxonomic levels and taxonomic proportions are consistent across all time periods.
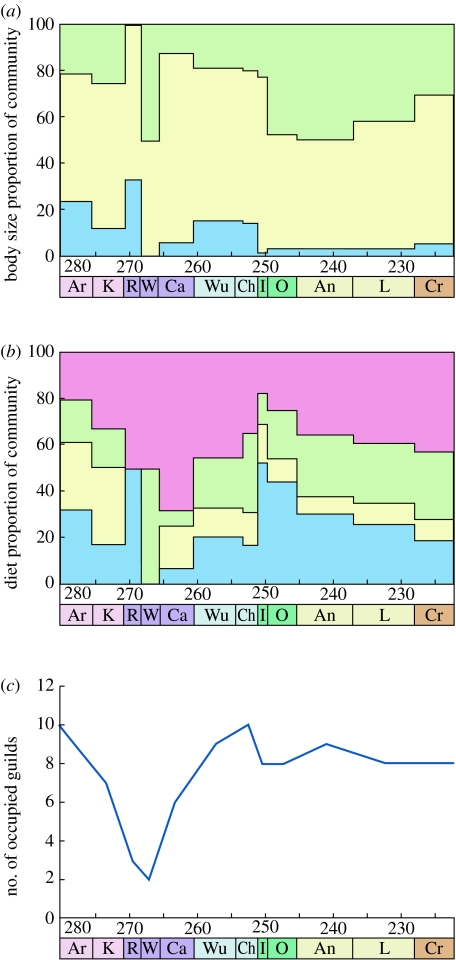
The ecological impact of the Guadalupian events is catastrophic; 8 (out of a possible 12) guilds are lost from the Artinskian high of 10 guilds. These are recovered in the last stages of the Permian before being devastated again by the end-Permian event (figure 3c). A dramatic change in diet type also occurs: proportions of piscivores, insectivores, predators and browsers are thrown out of balance during each extinction pulse (figure 3b). During the last two stages of the Permian, there is a movement towards pre-extinction diet proportions, but again the end-Permian event disrupts this recovery. Body size is affected in a similar manner: the proportion of small, medium and large animals is thrown out of balance and recovery towards a pre-extinction balance of roughly 20% small, 60% medium and 20% large tetrapods in the Late Permian follows (figure 3a).
Figure 3.
Ecological diversity of Permo-Triassic tetrapod communities. (a) Size (light green, large; light yellow, medium; sky blue, small) and (b) diet (pink, browsers; light green, predators; light yellow, insectivores; sky blue, piscivores) are expressed as a percentage of the total community. (c) The guilds are defined by body size and inferred diet. Error bars cannot be applied as the number of occupied guilds is not a mean number, rather a cumulative count of the guilds filled in each stage. Size of the animals is defined as small (with snout-vent length (SVL) less than 150 mm), medium (SVL from 150 mm to 1.5 m) and large (SVL greater than 1.5 m). Geological stages are the same as given in figure legend 1.
Olson's extinction was a dramatic extinction ‘trough’ that is a prolonged period of very low diversity after a long and sustained diversity rise and probably the result of prolonged environmental stress. Both Olson's extinction and the end-Guadalupian extinction experience a dramatic change in community diet proportions, body size and distinct faunal turnovers, from ‘pelycosaur’-rich communities to those dominated by the basal therapsid dinocephalians and finally giving way to more derived therapsids and pareiasaurs. Global and ecological diversities recover to pre-extinction levels by the end of the Permian. However, alpha diversity does not recover by reaching a pre-extinction balance; rather a new equilibrium is reached, significantly lower than the diversity of the Artinskian.
4. Across the Permo-Triassic boundary: ecosystem restructuring and recovery
(a) A fast global recovery and a slow community recovery
The global diversity rose sharply after each extinction pulse, probably the result of disaster taxa filling empty guilds. After the end-Permian event, this rapid refilling resulted in a return to pre-extinction taxonomic diversity by the Olenekian. However, this did not last, as there was a subsequent loss of nine families (figure 1).
