Abstract
Life-history theory posits a fundamental trade-off between number and size of offspring that structures the variability in parental investment across and within species. We investigate this ‘quantity–quality’ trade-off across primates and present evidence that a similar trade-off is also found across natural-fertility human societies. Restating the classic Smith–Fretwell model in terms of allometric scaling of resource supply and offspring investment predicts an inverse scaling relation between birth rate and offspring size and a −¼ power scaling between birth rate and body size. We show that these theoretically predicted relationships, in particular the inverse scaling between number and size of offspring, tend to hold across increasingly finer scales of analyses (i.e. from mammals to primates to apes to humans). The advantage of this approach is that the quantity–quality trade-off in humans is placed into a general framework of parental investment that follows directly from first principles of energetic allocation.
Keywords: quantity–quality trade-off, Smith–Fretwell model, number and size of offspring, natural-fertility human societies, primate life histories, quarter-power scaling
1. Introduction
Life-history models assume that parents make investment decisions that maximize reproductive success (fitness) in the face of constraints whereby energy, effort, resources or time invested in the provisioning of offspring cannot be invested in producing more offspring (Stearns 1992). As a consequence, one of the life's most fundamental trade-offs is between the number and size of offspring (Lack 1947; Roff 1992). Experimental studies that manipulate offspring number or the environment generally have been successful in revealing the quantity–quality trade-off (Roff 2002).
The quantity–quality trade-off has received considerable attention in the field of human behavioural ecology (Hill & Kaplan 1999; Borgerhoff Mulder 2000; Kaplan & Lancaster 2000; Mace 2000). Blurton Jones & Sibly (1978) tested a model where a 4-year interbirth interval was fitness-maximizing in the Ju/'hoansi of Botswana and Namibia. Strassmann & Gillepsie (2002) demonstrated a trade-off between female fertility and offspring survivorship for the Dogon of Mali. Hagen et al. (2006) showed that Shuar (Ecuador) children in families with fewer adults and more dependents have compromised growth and nutrition. Despite these and other demonstrated relationships, life-history trade-offs have been difficult to demonstrate in observational studies given the problem of unmeasured and confounding variables (Hill & Hurtado 1996). Since parental condition varies considerably within and across populations, the problem of phenotypic correlation between parental condition and current reproduction may mask the underlying trade-off (Reznick 1985; Stearns 1992). In such cases, a typical result is that parents with more children are also able to invest more in each child, presumably because healthier, higher-status individuals are better able to monopolize resources. For example, there is a positive relationship between childhood survival and number of siblings among Ache hunter-gatherers of Paraguay (Hill & Hurtado 1996) and Kipsigis agro-pastoralists of Kenya (Borgerhoff Mulder 2000). These relationships remain positive even after statistically controlling for body size or wealth, suggesting that other intrinsic differences among individuals may be obscuring the trade-off. Similarly, lifetime reproductive success as measured by number of grandchildren has been shown to increase with higher fertility in several populations, including the Ju/'hoansi (Pennington & Harpending 1988; cf. Blurton Jones & Sibly 1978), Ache (Hill & Hurtado 1996) and New Mexico men (Kaplan et al. 1995), contrary to the expectation based on trade-offs.
While attempts have been made to examine trade-offs within human populations, to our knowledge no attempt has been made to uncover the trade-off between number and size of offspring across human societies, perhaps due to the problems described above. A comparative study with humans is important because some derived human traits, such as cultural norms, food sharing, extended provisioning and extra-maternal care, may affect reproductive effort in ways that make the quantity–quality trade-off different than the trade-off in other animals. For example, equitable pooling of resources could hypothetically erase the trade-off within a population, though the trade-off may still be visible across populations. Moreover, given the problem of phenotypic correlation, it is probable that no quantity–quality trade-off will be visible if birth rate is not adjusted by mother's reproductive energy budget. Our analytical approach was developed by Smith & Fretwell (1974) and Charnov & Ernest (2006) in a model that accounts for the phenotypic correlation by controlling for mother's mass raised to the ¾ power, a proportionality of energy available for reproduction. An advantage of this approach is that the quantity–quality trade-off in human populations can be analysed from the same underlying framework as other animals. We make the parsimonious prediction that a trade-off will emerge across humans that is similar to the inverse relationship between quantity and quality seen in other mammals and driven by the same first principles of energetic allocation.
