Abstract
The recent degradation of coral reefs worldwide is increasingly well documented, yet the underlying causes remain debated. In this study, we used a large-scale database on the status of coral reef communities in the Caribbean and analysed it in combination with a comprehensive set of socioeconomic and environmental databases to decouple confounding factors and identify the drivers of change in coral reef communities. Our results indicated that human activities related to agricultural land use, coastal development, overfishing and climate change had created independent and overwhelming responses in fishes, corals and macroalgae. While the effective implementation of marine protected areas (MPAs) increased the biomass of fish populations, coral reef builders and macroalgae followed patterns of change independent of MPAs. However, we also found significant ecological links among all these groups of organisms suggesting that the long-term stability of coral reefs as a whole requires a holistic and regional approach to the control of human-related stressors in addition to the improvement and establishment of new MPAs.
Keywords: fishes, macroalgae, corals, ocean warming, overfishing, agricultural land use
1. Introduction
The human demographic expansion and associated increases in fishing, nutrient loadings and ocean warming, compounded with environmental variables, have been broadly debated to explain the increasing degradation of coral reefs worldwide (Hughes 1994; Hoegh-Guldberg 1999; McCook 1999; Micheli 1999; Jackson et al. 2001; Friedlander & DeMartini 2002; Hughes et al. 2003; Bellwood et al. 2004; Gardner et al. 2005; Aronson & Precht 2006; Cinner & McClanahan 2006; Mumby et al. 2006; Lesser et al. 2007; Markey et al. 2007; Mora et al. 2007). As the intensity of human activities is expected to increase (Cohen 2003; IPCC 2007), discriminating among stressors will be crucial to emphasize conservation strategies and effectively revert the degradation of coral reefs (Palumbi 2005; Aronson & Precht 2006). Unfortunately, rigorous testing and discrimination among possible causes have proved to be difficult and controversial (e.g. Aronson et al. 2004; Grigg et al. 2005; Chapman et al. 2006; Mora et al. 2007).
Determining the actual drivers of coral reef degradation has been challenging for several reasons. First, it is the fact that different ecological groups of reef organisms may respond differently to the same stressors (e.g. Micheli 1999; Mumby et al. 2006). Therefore, while any given stressor may negatively impact some groups of organisms, others may be favoured or not affected at all. This raises the need for a broad assessment of communities. Secondly, the results of localized studies may not necessarily scale to an entire region (Levin 1992; Guidetti & Sala 2007). It is known that the variation in a given response is usually (almost always) larger at a regional scale than that between neighbouring sites; as a result the effect of certain factors may diminish, to the possible point of losing their significance relative to other factors, when analysed at a regional scale (e.g. Guidetti & Sala 2007). Moreover, at a fine scale, stochastic phenomena or local differences that complicate proper replication may make the system of interest unpredictable (Levin 1992) and the results particularly prone to type I errors (i.e. false positives; e.g. Guidetti & Sala 2007). The complications described above highlight the need for the comprehensive assessment of coral reef communities and the test of hypotheses using databases collected over large geographical scales.
Another difficulty in identifying the causality of stressors is the test of hypotheses using standard analytical techniques. It is increasingly acknowledged that most ecological data are prone to the effects of spatial autocorrelation (i.e. lack of independence among sampled units) and multicollinearity (i.e. covariation among multiple predictive variables). The former increases standard errors and therefore inflates type I errors (Lichstein et al. 2002), whereas the latter may lead to spurious correlations if not all potential variables are considered and if inappropriate statistical tests are used (Graham 2003). Here we surveyed the status of the most important groups of coral reef organisms (i.e. herbivorous and carnivorous fishes, corals, macroalgae) in locations broadly distributed in the Caribbean Sea. We also used an extensive set of socioeconomic and environmental variables and statistical approaches to deal with spatial autocorrelation and multicollinearity. This study represents one of the first quantitative measurements of the relative role and scales of incidence of human- and environment-related variables on coral reef health.
