Abstract
Emulation has been distinguished from imitation as a form of observational learning because it focuses not on the model's actions but on the action's environmental results. Whether a species emulates, imitates or displays only simpler observational learning is expected to have profound implications for its capacity for cultural transmission. Chimpanzees' observational learning has been suggested to be primarily emulative, but this is an inference largely based upon low fidelity copying in experiments when comparing chimpanzees with humans rather than direct testing. Here we test directly for emulation learning by chimpanzees and children using a ‘ghost’ condition in which a sliding door obscuring a reward was moved to left or right with no agent visible, a context associated with the only published evidence for emulation learning in a non-human species (pigeons). Both children and chimpanzees matched the observed direction of ghost door movement on their first test trial. This is the first evidence for emulation in a non-human primate in the restricted context of a ghost condition. However, only the children continued to match in later trials. Individuals of both species continued to match with 99% or better fidelity when viewing a conspecific model operates the door. We conclude that chimpanzees can and will display emulation learning when the task is as simple as the present one, which contrasts with a failure to do so in a more complex manipulative task tested earlier. However, even with a simple task, emulation alone creates only fleeting fidelity compared with the opportunity to copy a conspecific, when considerable conformity is displayed.
Keywords: social learning, emulation, ghost condition, chimpanzees, children
1. Introduction
Much evidence has accumulated for local behavioural traditions in fishes, birds and mammals, generating a growing literature which overlaps with that concerning the evolution of culture in humans (Fragaszy & Perry 2003; Mesoudi et al. 2006; Whiten & van Schaik 2007). Investigation of underlying social learning mechanisms has over a century's history (Whiten & Ham 1992) but has likewise expanded greatly in recent years (Galef & Heyes 2004; Whiten et al. 2004; Hurley & Chater 2005; Zentall 2006).
An influential distinction in recent analyses of social learning has been between imitation (copying another's actions) and emulation (Wood 1989) a term promoted by Tomasello (1990) to label learning about the environmental results of another's actions. Tomasello and colleagues have concluded that their experiments show chimpanzees are emulators rather than true imitators, contrasting with children, who imitate with high fidelity (Nagell et al. 1993; Tomasello 1999; Call et al. 2005). This has implications for the evolution of culture, by its nature; emulation seems less likely than imitation to provide high fidelity behavioural transmission (Tomasello 1999).
However, emulation has been inferred in chimpanzee studies largely on the basis that the observer (i) learns more than can be explained by mere stimulus enhancement, in which an observer's attention is simply drawn to relevant stimuli, yet (ii) fails to show evidence for detailed imitative matching of the model's action. In other words, ‘emulation’ was inferred simply when imitative fidelity was low, which led Byrne (2002) to entitle his response to Want & Harris (2002) Emulation in Apes: Verdict ‘Not Proven’.
Call et al. (2005) offered more positive evidence for emulation in chimpanzees insofar as subjects showed evidence for learning after witnessing only the end result of an action. However, others have argued that a more direct test for emulation would be to allow a potential learner to watch the events normally caused by the model but with no model visibly causing them. A small number of such experiments have been conducted in recent years, generally referred to as ‘ghost’ conditions because the manipulanda move as if guided by an invisible ghostly agent (Fawcett et al. 2002). This approach is consistent with an example provided by Tomasello (1999) to explain his conception of emulation: ‘if a mother [chimpanzee] rolls a log and eats the insects underneath, her child will very likely follow suit…the youngster would have learned the same thing if the wind, rather than the mother, had caused the log to roll over and expose the ants.’ (p. 29). To this extent, we might say that emulation takes the ‘social’ out of social learning—and that is exactly what a ghost experiment does. However, Tomasello and others continue to class emulation as a form of social learning; owing to course in the normal state of affairs, the observer witnesses the crucial outcomes only through the actions of an agent, usually a conspecific. In a ghost experiment, this latter element is dissected out.
Two such studies with human children have provided evidence for emulation learning (Thompson & Russell 2004; Tennie et al. 2006). Ghost experiments with non-human subjects have produced more mixed results. In two, the task proved inappropriate for demonstrating social learning in the first place (rats, Heyes et al. 1994, Mitchell et al. 1999; chimpanzees, Tennie et al. 2006). Of the remaining five non-human studies, four have generated negative results in the emulation condition (Japanese quail, Akins et al. 2002; starlings, Fawcett et al. 2002; rhesus macaques, Subiaul et al. 2004; chimpanzees, Hopper et al. 2007).