(a) Analysing the quantity–quality trade-off
Smith & Fretwell (1974) developed a general model of the trade-off between number and size of offspring. A parent with R resources to devote to reproduction must divide them among C offspring in a clutch, creating a limited investment per offspring, I, such that
| (1.1) |
Most primates have only one offspring per litter, but C can be interpreted as the fertility rate (births per year) and R as the flow of total resources allocated to reproduction (offspring production rate). Then, time cancels out of the ratio such that I describes the energetic investment of parents to an average offspring.
The trade-off between the investment, I, and fertility rate, C, can be analysed under the assumption that offspring production rate, R, is constant across populations or species. However, as discussed in Charnov's (1993) general life-history model, R scales approximately as the ¾ power of adult body mass, M, across mammals (Charnov 2001). Daily milk yield and lactational capacity, perhaps the most direct measures of mammalian R, scale as approximately the ¾ power of body size (Martin 1984; Oftedahl 1984). Given that R∝M3/4, the following equation applies (Charnov & Ernest 2006):
| (1.2) |
The l.h.s. of equation (1.2) is mother's-energy-adjusted fertility rate and is expected to be inversely proportional to investment per offspring, I, estimated as size at independence. The predicted inverse relationship between number and size of offspring is strong across mammals (Charnov & Ernest 2006), a remarkable result given that different species probably express a variety of relationships between offspring fitness and parental investment (Charnov & Downhower 1995).
Re-expressing the Smith–Fretwell model in terms of allometric scaling predicts the −¼ power scaling of fertility rates with body size, provided resources invested per offspring is proportional to the mass of the mother. Across a wide spectrum of mammals, including primates, weaning size is approximately one-third of mother's size (Charnov 1993; Alvarez 2000). Given that R∝M3/4 and I∝M1, fertility rate, C, as a function of body mass, M, becomes
| (1.3) |
Thus, offspring production increases with mass slower than offspring investment increases with mass, and fertility rate scales to the −¼ power of body mass.
The elegance of the Smith–Fretwell model with the Charnov–Ernest correction is that it can be used to investigate the trade-off between offspring number and size both across and within species where phenotypic correlation is expected. Here we apply the model to study the trade-off across primate species and human societies, exposing variability in fertility decisions and shifts towards differential life-history strategies that favour more or less quantity versus quality of offspring. We have compiled a comparative database of 16 natural-fertility human societies and a primate database that we analysed with both conventional and phylogenetically controlled methods. Our primary hypotheses are that (i) fertility rate will scale as the −¼ power of body size and (ii) fertility rate adjusted by offspring production rate will be an inverse function of offspring size in both humans and other primates.
2. Material and methods
Evaluation of the Smith–Fretwell model with the Charnov–Ernest correction requires estimates of the following life-history components: (i) fertility rate, C, (ii) M3/4 to adjust for differences in maternal resource budgets allocated to reproduction, R, and (iii) offspring size, I, at some age (e.g. weaning, 5 and 10). We calculated 95% CIs of the trade-off exponent using ordinary least squares (OLS) and reduced major axis (RMA) regression. Nonlinear multiple regressions of C on R and I (and I on R and C) across natural-fertility societies were performed using SPSS v. 15.0 (SPSS, Inc., Chicago, IL).
(a) Non-human primates and mammals
Primate data (n=101 species) are from Lindenfors (2002). Data for non-volant, eutherian mammals (n=610 species excluding primates) are from Ernest (2003). Fertility rate was calculated by multiplying births per year by the average number of offspring per litter. Adult body sizes for primates were taken from Smith & Jungers (1997) with an emphasis on wild weights where available. Sizes at weaning for primates (n=5 apes, n=30 other haplorhines) included estimates from growth curves (Lee 1999; Lindenfors 2002). Analyses were conducted with primate species as independent data points and using independent contrasts that adjust for phylogenetic constraints (Nunn & Barton 2001) because phylogenetically close species may be similar only because they share a recent common ancestor (Felsenstein 1985). The independent contrasts module of PDTree by Garland et al. (1993) with the phylogenetic tree and branch lengths of Bininda-Emonds et al. (2007) were used to construct independent contrasts. Diagnostics available in PDTree were examined to ensure homoscedasticity in residuals and that branch lengths were statistically appropriate.
