Abstract
Ground squirrels (Spermophilus spp.) have evolved a battery of defences against the rattlesnakes (Crotalus spp.) that have preyed on them for millions of years. The distinctive behavioural reactions by these squirrels to rattlesnakes have recently been shown to include self-application of rattlesnake scent—squirrels apply scent by vigorously licking their fur after chewing on shed rattlesnake skins. Here, we present evidence that this behaviour is a novel antipredator defence founded on exploitation of a foreign scent. We tested three functional hypotheses for snake scent application—antipredator, conspecific deterrence and ectoparasite defence—by examining reactions to rattlesnake scent by rattlesnakes, ground squirrels and ectoparasites (fleas). Rattlesnakes were more attracted to ground squirrel scent than to ground squirrel scent mixed with rattlesnake scent or rattlesnake scent alone. However, ground squirrel behaviour and flea host choice were not affected by rattlesnake scent. Thus, ground squirrels can reduce the risk of rattlesnake predation by applying rattlesnake scent to their bodies, potentially as a form of olfactory camouflage. Opportunistic exploitation of heterospecific scents may be widespread; many species self-apply foreign odours, but few such cases have been demonstrated to serve in antipredator defence.
Keywords: antipredator behaviour, camouflage, chemical defence, ground squirrels, rattlesnakes
1. Introduction
Among the diverse ways that prey defend themselves against predation, crypsis and Batesian mimicry serve the important functions of reducing detection and recognition by predators (Edmunds 1974; Caro 2005). Most reported cases of crypsis and Batesian mimicry involve visual traits of the prey (Cott 1957), but these features might leave prey vulnerable to predators that use other sensory modalities, such as olfaction (Conover 2007). In such situations, prey could benefit from matching ‘background odour’ of the environment or mimicking an aversive scent (‘olfactory mimicry’ sensu Eisner & Grant 1981). Nevertheless, few cases have been reported of such exploitation of odours by vertebrate prey species.
Many vertebrates self-apply odiferous foreign substances to their integument (see Clucas et al. 2008). Rodents, for example, self-apply the scent of their predators by chewing on the scent source and licking their bodies. Chipmunks, ground squirrels, and grasshopper mice apply snake scent in this way (Kobayashi & Watanabe 1986; Clucas et al. 2008; M. Rowe 2000, unpublished data), and rats and ground squirrels apply weasel anal gland secretions (Xu et al. 1995; B. Clucas 2006, unpublished data). These commandeered odours may reduce predation (Brodie 1977; Kobayashi & Watanabe 1986; Xu et al. 1995; Clucas et al. 2008), but the effects of the applied scent on predators have not yet been tested systematically.
Some ground squirrels (Spermophilus spp.) have evolved remarkable defences against rattlesnakes (Crotalus spp.; Owings & Coss in press), forcing rattlesnakes to eat primarily squirrel pups rather than adults. Adult California ground squirrels (Spermophilus beecheyi) and rock squirrels (Spermophilus variegatus), for example, are resistant to sympatric rattlesnake venom and actively harass and even occasionally attack rattlesnakes (Owings & Coss 1977; Poran et al. 1987; Biardi 2000; Owings et al. 2001). A tail-flagging signal is always paired with such harassment (Hennessy et al. 1981; Owings et al. 2001), and California ground squirrels add infrared ‘illumination’ to the tail to augment the deterrent effects of tail-flagging while dealing with rattlesnakes, an infrared-sensitive taxon (Rundus et al. 2007). These anti-rattlesnake tactics are all deployed after ground squirrels have been detected and recognized by rattlesnakes, well into the predator–prey sequence (see Lima & Dill 1990). If snake scent application affects detection and recognition by rattlesnakes, ground squirrels could also benefit from reducing predation risk at these earlier stages.
Rattlesnake scent application could reduce detection or recognition by rattlesnakes in two ways. Snake scent may ‘camouflage’ ground squirrels, making them olfactorily cryptic while in their burrows. Alternatively, applying rattlesnake scent may mimic the presence of a rattlesnake (cf. Vane-Wright 1980) and discourage entry into the squirrel's burrow by rattlesnakes and other predators motivated to avoid rattlesnakes (cf. Rowe et al. 1986). A third possibility is that snake scent application could reduce predation risk by targeting conspecifics. The snake scent applier may prime other squirrels to detect and respond to rattlesnakes, and use these alerted squirrels as an early warning system (cf. Hersek & Owings 1993).