The only non-human study to provide the positive and significant evidence for emulation in a ghost condition is Klein & Zentall (2003). Pigeons were tested in one of four experimental conditions designed to discriminate imitation, emulation (or ‘affordance’) learning and effect of mere conspecific presence. In this study, a screen door could slide either left or right to reveal a food reward. In the ‘push’ condition, a conspecific model moved the door either left or right, whereas in a ‘no-demo’ ghost condition, the door was moved discretely using fishing line, with no conspecific present. ‘no push’ was the same but with a passive conspecific present. A ‘vision blocked’ condition replicated push but with a screen preventing the subject seeing the other pigeon to control for olfactory and gustatory cues. Pigeons tended to match the direction in which the model slid the door in both the push and no-demo conditions, the latter with 74% matching. The authors thus concluded that emulation (affordance) learning had occurred.
The literature is thus somewhat paradoxical. The earlier conclusion that chimpanzees are primarily emulators rather than imitators is contested by the negative results of the ghost experiment of Hopper et al. (2007). Conversely, the positive evidence for emulation has been offered for pigeons and human children. Accordingly, for clarity we sought to replicate Klein & Zentall (2003) study with both chimpanzees and children. The results of the child studies cited previously predict that children would match in the ghost condition and thus validate the paradigm for investigating emulation in chimpanzees, whereas the negative chimpanzee results of Hopper et al. would predict that chimpanzees will not learn in a ghost condition. However, the tool-use task used by Hopper et al. was sufficiently challenging that none of 18 individuals performed it when tested without benefit of observing a model (Whiten et al. 2005; Hopper et al. 2007). Klein & Zentall's apparently simpler task might be predicted to be learnable via emulation as with pigeons, and its simplicity for chimpanzees could be checked with a no-model control. Such a result might also be compatible with the findings of Call et al. (2005) given the simplicity of their task, which involved only pulling or breaking apart a two-part tube.
2. Material and methods
(a) Participants and testing environment
(i) Chimpanzees
Chimpanzee participants were 25 females and 15 males aged 11–44 years (mean 30.5 years). Of these, 8 acted as ‘demonstrators’ and 32 as ‘observers’ (see §2c). They were housed at The University of Texas M. D. Anderson Cancer Center, Texas (see appendix A in the electronic supplementary material for demographic information). Chimpanzees were tested in one-half of their inside cage, measuring 2.4×2.4×1.8 m3. At other times, they lived in social groups with access to outside corrals (21.3 m diameter).
(ii) Children
Child participants were 18 females and 22 males aged 3 years 2 months to 4 years 10 months (mean 4 years 2 months). Of these, 8 acted as demonstrators and 32 as observers (see §2c, and appendix B in the electronic supplementary material for demographic information). Children were tested with parental consent in rooms familiar to them at Scottish nursery schools.
(b) Apparatus
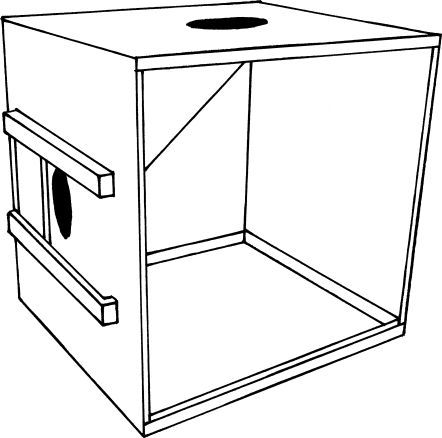
The ‘slide box’ (figure 1) was designed to replicate the methodology of Klein & Zentall (2003). From the top panel of an opaque, acrylic cube measuring 32 cm on each side, a reward chute led to a 4 cm diameter hole in the centre of the front panel. On the front panel, there was an 8×8 cm2 acrylic door that could slide left or right with equal ease. When in the centre, the door hid the reward-chute hole.
Figure 1.
The slide box apparatus. Here the door is slid to the left, revealing the hole from which the reward can be retrieved. See text for further information.