2. Material and methods
(a) Biological data
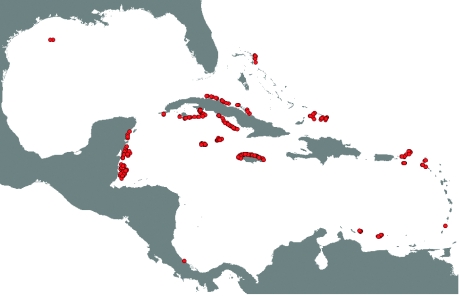
All biological data were obtained from the Atlantic and Gulf Rapid Reef Assessment Project, which uses a consistent methodology to sample the status of different coral reef guilds all throughout the Caribbean (www.agraa.org). In this paper, we used the surveys carried out in the shortest interval of consecutive years that had the largest number of locations sampled to avoid temporal variations in the guilds while increasing sampling size. In the light of this condition, our analysis has to be interpreted as a snapshot of the potential drivers of coral reef change. The surveys used were those carried out between 1999 and 2001. Those surveys were located in 322 reef sites belonging to 13 countries of the Caribbean (figure 1). The sampling comprises the broad variety of sites that may be found in the region like coastal, island and oceanic reefs with varying degrees of human densities and exposure to environmental factors. Such a sampling is likely to maximize statistical variation, and therefore provides a good proxy to assess the general causes of coral reef change in the Caribbean. We only used data from the sites located in the forereef to have a standard comparison among sites. We also included species richness in the analyses to assess potential variations in community structure along gradients of coral reef diversity. For each coral reef community, we quantified the biomass of carnivorous and herbivorous fishes, coral mortality and the abundance of macroalgae and Diadema antillarum (see extended details in the electronic supplementary material).
Figure 1.
Map of the Caribbean indicating the locations sampled. Filled circles, sampled sites.
(b) Environmental and socioeconomic data
For each of the locations sampled, we gathered data from a variety of sources on human population density, area of cultivated land, coastal development, effectiveness of marine protected areas (MPAs; i.e. an index that combines the scores received by each MPA in its regulations about fishing, levels of poaching, size and isolation; see the electronic supplementary material), sea surface temperature, frequency and intensity of hurricanes, and chlorophyll concentration. Here we expand the description of the socioeconomic variables, whereas the extended details of all variables are shown in the electronic supplementary material.
It has been previously argued that it is not the number of people per se but their activities (e.g. harvesting intensity, land use intensity) that lead to the degradation of ecosystems (Sanderson et al. 2002). In most cases, however, the number of people will correlate with the intensity of their activities (table 1 in the electronic supplementary material; Sanderson et al. 2002), which has been the main reason to use human population density as the only surrogate to assess the human effect. By doing so, however, the actual mechanism related to humans will remain open to discussion. Here we used statistical tests that account for collinearity among predictors (see below), which allowed us to test two potential underlying mechanisms associated with humans: coastal development and agricultural land use. Coastal development is an index that incorporates accessibility (i.e. roads) and electrical power, both of which are good surrogates for fishing pressure because they facilitate the storage of fishing products and transportation to external markets (Sanderson et al. 2002; Hughes et al. 2003; Cinner & McClanahan 2006). By contrast, the area of cultivated land leads to terrestrial run-offs such as sedimentation, nutrients and agrochemical pollutants, all of which can affect corals and macroalgae (e.g. McCook 1999; Markey et al. 2007).
(c) Statistical tests
We used trend surface analysis to differentiate the spatial scales (i.e. regional or local) at which any given independent variable exerted its effect. In general terms, the trend surface analysis uses a third-order polynomial of the geographical position of the sites to describe the large-scale (or regional) trend in the response variable. The variation explained by a given predictor that is also explained by the ‘trend’ is defined as the effect exerted at the regional scale (i.e. regional component). The variation explained by the predictor after partialling out the trend is defined as the local component (figure 2; a detailed description of the method can be found in Legendre & Legendre (1998) and Lichstein et al. (2002)). The significance and direction of the relationships were quantified using spatial regression analysis based on the simultaneous autoregressive models. These models were chosen to reduce type I errors due to spatial autocorrelation (Lichstein et al. 2002). We also used the sequential Bonferroni method to adjust p-values and reduce type I errors due to multiple comparisons. We confirmed the significance of the relationships using structural equation modelling to control for spurious correlations arising from collinearity among predictors (figure 3; a detailed description of the method is found in Ullman (1996)). We preferred the structural equation modelling over traditional multiple regression methods because the later can increase type II errors (i.e. in the face of collinearity, multiple regression analysis can lead to the exclusion of predictors weakly related to the response, even if such relationships are causal; see review by Graham 2003).