(b) Natural-fertility human societies
The sample used here is 16 subsistence-based societies including foragers, horticulturalists and one pastoral society. To our knowledge, it is the most complete sample of human populations that have limited access to modern contraception and health care and whose economies are primarily subsistence-based. We focus here on subsistence-based societies because most resources are invested as somatic capital in human bodies (i.e. body size and fertility) as opposed to stored, inherited wealth. Fertility rate was estimated as the inverse of closed interbirth intervals for the Ache (Hill & Hurtado 1996), Aeta (Migliano et al. 2007), Agta (Early & Headland 1998), Aborigines in Arnhem land (Billington 1948; Hamilton 1981), Baka (Yamauchi et al. 2000), Gambian villagers (Billewicz & McGregor 1981; Sear et al. 2003), Guaja (G. Djurovic 2005, unpublished data), Hadza (Blurton Jones et al. 1992), Hiwi (Hurtado & Hill 1987), Ju/'hoansi (Howell 1979), Maku-Nadeb (R. S. Walker 2003, unpublished data), Toba (Bove et al. 2002), Tsimane (M. Gurven 2003, unpublished data), Turkana (Little et al. 1983), West African ‘Pygmies’ (Cavallli-Sforza 1986) and Yanomamo (Neel & Weiss 1975). The Ache were allowed to enter the sample twice, as hunter-gatherers in earlier research and as horticulturalists on a reservation in more recent research. Female adult body sizes and average male and female sizes at ages 3, 5 and 10 were either available from the original sources above or from Walker et al. (2006). Comparative studies across societies may also suffer from problems of phylogenetic non-independence. We addressed this issue by adjusting for geographical location (Africa, South America, Australia and Southeast Asia), but the effect was very weak and not significant in any of the multiple regressions.
3. Results
(a) Fertility allometry
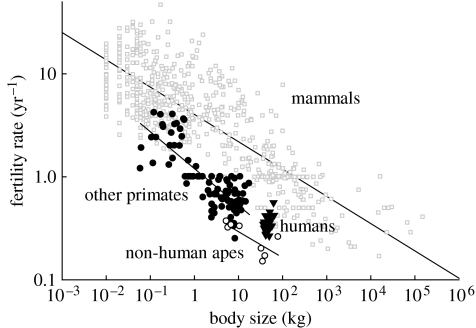
Fertility rates decline with body size to the −¼ power across mammals (−0.26±0.01, slope±95% CI; figure 1; Charnov 1993). The fertility rates of non-ape primates demonstrate an allometry steeper than −¼ (−0.36±0.06), driven by shifts towards slower reproduction in larger species. Using independent contrasts across primates yields a slope of −0.32±0.11 (n=95). Non-human apes (n=8) strongly shift towards a slower life history (i.e. lower intercept) yet scale as −¼ (±0.22; figure 1). In addition, there is approximately a −¼ power scaling for fertility rates across large haplorhines using data from only wild studies (n=8; data from Barrickman et al. in press). Humans have reproductive rates that are faster than those of other apes but, contrary to the expectation of negative allometry, human fertility rates increase with body size across natural-fertility populations (0.55±0.39; figure 1). Based on the ratio of scaling intercepts (ratio of geometric means assuming −¼ scaling), an average mammal reproduces at a rate 3.6 times faster than non-ape primates, 4.5-fold faster than humans and 7.7-fold faster than non-human apes. Humans reproduce on average at rates of approximately 1.7-fold faster than non-human apes and only 19% slower than non-ape primates.
Figure 1.
The allometry of birth rates across eutherian mammals on log–log axes. In Smith–Fretwell notation, this relationship is equivalent to regressing C on R·I−1 from equation (1.3). Mammals and apes scale as approximately the −¼ power of body size. The ‘other primates’ include strepsirhines and haplorhines as both clades show similar scaling. Natural-fertility human societies are the only sample here where fertility increases with body size.
(b) Trade-off between number and size of primate offspring
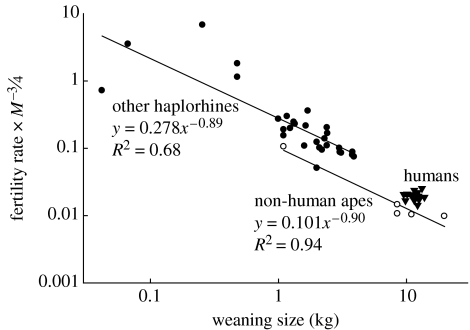
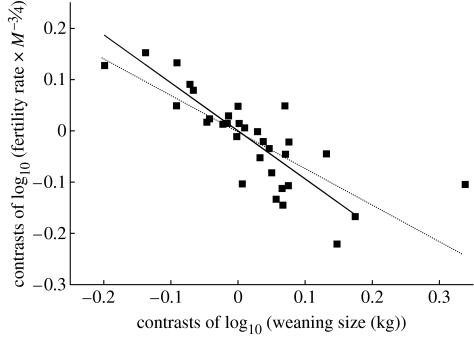
The negative relationship between log energy-adjusted fertility (C·R−1) and log weaning size (I) indicates a quantity–quality trade-off across primates (figure 2). The slope and 95% CIs are −0.90±0.43 for non-human apes and −0.89±0.23 for other haplorhines. Non-human apes demonstrate a shift towards slower fertility for a given weaning size at rates of only 36% of an average haplorhine, yet the expected trade-off holds despite the limited number of species. The trade-off between energy-corrected fertility rate and weaning size using independent contrasts yields a slope of −0.71±0.20 across primates, but after removing one contrast with considerable leverage the slope is −0.94±0.22 (figure 3). The wide CIs are unavoidable in this sample but nonetheless suggest that the trade-off is near the theoretically predicted value of −1.
