Abstract
Physiological costs of compensatory growth are poorly understood, yet may be the key components in explaining why growth rates are typically submaximal. Here we tested the hypothesized direct costs of compensatory growth in terms of oxidative stress. We assessed oxidative stress in a study where we generated compensatory growth in body mass by exposing larvae of the damselfly Lestes viridis to a transient starvation period followed by ad libitum food. Compensatory growth in the larval stage was associated with higher oxidative stress (as measured by induction of superoxide dismutase and catalase) in the adult stage. Our results challenge two traditional views of life-history theory. First, they indicate that age and mass at metamorphosis not necessarily completely translate larval stress into adult fitness and that the observed physiological cost may explain hidden carry-over effects. Second, they support the notion that costs of compensatory growth may be associated with free-radical-mediated trade-offs and not necessarily with resource-mediated trade-offs.
Keywords: antioxidant enzymes, carry-over costs, damselfly larvae, life-history trade-offs, oxidative stress, rapid growth
1. Introduction
Life-history traits are often plastic and understanding the limits to their plasticity is a challenge for evolutionary ecology (Roff 2002). Plasticity in growth rate receives much attention as it directly links age and size at maturity, the two key life-history traits (Nylin & Gotthard 1998). One particularly intriguing and widespread form of rapid growth is compensatory growth where animals accelerate growth to catch up after a period of low growth, e.g. caused by food shortage (Metcalfe & Monaghan 2001). Recently, Mangel & Munch (2005) hypothesized that an important cost of compensatory growth, and therefore also an evolutionary constraint on growth rate in general, is that growth leads to the accumulation of damage at the cellular level that is expressed at the level of the organism (see also Metcalfe & Monaghan 2003). One such suggested cost of compensatory growth is oxidative damage due to reactive oxygen species (ROS; Mangel & Munch 2005). ROS are the by-products of normal metabolic activities that damage key biomolecules like DNA, proteins and lipids (Finkel & Holbrook 2000).
Costs of compensatory growth in terms of oxidative damage can result from two non-exclusive mechanisms. First, compensatory growth may impair the antioxidant defence system making organisms more sensitive when challenged with oxidants. Second, compensatory growth may itself cause oxidative stress. When expressing rapid growth animals have high oxygen consumption (Fischer et al. 2004; Stoks et al. 2006). As higher oxygen consumption has been linked to greater ROS production (Loft et al. 1994), the latter is a probable physiological cost of compensatory growth. Indirect support for the first mechanism is given by Blount et al. (2003) reporting that after a period of low-quality nutrition zebra finch showed lower lipophilic antioxidant levels suggesting that they assimilated less lipophilic antioxidants from the diet. Unfortunately, compensatory growth was not quantified here. Direct proof of the second mechanism, rapid (compensatory) growth leading to more oxidative stress, is absent, although growth rate and free-radical processes are causally linked by cellular transduction systems (Rollo 2002). Crescenzo et al. (2006) showed compensatory growth in fat, but not in mass, after a period of semi-starvation in rats, and a higher superoxide dismutase (SOD) activity (reflecting oxidative stress) in previously semi-starved rats after they were fed ad libitum again. However, in this study semi-starvation itself also increased SOD, making it hard to disentangle the costs of semi-starvation and compensatory growth. Alonso-Alvarez et al. (2007) demonstrated a higher susceptibility to oxidative damage after compensatory growth in zebra finches when challenged with an oxidant, but could not disentangle both mechanisms as they assessed combined effects of the exogenous (oxidant) and endogenous (due to compensatory growth) sources of ROS. Therefore, we lack experimental studies explicitly demonstrating the second mechanism: an increase in oxidative stress as a direct (without external oxidative challenge) effect of compensatory growth.