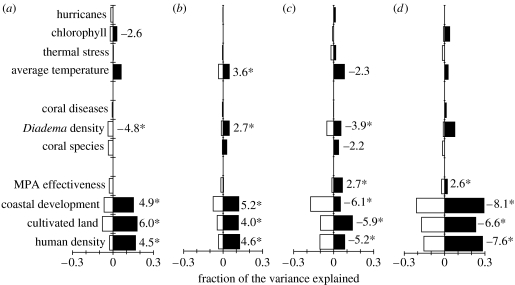
Figure 2.
Spatial fractioning of the variance in the different guilds accounted for by different factors. (a) Macroalgae, (b) coral mortality, (c) herbivore biomass, (d) carnivore biomass. Differentiation between localized and regional variances was calculated by introducing a spatial trend (i.e. a third-order polynomial of the geographical position of the locations) into the regression model. Regional variation (black bars) was the variation accounted for by the predictor that was also accounted for by the spatial trend. Localized variation (white bars) was the variation accounted for by the predictor after partialling out the trend. Tests of significance of each regression were calculated using spatially autoregressive models to control for spatial autocorrelation. The t-values are shown only for those regressions that were significant. The t-values with an asterisk indicate values that remained significant after sequential Bonferroni adjustment of the p-values (see §2 for extended details).
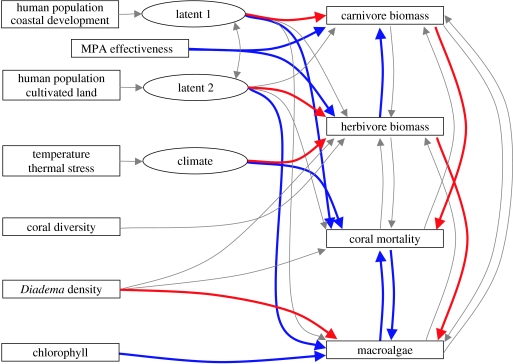
Figure 3.
Confirmatory structural equation model of the links analysed in this study. We used structural equation modelling to confirm the significance of relationships while controlling for collinearity among predictors. The relationships or links (all arrows) were incorporated into the models based on the independent analysis of the variables and our understanding of ecological communities and their interaction with the environment. Levels of significance were established at p<0.01. This p-value has been recommended as a conservative measure to adjust for increased type I errors due to preconceived incorporation of parameters. Significant negative relationships are shown in red and significant positive relationships in blue. We created two latent variables for the human effect: coastal development (i.e. roads and electricity) and area of cultivated land. We assume that both variables are related via human density and so we included a correlation link between the two variables in the model.
3. Results
Among the set of hypotheses tested here, those related to humans showed the strongest effects on coral reefs at both small and large geographical scales (figure 2). We found that increases in the number of people were positively related to macroalgae abundance (t=4.5, p<0.0001) and coral mortality (t=4.6, p<0.0001) and negatively related to the biomass of herbivorous (t=−5.9, p<0.0001) and carnivorous (t=−7.6, p<0.0001) fishes (figure 2). Our results indicate that coastal development showed their main effect on the biomass of carnivorous fishes (t=−8.1, p<0.0001) and mortality of corals (t=5.2, p<0.0001; figures 2 and 3), whereas the area of cultivated land near the reefs exerted its main effect on macroalgae abundance (t=6.0, p<0.0001; figures 2 and 3). It is important to note that our results indicate that herbivorous fishes (t=−3, p<0.01; figure 3) and Diadema (t=−4.9, p<0.001) are also significant drivers of macroalgae abundance, although their relative roles are smaller than that caused by cultivated land (t=6.0, p<0.0001; the comparison of the regional/local variation explained by all variables is shown in figure 2).