Figure 2.
Energy-corrected fertility rate as a function of weaning mass for primates on log–log axes. In Smith–Fretwell notation, this relationship is C·R−1 regressed on I. The slopes are close to −1 (apes: −0.90±0.43; other haplorhines −0.89±0.23). While there is some uncertainty in the data for five smaller haplorhines, their removal has little effect on the scaling (−0.88±0.36). These primate lines are downshifted (i.e. lower intercept) in comparison to the trade-off in other mammals (Charnov & Ernest 2006). Size at age 3 is shown for humans.
Figure 3.
Independent contrasts (n=32) of the trade-off between energy-corrected fertility rate and weaning size (both on log-scale) for primates (humans excluded and no data for strepsirhines). In the Smith–Fretwell model, this is C·R−1 regressed on I. The slope of this relationship that is constrained through the origin is −0.71±0.20 across primates (dotted line), but one particular contrast (Miopithecus talapoin and Cercopithecus, right-most side of graph) has considerable leverage and upon removal the slope is −0.94±0.22 (solid line).
(c) Trade-off between number and size of human offspring
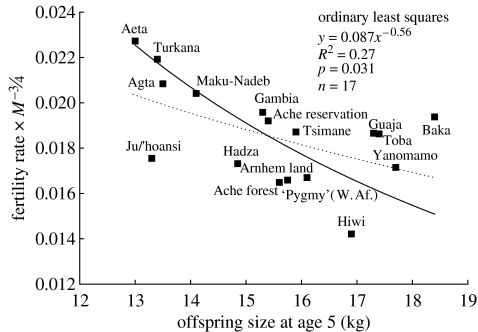
Humans have a higher mother's-energy-adjusted fertility rate given weaning size than do other apes. Since weaning size is not a perfect surrogate for energetic independence in humans, their true size at independence, which is indicative of actual maternal investment, should be farther to the r.h.s. in figure 2 and even more divergent from other apes. In other words, the high fertility rate for weaning size in humans is surprising because weaning size actually underestimates the energetic investment per offspring. The trade-off is not seen with size at age 3, either because the sample size is small or because the long, continued investment by human parents is better indexed by size at later ages. There are more data available for sizes at age 5 and 10 and a trade-off is apparent between mother's-energy-adjusted fertility rate and size at these ages. Using OLS regression, the trade-off is shallow (e.g. with size at age 5 the slope is −0.56±0.51; figure 4). However, if there is error in the estimates of offspring size, then OLS regression will underestimate the slope of the trade-off. The exact relationship between maternal investment and age/size of offspring is difficult to estimate for humans and may further increase uncertainty in empirical estimates of the trade-off. Using an RMA regression that adjusts for such error in size at age 5 yields a trade-off with an exponent of −1.15±0.86. Using size at age 10 as a measure of offspring investment, an RMA regression gives a slope of −0.87±0.72.
Figure 4.
Energy-corrected fertility rate as a function of offspring size at age 5 across natural-fertility human societies. Axes are not logged; the fits are power equations. Following the Smith–Fretwell model, this graph is C·R−1 regressed on I. Dashed line is the ordinary least-squares fit with an exponent of −0.56±0.51. However, given that error is probably present in our estimates of offspring size, RMA regression may be more appropriate (solid line, exponent=−1.15±0.86). Similar results are found for size at age 10.