(c) Procedure
As in Klein & Zentall (2003), there were three experimental conditions and one control condition. Klein & Zentall termed conditions push, no push and no demo, which we feel are more clearly described in our study as ‘push demo’, ‘enhanced ghost’ and ‘ghost’, respectively. Klein & Zentall tested their subjects in adjacent cages, while it was more appropriate to test the chimpanzees and children with no barrier between them. Demonstrator chimpanzees were of higher rank than observers so that they could complete the task without being displaced. Children were given a chair each and asked to sit throughout testing. For each experimental condition, four subjects observed the door moved to the right and four to the left. For each push-demo and enhanced-ghost condition, two conspecific demonstrators were ‘push-right’ models and two were ‘push-left’. This was in accordance with Klein & Zentall's method controlling for individual demonstrator effects.
Chimpanzees were tested in one of four conditions.
(i) Push demo
A trained conspecific demonstrated sliding the door in one direction and gaining food 58 times (the same number as in Klein & Zentall (2003)).
(ii) Enhanced ghost
For this condition and the next, fishing line was attached at each side of the door and fed to the back of the slide box. The experimenter (LMH) used this to slide the door either left or right 58 times, her actions occluded from the chimpanzee's view by the slide box, although the top part of her body remained visible. A chimpanzee, trained to sit in front of the apparatus, retrieved each reward, while the observer chimpanzee watched.
(iii) Ghost
An observer chimpanzee, alone in the cage, observed the door being moved as in the enhanced-ghost condition. For each demonstrated slide, the food reward could be seen to drop into a pipe that led into a bucket outside the cage, so that as in the other conditions, the observer was not rewarded.
After each set of observation periods, the observer chimpanzee was given a single free-access period with the baited slide box for 20 min by itself. Subjects recovered a grape for each door slide regardless of the direction in which they moved it.
(iv) No-info condition
No information was provided. The subject was free to act on the apparatus for 20 min.
For the children, minor procedural changes were (i) the reward was a ‘sticker’ in a plastic capsule, (ii) based on pilot studies, the children's level of interest was maintained by reducing the number of demonstrations given in each condition to 15, and (iii) for the same reason, the test phase was run only up to the first 15 responses.
All test and response sessions were recorded using a Sony MiniDV Digital Handycam (DCR-HC35E). The direction of each door slide was recorded and the proportion of door slides that matched the direction demonstrated was calculated.
3. Results
(a) Response rates in experimental and control conditions
Each child's responses were limited to a maximum of 15 to maintain their level of interest. In contrast, the chimpanzees had a 20 min free-access period. Table 1 shows chimpanzees' response rates. Regardless of matching the direction demonstrated (DD) in the push-demo and ghost conditions all eight chimpanzees responded, while seven of eight did so in the enhanced ghost. In contrast, in the no-info condition, only three of eight chimpanzees moved the slide door (two to the left and one to the right) and retrieved grapes. Significantly more chimpanzees responded in each experimental condition than in the no-info condition (Fisher's exact test, p≤0.05).
Table 1.
The mean number of actions (a) across all chimpanzees tested and (b) by chimpanzees that responded successfully in the three 20 min experimental and no-info conditions.
| push-demo condition | enhanced-ghost condition | ghost | no-info condition | |
|---|---|---|---|---|
| a | 47.8 | 54.5 | 69.9 | 19.8 |
| b | 47.8 | 62.3 | 69.9 | 52.7 |
Six of eight children acted successfully in the no-info condition not significantly different from the full response rate in the experimental conditions. Three moved the door to the left and three to the right.
(b) First responses
Results are summarized in table 2. In the push-demo condition, both chimpanzee and child observers matched the DD (binomial test: p≤0.05 and p≤0.01, respectively). Chimpanzees also significantly matched the DD with their first response in the enhanced-ghost condition (p≤0.05). For the remaining conditions, the chimpanzees and children did not show significant matching.
Table 2.