The analysis of environmental variables indicated that warmer environments have had higher coral mortality (t=3.6, p<0.0001; figures 2b and 3) with a variation similarly distributed between local and regional scales. We also found that increased temperature is related to reduced herbivore biomass (t=−2.3, p<0.01), although most of this pattern occurred at the regional scale (figure 2). The changes in temperature did not affect macroalgae or carnivore biomass. Chlorophyll (a surrogate for productivity) only had a small effect on macroalgae abundance (figures 2 and 3) and no effect on the status of any other group (figure 2). The number and intensity of hurricanes showed no relationship with any of the studied groups (figures 2 and 3).
Surrogates for stress and resilience such as coral diseases and coral richness showed no systematic relationship with fish biomass, coral mortality or macroalgae abundance (figures 2 and 3). We found no support for a region-wide effect of carnivores on Diadema densities as had been previously reported in certain locations (McClanaham et al. 2002). We presume, however, that this difference occurs because only a small subset of the carnivorous species analysed here prey upon Diadema. Our results, however, confirm the significance of several ecological links among the groups of organisms studied (figure 3; table 1 in the electronic supplementary material). We found herbivorous fishes affecting the biomass of carnivorous fishes and the abundance of macroalgae and a reciprocal and negative relationship between corals and macroalgae (figure 3; table 1 in the electronic supplementary material). We also found that increased carnivore biomass was related to reduced coral mortality (figure 3).
Finally, we found that MPA effectiveness was related to reef sites with larger biomass of both herbivorous (t=2.7, p<0.0001; figure 2c) and carnivorous (t=2.6, p<0.03; figure 2d) fishes but showed no relationship with coral mortality (t=−0.6, p=0.5; figure 2b) or macroalgae abundance (t=0.06, p=0.9; figure 2a).
4. Discussion
Human population density per se may be the best surrogate of anthropogenic impacts and has been previously related to the loss of biodiversity in terrestrial ecosystems (Forester & Machlis 1996; Brashares et al. 2001). For coral reefs, it has been argued that human density should not be related to coral reef status, given that isolated areas often show symptoms of deterioration similar to reefs near human settlements (Aronson & Precht 2006); however, until now an actual test of the hypothesis has not been performed. In testing the human population effect, we found that humans as in the terrestrial ecosystems have had an effect on coral reefs. It is probable that the claim that coral reefs are not affected by humans rests on few localized observations, which as indicated in the introduction may lead to misleading conclusions. Additionally, there is a lack of studies actually discriminating among the effects of contrasting hypotheses. As we found here, there are several important drivers of coral reef mortality, which do not disprove the human population effect. In regards to the scale of incidence of the human effect, we found that the responses in all ecological groups to such an effect occurred mostly over large geographical scales (figure 2). This indicates a common response of coral reefs and a broad effect of human settlements throughout the Caribbean, which is not surprising considering that approximately 121 million people live broadly distributed along the Caribbean coasts (figure S1 in the electronic supplementary material).
Explaining the human population effect for coral reefs is complex because it may affect coral reef communities at different trophic levels via different mechanisms (e.g. higher trophic groups may be more affected by fishing, whereas lower trophic levels may be more affected by the terrestrial run-offs associated with land use; Micheli 1999, Mumby et al. 2006). Indeed, we found that coastal development, a surrogate for fishing pressure, has led to decreased fish biomass, whereas cultivated land, a surrogate for terrestrial run-offs, to increased macroalgae abundance. We also found coastal development being related to coral mortality, which may be explained by increased sewage, which is particularly lethal to corals (note that these hypotheses are significant after controlling for the potential effect of collinearity among predictors; figure 3).