We tested for oxidative stress induced by compensatory growth in larvae of the damselfly Lestes viridis after exposure to transient food shortage followed by ad libitum food. To disentangle the costs of food shortage and compensatory growth, we measured oxidative stress at several key moments: before and after the period of starvation, just before emergence and after emergence. The measurement directly after starvation allows evaluating the effect of starvation without interference by compensatory growth. Any successive change in the oxidative stress levels after starvation can then be attributed to compensatory growth. To quantify oxidative stress, we measured the activity of the two key enzymes in insects involved in the defence against two important ROS: superoxide anions and hydrogen peroxide. SOD is the enzyme that dismutates superoxide anions to hydrogen peroxide. This ROS is normally dismutated to water through two enzymes, catalase (CAT) and glutathione peroxidase, of which the latter is less important in insects (Felton & Summers 1995; Korsloot et al. 2004). Therefore, we focused on SOD and CAT. Upon exposure to environmental stressors or when metabolism is increased, extra amounts of ROS may be generated, which can accumulate to toxic levels. Under oxidative stress, insects therefore show increased activities of SOD and CAT (e.g. Krishnan & Kodrik 2006; Mittapalli et al. 2007).
2. Material and methods
Freshly hatched larvae were placed in groups of 10 in plastic containers (diameter 8 cm, height 5 cm). After 14 days, larvae were placed individually in 100 ml plastic cups. Throughout the experiment, larvae were kept in a room at 23°C under natural photoperiod. Larvae were fed one portion of Artemia nauplii (mean 328 nauplii, s.e.m.=36, n=8) daily until they entered the final instar.
Larvae were randomly assigned to one out of the two starvation treatments when they entered the final instar. Starved larvae did not receive food for 8 days (=starvation period), while non-starved larvae received three chironomids daily. During the post-starvation period, all larvae received this daily portion of three chironomids. The post-starvation period ended when larvae stopped eating to prepare for emergence. This emergence period takes approximately 3 days (Stoks et al. 2006). From a previous experiment with the same set-up, we know that larvae show compensatory growth in body mass during the post-starvation period, which results in full mass compensation at the end of the post-starvation period (Stoks et al. 2006). We confirmed this growth pattern in the current experiment on a separate set of larvae not used for oxidative stress assessment, by measuring wet mass of 30 individuals of both the starvation treatments at the start (day 1) and the end (day 8) of the starvation period, and at the end of the post-starvation period and 1 day after emergence. Masses were taken to the nearest 0.01 mg when animals had empty guts (24 hours after the last feeding occasion). Daily growth rates were calculated per period (pre-starvation, starvation, post-starvation and emergence period) as (ln(massend)−ln(massini))/(tend−tini), where massend and massini are larval masses (mg) at the final (tend) and initial (tini) days of a given period, respectively. This measure of growth rate takes into account the exponential growth curves of Lestes damselfly larvae and is therefore independent of initial mass (Stoks & McPeek 2003).
To monitor oxidative stress, we randomly selected animals of each starvation treatment at the same moments at which we measured wet mass. Animals were flash frozen in liquid nitrogen and kept at −80°C prior to analysis. Both CAT and SOD were determined colorometrically. To avoid enzyme degradation, assays were run within three weeks. Each endpoint was assayed in duplicate during the same assay and the mean of the duplicate readings was taken as the measurement for that individual. The total number of animals assessed was 165 (figure 2). To quantify the activity of both antioxidant enzymes, each animal was homogenized in PBS (pH 7.4; 1/23 w/v) in a microcentrifuge tube while on ice. Samples were centrifuged at 1310g for 10 min at 4°C. SOD activity was quantified closely following the protocol described in the SOD Assay Kit-WST (Fluka, Buchs, Austria), which measures the formation of a formazan dye upon reduction of the tetrazolium salt WST-1 with superoxide anions. We added 200 μl of WST working solution and 20 μl of enzyme working solution to 20 μl of the supernatant. This mixture was incubated for 20 min at 37°C followed by reading the absorbance at 450 nm. SOD activity was expressed in units (U), where one unit of SOD is defined as the amount of sample causing 50% inhibition of the colorimetric reaction per mg protein. The activity of CAT was quantified following the protocol of Aebi (1984). The supernatant was further diluted 16 times with PBS (pH 7.4). To 20 μl of the diluted supernatant, we added 80 μl of PBS (pH 7.4) and 100 μl of 20 mM H2O2. We measured the removal of H2O2 as reduction in absorbance at 240 nm at 25°C within 2 min. One unit of CAT was defined as the activity of the enzyme that catalysed the reduction of 1 μmol of H2O2 per min per mg protein. Note that by expressing SOD and CAT in these units, we corrected for protein content. Intra-assay CVs for SOD and CAT were 4.2 and 3.6%, respectively.
