Abstract
Background
Bilirubin inhibits experimental atherosclerosis, is inversely associated with carotid plaque burden, and confers neuroprotection in experimental stroke. Clinical data addressing the association of bilirubin with stroke are not available. We hypothesized that higher bilirubin levels would be associated with reduced stroke prevalence and improved stroke outcomes.
Methods
We used the National Health and Nutrition Examination Survey 1999–2004, a nationally representative cross-sectional examination of the United States civilian population, to examine the association of bilirubin with stroke. Of 13,214 adult participants with data on stroke history, serum total bilirubin level, and stroke risk factors, 453 reported a history of stroke. Of these, 138 reported an adverse stroke outcome, defined as a long-term health problem or disability due to stroke. We performed multivariable logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CI) with adjustment for demographic characteristics and stroke risk factors.
Results
After multivariable adjustment, a 1.71 umol/L (0.1 mg/dL) increment in bilirubin level was associated with a 9% reduced odds of stroke (OR 0.91, 95% CI: 0.86, 0.96) among all participants, and was associated with a 10% reduced odds of an adverse stroke outcome (OR 0.90, 95% CI: 0.80, 1.00) among participants with a history of stroke.
Conclusions
These results suggest that higher serum total bilirubin level is associated with reduced stroke prevalence and improved stroke outcomes. Our findings support the hypotheses that bilirubin may protect from stroke events and from neurologic damage in stroke.
Keywords: Stroke, bilirubin, epidemiology, nutrition survey, National Health and Nutrition Examination Survey, heme oxygenase
Stroke is the third leading cause of death and a leading cause of disability in the United States1. While much progress has been made in identifying risk factors for stroke, little is known about factors that modulate stroke risk. Bilirubin, long considered merely metabolic waste but now recognized as having important antioxidant, anti-inflammatory, and neuroprotectant properties, has emerged as a possible endogenous defense mechanism against stroke2–4. The inhibitory actions of bilirubin in experimental atherosclerosis, the ability of bilirubin to prevent arterial thrombosis, and the inverse association of bilirubin with carotid atherosclerotic plaque suggest that higher bilirubin levels may be associated with a reduced risk of stroke5–7. Additionally, the ability of bilirubin to protect from neuronal injury in experimental acute ischemic stroke suggests that bilirubin may limit neurologic damage once a stroke has occurred3, 8. We therefore hypothesized that individuals with higher serum total bilirubin levels would be less likely to have suffered a stroke event, and that higher bilirubin levels would be associated with a more favorable stroke outcome among stroke survivors. To test these hypotheses, we examined the cross-sectional association of serum total bilirubin level with prevalent stroke and adverse stroke outcomes in the National Health and Nutrition Examination Survey (NHANES), a nationally representative cross-sectional examination of the United States population.
MATERIALS AND METHODS
The NHANES, conducted by the National Center for Health Statistics (NCHS), is a program of studies designed to assess the health and nutritional status of children and adults in the United States. The 1999–2004 NHANES was reviewed and approved by the NCHS Institutional Review Board. Informed consent was obtained from all participants. The NHANES detailed interview includes demographic, socioeconomic, dietary, and health-related questions. The examination component consists of medical examinations, physiological measurements, and laboratory tests administered by highly trained medical personnel. Of the 15,332 participants aged ≥ 20 years, 21 were missing data regarding stroke history and an additional 2,041 were missing serum total bilirubin level. Fifty-six of the remaining participants were missing data for a key covariate, allowing 13,214 participants to be included in this analysis.
Laboratory methods
Serum total bilirubin, creatinine, alanine aminotransfersase (ALT), aspartate aminotransferase (AST), albumin and glucose were determined by automated biochemical profiling (Beckman Synchron LX20); fractionation of total bilirubin was not performed in NHANES, and bilirubin levels were recorded in mg/dL. C-reactive protein (CRP) was quantified by latex-enhanced nephelometry. Total cholesterol and high-density lipoprotein (HDL) cholesterol were measured by automated enzymatic assay (Hitachi 704 Analyzer serviced by Roche Diagnostics, Indianapolis). Total homocysteine was measured by the Abbott Homocysteine assay. Hemoglobin was measured by the Beckman Coulter MAXM Instrument.
Definitions
Stroke prevalence and adverse stroke outcomes
Prevalent stroke was defined based upon the response to the question, ‘Has a doctor or other health professional ever told you that you have had a stroke’. We defined a participant as having an adverse stroke outcome if the participant reported that stroke was the source of a long-term health problem or disability. As part of the ‘Physical Function’ questionnaire of the home interview portion of the examination, participants were asked a series of questions to determine whether they had a long-term health problem (not including pregnancy) that caused any long-term physical, mental, or emotional problem or illness9. If a subject reported a health problem, he or she was asked to identify the source of this; stroke was included in the list of health problems for the subject to choose from. Examples of health problems specifically queried are difficulty with working, memory, managing money, and walking a quarter mile. The online supplement contains full details of the questionnaire.