The causes of variation in macroalgae abundance have been broadly debated between those caused by herbivory and those caused by nutrients and/or terrestrial run-offs (e.g. Hughes 1994 versus Lapointe 1999). Our results support the idea of a simultaneous and significant effect of both, although they also indicated that terrestrial run-offs have a relatively stronger effect. Our argument to explain this result is as follows. It has been found that herbivores may not be able to cope with increases in macroalgae beyond a given threshold of macroalgae coverage (see Williams & Polunin 2001). For the Caribbean, increasing coral mortality (e.g. Gardner et al. 2003) has probably opened large areas of substrate for algae growth, which may be surpassing the threshold of herbivory control. This, in turn, may be allowing another factor such as terrestrial run-offs to drive macroalgae abundance. Furthermore, such a threshold of herbivory control may be particularly low for the Caribbean, given the diminished populations of herbivores since historical times (see Pandolfi et al. 2003).
Climate change is regarded as one of the major threats to the future of coral reefs (Hoegh-Guldberg 1999; Hughes et al. 2003), being incontrovertibly evident during regional- to global-scale episodes of coral bleaching when temperature increases only by a few degrees (Hoegh-Guldberg 1999). We found that reef sites in warmer environments indeed have had higher coral mortality. We also found that the net effect of temperature was evenly distributed between the local and regional scales. This suggests that temperature has a similar effect on reefs throughout the region, although there is an intrinsic local variation in this factor (perhaps due to local hydrodynamics) to which coral reefs are also responsive. This later result indicates that MPAs can gain benefits by spatially avoiding the effects of ocean warming (see West & Salm 2003). We also found that temperature was negatively related to the biomass of herbivorous fishes. Negative relationships between herbivore biomass and temperature have been described previously and attributed to thermophysiological constraints in herbivores (i.e. it seems that the digestion of macroalgae may be restricted by temperature, which may impose geographical limitations in the distribution of herbivores, e.g. Floeter et al. 2005). The fact that the herbivore response to temperature occurred almost completely at the regional scale supports the existence of such a biogeographical pattern.
Diadema antillarum was once an abundant and widely distributed grazer in the Caribbean (Lessios 2005). The phase shift, from coral to algae dominance, in the Caribbean reefs after the Diadema die-offs in the early 1980s is one of the best documented examples of the keystone role of grazing on coral reefs (Lessios 2005). In the Caribbean, the recovery of Diadema has been spatially variable, perhaps due to hydrodynamic processes underlying the supply of propagules to benthic populations (Lessios 2005). Until 2000, the average density of Diadema in the sampled sites was 0.06 individuals m−2 (s.d.=0.3), which is relatively similar to more recent surveys (0.02 individuals m−2; Newman et al. 2006) but lower by orders of magnitude than the densities prior to their die-off in the 1980s (1–10 individuals m−2; Lessios 2005). Despite their diminished populations, Diadema densities were significantly correlated with macroalgae abundance. Reduced macroalgae where Diadema was more abundant is very likely a response to grazing. Our structural equation model indicates that the interaction between coral mortality and macroalgae abundance is significant in both directions, which in turn highlights the potential for Diadema to facilitate the recovery of reefs by reducing the negative effect of macroalgae on corals.
MPAs are among the main approaches implemented for the conservation of coral reefs (Hughes et al. 2003; Bellwood et al. 2004; Mora et al. 2006) and have been shown to have positive effects on different groups of fishes (Cote et al. 2001; Gell & Roberts 2003; Guidetti & Sala 2007). In support of this, we found that the effectiveness of MPAs in the Caribbean has been related to larger biomass of both herbivorous and carnivorous fishes, although MPAs showed no effect on the mortality of corals and abundance of macroalgae. As we have shown before, among the main drivers of coral mortality were temperature and potentially sewage from coastal development. As far as we know, the regional network of MPAs in the Caribbean has not been designed to account for the effects of warming and many do not control external threats such as sewage (e.g. Mora et al. 2006), which may explain their general failure to prevent coral mortality. A recent localized study (Mumby et al. 2006) found that the variation in herbivore biomass between an MPA and neighbouring non-protected sites was sufficient to cause a significant variation in macroalgae abundance between protected and unprotected sites. By extending the spatial scale of study, we found that at a regional scale, existing MPAs in the Caribbean do not lead to significant variations in macroalgae biomass. The contrasting results between our study and that by Mumby et al. (2006) may be explained, in part, by differences in scale between the studies (see §1). In support of our result, it should be mentioned that cultivated land, which was the main driver of macroalgae abundance, remains largely uncontrolled by most MPAs of the Caribbean. In summary, our results indicate the positive effect of enforcing reductions in fishing pressure on fish populations and also that the stressors responsible for variations in macroalgae abundance and coral mortality remain poorly, if at all, controlled inside MPAs. Although our results indicate that fishes will gain benefits from increasing effective MPA coverage in the region, they also show that in addition to fishing other human impacts need to be managed or regulated. Unfortunately, failure to account for threats that affect corals and macroalgae may ultimately defeat the results of MPAs on fish populations. This will occur because many fishes depend on the coral reef matrix, and so they can be at risk in the long term due to uncontrolled threats degrading coral reefs inside MPAs (see also Jones et al. 2004).