Alternatively, snake scent application could serve a function other than antipredator defence. Snake scent might be targeted on conspecifics to repel or distract them during aggressive interactions (e.g. Drea et al. 2002), or serve in defence against ectoparasites by repelling them or masking host odour (see Weldon et al. 2003; Carroll et al. 2005).
We evaluate these functional hypotheses by assessing rattlesnake, ground squirrel and flea reactions to rattlesnake scent. In experiment 1, northern Pacific rattlesnakes (Crotalus oreganus) were tested in a foraging arena to compare their behaviour towards squirrel scent, rattlesnake scent and squirrel plus rattlesnake scent. In experiment 2, we compared the social and anti-snake behaviour of California ground squirrels and rock squirrels before and after snake scent application. Experiment 3 gave fleas a choice between snake- and water-scented squirrels to assess the impact of snake scent on host choice.
2. Material and methods
(a) Experiment 1
(i) Study species
We used eight wild-caught northern Pacific rattlesnakes (three males and five females, 185–585 g, captured March–August 2004, each housed separately at approximately 26–27°C and a 12 L : 12 D light cycle). All rattlesnakes were likely to have interacted with and preyed upon California ground squirrels. Snakes were fed one pre-killed mouse twice per month, but were not fed for 18–22 days prior to testing to increase foraging motivation.
(ii) Trials and treatment stimuli
Trials were conducted in two rooms with identical foraging arenas (figure 1) from June to July 2005, and each snake was always tested in the same arena. Rattlesnakes received three different scent trials (squirrel, rattlesnake and squirrel+rattlesnake, see below), and each scent stimulus was paired with a water control stimulus. Treatment orders were assigned randomly to individuals. Four of the six possible stimulus sequences were used once and two were used twice (figure 1). Trials began between 17.00 and 18.00, and consecutive presentations for each snake were conducted at 3-day interval. Arena rooms were kept approximately at 27°C with light levels at 0.05 μmol s−1 m−2 μA−1 to simulate dusk. The foraging arena was sprayed down with water, wiped and allowed to dry after each trial, and lined with fresh white butcher paper before the next trial. Treatment stimuli were made as follows.
Squirrel. California ground squirrel scent was collected between 08.00 and 12.00 the day of the trial from wild-caught juvenile squirrels. Filter paper (12.5 cm in diameter) was rubbed on the squirrels' ventrum, paws and face, and then sealed into a plastic bag with hair cut from the tail. These filter papers were then used as treatment stimuli, with 100 cm3 of water added 2 hours prior to trials. Hairs were removed just before testing to eliminate visual cues. The filter papers were always handled with latex gloves.
Rattlesnake. Northern Pacific rattlesnake shed skins were collected from the captive population during the three months prior to the trials and kept frozen until the day of the trial. The morning of the trial, approximately 10 cm of a shed skin was cut into pieces and added to a plastic bag containing a filter paper. Two hours prior to starting the trial, 100 cm3 of water was added. Just prior to testing, pieces of shed skin were removed to eliminate visual cues. Each individual rattlesnake was tested with the odour of a different snake of similar size and of the same sex.
Squirrel+rattlesnake. Shed skins (10 cm) of northern Pacific rattlesnake were cut into pieces and combined in a plastic bag with squirrel-scented filter paper (see above) and 100 cm3 of water added 2 hours prior to the trial. Pieces of shed and hair were removed just before testing. Each rattlesnake was tested with the odour of a different snake of similar size and of the same sex.
Water controls. Unscented filter paper was placed in a plastic bag with 200 cm3 of water 2 hours prior to starting a trial.
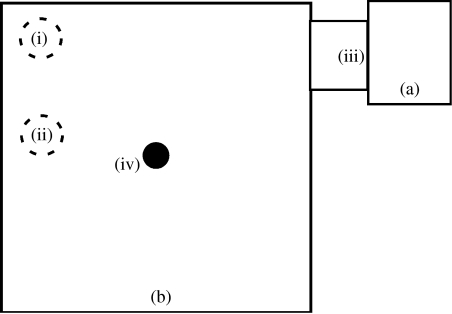
Figure 1.