Until the Carnian, community diversity never reached the highest observed in Artinskian faunas. Though the end-Permian extinction pulse had the most dramatic global effect, it does not appear to have impacted the diversity within individual communities as badly as the first Guadalupian pulse. It seems to have ‘thinned out’ families rather than destroyed them completely and the average number of tetrapod families lost is only 1.5 from each community (figure 1).
A look at the community diversification rate helps explain the slow recovery of tetrapods in the Triassic (figure 2). Since the Viséan, there have been three distinct periods of diversification interrupted by three major extinctions, in the Late Pennsylvanian, Middle Permian and End Permian. During the periods of diversification (with the exception of the Moscovian), fewer than one family was added every Myr, representing a slow but steady rate of community-level diversification. For the four stages following the Late Pennsylvanian extinction, an average of one family was added every 4 Myr. Then for four stages after Olson's extinction, this rate increased to one family added every 3 Myr. But oddly, for the four stages immediately following the end-Permian extinction, a period of 35 Myr, the familial diversification rate dips in and out of the negative realm and results in an average of one additional family every 25 Myr. Outside the three mass extinction events, this is the lowest rate of tetrapod diversification since their origin in the Frasnian.
Figure 2.
Rate of alpha diversification (the first derivative of diversity through time) of mean number of tetrapod families in communities, calculated stage-by-stage from the Mississippian to the Late Triassic; error bars cannot be applied. Miss, Mississippian; Penn, Pennsylvanian; Cisur, Cisuralian; Guad, Guadalupian; Lopin, Lopingian; E. Tri, Early Triassic; M. Tri, Middle Triassic; L. Tri, Late Triassic.
(b) Ecological recovery
Communities were slow to recover ecologically as well as numerically, and body size and diet ratios show disruptions at all extinction pulses (figure 3). The Permian extinctions created a ‘Lilliput effect’ (Urbanek 1993) in which few large species survived and, in addition, many small species were also extinguished. The large animals included the disaster taxon Lystrosaurus that dominated terrestrial communities, capitosaurs and Proterosuchus, which were populous in semi-aquatic environments. Further into the Early Triassic, larger animals became more common as dicynodonts such as Kannemeyeria replaced Lystrosaurus, and large carnivores reappeared including Cynognathus, Garjainia and Erythrosuchus.
The end-Permian event dramatically restructured communities with the loss of browsers and predators and an increase in piscivores. The loss of browsers is, no doubt, linked to changes in vegetation. A rapid loss of sediment-binding vegetation was responsible for a basin-wide change from low-energy meandering streams to high-energy braided rivers in the Early Triassic of Russia, South Africa, Australia, India and Spain (Newell et al. 1999; Ward et al. 2000; Benton in press). Also, the 7 Myr ‘coal gap’ is the result of an insufficient amount of plant material to form coal deposits, and hence little food for large browsing animals. Permian levels of plant diversity were not reached again until the Late Triassic (230 Myr ago; Retallack et al. 1996). Tracking diet proportions through the first five stages of the Triassic shows a clear trend towards reaching a pre-extinction balance of communities dominated by predators and browsers with a smaller proportion of piscivores and insectivores (figure 3b).
Overall, the recovery of the eight guilds lost in the Guadalupian occurred in 10–15 Myr, relatively quickly compared with the original 100 Myr it took to colonize them by the first tetrapods (Sahney & Benton in preparation), perhaps because sufficient representatives of the relevant lineages and guilds survived the event in some form. These broad guilds had just been refilled by the end of the Permian, when the end-Permian extinction pulse caused the loss of two major guilds (small piscivores and small browsers). These two are not regained by the end of the Carnian (table 1).
Table 1.