Other factors analysed in this study including hurricanes and surrogates for stress and resilience such as coral diseases and coral diversity showed no relationship with fish biomass, coral mortality or macroalgae abundance. For the Caribbean, recent meta-analyses on the effect of hurricanes showed not only that coral reefs are capable of fast recovery but also that in recent times coral declines have been similar between reef areas with and without hurricanes, indicating that other stressors are having more pervasive consequences on coral reefs (Gardner et al. 2005). The idea that pathogens cause direct mortality of corals has been challenged by the alternative that more chronic stressors (e.g. those anthropogenic in nature) cause physiological stress that leaves corals vulnerable to infections by opportunistic pathogens (Lesser et al. 2007). Thus, it is possible that the effects of diseases can be a consequence rather than a cause of the degradation of coral reefs. Intriguingly, we also found that sites with high carnivore biomass had significantly lower coral mortality. Although it has been previously suggested that the loss of coral reefs will reduce the biomass of fishes (Jones et al. 2004), our structural equation model indicated that carnivore biomass reduced coral mortality. It is possible that carnivore biomass triggers an indirect mechanism increasing the survival of coral reefs (i.e. high carnivore biomass is a good predictor of intact ecosystems, which are more resistant to stressors and diseases). However, it is also possible that both carnivores and corals are casually correlated because they are strongly affected by common factors (e.g. see the results about coastal development; figure 2).
Human settlements have been inevitably accompanied by the changes in land use and exploitation of natural resources which have caused widespread and profound changes in the structure of coral reef communities. The increasing production of greenhouse gases to supply an increasing demand for energy is also leading to increases in temperature (IPCC 2007), which causes bleaching and coral mortality and indirectly threatens other reef organisms as the coral reef matrix becomes degraded. The expected increase in human population from 6 billion people today to 9 billion for the year 2050 (Cohen 2003) and a probable 1.8–4°C temperature increase over the same time period (IPCC 2007) suggest that coral reefs are likely to witness a significant ecological crisis in the coming half century. Fortunately, the solutions are already available, which include the use of enforced no-take MPAs definitely complemented with strategies to regulate the effects of land use and international commitment to reduce the emission of greenhouse gases, and finally the implementation of strategies to reduce or stabilize the ultimate cause of all these stressors, the world's human population.
Acknowledgments
I thank all researchers who collaborated with the fieldwork. Funding was provided by the Caribbean Environmental Program of the United Nations Environmental Program, the Bacardi Family Foundation, National Geographic, the Hachette-Filipacchi Foundation, the Ocean Research and Education Foundation, the National Oceanographic and Atmospheric Administration Coral Health and Monitoring Program, American Airlines and the Sloan Foundation through the Future of Marine Animal Populations project. I thank Robert Ginsburg, Boris Worm, Philip Kramer, Jana McPherson and Judith Lang for their comments on the manuscript.