Rattlesnake foraging arena: rattlesnakes were first placed into the (a) starting chamber (0.63×0.51×0.80 m) and then a divider (iii) was lifted remotely, giving access to the (b) foraging arena (1.20×1.20×0.80 m). For each trial, the arena contained one of the three scent types—squirrel (S), squirrel+rattlesnake (S+R) or rattlesnake (R) scent presented on filter paper—paired with a water control filter paper on the opposite side. Possible treatment orders were: S→R→S+R, S→S+R→R, R→S→S+R, R→S+R→S, S+R→S→R and S+R→R→S. Placement of scents (sides (i) or (ii)) was counterbalanced by assigning half the snakes the order (i)→(ii)→(i) and the other half (ii)→(i)→(ii) for their three trials. The trials were filmed with a closed circuit camera (iv) affixed to the plexiglass arena cover that fed into a VHS recorder outside the room.
Trials were recorded onto a VHS system located just outside the rooms using closed circuit cameras (Sony SSC-M383) that were affixed to the plexiglass arena covers, each capturing its entire arena. The trial scent stimulus and water control were stapled to arena floors, on right or left sides (figure 1). Rattlesnakes were transported via their terrarium into the arena room and transferred to the starting chamber with a snake hook. After 10 min, we began the trial by remotely lifting the divider between the starting chamber and arena.
(iii) Data collection and analysis
Trials were filmed for 30 min and the following measures were later scored: (i) latency to encounter scent and water stimuli (from the time the rattlesnakes left the start chamber), (ii) time spent over scent and water stimuli (total time head over filter paper), and (iii) tongue flicks over scent and water stimuli. The video scorer was blind to both the scent type involved and the sides on which scent and water were located.
We first compared the three scent stimuli separately with their water controls (paired t-tests). We then tested for an effect of stimulus scent type using a repeated measures multivariate general linear model (GLM) and planned contrasts to compare squirrel scent with rattlesnake and rattlesnake plus squirrel scents.
(b) Experiment 2
(i) Study species
We studied adult rock squirrels (S. variegatus) at Caballo Lake State Park, New Mexico in August 2004, and adult California ground squirrels (S. beecheyi) at Lake Solano County Park, Winters, California in July–August 2004. Squirrels were trapped and released after each had been weighed, anaesthetized, dye-marked with a number, sexed and aged (see Clucas et al. 2008 for details).
(ii) Trials and treatment stimuli
Behaviour of each individually marked squirrel was observed for a 20 min ‘pre’-trial and then 3–4 days later for a 20 min ‘post’-trial. Midway between pre- and post-trials, ground squirrels were randomly selected to be ‘scent appliers’ (allowed to apply snake scent) or controls (no chance to apply scent). All but one designated scent applier applied scent; the single non-applier was placed in the control group. Sympatric rattlesnake shed skins (Caballo=western diamondback rattlesnake (Crotalus atrox) and Solano=northern Pacific rattlesnake (C. oreganus)) were staked down at sites 3 m from squirrels' burrows and left there for approximately 1 hour after the squirrel initiated snake scent application.
(iii) Data collection and analysis
Focal squirrel behaviour was narrated into an audio recorder during pre- and post-scent application trials for scent appliers (rock squirrels, n=13 and California ground squirrels, n=15) and non-appliers (rock squirrels, n=16 and California ground squirrels, n=15). Instantaneous time samples of the focal squirrel's major behaviour were entered every 30 s, including foraging, moving, grooming, resting, vigilant (bipedal) and out of view. All occurrences were recorded of antipredator behaviour (tail-flagging) and conspecific interactions (aggressive: chasing, biting and shoving; tolerant: within a squirrel length from conspecific with no aggressive behaviour). Narrations were later entered into an event recorder (JWatcher, Blumstein et al. 2006). These data were then transformed either into proportions of time samples (out of 40) for major behaviours, or rates per second (out of 1200) for conspecific interactions and antipredator behaviour.