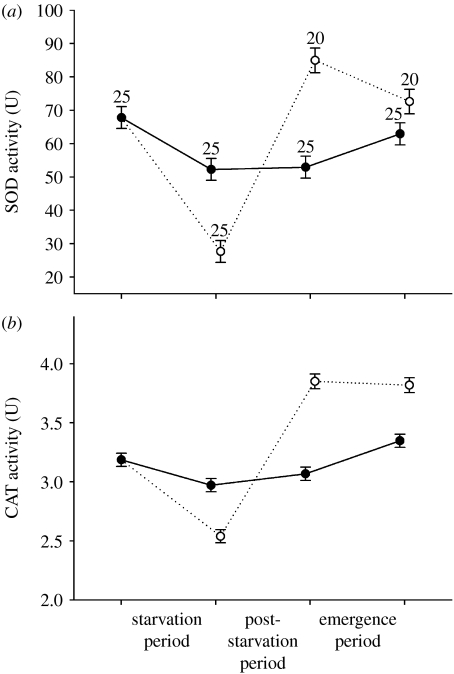
Figure 2.
Oxidative stress response variables (a) SOD and (b) CAT of the damselfly L. viridis as a function of starvation status through time. CAT was log transformed. The categories starved and non-starved refer to the treatment applied during the starvation period. Values are means±1 s.e.m. Numbers above the symbols in (a) denote sample sizes. Solid line with filled circles, non-starved; dotted lines with open circles, starved.
Because we had repeated observations per larva for daily growth rates, age and body mass, we analysed the effects of the starvation treatment with repeated-measures ANOVAs (rm-ANOVAs) with starvation as between-subject variable and period as within-subject variable. Because there were no repeated readings per larva for the antioxidant enzymes and no starvation groups at the start of the starvation period, we analysed the physiological data in two steps. First, we tested for an effect of starvation on the proportional change in the activity of both SOD and CAT during the starvation period, calculated as ((activity at the end of the starvation period)−(mean activity at the start of the starvation period))/(mean activity at the start of the starvation period), with a MANOVA. Second, we tested for the effects of starvation and period (i.e. end starvation period, end post-starvation period, emergence) with a MANOVA (SOD and CAT activity were dependent variables). To meet ANOVA assumptions, CAT activity was log transformed. Sex was also tested in the models but was removed as a non-significant term (all p>0.34) except when analysing body mass. Initially, we included mass and age as covariates when analysing oxidative stress but excluded them as they were never significant (all p>0.08) and did not affect the significance of the main effects and their interaction in the MANOVA. Therefore, all effects on antioxidant enzymes were not affected by body mass and age at a given period. To interpret the effects, MANOVAs were followed by univariate ANOVAs. When the starvation treatment or its interaction with period was significant, we tested for the differences between means using Duncan's post hoc tests. For non-significant treatment effects, we report the 95% confidence interval (CI) on the observed effect size (mean difference) as advocated by Steidl & Thomas (2001).