An alternative method to uncover the trade-off is with a nonlinear regression of fertility rate (C) as a power function of size at age 5 or 10 (I) and adult body mass (M), which requires a re-arrangement of the variables into the form where c is a constant and a and b are exponents. The results from this regression are similar to those from the graphical method, yielding the equation . Comparable results are obtained using size at age 10 and switching the dependent and independent variables (table 1), which is justified because a trade-off implies bi-directional causality (i.e. higher fertility leads to decreased investment and higher investment leads to decreased fertility). These consistent results make us confident that the trade-off is not simply the result of a positive relationship between mother and offspring size. As expected, there is no evidence for a trade-off if we do not adjust fertility rate by female body mass. This is due to the positive correlation between female size and current reproduction mentioned above. Thus our methods, which adjust for energy budget via maternal mass, at least partially solve the problem of phenotypic correlation that would otherwise hide the underlying trade-off between size and number of offspring.
Table 1.
Nonlinear multiple regressions of the quantity–quality trade-off across natural-fertility human societies (n=17). (The first two models correspond to C (fertility rate) as a power function of M (maternal mass) and I (investment in offspring), and the second two are I as a power function of M and C (equation (1.1)).)
| dependent variable | independent variables | predicted exponent | exponent | bootstrapped 95% CI | R2 |
|---|---|---|---|---|---|
| fertility rate | (constant) | (0.07) | 0.55 | ||
| mother's mass | 0.75 | 0.89 | 0.49, 1.29 | ||
| offspring size age 5 | −1 | −0.65 | −1.24, −0.07 | ||
| fertility rate | (constant) | (0.03) | 0.55 | ||
| mother's mass | 0.75 | 1.23 | 0.62, 1.87 | ||
| offspring size age 10 | −1 | −0.68 | −1.40, −0.10 | ||
| offspring size age 5 | (constant) | (1.01) | 0.35 | ||
| mother's mass | 0.75 | 0.59 | 0.32, 0.86 | ||
| fertility rate | −1 | −0.41 | −0.72, −0.09 | ||
| offspring size age 10 | (constant) | (0.19) | 0.63 | ||
| mother's mass | 0.75 | 1.11 | 0.82, 1.40 | ||
| fertility rate | −1 | −0.52 | −0.76, −0.28 |
4. Discussion
Our results show the predicted energetic trade-off between the number and size of primate offspring using both conventional analysis and independent contrasts. Importantly, the quantity–quality trade-off in primates, and especially apes, is downshifted (i.e. lower intercept towards a slower life history) in comparison to that of other mammals. Primates, and especially larger-bodied species, reproduce at slower rates for a given weaning size than most other mammals, probably reflecting the larger brains of primate offspring and generally slower life histories. That some smaller primates often exhibit scaling more similar to the typical mammalian pattern, whereas apes have slow life histories, may be important for explaining why allometric slopes are sometimes steeper than predicted values. When all primates are included in the same dataset, we are lumping fast and slow life histories together.
In contrast to mixed results of within-population studies mentioned in §1, the quantity–quality trade-off consistently emerges across human populations provided an adjustment is made for mother's energy budget. Here we used the same budget adjustment for both within- and across-species analyses. Our OLS estimates of the trade-off exponent for humans are consistently less negative than the predicted −1. However, OLS might underestimate the true slope as it is reasonable to assume that there is also error in the estimates of offspring size and there may be considerable variation in the true caloric investment per offspring. Indeed, RMA regression gives exponents that are closer to the predicted value of −1 (albeit with wide CIs). Alternatively, some derived human traits like extra-maternal care and food sharing may compensate for mother's care and adjust the trade-off accordingly. Nonetheless, that a consistent trade-off is found is remarkable. In fact, the approximately inverse relationship between fertility rate and offspring investment appears to hold at increasingly finer scales of analyses (mammals to primates to apes to humans). The quantity–quality trade-off across human populations may follow the same pattern as seen in other animals and suggests that reproductive variation in natural-fertility societies can be understood from first principles of energetic allocation.
Importantly, the −¼ fertility allometry generally holds across eutherian mammals and also suggests the existence of a fundamental trade-off between number and size of offspring following directly from the Smith–Fretwell model (equation (1.3)). The increase in fertility rate with body size across human societies seems to contradict the −¼ power fertility allometry seen across species. The shape of the relationship between offspring quality and parental investment may be important to understanding positive fertility scaling across humans. The optimal investment in offspring depends on the rate at which returns to offspring quality (fitness) diminish with each additional unit of investment (Kaplan et al. 1995). Natural-fertility societies with large-bodied individuals and fast population growth are pushing the biological envelope in terms of reproductive output with interbirth intervals as short as 2 years, but these offspring are still able to grow to the size of normal adults. Most likely there are quickly diminishing returns on investments to offspring quality in these societies, perhaps even a fixed threshold investment, such that investment per offspring increases sublinearly for larger mothers (I scales less than M1) and surplus energy is funnelled to fertility. The common result of a positive effect of maternal body size on fertility rates within other species (Roff 2002) and within human populations may also reflect a similar phenomenon (e.g. New Guinea Highlanders, Brush et al. 1983; Ache, Hill & Hurtado 1996; Gambian villagers, Sear et al. 2003).