Other characteristics
Self-reported race/ethnicity was defined as non-Hispanic white, non-Hispanic black, Mexican-American, or other. A diagnosis of hypertension, hypercholesterolemia, and diabetes mellitus were defined as previously described10. Body weight was considered normal, overweight, or obese if the body mass index (BMI) was < 25, 25–29.9, or ≥ 30 kg/m2, respectively. CRP level was categorized as low (< 1 mg/L), intermediate (1–3 mg/L), or high (> 3 mg/L) as suggested by Centers for Disease Control and Prevention / American Heart Association guidelines11. Participants were characterized as active, former, or never smokers10. The presence of active liver disease was determined by the subject’s answer to the questions ‘Has a doctor or other health professional ever told you that you have liver disease?’ and ‘Do you still have a liver condition?’. For further information about data collection, the reader is referred to the NHANES homepage (http://www.cdc.gov/nchs/nhanes.htm).
Statistical analysis
Analyses were performed with SAS version 9.1 (SAS Institute Inc) and SUDAAN version 9.0.1 (Research Triangle Institute) to account for the complex sample structure. Subject characteristics are reported as percentage and 95% confidence intervals (CI) or as mean and 95% CI. We examined the likelihood of stroke associated with a 1.71 µmol/L (0.1 mg/dL) increase in bilirubin level and with a 1-standard deviation increment bilirubin level; the standard deviation of bilirubin was 5.3 µmol/L (0.31 mg/dL). The association of bilirubin with stroke was also examined by tertiles of bilirubin level. We chose to use tertiles of bilirubin due to the relatively small number of events. We used logistic regression to estimate odds ratios (ORs) and 95% CI. Covariates in multivariable analysis were age, race/ethnicity, gender, smoking status, hypertension, diabetes, and total cholesterol to HDL cholesterol ratio. We individually considered BMI, CRP, hemoglobin and homocysteine as confounders, with a predetermined cutoff of a change in the bilirubin effect estimate of ≥ 20% indicating significant confounding. Additional adjustment for a quadratic term did not demonstrate an important non-linear relationship between bilirubin and stroke, and log transformation did not improve the fit of bilirubin in determining odds of stroke.
A priori we analyzed the interaction between bilirubin level and gender and between bilirubin level and smoking10. To address a possible influence from liver disease, we repeated the analysis first excluding participants who reported active liver disease, and second by additionally excluding participants with laboratory evidence suggestive of undiagnosed liver disease (AST or ALT > 2x gender-specific upper limit of normal, bilirubin > 34.2 µmol/L (2.0 mg/dL), or albumin < 35 g/L (3.5 mg/dL)). We additionally adjusted for alcohol intake in participants who had this data available (n = 12,361).