Guilds (defined by body size and diet) filled by Permo-Triassic tetrapods.
| stage | midpoint (Myr ago) | piscivores | insectivores | browsers | predators | guilds occupied | guild loss/gain | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | M | L | S | M | L | S | M | L | S | M | L | ||||
| Artinskian | 280 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 10 | |||
| Kungurian | 273.1 | Y | Y | Y | Y | Y | Y | Y | 7 | −3 | |||||
| Roadian | 269.3 | Y | Y | Y | 3 | −4 | |||||||||
| Wordian | 266.9 | Y | Y | 2 | −1 | ||||||||||
| Capitanian | 263.1 | Y | Y | Y | Y | Y | Y | 6 | 4 | ||||||
| Wuchiapingian | 257.1 | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9 | 3 | |||
| Changhsingian | 252.4 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 10 | 1 | ||
| Induan | 250.35 | Y | Y | Y | Y | Y | Y | Y | 7 | −3 | |||||
| Olenekian | 247.35 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | 1 | ||||
| Anisian | 241 | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9 | 1 | |||
| Ladinian | 232.5 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | −1 | ||||
| Carnian | 222.25 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | 0 | ||||
A slow Triassic recovery may initially seem at odds with Botha & Smith's (2006) analysis of the Karoo Basin, which indicates an initial rapid recovery of the tetrapod fauna after the end-Permian event. They noted that nearly half (43%) of the Early Triassic genera appeared ‘rapidly’, that is within 20 m of the Permo-Triassic boundary. As they state, however, they are documenting the onset of recovery, rather than the establishment of mature ecosystems. Botha & Smith (2006) defined recovery as a ‘new balance’ rather than a return to pre-existing conditions, something that could not be observed in the Karoo alone as this sequence begins in the Guadalupian, after the first extinction pulse.
A longer sequence of tetrapod faunas is found in the Southern Urals of Russia, where a continuous record of tetrapod faunas spanning 30 Myr from the Kungurian to the Ladinian reveals low taxonomic and ecological diversity in the Early Triassic. Even after 15 Myr of rebuilding ecosystems, many guilds were still unfilled, such as those of small piscivores, small insectivores, large herbivores and top predators (Benton et al. 2004).
(c) Cosmopolitanism
Cosmopolitanism is the degree to which a species or a clade is distributed worldwide; it can be measured simply as the mean alpha diversity of communities divided by global diversity. The Pennsylvanian extinction, Guadalupian pulses and end-Permian event experience significant rises in cosmopolitanism followed by dramatic losses of 40–50% (figure 4). This rise and fall of cosmopolitanism following an extinction event is in keeping with established ecological models, which predict that when endemic/regional faunas are devastated, the survivors, especially disaster taxa, become cosmopolitan, at least in the immediate aftermath of a mass extinction (Hallam & Wignall 1997; Benton 2003; Erwin 2006). This is well illustrated among tetrapods by Lystrosaurus, a bulky herbivore that thrived in the harsh arid conditions of the Induan. Scroungers such as Lystrosaurus were then rapidly overtaken by the evolution of more stable regionally distinct faunas that were to establish the longer lasting ecosystems of the later Triassic.
Figure 4.
Cosmopolitanism of tetrapods through the Carboniferous, Permian and Triassic. Cosmopolitanism (C) is measured as mean alpha diversity divided by global diversity (Tt), according to the formula . Note the overall decline of cosmopolitanism through this time interval, perhaps related to increasing taxonomic and ecological diversity of tetrapods, but also note the coupled rises and falls in cosmopolitanism following major extinction events, especially (1) Olson's extinction, (2) the end-Guadalupian extinction and (3) the end-Permian extinction. Abbreviations are the same as given in figure legend 2.
There is a repeated pattern of coupled rises and falls in cosmopolitanism during times of extinction, though in each case the fall is far more dramatic than the rise, creating an overall downward trend. Cosmopolitanism was slow to recover in the Triassic, rising just 7% from the Olenekian to the Carnian. There is no other time in tetrapod history that cosmopolitanism is sustained at such a low level for so long. Continued low cosmopolitanism in the Triassic is difficult to envisage owing to the existence of the global supercontinent, Pangaea and equable climates: terrestrial organisms should have been able to migrate nearly worldwide without major barriers.