We tested for differences between pre- and post-trials separately for each species. Differences in major behaviour types were analysed with a repeated measures GLM with time (pre- or post-observation trial) and treatment (scent applied or not applied) as the main effects. Non-normal data were square root transformed to meet assumptions for parametric statistics. Owing to a large number of zeros in the data, we used Wilcoxon signed-rank tests to compare the antipredator behaviour and conspecific interactions of scent appliers and non-appliers.
(c) Experiment 3
(i) Study species
Fifty-six fleas (Oropsylla montana and Hoplopsyllus anomales; Bursten et al. 1997) were collected into individual glass vials from 19 California ground squirrels at Solano (see §2b) from June to September 2006. Each flea was tested separately in the field within 30 min of collection.
(ii) Trials and treatment stimuli
Flea host choice was tested in an enclosed arena lined with white butcher paper. A snake-scented squirrel was placed on one side and a water-scented squirrel on the other (figure S1 in the electronic supplementary material). Four pairs of same-sex anaesthetized juvenile ground squirrels in Tomahawk traps served as stimulus hosts. Monitoring of the subject flea was not impaired by fleas on these squirrels as none left squirrels during trials. Each squirrel pair was used for approximately 30 min, during which we were able to test 9–23 fleas. Shed skins from northern Pacific rattlesnakes mixed with water were used to scent experimental squirrels while only water was used on controls. Trials began by placing a flea's vial in the centre of the arena and releasing it, and ended either after the flea had made a choice or 10 min had elapsed. A new paper lining was used for each flea.
(iii) Data collection and analysis
Flea behaviour scored was (i) choice (snake- or water-scented squirrel) and (ii) latency to choose (time when flea crosses the ‘choice’ line; figure S1 in the electronic supplementary material; see Krasnov et al. 2002).
Choice was compared with a Χ2-test. Independent t-tests were used to compare latencies between those fleas that chose snake-scented squirrels and those that chose water-scented squirrels. In addition, we tested for effects of three additional factors on flea choice and latency—the sex and age of the fleas' prior squirrel host, and the sex of stimulus squirrels. All statistical tests were conducted using SPSS v. 11.0.2 (SPSS, Inc. 2002).
3. Results
(a) Experiment 1
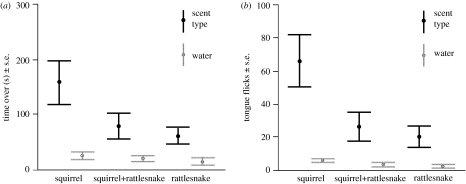
Rattlesnakes spent more time and tongue-flicked more over all three treatment stimuli than over water controls (paired t-tests, time: squirrel scent: t7=3.183, p=0.015; squirrel+rattlesnake scent: t7=2.852, p=0.025 and rattlesnake scent: t7=7.942, p<0.0001; tongue flicks: squirrel scent: t7=3.969, p=0.005; squirrel+rattlesnake scent: t7=2.711, p=0.030 and rattlesnake scent: t7=3.157, p=0.016; figure 2). Time to encounter stimuli versus water controls did not differ for any of the treatments (squirrel scent: t7=−1.553, p=0.164; squirrel+rattlesnake scent: t7=−1.603, p=0.153; rattlesnake scent: t7=1.629, p=0.147).
Figure 2.
Rattlesnake foraging behaviour. (a) Time spent over the scent stimuli and their water controls and (b) total tongue-flick counts over the scent stimuli and their water controls. Rattlesnakes spent more time and tongue-flicked more over all three scent types than their water controls (all p<0.05). Rattlesnakes spent more time over squirrel scent than squirrel+rattlesnake and rattlesnake scent (p=0.048 and 0.042) and tended to tongue-flick more over squirrel scent than squirrel+rattlesnake and rattlesnake scent (p=0.070 and 0.030).
Rattlesnake behaviour varied significantly with type of treatment stimulus (multivariate repeated measures GLM: F3,13=3.850, p=0.036). Both time and tongue flicking over stimuli differed significantly across treatments (time: F2,14=4.478, p=0.031 and tongue flicks: F2,14=4.667, p=0.028; figure 2) while time to encounter stimuli did not differ (F2,14=2.180, p=0.183).