3. Results
(a) Life history
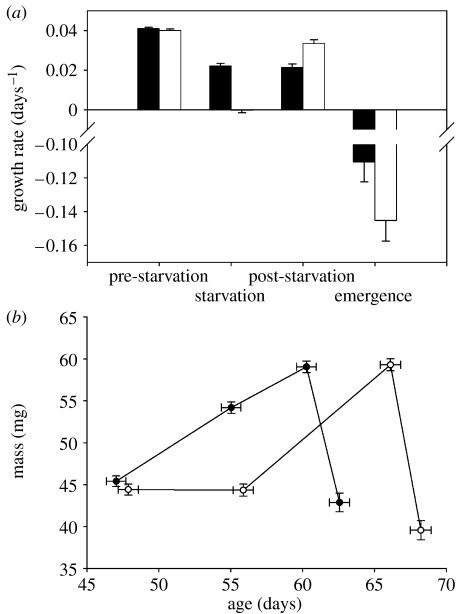
Starvation affected daily growth rate in a time-dependent way (rm-ANOVA, starvation×period, F3,174=5.56, p=0.0012; figure 1a). Daily growth rates did not differ between starvation treatments before starvation (ANOVA, F1,58=0.70, p=0.42, effect size CI (−0.0011,0.0028)). During the starvation period, starved larvae had much lower daily growth rates than non-starved larvae (ANOVA, F1,58=161.41, p<0.0001). Previously starved larvae grew faster than non-starved larvae during the post-starvation period (F1,58=24.99, p<0.0001) and tended to have more negative daily growth rates during the emergence period (F1,58=3.55, p=0.064, effect size CI (−0.002,0.065)).
Figure 1.
(a) Growth rate (black bar, non-starved; white bar, starved) and (b) age and mass of the damselfly L. viridis as a function of starvation status through time (filled circles, non-starved; open circles, starved). The categories starved and non-starved refer to the treatment applied during the starvation period. Values are means±1 s.e.m. Successive points in (b) give bivariate means for age and mass at the start and the end of the starvation period, and at the end of the post-starvation period and at emergence, respectively. Slopes of the age versus mass line during the post-starvation period: non-starved larvae, 0.93 mg d−1 and starved larvae: 1.46 mg d−1.
At the start of the starvation period, mass did not differ between starvation treatments, but at the end of the starvation period mass was much lower in starved larvae (figure 1b). This mass difference had disappeared at the end of the post-starvation period, but re-appeared, although less pronounced, at emergence (rm-ANOVA, starvation×period, F3,171=29.63, p<0.0001; ANOVA mass end post-starvation period, F1,57=0.07, p=0.80, effect size CI (−3.14,1.33); mass at emergence, F1,57=4.54, p=0.037).
At the start and the end of the starvation period, larvae did not differ in age, but previously starved larvae had an extended post-starvation period (rm-ANOVA, starvation×period, F3,174=81.65, p<0.0001; ANOVA, end post-starvation period, F1,58=36.86, p<0.0001; figure 1b). As a result, age at emergence was higher in previously starved larvae (F1,58=32.14, p<0.0001).
(b) Oxidative stress
The proportional change in the activity of both antioxidant enzymes during the starvation period differed between starvation treatments (MANOVA: F2,47=20.69, p<0.0001; SOD: F1,48=35.66, p<0.0001; CAT: F1,48=19.03, p<0.0001; figure 2). The activities of SOD and CAT decreased considerably in starved larvae, and less so (SOD) or not (CAT) in non-starved larvae. The starvation treatment affected both variables in a similar time-dependent way, also when correcting for age and mass (MANOVA, starvation×period, F4,266=28.79, p<0.0001; SOD: F2,134=42.26, p<0.0001; CAT: F2,134=56.47, p<0.0001; MANCOVA, mass, F2,131=2.61, p=0.077; age, F2,131=1.80, p=0.17; starvation×period, F4,262=23.02, p<0.0001; SOD: F2,132=33.78, p<0.0001; CAT: F2,132=41.83, p<0.0001; figure 2). At the end of the starvation period, SOD and CAT were much lower in starved larvae compared with non-starved larvae (both p<0.0001; figure 2). During the post-starvation period, the levels remained constant in non-starved larvae (both p>0.25, effect size CI SOD: (−6.60,5.33), CAT: (−0.26,0.07)) and drastically increased in previously starved larvae (both p<0.0001), resulting in higher activities in previously starved larvae at the end of the post-starvation period (both p<0.0001). During the emergence period, activities slightly increased in non-starved larvae (both p<0.025) and decreased (SOD, p=0.0054) or remained constant (CAT, p=0.71, effect size CI (−0.15,0.22)) in previously starved larvae. At emergence, SOD and CAT levels were higher in previously starved larvae (both p<0.030).