5. Conclusions
The Permo-Triassic extinction events halted the growth of tetrapod communities. Extinction in the Guadalupian had a much more severe impact at the community level than the terminal end-Permian event. Faunas were recovering from the Guadalupian events at reasonably high rates and ecologically communities had recovered when the end-Permian event hit. Though globally tetrapods recovered quickly, the dramatic restructuring that occurred at the community level was not permanent and communities did not recover numerically or ecologically in the Early and Middle Triassic. It would not be until the great diversity of the Late Triassic, which included dinosaurs, pterosaurs, crocodilians, rauisuchids, aetosaurs, rhynchosaurs, trilophosaurs, sphenodonts, amphibians and mammals, some 30 Myr after the end-Permian event, that terrestrial tetrapod community diversity was restored.
Acknowledgments
We thank Paul Ferry for making the ADD available online and his continued technical support. Also, appreciated is the advice of Saswati Bandyopadhyay, Max Langer, Graeme Lloyd, Rainer Schoch, and Anne Warren, on Triassic communities. This work was partially funded by NERC grant NE/C518973/1 to M.J.B. and supported by grants to S.S. from the Bob Savage Fund, the Natural Environment Research Council, the Palaeontological Association, and the University of Bristol Alumni Foundation.
Supplementary Material
Summary of the localities and key references
References
- Alroy J, et al. Effects of sampling standardization on estimates of Phanerozoic marine diversification. Proc. Natl Acad. Sci. USA. 2001;98:6261–6266. doi: 10.1073/pnas.111144698. doi:10.1073/pnas.111144698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambach R.K. Classes and adaptive variety: the ecology of diversification in marine faunas through the Phanerozoic. In: Valentine J.W, editor. Phanerozoic diversity patterns. Princeton University Press; Princeton, NJ: 1985. pp. 191–253. [Google Scholar]
- Bambach R.K, Knoll A.H, Wang S.C. Origination, extinction, and mass depletions of marine diversity. Paleobiology. 2004;30:522–542. doi:10.1666/0094-8373(2004)030<0522:OEAMDO>2.0.CO;2 [Google Scholar]
- Bambach R.K, Bush A.M, Erwin D.H. Autecology and the filling of ecospace: key metazoan radiations. Palaeontology. 2007;50:1–22. doi:10.1111/j.1475-4983.2006.00611.x [Google Scholar]
- Benton M.J. Dinosaur success in the Triassic—a noncompetitive ecological model. Q. Rev. Biol. 1983;58:29–55. doi:10.1086/413056 [Google Scholar]
- Benton M.J. Mass extinction among non-marine tetrapods. Nature. 1985;316:811–814. doi:10.1038/316811a0 [Google Scholar]
- Benton M.J. Mass extinctions among tetrapods and the quality of the fossil record. Phil. Trans. R. Soc. B. 1989;325:369–386. doi: 10.1098/rstb.1989.0094. doi:10.1098/rstb.1989.0094 [DOI] [PubMed] [Google Scholar]
- Benton M.J. Chapman & Hall; London, UK: 1993. The fossil record 2. [Google Scholar]
- Benton M.J. Diversification and extinction in the history of life. Science. 1995;268:52–58. doi: 10.1126/science.7701342. doi:10.1126/science.7701342 [DOI] [PubMed] [Google Scholar]
- Benton M.J. Testing the roles of competition and expansion in tetrapod evolution. Proc. R. Soc. B. 1996;263:641–646. doi:10.1098/rspb.1996.0096 [Google Scholar]