Matching to direction witnessed in first responses by chimpanzees and children (probability levels are for results this extreme or more so).
| species | condition | first response match demo | first response match demo enhanced ghost and ghost | total match demo responses compared with chance |
|---|---|---|---|---|
| chimpanzees | push demo | 7/8 (p=0.04) | 8/8 (p=0.01) | |
| enhanced ghost | 6/7 (p=0.06) | 12/15 (p=0.02) | 7/7 (p=0.01) | |
| ghost | 6/8 (p=0.15) | 4/7 (p=0.27) | ||
| children | push demo | 7/7 (p=0.01) | 7/7 (p=0.01) | |
| enhanced ghost | 7/8 (p=0.04) | 13/16 (p=0.01) | 8/8 (p=0.01) | |
| ghost | 6/8 (p=0.15) | 6/8 (p=0.11) |
However, since there was no significant difference between the results for the enhanced-ghost and ghost conditions, and both are ghost conditions (in neither condition the door was operated by a conspecific model), the data were collapsed across these two conditions. When combined, both the chimpanzees and the children significantly matched the DD (binomial test: p≤0.05 for both: table 2). Thus, there was evidence of matching for both children and chimpanzees in their first trial, whether watching a model or a ghost condition.
(c) Total responses compared to chance
The total responses of each subject were found to be distributed in a bimodal manner, with the proportion of matching was always either above 0.70 or below 0.05. Accordingly, the results were analysed by classing each subject as ‘matching’ if their responses had a mean proportion of matching 0.50 or more and ‘non-matching’ if 0.50 or less. More chimpanzees and children in push demo matched the DD than chance (binomial test: p≤0.01 for both; table 2). Conversely, only the children showed greater matching than chance in the enhanced ghost (binomial test: p≤0.01). Neither species matched the DD in the basic ghost condition.
(d) Comparisons between conditions
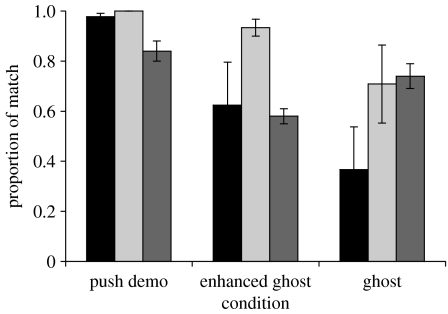
The overall proportions of matching responses by the chimpanzees and the children in all three conditions are shown in figure 2 along with the responses of the pigeons tested by Klein & Zentall (2003) for comparison. Figure 2 shows parametric results for comparison with the published pigeon results, but owing to the sample size and particularly the bimodal distributions, it was judged safer to apply non-parametric statistics to our chimpanzee and child data. Chimpanzees showed a significantly greater proportion of matching to the DD in the push demo (median 1.00) than in the ghost condition (median 0.04: Mann–Whitney U test: U=11.0 N1=8, N2=8; p≤0.05). A significant, although much smaller, difference was found for children between the push-demo condition (median 1.00) and ghost conditions (median 0.90: U=10.5 N1=7, N2=8; p≤0.05). The children, unlike the chimpanzees, also showed significantly greater matching in the push-demo condition compared with the enhanced-ghost condition (median 0.97: U=14.0 N1=7, N2=8; p≤0.05). Neither species showed a significant difference in level of matching between the enhanced-ghost and ghost conditions.
Figure 2.
Overall matching responses. Mean proportion of responses made by chimpanzees and children which matched the direction of the door movement demonstrated in the 20 min free-access period. Means and standard errors are shown to facilitate direct comparison with results for the pigeons tested by Klein & Zentall (2003). Standard errors for the latter were provided by the author (T. Zentall 2007, personal communication). Black bar, chimpanzees; light grey, children; dark grey bar, pigeons. Also, for comparison with the results of Klein, Zentall, one-sample t-test results for the chimpanzees and children are provided in the electronic supplementary material, appendix C.
(e) Pattern of responses
In summary, high levels of matching in the first responses by chimpanzees were followed by failure to match closely in the later trials for all but the push-demo condition. This was also true in the ghost condition for children. The fluctuations involved are charted in figure 3. The most noticeable contrast is between chimpanzees in the push-demo condition, who matched consistently, and those in the ghost condition, four of whom matched on their first response but then went on to explore alternative responses.
Figure 3.
Fluctuations in matching. Matching and non-matching pushes made during the free-access period by (a) chimpanzees ((i) push demo, (ii) enhanced ghost and (iii) ghost conditions) and (b) children ((i) push demo, (ii) enhanced ghost and (iii) ghost) in three conditions. Black, percentage of matching responses; grey, percentage of responses in the direction opposite to those demonstrated (n=58 for chimpanzees, n=15 for children).