4. Discussion
After reduced growth rates during the period of starvation, animals showed the expected increased growth rates, resulting in a full mass compensation during the larval stage. Compared with non-starved larvae, starved larvae had lower levels of SOD and CAT at the end of the starvation period. Similar effects of starvation have been reported in other invertebrates for SOD (e.g. Abele et al. 1998) and CAT (e.g. Sandrini et al. 2006). Thereafter, the pattern in SOD and CAT levels switched with higher levels in previously starved larvae at the end of the post-starvation period. This is consistent with increased oxidative stress as a result of compensatory growth and not as a result of starvation. Note that these patterns were not affected by age and mass (see §2), and therefore cannot be explained by differences between treatment groups in age or mass at a given period. This temporal pattern in SOD and CAT can be explained by the lowered metabolism during starvation, followed by an increased metabolism in animals showing compensatory growth as suggested previously by associated changes in respiration rate in L. viridis (Stoks et al. 2006). A higher respiration may generate a higher production of ROS (Loft et al. 1994). More specifically, our data suggest that increased respiration as a result of compensatory growth increased the production of the superoxide anion, which was dismutated by the induced SOD levels to hydrogen peroxide. The subsequently raised hydrogen peroxide levels may have induced CAT levels and were converted to water. Two types of antioxidant enzyme induction by oxidative stress have been described: increased activity due to activation of a pre-existing pool of inactive enzyme and de novo synthesis of the enzyme (Rojas & Leopold 1996 and references therein). Alternatively, the higher SOD and CAT levels in previously starved larvae may reflect their higher antioxidant power. The decrease during starvation of these antioxidant enzymes, however, strongly suggests that these are costly. Therefore, it seems unlikely that previously starved larvae, which are relatively resource limited, show costly higher SOD and CAT levels unless they are forced to defend themselves against increased ROS production.
Recently, Mangel & Munch (2005) argued that an important cost of compensatory growth, and therefore also an evolutionary constraint on growth rate in general, is that growth leads to the accumulation of damage at the cellular level that is expressed at the level of the organism (see also Metcalfe & Monaghan 2003). An indication for a first mechanism linking compensatory growth to oxidative damage, namely through impaired antioxidant defences, was given by Alonso-Alvarez et al. (2007). Our results complement those findings by providing the first evidence of the second mechanism, a direct (i.e. without external oxidant challenge) cost of compensatory growth in terms of oxidative stress. Interestingly, this cost seemed to persist after metamorphosis as SOD and CAT levels at emergence remained higher in animals that showed compensatory growth. Probably, these animals maintained a higher metabolism (see Fischer et al. 2004; Stoks et al. 2006). For example, zebra finches reared in a large brood, hence at low food, showed higher metabolic rates when 1-year-old compared with those reared in a small brood (Verhulst et al. 2006). Higher ROS levels negatively affect several components of adult fitness in insects including fecundity and adult longevity (Ahmad 1992). Note that, although our results provide proof that rapid compensatory growth induces ROS production, they do not allow us to identify whether the elevated levels of SOD and CAT were effective in offsetting higher oxidative damage.
Taken together, the observed cost of rapid compensatory growth in terms of increased oxidative stress (as indicated by induced levels of SOD and CAT) may contribute to explaining why animals typically do not grow at their maximal rates (Arendt 1997; Metcalfe & Monaghan 2001). Furthermore, our results challenge two traditional views of life-history theory. First, life-history models typically assume that only age and mass at metamorphosis are optimized and translate larval stressors to adult fitness (e.g. Rowe & Ludwig 1991), and therefore are good proxies for adult fitness. Yet, the here-identified physiological cost of rapid growth after starvation remained in the adult stage, and this is independent of age and mass at emergence. Therefore, oxidative stress may contribute to hidden carry-over effects bridging metamorphosis (De Block & Stoks 2005). Second, a greater ROS production as suggested by our results may explain many of the observed costs of compensatory growth, which are typically thought of in terms of a trade-off in energy allocation (see also Alonso-Alvarez et al. 2004). Note that, in contrast, under the first mechanism, where the antioxidant defence system is impaired due to compensatory growth, energy allocation away from investment in antioxidant defence may still play a role. Our results are linked for example with the free-radical theory of ageing, stating that a higher metabolism will result in a greater production of ROS and hence a shorter lifespan (Finkel & Holbrook 2000). Interestingly, a reduced lifespan after compensatory growth has been observed (Metcalfe & Monaghan 2001). Also, other costs of compensatory growth like reduced swimming performance (Metcalfe & Monaghan 2001) and the recently reported impaired learning capabilities (Fisher et al. 2006) may be due to oxidative damage to cells and tissues. For example, oxidative stress can cause neurodegeneration and disruption in signalling processes in brain tissue, thereby inducing cognitive decline (Droge & Schipper 2007). Future studies on compensatory growth should therefore benefit from unravelling direct links between induced oxidative stress and these other more typically studied costs.