- Benton M.J. The quality of the fossil record of vertebrates. In: Donovan S.K, Paul C.R.C, editors. The adequacy of the fossil record. Wiley; New York, NY: 1998. pp. 269–303. [Google Scholar]
- Benton M.J. Thames & Hudson; London, UK: 2003. When life nearly died: the greatest mass extinction of all time. [Google Scholar]
- Benton M.J. Blackwell Science; Oxford, UK: 2005. Vertebrate palaeontology. [Google Scholar]
- Benton, M. J. In press. The end-Permian mass extinction: events on land in Russia. Proc. Geol. Assoc.119
- Benton M.J, Twitchett R.J. How to kill (almost) all life: the end-Permian extinction event. Trends Ecol. Evol. 2003;18:358–365. doi:10.1016/S0169-5347(03)00093-4 [Google Scholar]
- Benton M.J, Benton M.J, Tverdokhlebov V.P, Surkov M.V. Ecosystem remodelling among vertebrates at the Permian–Triassic boundary in Russia. Nature. 2004;432:97–100. doi: 10.1038/nature02950. doi:10.1038/nature02950 [DOI] [PubMed] [Google Scholar]
- Botha J, Smith R.M.H. Rapid vertebrate recuperation in the Karoo Basin of South Africa following the end-Permian extinction. J. Afr. Earth Sci. 2006;45:502–514. doi:10.1016/j.jafrearsci.2006.04.006 [Google Scholar]
- Carroll R.L, Bossy K.A, Milner A.C, Andrews S.M, Wellstead C.F. Dr Friedrich Pfeil; München, Germany: 1998. Encyclopedia of paleoherpetology: Lepospondyli. [Google Scholar]
- Erwin D.H. The end-Permian mass extinction. Annu. Rev. Ecol. Syst. 1990;21:69–91. doi:10.1146/annurev.es.21.110190.000441 [Google Scholar]
- Erwin D.H. Princeton University Press; Princeton, NJ: 2006. Extinction: how life on Earth nearly ended 250 million years ago. [Google Scholar]
- Foote M. Morphological diversification of Paleozoic crinoids. Paleobiology. 1995;21:273–299. [Google Scholar]
- Gradstein, F. M. & Ogg, J. G. 2004 Geologic time scale 2004—why, how, and where next! Lethaia37, 175–181.
- Grauvogel-Stamm L, Ash S.R. Recovery of the Triassic land flora from the end-Permian life crisis. C. R. Palevol. 2005;4:593–608. doi:10.1016/j.crpv.2005.07.002 [Google Scholar]
- Hallam A, Wignall P.B. Oxford University Press; New York, NY: 1997. Mass extinctions and their aftermath. [Google Scholar]
- Jin Y.G, Zhang J, Shang Q.H. Two phases of the end-Permian mass extinction. In: Beauchamp B, editor. Pangea: global environments and resources. Canadian Society of Petroleum Geologists, Memoir 17. Canadian Society of Petroleum Geologists; Calgary, Canada: 1994. pp. 813–822. [Google Scholar]
- King G.M. Terrestrial tetrapods and the end Permian event: a comparison of analysis. Hist. Biol. 1991;5:239–255. [Google Scholar]
- Labandeira C.C, Sepkoski J.J. Insect diversity in the fossil record. Science. 1993;261:310–315. doi: 10.1126/science.11536548. doi:10.1126/science.11536548 [DOI] [PubMed] [Google Scholar]
- Lucas S.G. A global hiatus in the Middle Permian tetrapod fossil record. Stratigraphy. 2004;1:47–64. [Google Scholar]
- Lucas S.G, Heckert A.B. Olson's gap: a global hiatus in the record of Middle Permian tetrapods. J. Vertebr. Paleontol. 2001;21:75A. doi:10.1671/0272-4634(2001)021[0397:MLTFTU]2.0.CO;2 [Google Scholar]
- Newell A.J, Tverdokhlebov V.P, Benton M.J. Interplay of tectonics and climate on a transverse fluvial system, Upper Permian, Southern Uralian Foreland Basin, Russia. Sediment. Geol. 1999;127:11–29. doi:10.1016/S0037-0738(99)00009-3 [Google Scholar]