Supplementary Material
References
- Aronson R.B, Precht W.F. Conservation, precaution, and Caribbean reefs. Coral Reefs. 2006;25:441–450. doi:10.1007/s00338-006-0122-9 [Google Scholar]
- Aronson R.B, et al. Causes of coral reef degradation. Science. 2004;302:1502–1504. doi: 10.1126/science.302.5650.1502b. doi:10.1126/science.302.5650.1502b [DOI] [PubMed] [Google Scholar]
- Bellwood D.R, Hughes T.P, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. doi:10.1038/nature02691 [DOI] [PubMed] [Google Scholar]
- Brashares J.S, Arcese P, Sam M.K. Human demography and reserve size predict wildlife extinction in West Africa. Proc. R. Soc. B. 2001;268:2473–2478. doi: 10.1098/rspb.2001.1815. doi:10.1098/rspb.2001.1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D.D, et al. Marine parks need sharks. Science. 2006;312:526–528. doi: 10.1126/science.312.5773.526d. doi:10.1126/science.312.5773.526d [DOI] [PubMed] [Google Scholar]
- Cinner J.E, McClanahan T.R. Socioeconomic factors that lead to overfishing in small-scale coral reef fisheries of Papua New Guinea. Environ. Conserv. 2006;33:73–80. doi:10.1017/S0376892906002748 [Google Scholar]
- Cohen J.E. Human population: the next half century. Science. 2003;302:1172–1175. doi: 10.1126/science.1088665. doi:10.1126/science.1088665 [DOI] [PubMed] [Google Scholar]
- Cote I.M, Mosqueira I, Reynolds J.D. Effects of marine reserve characteristics on the protection of fish populations: a meta-analysis. J. Fish Biol. 2001;59:178–189. [Google Scholar]
- Floeter S.R, Behrens M.D, Ferreira C.E.L, Paddack M.J, Horn M.H. Geographical gradients of marine herbivorous fishes: patterns and processes. Mar. Biol. 2005;147:1435–1447. doi:10.1007/s00227-005-0027-0 [Google Scholar]
- Forester D.J, Machlis G.E. Modeling human factors that affect the loss of biodiversity. Conserv. Biol. 1996;10:1253–1263. doi:10.1046/j.1523-1739.1996.10041253.x [Google Scholar]
- Friedlander A.M, DeMartini E.E. Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators. Mar. Ecol. Prog. Ser. 2002;230:253–264. doi:10.3354/meps230253 [Google Scholar]
- Gardner T.A, Cote I.M, Gill J.A, Grant A, Watkinson A.R. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. doi:10.1126/science.1086050 [DOI] [PubMed] [Google Scholar]
- Gardner T, Cote I.M, Gill J.A, Grant A, Watkinson A.R. Hurricanes and Caribbean coral reefs: impacts, recovery patterns, and role in long-term decline. Ecology. 2005;86:174–184. doi:10.1890/04-0141 [Google Scholar]
- Gell F.R, Roberts C.M. Benefits beyond boundaries: the fishery effects of marine reserves. Trends Ecol. Evol. 2003;18:448–454. doi:10.1016/S0169-5347(03)00189-7 [Google Scholar]
- Graham M.H. Confronting multicollinearity in ecological multiple regression. Ecology. 2003;84:2809–2815. doi:10.1890/02-3114 [Google Scholar]
- Grigg R.W, et al. Reassessing U.S. coral reefs. Science. 2005;308:1740–1742. doi: 10.1126/science.308.5729.1740c. doi:10.1126/science.308.5729.1740c [DOI] [PubMed] [Google Scholar]
- Guidetti P, Sala E. Community-wide effects of marine reserves in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2007;335:43–56. doi:10.3354/meps335043 [Google Scholar]
- Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshw. Res. 1999;50:839–866. doi:10.1071/MF99078 [Google Scholar]
- Hughes T.P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. doi:10.1126/science.265.5178.1547 [DOI] [PubMed] [Google Scholar]
- Hughes T.P, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. doi:10.1126/science.1085046 [DOI] [PubMed] [Google Scholar]
- IPCC 2007 See www.ipcc.ch/SPM6avr07.pdf
- Jackson J.B.C, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. doi:10.1126/science.1059199 [DOI] [PubMed] [Google Scholar]