Many human societies in our sample are undergoing population expansion and this may affect our analyses. To adjust for this problem, we can focus on hunter-gatherers living at high population densities and near zero population growth. The Hiwi, for example, have large offspring and low fertility while other foragers, also near carrying capacity, have small offspring and relatively high fertility (e.g. Agta, Aeta; figure 4), supporting the trade-off prediction. Even these human populations that are nearly stationary, including the Hiwi, reproduce at mass-adjusted rates that are faster for their weaning size than those of other apes (except perhaps gorillas). Population expansion in the sample may affect our ability to accurately reveal the true fertility scaling within humans, and it is unclear exactly how differential population growth affects the trade-off and the extent to which controlling for mother's body size also adjusts for population growth (see electronic supplementary material for population growth rates).
Our primary hypothesis of an inverse relationship between fertility rate and offspring size across natural-fertility human societies cannot be rejected. Controlling for mother's energy availability, primate species and human societies with faster fertility rates have predictably smaller offspring, and vice versa. However, humans have a higher than expected fertility rate for weaning size than other apes when adjusting for mother's energy budget, suggesting that energy available for human reproduction is probably increased by technological advances, slow offspring growth (Gurven & Walker 2006) and/or extra-maternal provisioning (e.g. grandparents: Hawkes et al. 1998, husbands: Kaplan et al. 2000). Humans as large, long-lived, cooperative mammals that reproduce at a fast rate create a formidable life-history combination that probably figured prominently in the successful colonization of hunter-gatherers around the globe.
Acknowledgments
We thank Melanie Moses, Ric Charnov, Kim Hill, Eric Schniter, Chris Von Rueden and several anonymous reviewers for their helpful comments. We also thank Gradimir Djurovic for providing unpublished data for the Guaja. The National Science Foundation, LSB Leakey Foundation and Wenner-Gren Foundation provided dissertation research grants to R.S.W. that supported fieldwork with the Maku-Nadeb.
Supplementary Material
Population growth rates
References
- Alvarez H.P. Grandmother hypothesis and primate life histories. Am. J. Phys. Anthropol. 2000;113:435–450. doi: 10.1002/1096-8644(200011)113:3<435::AID-AJPA11>3.0.CO;2-O. doi:10.1002/1096-8644(200011)113:3<435::AID-AJPA11>3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- Barrickman, N. L., Bastian, M. L., Isler, K. & van Schaik, C. P. In press. Life history costs and benefits of increased brain size: A comparative test using data from long-term studies of primates in the wild. J. Hum. Evol [DOI] [PubMed]
- Billewicz W.Z, McGregor I.A. The demography of two West African (Gambian) villages, 1951–75. J. Biosoc. Sci. 1981;13:219–240. doi: 10.1017/s0021932000013390. [DOI] [PubMed] [Google Scholar]
- Billington B.P. Food consumption and dietary levels of groups of Aborigines living on naturally occurring foods. In: Mountford C.P, editor. Records of the American–Australian scientific expedition to Arnhem Land. Anthropology and nutrition. vol. 2. Melbourne University Press; Melbourne, Australia: 1948. pp. 90–143. [Google Scholar]
- Bininda-Emonds O.R.P, et al. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. doi:10.1038/nature05634 [DOI] [PubMed] [Google Scholar]
- Blurton Jones N, Sibly R.M. Testing adaptiveness of culturally determined behavior: do bushman women maximise their reproductive success by spacing births widely and foraging seldom? In: Blurton Jones N, Reynolds V, editors. Human behavior and adaptation: society of study of human biology symposium no. 18. Taylor and Francis; London, UK: 1978. pp. 135–158. [Google Scholar]