Acknowledgments
We thank the reviewers for their constructive comments. M.D.B. is a postdoctoral fellow of the Research Foundation-Flanders (FWO-Vlaanderen). Financial support was provided by the research grants of FWO and the KULeuven Research Fund.
References
- Abele D, Burlando B, Viarengo A, Pörtner H.-O. Exposure to elevated temperatures and hydrogen peroxide elicits oxidative stress and antioxidant response in the Antarctic intertidal limpet Nucella concinna. Comp. Biochem. Physiol. B. 1998;120:425–435. doi:10.1016/S0305-0491(98)10028-7 [Google Scholar]
- Aebi H. Catalase in vitro. Meth. Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahmad S. Biochemical defense of prooxidant plant allelochemicals by herbivorous insects. Biochem. Syst. Ecol. 1992;20:269–296. doi:10.1016/0305-1978(92)90040-K [Google Scholar]
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol. Lett. 2004;7:363–368. doi:10.1111/j.1461-0248.2004.00594.x [Google Scholar]
- Alonso-Alvarez C, Bertrand S, Faivre B, Sorci G. Increased susceptibility to oxidative damage as a cost of accelerated somatic growth in zebra finches. Funct. Ecol. 2007;21:873–879. doi:10.1111/j.1365-2435.2007.01300.x [Google Scholar]
- Arendt J.D. Adaptive intrinsic growth rates: an integration across taxa. Q. Rev. Biol. 1997;72:149–177. doi:10.1086/419764 [Google Scholar]
- Blount J.D, Metcalfe N.B, Arnold K.E, Surai P.F, Devevey G.I, Monaghan P. Neonatal nutrition, adult antioxidant defences and sexual attractiveness in the zebra finch. Proc. R. Soc. B. 2003;270:1691–1696. doi: 10.1098/rspb.2003.2411. doi:10.1098/rspb.2003.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescenzo R, Lionetti L, Mollica M.P, Ferraro M, D'Andrea E, Mainieri D, Dulloo A.G, Liverini G, Iossa S. Altered skeletal muscle subsarcolemmal mitochondrial compartment during catch-up fat after caloric restriction. Diabetes. 2006;55:2286–2293. doi: 10.2337/db06-0312. doi:10.2337/db06-0312 [DOI] [PubMed] [Google Scholar]
- De Block M, Stoks R. Fitness effects from egg to reproduction: bridging the life history transition. Ecology. 2005;86:185–197. doi:10.1890/04-0116 [Google Scholar]
- Droge W, Schipper H.M. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. doi:10.1111/j.1474-9726.2007.00294.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton G.W, Summers C.B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 1995;29:187–197. doi: 10.1002/arch.940290208. doi:10.1002/arch.940290208 [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. doi:10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- Fischer K, Zeilstra I, Hetz S.K, Fiedler K. Physiological costs of growing fast: does accelerated growth reduce pay-off in adult fitness? Evol. Ecol. 2004;18:343–353. doi:10.1007/s10682-004-2004-3 [Google Scholar]
- Fisher M.O, Nager R.G, Monaghan P. Compensatory growth impairs adult cognitive performance. PLoS Biol. 2006;4:e251. doi: 10.1371/journal.pbio.0040251. doi:10.1371/journal.pbio.0040251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsloot A, van Gestel C.A.M, van Straalen N.M. CRC Press; London, UK: 2004. Environmental stress and cellular response in arthropods. [Google Scholar]