Rattlesnakes spent more time over ground squirrel scent than the two rattlesnake-scented stimuli (planned contrasts: squirrel versus squirrel+rattlesnake: F1,7=6.147, p=0.048; squirrel versus rattlesnake: F1,7=5.726, p=0.042; figure 2a). Tongue flicking over squirrel scent was greater than for rattlesnake scent (F1,7=7.431, p=0.030) and tended to be greater than for squirrel+rattlesnake, but was not statistically significant (F1,7=4.426, p=0.070; figure 2b).
(b) Experiment 2
(i) Rock squirrels
Application of snake scent did not affect rock squirrel behaviour. Neither time (pre- versus post-sample) nor the interaction of time and condition (snake scent application versus no application) was significantly related to major behaviour (repeated measures GLM: time: F5,25=1.030, p=0.421; time×condition: F5,25=0.897, p=0.498; see table S1 in the electronic supplementary material). Antipredator behaviour and conspecific interactions were rare, and did not differ between pre- and post-samples for either scent application or no application conditions (Wilcoxon signed-rank test, scent application: tail-flagging: Z=−0.736, p=0.461; aggression: Z=−1.103, p=0.270 and tolerance: Z=−0.137, p=0.891 and no application: tail-flagging: Z=0.00, p=1.000; aggression: Z=−0.059, p=0.953 and tolerance: Z=−0.921, p=0.357).
(ii) California ground squirrels
Squirrels that applied snake scent significantly modified their subsequent behaviour (time×condition; repeated measures GLM: F5,24=5.206, p=0.002; see table S1 in the electronic supplementary material). Application of snake scent was associated with a reduction in resting and an increase in grooming during post-trials (mean±s.e. (post–pre)=−0.1394±0.061; F1,28=5.234, p=0.030 and 0.1224±0.038; F1,28=6.192, p=0.019 for resting and grooming, respectively), but squirrels which applied no scent exhibited no such change in behaviour (mean±s.e. (post–pre)=0.039±0.100 and −0.045±0.095 for resting and grooming, respectively). Both antipredator behaviour and conspecific interactions were more common for this species than for rock squirrels, but neither type of behaviour differed between pre and post for either scent application or no application conditions (Wilcoxon signed-rank test, scent application: tail-flagging: Z=−0.730, p=0.465; aggression: Z=−0.114, p=0.910 and tolerance: Z=−1.369, p=0.171; no application: tail-flagging: Z=−0.700, p=0.484; aggression: Z=−1.364, p=0.173 and tolerance: Z=−1.532, p=0.126).
(c) Experiment 3
Snake scent had no effect on flea host choice behaviour (choice: snake=25, water=30; Χ12=0.455, N=56, p=0.500). Latency to choose did not differ significantly between fleas that chose the snake- and the water-scented squirrels (mean±s.e.: snake=17.96±4.95 s, water=24.90±8.75 s; independent t-test: t53=0.030, p=0.976). Neither host choice nor latency was significantly associated with prior host sex and age, or sex of stimulus.
4. Discussion
Rattlesnake scent affected rattlesnake foraging behaviour but did not modify ground squirrel social and antipredator behaviour or flea host choice. Rattlesnakes spent the most time and tongue flicked more over ground squirrel scent than over rattlesnake or rattlesnake plus squirrel scent. These results suggest that rattlesnake-scented ground squirrels in burrows may experience a reduced probability that a rattlesnake will detect and/or recognize them as prey. Indeed, previous studies have found that rattlesnake species use prey odour when selecting habitat (Theodoratus & Chiszar 2000) and ambushing sites (Clark 2004).
This study and previous research most strongly support an antipredator function of snake scent application in ground squirrels. Not only does rattlesnake scent reduce rattlesnake foraging behaviour but also the squirrels that are most vulnerable to the effects of predation, the juveniles and adult females, spend the most time applying snake scent (Clucas et al. 2008). In contrast, there is no support for either the conspecific deterrence or ectoparasite defence hypotheses. The duration of application is not related to flea loads in ground squirrels (Clucas et al. 2008) and rattlesnake scent had no impact on squirrel social and antipredator behaviour, or flea host choice.