4. Discussion
Our results allow a fairly direct comparison between the responses of chimpanzees, children and pigeons studied by Klein & Zentall (2003). Interestingly, all three species behaved similarly in some respects, yet each differed from the others in at least one respect. Of course, testing conditions will never be identical for such different species: for example, the pigeons are operating the device with their beak; the primates with their hands; and the children are acting in the context of an experiment run by a conspecific making verbal requests of them. Nevertheless, moving the door appears motorically easy for all three species, making this a reasonable comparative test of emulation.
In the critical ghost conditions (enhanced ghost and ghost), our results revealed emulation in the first responses of both chimpanzees and children. This is the first direct evidence for emulation learning in a non-human primate in the strict conditions of a ghost experiment. Our positive results contrast with previous negative ones in ghost condition studies with non-human primates (Subiaul et al. 2004; Hopper et al. 2007). We suggest an explanation may be that our earlier study employed a complex tool-use technique that chimpanzees never discovered in no-model control conditions (Whiten et al. 2005; Hopper et al. 2007), whereas in the present study three of eight control subjects completed the task. Although motorically simple, the task employed by Subiaul et al. was also complex involving four steps. We tentatively conclude that chimpanzees may be capable of emulation learning in tasks relatively simple in their cognitive demands, but not in more complex tasks, where observers instead need to watch a model act. Precisely what factors are critical in determining such a difference thus becomes an important question for future research.
With repeated trials, chimpanzees ceased to match what they had seen in enhanced-ghost and ghost conditions. In this respect, chimpanzees differed from both children and pigeons, who showed significant matching in the ghost condition. Chimpanzees thus appeared more innovative, exploring alternatives in the ghost conditions, as illustrated in figure 3. This tendency contrasts with the remarkable median 100% (mean 99%) match in the push-demo condition, indicating a strong tendency to conform to the consistent actions of a conspecific.
Children differed from the other species in showing a strong tendency to match even when the ‘model’ did not actually make the door slide (enhanced ghost). We know no previous work in developmental psychology that helps explain this. Children might be more familiar with actions that can create effects remotely, as in a light switch: however, in the enhanced-ghost condition there was no action at all. We can only note that our child subjects were sufficiently sensitive to social cues that the mere presence of another child, even though passive, was sufficient to elicit matching to the ghost event witnessed.
Pigeons differed from the other species in that, by contrast with the significant matching shown in the ghost condition, they showed none in the enhanced-ghost condition. Klein & Zentall (2003) offered no explanation for this. Possibly the pigeons were either distracted by the non-acting pigeon or copied its passivity, but it is unclear why such biases should affect only the pigeons. This, and the other species differences outlined above, lays foundations for future comparative work that may further explain underlying causes.
Klein & Zentall (2003) interpreted their finding that matching was significantly greater in the push demo than in the enhanced-ghost condition as implying imitation. We question this conclusion. Given that the significant matching recorded in the ghost condition showed that pigeons could learn from watching the screen move alone (i.e. emulate), this might also account for their push-demo success. A significantly higher degree of matching in this condition would be required to infer imitative effects over and above those expected through emulation. Thus, we conclude emulation was demonstrated in the pigeons, but not imitation. By contrast, the chimpanzees we tested did show a greater tendency, overall, to match in the push-demo condition compared with either of the ghost conditions. Whether this implies imitation depends on how imitation is defined. The fact that emulation was sufficient to explain chimpanzees' first matching responses means that we cannot say whether or not imitation was also occurring in the push-demo condition, in the sense of learning specifically from a model's actions on the door, as opposed to how the door moved. However, the striking tendency of chimpanzees to continue to match specifically in the push-demo condition suggests imitation in the broad sense of a motivation to match what a conspecific consistently continues to do.