- Blurton Jones N, Smith L, O'Connell J, Hawkes K, Kamusora C.L. Demography of the Hadza, an increasing and high density population of savanna foragers. Am. J. Phys. Anthropol. 1992;89:159–181. doi: 10.1002/ajpa.1330890204. doi:10.1002/ajpa.1330890204 [DOI] [PubMed] [Google Scholar]
- Borgerhoff Mulder M. Optimizing offspring: the quantity–quality tradeoff in agropastoral Kipsigis. Evol. Hum. Behav. 2000;21:391–410. doi: 10.1016/s1090-5138(00)00054-4. doi:10.1016/S1090-5138(00)00054-4 [DOI] [PubMed] [Google Scholar]
- Bove R.B, Valeggia C.R, Ellison P.T. Girl helpers and time allocation of nursing women among the Toba of Argentina. Hum. Nat. 2002;13:457–472. doi: 10.1007/s12110-002-1003-8. doi:10.1007/s12110-002-1003-8 [DOI] [PubMed] [Google Scholar]
- Brush G.A, Boyce J, Harrison G.A. Associations between anthropometric variables and reproductive performance in a Papua New Guinea highland population. Ann. Hum. Biol. 1983;10:223–234. doi: 10.1080/03014468300006391. doi:10.1080/03014468300006391 [DOI] [PubMed] [Google Scholar]
- Cavallli-Sforza L.L. Academic Press; Orlando, FL: 1986. African pygmies. [Google Scholar]
- Charnov E.L. Oxford University Press; Oxford, UK: 1993. Life history invariants: some explorations of symmetry in evolutionary ecology. [Google Scholar]
- Charnov E.L. Evolution of mammal life histories. Evol. Ecol. Res. 2001;3:521–535. [Google Scholar]
- Charnov E.L, Downhower J.F. A trade-off-invariant life-history rule for optimal offspring size. Nature. 1995;376:418–419. doi: 10.1038/376418a0. doi:10.1038/376418a0 [DOI] [PubMed] [Google Scholar]
- Charnov E.L, Ernest S.K.M. The offspring-size/clutch-size trade-off in mammals. Am. Nat. 2006;167:578–582. doi: 10.1086/501141. doi:10.1086/501141 [DOI] [PubMed] [Google Scholar]
- Early J.D, Headland T.N. University Press of Florida; Gainsville, FL: 1998. Population dynamics of a Philippine rain forest people. [Google Scholar]
- Ernest S.K.M. Life history characteristics of non-volant placental mammals. Ecology. 2003;84:3402. doi:10.1890/02-9002 [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. doi:10.1086/284325 [DOI] [PubMed] [Google Scholar]
- Garland T, Dickerman A.W, Janis C.M, Jones J.A. Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 1993;42:265–292. doi:10.2307/2992464 [Google Scholar]
- Gurven M, Walker R.S. Energetic demand of multiple dependents and the evolution of slow human growth. Proc. R. Soc. B. 2006;273:835–841. doi: 10.1098/rspb.2005.3380. doi:10.1098/rspb.2005.3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen E.H, Barrett H.C, Price M.E. Do human parents face a quantity–quality tradeoff?: evidence from a Shuar community. Am. J. Phys. Anthropol. 2006;130:405–418. doi: 10.1002/ajpa.20272. doi:10.1002/ajpa.20272 [DOI] [PubMed] [Google Scholar]
- Hamilton A. Australian Institute of Aboriginal Studies; Canberra, Australia: 1981. Nature and nurture: aboriginal child-rearing in North Central Arnhem Land. [Google Scholar]
- Hawkes K, O'Connell J.F, Blurton Jones N.G, Alvarez H, Charnov E.L. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. doi:10.1073/pnas.95.3.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Hurtado A.M. Aldine de Gruyter; New York, NY: 1996. Ache life history: the ecology and demography of a foraging people. [Google Scholar]
- Hill K, Kaplan H. Life history traits in humans: theory and empirical studies. Annu. Rev. Anthropol. 1999;28:397–430. doi: 10.1146/annurev.anthro.28.1.397. doi:10.1146/annurev.anthro.28.1.397 [DOI] [PubMed] [Google Scholar]
- Howell N. Academic Press; New York, NY: 1979. Demography of the Dobe !Kung. [Google Scholar]
- Hurtado A.M, Hill K. Early dry season subsistence ecology of Cuiva (Hiwi) foragers of Venezuela. Hum. Ecol. 1987;15:163–189. doi:10.1007/BF00888379 [Google Scholar]
- Kaplan H, Lancaster J.B. The evolutionary economics and psychology of the demographic transition to low fertility. In: Cronk L, Chagnon N.A, Irons W, editors. Human behavior and adaptation: an anthropological perspective. Aldine de Gruyter; New York, NY: 2000. pp. 283–322. [Google Scholar]