We proposed two mechanisms whereby applied rattlesnake scent might reduce predation—mimicry and crypsis. The mimicry mechanism implies that rattlesnakes should avoid the odour of conspecifics, a prediction that is not compatible with our results. Rattlesnakes actually exhibited greater interest in rattlesnake scent than their water controls, both for rattlesnake scent alone and mixed with squirrel scent. These observations are consistent with evidence that other rattlesnake species follow conspecific scent trails (timber rattlesnakes Crotalus horridus: Clark 2007). However, rattlesnakes exhibited more foraging behaviour towards squirrel scent alone, indicating that applied snake scent most likely serves as olfactory camouflage, disguising the odour of ground squirrels.
Nevertheless, snake scent application may work in multiple ways to reduce vulnerability to predators. Applied snake scent may also reduce predation risk by modifying the behaviour of squirrels. California ground squirrels in this study, for example, groomed more and rested less after applying snake scent. Such an effect could have been mediated by a stress response to the self-applied predator scent (see Spruijt et al. 1992), a common reaction to predator odours in rodents (Zhang et al. 2003; Apfelbach et al. 2005) that could elevate the level of alertness of the applier. In addition, 10 of 15 California ground squirrels tail-flagged during snake scent application trials, whereas none of the rock squirrels did, and tail-flagging is known to increase the alertness of conspecifics (Hersek & Owings 1993). This species difference could reflect the greater sociality of California ground squirrels (Owings et al. 2001), which would provide this species with more opportunities to protect themselves by alerting conspecifics. Further assessments of species differences and the changes in ground squirrel behaviour associated with snake scent application are needed to examine their adaptive potential.
Ground squirrels have evolved a complex antipredator system to deal with rattlesnakes at many stages of the predator–prey sequence (see Lima & Dill 1990). Their repertoire for dealing with actual encounters includes tail-flagging, harassment, venom resistance, probing assessment of rattlesnake size and body temperature, management of cue leakage to hunting snakes, burrow plugging and pup transfer to alternative burrow systems (Owings & Coss in press). However, these squirrels are also sensitive to the prospects of a rattlesnake encounter and regulate their behaviour accordingly (Hersek & Owings 1993). Here, we have added to our knowledge of the prospective features of this antipredator system, showing that rattlesnake scent application may reduce detection and recognition of ground squirrel odour by rattlesnakes. Ground squirrels may have evolved this cryptic defence to reduce the probability that rattlesnakes will choose ambush sites near their burrows (e.g. Hennessy & Owings 1988), or as a defence against nocturnally hunting rattlesnakes when squirrels are asleep in their burrows. Finally, it is also possible that snake scent application affects other olfactory-oriented predators, such as weasels or coyotes.
The use of olfactory camouflage or even ‘olfactory aposematism’ (sensu Eisner & Grant 1981) may not be uncommon. Some bird species, for example, use odiferous non-structural material such as carnivore scat in their nests for camouflage or as a repellent (Schuetz 2005), and many animals are known to self-apply odiferous heterospecific substances to their bodies (see Weldon 2004; Clucas et al. 2008). Scent application in animals appears to be analogous to other behavioural phenomena such as tool use or habitat construction, in which animals opportunistically make use of substances in their environments. Nevertheless, the functions of only a few of these application behaviours are known (e.g. Weldon et al. 2003) and none have been clearly demonstrated to serve an antipredator function. Thus, snake scent application in ground squirrels may be the first demonstration of an animal's use of another species' scent for predator defence. Such a defensive tactic may have many opportunities to be adaptive, given the widespread dependence by predators on olfaction.
Acknowledgments
We thank Tim Caro and two anonymous reviewers for helpful comments, Aaron Rundus for use of rattlesnakes and foraging arenas in Experiment 2, Duane Davis and Phillip McClelland for access to ground squirrels at Lake Solano County Park and Caballo Lake State Park, respectively, and also Pat Arrowood, Doug Dinero and Terry Ord for help in the field. The research was funded by an NSF Predoctoral Fellowship to B.C. All uses of animals were approved by the University of California Davis Animal Care and Use Committee, protocol nos. 10485, 9145 and 10734.