- Krishnan N, Kodrik D. Antioxidant enzymes in Spodoptera littoralis (Boisduval): are they enhanced to protect gut tissues during oxidative stress? J. Insect Physiol. 2006;52:11–20. doi: 10.1016/j.jinsphys.2005.08.009. doi:10.1016/j.jinsphys.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Loft S, Astrup A, Buemann B, Poulsen H.E. Oxidative DNA-damage correlates with oxygen-consumption in humans. FASEB J. 1994;8:534–537. doi: 10.1096/fasebj.8.8.8181672. [DOI] [PubMed] [Google Scholar]
- Mangel M, Munch S.B. A life-history perspective on short- and long-term consequences of compensatory growth. Am. Nat. 2005;166:E155–E176. doi: 10.1086/444439. doi:10.1086/444439 [DOI] [PubMed] [Google Scholar]
- Metcalfe N.B, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. doi:10.1016/S0169-5347(01)02124-3 [DOI] [PubMed] [Google Scholar]
- Metcalfe N.B, Monaghan P. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 2003;38:935–940. doi: 10.1016/s0531-5565(03)00159-1. doi:10.1016/S0531-5565(03)00159-1 [DOI] [PubMed] [Google Scholar]
- Mittapalli O, Neal J.J, Shukle R.H. Antioxidant defense response in a galling insect. Proc. Natl Acad. Sci. USA. 2007;104:1889–1894. doi: 10.1073/pnas.0604722104. doi:10.1073/pnas.0604722104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylin S, Gotthard K. Plasticity in life-history traits. Annu. Rev. Entomol. 1998;43:63–83. doi: 10.1146/annurev.ento.43.1.63. doi:10.1146/annurev.ento.43.1.63 [DOI] [PubMed] [Google Scholar]
- Roff D.A. Sinauer Associates; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Rojas R.R, Leopold R.A. Chilling injury in the housefly: evidence for the role of oxidative stress between pupariation and emergence. Cryobiology. 1996;33:447–458. doi:10.1006/cryo.1996.0045 [Google Scholar]
- Rollo C.D. Growth negatively impacts the life span of mammals. Evol. Dev. 2002;4:55–61. doi: 10.1046/j.1525-142x.2002.01053.x. doi:10.1046/j.1525-142x.2002.01053.x [DOI] [PubMed] [Google Scholar]
- Rowe L, Ludwig D. Size and timing of metamorphosis in complex life cycles: time constraints and variation. Ecology. 1991;72:413–427. doi:10.2307/2937184 [Google Scholar]
- Sandrini J.Z, et al. Short-term responses to cadmium exposure in the estuarine polychaete Laeonereis acuta (Polychaeta, Nereididae): subcellular distribution and oxidative stress generation. Environ. Toxicol. Chem. 2006;25:1337–1344. doi: 10.1897/05-275r.1. doi:10.1897/05-275R.1 [DOI] [PubMed] [Google Scholar]
- Steidl R.J, Thomas L. Power analysis and experimental design. In: Scheiner S.M, Gurevitch J, editors. Design and analysis of ecological experiments. Chapman & Hall; London, UK: 2001. pp. 14–36. [Google Scholar]
- Stoks R, McPeek M.A. Antipredator behavior and physiology determine Lestes species turnover along the pond-permanence gradient. Ecology. 2003;84:3327–3338. doi:10.1890/02-0696 [Google Scholar]
- Stoks R, De Block M, McPeek M.A. Physiological costs of compensatory growth in a damselfly. Ecology. 2006;87:1566–1574. doi: 10.1890/0012-9658(2006)87[1566:pcocgi]2.0.co;2. doi:10.1890/0012-9658(2006)87[1566:PCOCGI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Verhulst S, Holveck M.J, Riebel K. Long-term effects of manipulated natal brood size on metabolic rate in zebra finches. Biol. Lett. 2006;2:478–480. doi: 10.1098/rsbl.2006.0496. doi:10.1098/rsbl.2006.0496 [DOI] [PMC free article] [PubMed] [Google Scholar]