- Niklas K.J, Tiffney B.H, Knoll A.H. Patterns in vascular land plant diversification. Nature. 1983;303:614–616. doi:10.1038/303614a0 [Google Scholar]
- Padian K, Clemens W.A. Terrestrial vertebrate diversity: episodes and insights. In: Valentine J.W, editor. Phanerozoic diversity patterns. Princeton University Press; Princeton, NJ: 1985. pp. 41–96. [Google Scholar]
- Peters S.E, Foote M. Biodiversity in the Phanerozoic: a reinterpretation. Paleobiology. 2001;27:583–601. doi:10.1666/0094-8373(2001)027<0583:BITPAR>2.0.CO;2 [Google Scholar]
- Raup D.M. Taxonomic diversity during Phanerozoic. Science. 1972;177:1065–1071. doi: 10.1126/science.177.4054.1065. doi:10.1126/science.177.4054.1065 [DOI] [PubMed] [Google Scholar]
- Retallack G.J, Veevers J.J, Morante R. Global coal gap between Permian–Triassic extinction and Middle Triassic recovery of peat-forming plants. Bull. Geol. Soc. Am. 1996;108:195–207. doi:10.1130/0016-7606(1996)108<0195:GCGBPT>2.3.CO;2 [Google Scholar]
- Retallack G.J, Metzger C.A, Greaver T, Jahren A.H, Smith R.M.H, Sheldon N.D. Middle–Late Permian mass extinction on land. Bull. Geol. Soc. Am. 2006;118:1398–1411. doi:10.1130/B26011.1 [Google Scholar]
- Rohde R.A, Muller R.A. Cycles in fossil diversity. Nature. 2005;434:208–210. doi: 10.1038/nature03339. doi:10.1038/nature03339 [DOI] [PubMed] [Google Scholar]
- Roy K, Foote M. Morphological approaches to measuring biodiversity. Trends Ecol. Evol. 1997;12:277–281. doi: 10.1016/s0169-5347(97)81026-9. doi:10.1016/S0169-5347(97)81026-9 [DOI] [PubMed] [Google Scholar]
- Schoch R.R, Milner A.R. Dr Friedrich Pfeil; München, Germany: 2000. Encyclopedia of paleoherpetology: Stereospondyli. [Google Scholar]
- Smith A.B. Large-scale heterogeneity of the fossil record: implications for Phanerozoic biodiversity studies. Phil. Trans. R. Soc. B. 2001;356:1–17. doi: 10.1098/rstb.2000.0768. doi:10.1098/rstb.2000.0768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.B, McGowan A.J. Cyclicity in the fossil record mirrors rock outcrop area. Biol. Lett. 2005;1:443–445. doi: 10.1098/rsbl.2005.0345. doi:10.1098/rsbl.2005.0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole R.V, Montoya J.M, Erwin D.H. Recovery after mass extinction: evolutionary assembly in large-scale biosphere dynamics. Phil. Trans. R. Soc. B. 2002;357:697–707. doi: 10.1098/rstb.2001.0987. doi:10.1098/rstb.2001.0987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S.M, Yang X. A double mass extinction at the end of the Paleozoic era. Science. 1994;266:1340–1344. doi: 10.1126/science.266.5189.1340. doi:10.1126/science.266.5189.1340 [DOI] [PubMed] [Google Scholar]
- Teichert C. The Permian–Triassic revisited. In: Kauffman E.G, Walliser O.H, editors. Extinction events in Earth history. Springer; Berlin, Germany: 1990. pp. 199–238. [Google Scholar]
- Urbanek A. Biotic crises in the history of Upper Silurian graptoloids: a palaeobiological model. Hist. Biol. 1993;7:29–50. [Google Scholar]
- Ward P.D, Montgomery D.R, Smith R. Altered river morphology in South Africa related to the Permian–Triassic extinction. Science. 2000;289:1740–1743. doi: 10.1126/science.289.5485.1740. doi:10.1126/science.289.5485.1740 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the localities and key references