Supplementary Material
Flea host choice arena
Ground squirrel behaviour before and after scent application or no application
References
- Apfelbach R, Blanchard C.D, Blanchard R.J, Hayes R.A, McGregor I.S. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev. 2005;29:1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. doi:10.1016/j.neubiorev.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Biardi, J. E. 2000 Adaptive variation and coevolution in California ground squirrel (Spermophilus beecheyi) and rock squirrel (Spermophilus variegatus) resistance to rattlesnake venom. PhD dissertation, University of California, Davis.
- Blumstein, D. T., Evans, C. & Daniels, J. C. 2006 JWatcher 1.0. See http://www.jwatcher.ucla.edu/
- Brodie E.D. Hedgehogs use toad venom in their own defence. Nature. 1977;268:627–628. doi:10.1038/268627a0 [Google Scholar]
- Bursten S.N, Kimsey R.B, Owings D.H. Ranging of male Oropsylla montana fleas via male California ground squirrel (Spermophilius beecheyi) juveniles. J. Parasitol. 1997;83:804–809. doi:10.2307/3284271 [PubMed] [Google Scholar]
- Caro T.M. University of Chicago Press; Chicago, IL: 2005. Antipredator defenses in birds and mammals. [Google Scholar]
- Carroll J.F, Kramer M, Weldon P.J, Robbins R.G. Anointing chemicals and ectoparasites: effects of benzoquinones from millipedes on the lone star tick, Amblyomma americanum. J. Chem. Ecol. 2005;31:63–75. doi: 10.1007/s10886-005-0974-4. doi:10.1007/s10886-005-0974-4 [DOI] [PubMed] [Google Scholar]
- Clark R.W. Timber rattlesnakes (Crotalus horridus) use chemical cues to select ambush sites. J. Chem. Ecol. 2004;30:607–617. doi: 10.1023/b:joec.0000018632.27010.1e. doi:10.1023/B:JOEC.0000018632.27010.1e [DOI] [PubMed] [Google Scholar]
- Clark R.W. Public information for solitary foragers: timber rattlesnakes use conspecific chemical cues to select ambush sites. Behav. Ecol. 2007;18:487–490. doi:10.1093/beheco/arm002 [Google Scholar]
- Clucas B, Rowe M.P, Owings D.H, Arrowood P.C. Snake scent application in ground squirrels, Spermophilus spp.: a novel form of antipredator behavior? Anim. Behav. 2008;75:299–307. doi:10.1016/j.anbehav.2007.05.024 [Google Scholar]
- Conover M.R. CRC Press; Boca Raton, FL: 2007. Predator prey dynamics: the role of olfaction. [Google Scholar]
- Cott H.B. Methuen & Co; London, UK: 1957. Adaptive coloration in animals. [Google Scholar]
- Drea C.M, Vignieri S.N, Cunningham S.B, Glickman S.E. Responses to olfactory stimuli in spotted hyenas (Crocuta crocuta): I. Investigation of environmental odors and the function of rolling. J. Comp. Psychol. 2002;116:331–341. doi: 10.1037/0735-7036.116.4.331. doi:10.1037/0735-7036.116.4.331 [DOI] [PubMed] [Google Scholar]
- Edmunds M. Longman Group Limited; New York, NY: 1974. Defense in animals. [Google Scholar]
- Eisner T, Grant R.P. Toxicity, odor aversion, and “olfactory aposematism”. Science. 1981;213:476. doi: 10.1126/science.7244647. doi:10.1126/science.7244647 [DOI] [PubMed] [Google Scholar]
- Hennessy D.F, Owings D.H. Rattlesnakes create a context for localizing their search for potential prey. Ethology. 1988;77:317–329. [Google Scholar]
- Hennessy D.F, Owings D.H, Rowe M.P, Coss R.G, Leger D.W. The information afforded by a variable signal: constraints on snake-elicited tail flagging by California ground squirrels. Behaviour. 1981;78:188–226. [Google Scholar]
- Hersek M.J, Owings D.H. Tail flagging by adult California ground squirrels: a tonic signal that serves different functions for males and females. Anim. Behav. 1993;46:129–138. doi:10.1006/anbe.1993.1168 [Google Scholar]
- Kobayashi T, Watanabe M. An analysis of snake-scent application behavior in Siberian chipmunks (Eutamias sibiricus asiaticus) Ethology. 1986;72:40–52. [Google Scholar]
- Krasnov B.R, Khokhlova I.S, Oguzoglu I, Burdelova N.V. Host discrimination by two desert fleas using an odour cue. Anim. Behav. 2002;64:33–40. doi:10.1006/anbe.2002.3030 [Google Scholar]
- Lima S.L, Dill L.M. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1990;68:619–640. [Google Scholar]
- Owings D.H, Coss R.G. Snake mobbing by California ground squirrels—adaptive variation and ontogeny. Behaviour. 1977;62:50–69. [Google Scholar]
- Owings, D. H. & Coss, R. G. In press. Hunting California ground squirrels: constraints and opportunities for northern Pacific rattlesnakes. In The biology of rattlesnakes (eds W. K. Hayes, K. R. Beaman, M. D. Cardwell & S. P. Bush). Loma Linda, CA: Loma Linda University Press.