- Kaplan H, Lancaster J.B, Bock J, Johnson S. Does observed fertility maximize fitness among New Mexico men?: a test of an optimality model and a new theory of parental investment in the embodied capital of offspring. Hum. Nat. 1995;6:325–360. doi: 10.1007/BF02734205. doi:10.1007/BF02734205 [DOI] [PubMed] [Google Scholar]
- Kaplan H, Hill K, Lancaster J, Hurtado A.M. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 2000;9:156–184. doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7 [Google Scholar]
- Lack D. The significance of clutch size 1. Intraspecific variation. Ibis. 1947;89:302–352. [Google Scholar]
- Lee P.C. Comparative ecology of postnatal growth and weaning among haplorhine primates. In: Lee P.C, editor. Comparative primate socioecology. Cambridge University Press; Cambridge, UK: 1999. pp. 111–139. [Google Scholar]
- Lindenfors P. Sexually antagonistic selection on primate size. J. Evol. Biol. 2002;15:595–607. doi:10.1046/j.1420-9101.2002.00422.x [Google Scholar]
- Little M.A, Galvin K, Mugambi M. Cross-sectional growth of nomadic Turkana pastoralists. Hum. Biol. 1983;55:811–830. [PubMed] [Google Scholar]
- Mace R. Evolutionary ecology of human life history. Anim. Behav. 2000;59:1–10. doi: 10.1006/anbe.1999.1287. doi:10.1006/anbe.1999.1287 [DOI] [PubMed] [Google Scholar]
- Martin R.D. Scaling effects and adaptive strategies in mammalian lactation. Symp. Zool. Soc. Lond. 1984;51:87–117. [Google Scholar]
- Migliano A.B, Vinicius L, Lahr M.M. Life history trade-offs explain the evolution of human pygmies. Proc. Natl Acad. Sci. USA. 2007;104:20 216–20 219. doi: 10.1073/pnas.0708024105. doi:10.1073/pnas.0708024105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J.V, Weiss K.M. The genetic structure of a tribal population, the Yanomama Indians. XII. Biodemographic studies. Am. J. Phys. Anthropol. 1975;42:25–51. doi: 10.1002/ajpa.1330420105. doi:10.1002/ajpa.1330420105 [DOI] [PubMed] [Google Scholar]
- Nunn C, Barton R. Comparative methods for studying primate adaptation and allometry. Evol. Anthropol. 2001;10:81–98. doi:10.1002/evan.1019 [Google Scholar]
- Oftedahl O.T. Milk composition, milk yield and energy output at peak lactation: a comparative review. Symp. Zool. Soc. Lond. 1984;51:33–85. [Google Scholar]
- Pennington R, Harpending H. Fitness and fertility among Kalahari !Kung. Am. J. Phys. Anthropol. 1988;777:303–319. doi: 10.1002/ajpa.1330770304. doi:10.1002/ajpa.1330770304 [DOI] [PubMed] [Google Scholar]
- Reznick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. doi:10.2307/3544698 [Google Scholar]
- Roff D.A. Chapman and Hall; New York, NY: 1992. The evolution of life histories. [Google Scholar]
- Roff D.A. Sinauer Associates; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Sear R, Mace R, McGregor I.A. A life-history analysis of fertility rates in rural Gambia: evidence for trade-offs or phenotypic correlations? In: Rodgers J, Kohler H.-P, editors. The biodemography of human reproduction and fertility. Kluwer Academic Press; Boston, MA: 2003. pp. 135–160. [Google Scholar]
- Smith C.C, Fretwell S.D. The optimal balance between number and size of offspring. Am. Nat. 1974;108:499–506. doi:10.1086/282929 [Google Scholar]
- Smith R.J, Jungers L. Body mass in comparative primatology. J. Hum. Evol. 1997;32:523–559. doi: 10.1006/jhev.1996.0122. doi:10.1006/jhev.1996.0122 [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Strassmann B.I, Gillepsie B. Life-history theory, fertility and reproductive success in humans. Proc. R. Soc. B. 2002;269:553–562. doi: 10.1098/rspb.2001.1912. doi:10.1098/rspb.2001.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R, et al. Growth rates and life histories in twenty-two small-scale societies. Am. J. Hum. Biol. 2006;18:295–311. doi: 10.1002/ajhb.20510. doi:10.1002/ajhb.20510 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Sato H, Kawamura K. Nutritional status, activity pattern, and dietary intake among the Baka hunter-gatherers in the village camps in Cameroon. Afr. Study Monogr. 2000;21:67–82. [Google Scholar]
Notice of correction
The references in §2b and the reference list are now presented in the correct form.
15 January 2008
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Population growth rates