- Jones G.P, McCormick M.I, Srinivisan B, Eagle J.V. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl Acad. Sci. USA. 2004;101:8251–8253. doi: 10.1073/pnas.0401277101. doi:10.1073/pnas.0401277101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe B.E. Simultaneous top-down and bottom-up forces control macroalgal blooms on coral reefs. Limnol. Oceanogr. 1999;44:1586–1592. [Google Scholar]
- Legendre P, Legendre L. 2nd English edn. Elsevier Science; Amsterdam, The Netherlands: 1998. Numerical ecology. [Google Scholar]
- Lesser M.P, Byhtell J.C, Gates R.D, Johnstone R.W, Hoegh-Guldberg O. Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. J. Exp. Mar. Biol. Ecol. 2007;346:36–44. doi:10.1016/j.jembe.2007.02.015 [Google Scholar]
- Lessios H.A. Diadema populations twenty years following mass mortality. Coral Reefs. 2005;24:125–127. doi:10.1007/s00338-004-0443-5 [Google Scholar]
- Levin S.A. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1947. doi:10.2307/1941447 [Google Scholar]
- Lichstein J.W, Simons T.R, Shriner S.A, Franzreb K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002;72:445–463. [Google Scholar]
- Markey K.L, Baird A.H, Humphrey C, Negri A.P. Insecticides and a fungicide affect multiple coral life stages. Mar. Ecol. Prog. Ser. 2007;330:127–137. doi:10.3354/meps330127 [Google Scholar]
- McClanaham T.R, Polunin N, Done T. Ecological states and the resilience of coral reefs. Conserv. Ecol. 2002;6:18–29. [Google Scholar]
- McCook L.J. Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef. Coral Reefs. 1999;18:357–367. doi:10.1007/s003380050213 [Google Scholar]
- Micheli F. Eutrophication, fisheries, and consumer-resource dynamics in marine pelagic ecosystems. Science. 1999;285:1396–1398. doi: 10.1126/science.285.5432.1396. doi:10.1126/science.285.5432.1396 [DOI] [PubMed] [Google Scholar]
- Mora C, Andréfouët S, Costello M, Kranenburg S, Rollo A, Veron J, Gaston K.J, Myers R.A. Coral reefs and the global network of marine protected areas. Science. 2006;312:1750–1751. doi: 10.1126/science.1125295. doi:10.1126/science.1125295 [DOI] [PubMed] [Google Scholar]
- Mora C, Metzker R, Rollo A, Myers R.A. Experimental simulations about the effects of habitat fragmentation and overexploitation on populations facing environmental warming. Proc. R. Soc. B. 2007;274:1023–1028. doi: 10.1098/rspb.2006.0338. doi:10.1098/rspb.2006.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby P.J, et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science. 2006;311:98–101. doi: 10.1126/science.1121129. doi:10.1126/science.1121129 [DOI] [PubMed] [Google Scholar]
- Newman M.J.H, Paredes G.A, Sala E, Jackson J.B.C. Structure of Caribbean coral reef communities across a large gradient of fish biomass. Ecol. Lett. 2006;9:1216–1227. doi: 10.1111/j.1461-0248.2006.00976.x. doi:10.1111/j.1461-0248.2006.00976.x [DOI] [PubMed] [Google Scholar]
- Palumbi S. Germ theory for ailing corals. Nature. 2005;434:713–714. doi: 10.1038/434713a. doi:10.1038/434713a [DOI] [PubMed] [Google Scholar]
- Pandolfi J.M, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. doi:10.1126/science.1085706 [DOI] [PubMed] [Google Scholar]
- Sanderson E.W, Jaiteh M, Levy M.A, Redford K, Wannebo A, Woolmer G. The human footprint and the last of the wild. Bioscience. 2002;52:891–904. doi:10.1641/0006-3568(2002)052[0891:THFATL]2.0.CO;2 [Google Scholar]
- Ullman, J. B. 1996 Structural equation modeling. In Using multivariate statistics (eds B. G. Tabachnick & L. S. Fidell), pp. 709–819, 3rd edn. New York, NY: Harper Collins College Publishers.
- West J.M, Salm R.V. Resistance and resilience to coral bleaching: implications for coral reef conservation and management. Conserv. Biol. 2003;17:956–967. doi:10.1046/j.1523-1739.2003.02055.x [Google Scholar]
- Williams I.D, Polunin N.V.C. Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs. 2001;19:358–366. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.