- Owings D.H, Coss R.G, McKernon D, Rowe M.P, Arrowood P.C. Snake-directed antipredator behavior of rock squirrels (Spermophilus variegatus): population differences and snake-species discrimination. Behaviour. 2001;138:575–595. doi:10.1163/156853901316924485 [Google Scholar]
- Poran N.S, Coss R.G, Benjamini E. Resistance of California ground-squirrels (Spermophilus beecheyi) to the venom of the northern Pacific rattlesnake (Crotalus viridis oreganus)—a study of adaptive variation. Toxicon. 1987;25:767–777. doi: 10.1016/0041-0101(87)90127-9. doi:10.1016/0041-0101(87)90127-9 [DOI] [PubMed] [Google Scholar]
- Rowe M.P, Coss R.G, Owings D.H. Rattlesnake rattles and burrowing owl hisses: a case of acoustic Batesian mimicry. Ethology. 1986;72:53–71. [Google Scholar]
- Rundus A.S, Owings D.H, Joshi S, Chinn E, Giannini N. Ground squirrels use an infrared signal to deter rattlesnake predation. Proc. Natl Acad. Sci. USA. 2007;104:14 372–14 376. doi: 10.1073/pnas.0702599104. doi:10.1073/pnas.0702599104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz J.G. Common waxbills use carnivore scat to reduce the risk of nest predation. Behav. Ecol. 2005;16:133–137. doi:10.1093/beheco/arh139 [Google Scholar]
- Spruijt B.M, Van Hooff J.A.R.A.M, Gispen W.H. Ethology and neurobiology of grooming behavior. Physiol. Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- SPSS 2002 SPSS for Mac OS X, rel. 11.0.2. Chicago, IL: SPSS, Inc.
- Theodoratus D.H, Chiszar D. Habitat selection and prey odor in the foraging behavior of western rattlesnakes (Crotalus viridis) Behaviour. 2000;137:119–135. doi:10.1163/156853900501908 [Google Scholar]
- Vane-Wright R.I. On the definition of mimicry. Biol. J. Linn. Soc. 1980;13:1–6. [Google Scholar]
- Weldon P.J. Defensive anointing: extended chemical phenotype and unorthodox ecology. Chemoecology. 2004;14:1–4. doi:10.1007/s00049-003-0259-8 [Google Scholar]
- Weldon P.J, Aldrich J.R, Klun J.A, Oliver J.E, Debboun M. Benzoquinones from millipedes deter mosquitoes and elicit self-anointing in capuchin monkeys (Cebus spp.) Naturwissenschaften. 2003;90:301–304. doi: 10.1007/s00114-003-0427-2. doi:10.1007/s00114-003-0427-2 [DOI] [PubMed] [Google Scholar]
- Xu Z.J, Stoddart D.M, Ding H.B, Zhang J. Self-anointing behavior in the rice-field rat, Rattus rattoides. J. Mammal. 1995;76:1238–1241. doi:10.2307/1382617 [Google Scholar]
- Zhang J, Cao C, Gao H, Yang Z, Sun L, Zhang Z, Wang Z. Effects of weasel odor on behavior and physiology of two hamster species. Physiol. Behav. 2003;79:549–552. doi: 10.1016/s0031-9384(03)00123-9. doi:10.1016/S0031-9384(03)00123-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flea host choice arena
Ground squirrel behaviour before and after scent application or no application