These results may have implications for the cultural transmission of behaviour patterns. If chimpanzees were predominantly emulators rather than imitators, the scope for fidelity of cultural transmission is accordingly less. However, our ghost condition results suggest that emulation, in the sense of learning from the environmental results of actions alone, may be employed by chimpanzees only for relatively simple events and then only fleetingly. By contrast, when a conspecific model was witnessed, a strong degree of conformity emerged—a quality that could clearly affect fidelity of transmission. This is not to suggest that a tradition of, say, ‘left pushing’ would be likely to be sustained by chimpanzees in a task as simple as the Klein & Zentall (2003) paradigm, but in the context of the more complex techniques that have been seen as candidate traditions in wild apes (Whiten & van Schaik 2007) and modelled in captive diffusion experiments (Whiten et al. 2007), such a tendency could play an influential role.
Acknowledgments
The chimpanzee study was conducted at the Department of Veterinary Sciences, The University of Texas M. D. Anderson Cancer Center, USA. Chimpanzees were never food deprived and had constant access to water. They were housed in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International, and in accordance with current United States Department of Agriculture, Department of Health and Human Services and National Institutes of Health regulations and standards. Support for the chimpanzee colony comes from NIH/NCRR U42-RR015090. Approval was given by Fife LEA and University of St Andrews Ethics Committee for the child studies, completed at nurseries in Fife, Scotland. The study was supported by the BBSRC (A.W., L.M.H.) and a Royal Society Leverhulme Trust Senior Research Fellowship to A.W. We thank Oscar Rousette and Emma Flynn for their logistical support, Andy Burnley and Bryan Paenitz for constructing the slide boxes, and Gillian Brown and Thomas Zentall for comments on earlier manuscripts. Author contributions. L.H. and A.W. designed the study and wrote the paper. L.H. conducted the study and analysed the data. This work constitutes part of her PhD dissertation research. S.S. and S.L. provided essential logistical guidance and support.
Supplementary Material
Chimpanzee ID (sex, age)
Children ID (sex, age)
One sample t-test values
References
- Akins C.K, Klein E.D, Zentall T.R. Imitative learning in Japanese quail (Coturnix japonica) using the bidirectional control procedure. Anim. Learn. Behav. 2002;30:275–281. doi: 10.3758/bf03192836. [DOI] [PubMed] [Google Scholar]
- Byrne R.W. Emulation in apes: verdict ‘not proven’. Dev. Sci. 2002;5:20–22. doi:10.1111/1467-7687.00198 [Google Scholar]
- Call J, Carpenter M, Tomasello M. Copying results and copying actions in the process of social learning: chimpanzees (Pan troglodytes) and human children (Homo sapiens) Anim. Cogn. 2005;8:151–163. doi: 10.1007/s10071-004-0237-8. doi:10.1007/s10071-004-0237-8 [DOI] [PubMed] [Google Scholar]
- Fawcett T.W, Skinner A.M.J, Goldsmith A.R. A test of imitative learning in starlings using a two-action method with an enhanced ghost control. Anim. Behav. 2002;64:547–556. doi:10.1006/anbe.2002.3092 [Google Scholar]
- Fragaszy D, Perry S. Cambridge University Press; Cambridge, UK: 2003. The biology of traditions: models and evidence. [Google Scholar]
- Galef B.G, Jr, Heyes C.M. Introduction. Learn. Behav. 2004;32:1–3. [Google Scholar]
- Heyes C.M, Jaldow E, Nokes T, Dawson G.R. Imitation in rats (Rattus norvegicus): the role of demonstrator action. Behav. Process. 1994;32:173–182. doi: 10.1016/0376-6357(94)90074-4. doi:10.1016/0376-6357(94)90074-4 [DOI] [PubMed] [Google Scholar]
- Hopper L.M, Spiteri A, Lambeth S.P, Schapiro S.J, Horner V, Whiten A. Experimental studies of traditions and underlying transmission processes in chimpanzees. Anim. Behav. 2007;73:1021–1032. doi:10.1016/j.anbehav.2006.07.016 [Google Scholar]
- Hurley S, Chater N. MIT Press; Cambridge, MA: 2005. Perspectives on imitation: from neuroscience to social science. [Google Scholar]
- Klein E.D, Zentall T.R. Imitation and affordance learning by pigeons (Columba livia) J. Comp. Psychol. 2003;117:414–419. doi: 10.1037/0735-7036.117.4.414. doi:10.1037/0735-7036.117.4.414 [DOI] [PubMed] [Google Scholar]
- Mesoudi A, Whiten A, Laland K.N. Towards a unified science of cultural evolution. Behav. Brain Sci. 2006;29:329–382. doi: 10.1017/S0140525X06009083. doi:10.1017/S0140525X06009083 [DOI] [PubMed] [Google Scholar]
- Mitchell C.J, Heyes C.M, Gardener M.R, Dawson G.R. Limitations of a bidirectional control procedure for the investigation of imitation in rats: odour cues on the manipulandum. Quart. J. Exp. Psych. B. 1999;52:193–202. doi:1080/713932705 [Google Scholar]
- Nagell K, Olguin R.S, Tomasello M. Processes of social learning in the tool use of chimpanzees (Pan troglodytes) and human children (Homo sapiens) J. Comp. Psychol. 1993;107:174–186. doi: 10.1037/0735-7036.107.2.174. doi:10.1037/0735-7036.107.2.174 [DOI] [PubMed] [Google Scholar]
- Subiaul F, Cantlon J.F, Holloway R.L, Terrace H.S. Cognitive imitation in rhesus macaques. Science. 2004;305:407–410. doi: 10.1126/science.1099136. doi:10.1126/science.1099136 [DOI] [PubMed] [Google Scholar]
- Tennie C, Call J, Tomasello M. Push or pull: imitation vs. emulation in great apes and human children. Ethology. 2006;112:1159–1169. doi:10.1111/j.1439-0310.2006.01269.x [Google Scholar]
- Thompson D.E, Russell J. The ghost condition: imitation versus emulation in young children's observational learning. Dev. Psychol. 2004;40:882–889. doi: 10.1037/0012-1649.40.5.882. doi:10.1037/0012-1649.40.5.882 [DOI] [PubMed] [Google Scholar]
- Tomasello M. Cultural transmission in the tool use and communicatory signaling of chimpanzees. In: Parker S.T, Gibson K.R, editors. ‘Language’ and intelligence in monkeys and apes: comparative developmental perspectives. Cambridge University Press; Cambridge, UK: 1990. pp. 274–311. [Google Scholar]
- Tomasello M. Harvard University Press; Cambridge, MA: 1999. The cultural origins of human cognition. [Google Scholar]
- Want S.C, Harris P.L. How do childern ape? Applying concepts from the study of non-human primates to the developmental study of ‘imitation’ in childern. Dev. Sci. 2002;5:1–13. doi:10.1111/1467-7687.00194 [Google Scholar]
- Whiten A, Ham R. On the nature and evolution of imitation in the animal kingdom: a reappraisal of a century of research. Adv. Study Behav. 1992;21:239–283. [Google Scholar]
- Whiten A, van Schaik C.P. The evolution of animal ‘cultures’ and social intelligence. Phil. Trans. R. Soc. B. 2007;362:603–620. doi: 10.1098/rstb.2006.1998. doi:10.1098/rstb.2006.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A, Horner V, Litchfield C.A, Marshall-Pescini S. How do apes ape? Learn. Behav. 2004;32:36–52. doi: 10.3758/bf03196005. [DOI] [PubMed] [Google Scholar]
- Whiten A, Horner V, de Waal F.B.M. Conformity to cultural norms of tool use in chimpanzees. Nature. 2005;437:737–740. doi: 10.1038/nature04047. doi:10.1038/nature04047 [DOI] [PubMed] [Google Scholar]
- Whiten A, Spiteri A, Horner V, Bonnie K.E, Lambeth S.P, Schapiro S.J, de Waal F.B.M. High-fidelity transmission of multiple traditions within and between groups of chimpanzees. Curr. Biol. 2007;17:1038–1043. doi: 10.1016/j.cub.2007.05.031. doi:10.1016/j.cub.2007.05.031 [DOI] [PubMed] [Google Scholar]
- Wood D. Social interaction as tutoring. In: Bornstein M.H, Bruner J.S, editors. Interaction in human development. Lawrence Erlbaum Associates; Hillsdale, NJ: 1989. pp. 59–80. [Google Scholar]
- Zentall T.R. Imitation: definitions, evidence, and mechanisms. Anim. Cogn. 2006;9:335–353. doi: 10.1007/s10071-006-0039-2. doi:10.1007/s10071-006-0039-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chimpanzee ID (sex, age)
Children ID (sex, age)
One sample t-test values