To determine whether bilirubin level was associated with adverse stroke outcome, multivariable logistic regression of adverse stroke outcome was performed among participants diagnosed with stroke. Multivariable adjustment and sensitivity analyses were the same as described above. Log transformation improved the fit of bilirubin in determining the odds of adverse stroke outcome in the multivariable adjusted model. For this outcome we therefore also report the results of the analysis using log-transformed bilirubin, but focus on the results of analysis using untransformed bilirubin so that the effect estimate is more easily understood.
RESULTS
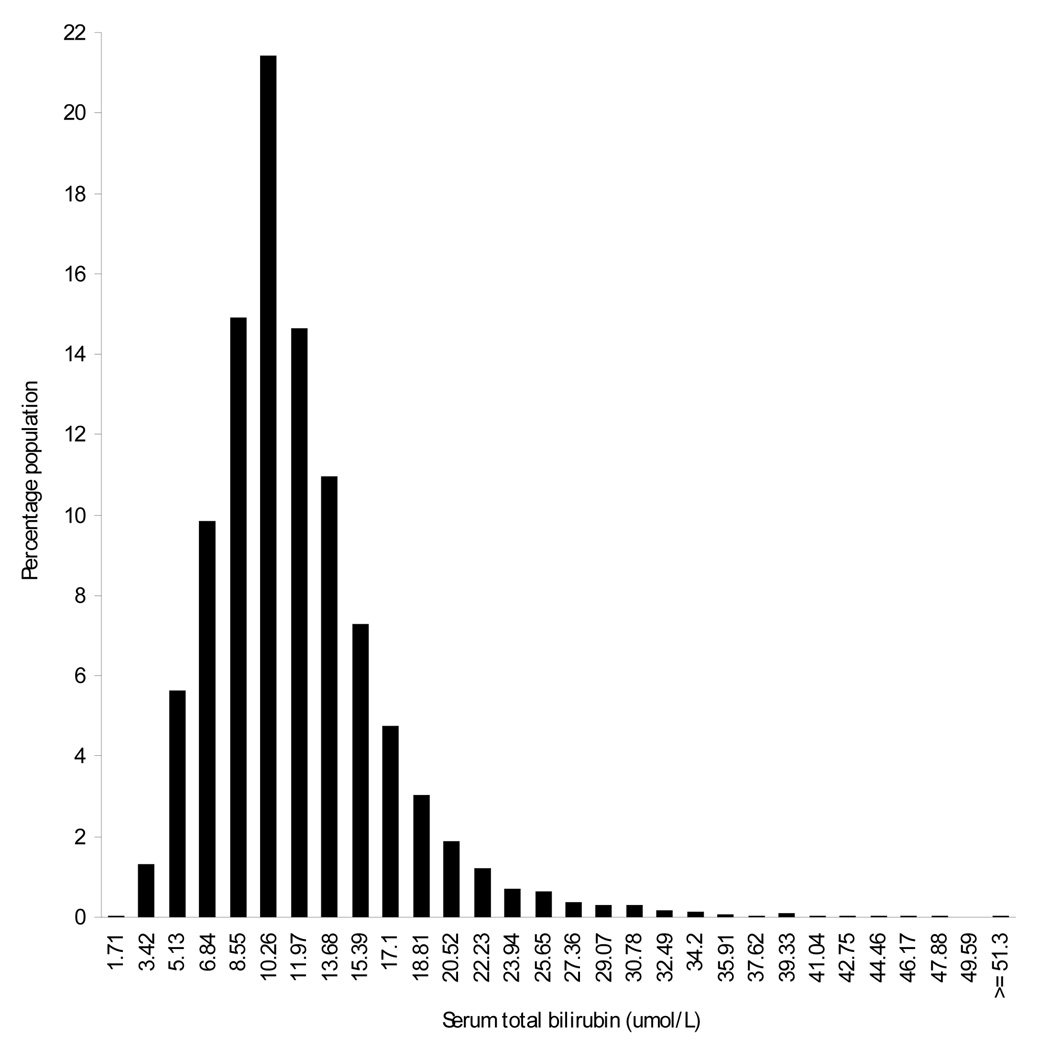
Participant characteristics associated with lower bilirubin levels included female sex, non-Hispanic black race/ethnicity, hypercholesterolemia, and active smoking (Table 1). The distribution of bilirubin levels demonstrated a small right tail (Figure 1). Participant characteristics associated with prevalent stroke included older age, hypertension, diabetes and hypercholesterolemia.
Table 1.
Association of serum bilirubin and prevalent stroke with participant characteristics.
| Characteristic | Frequency within study population*, mean (95% CI) | Serum total bilirubin level (umol/L)**, mean (95% CI) | Stroke prevalence (%), percentage (95% CI) |
|---|---|---|---|
| Age group (years) | |||
| 20–39 | 39.4 (37.8–40.1) | 12.1 (11.8–12.5) | 0.5 (0.3–0.8) |
| 40–59 | 38.3 (37.0–39.6) | 12.0 (11.8–12.1) | 1.7 (1.3–2.3) |
| 60 or over | 22.3 (21.2–23.5) | 12.1 (11.8–12.5) | 7.3 (6.4–8.3) |
| Gender | |||
| Male | 48.1 (47.4–48.9) | 13.7 (13.3–13.9) | 2.1 (1.8–2.5) |
| Female | 51.9 (51.1–52.6) | 10.6 (10.4–10.8) | 2.8 (2.3–3.4) |
| Race/ethnicity | |||
| Non-Hispanic White | 72.6 (69.1–75.9) | 12.3 (12.0–12.5) | 2.6 (2.2–3.1) |
| Non-Hispanic Black | 10.5 (8.7–12.7) | 11.1 (10.6–11.3) | 2.9 (2.3–3.7) |
| Mexican American | 7.3 (5.7–9.2) | 12.0 (11.6–12.3) | 1.2 (0.8–1.7) |
| Other | 9.6 (7.3–12.4) | 11.8 (11.1–12.7) | 1.9 (1.1–3.3) |
| Hypertension | |||
| No | 65.1 (63.5–66.6) | 12.1 (12.0–12.5) | 0.9 (0.6–1.2) |
| Yes | 34.9 (33.4–36.5) | 12.0 (11.6–12.1) | 5.5 (4.8–6.3) |
| Diabetes mellitus | |||
| No | 91.4 (90.7–92.1) | 12.1 (11.8–12.3) | 2.0 (1.7–2.4) |
| Yes | 8.6 (7.8–9.2) | 11.6 (11.3–12.0) | 7.5 (6.0–9.2) |
| Hypercholesterolemia | |||
| No | 64.3 (63.0–65.5) | 12.3 (12.0–12.5) | 1.7 (1.4–2.1) |
| Yes | 35.3 (34.1–36.5) | 11.6 (11.5–12.0) | 3.9 (3.2–4.7) |
| Smoking status | |||
| Never | 49.1 (47.3–50.9) | 12.3 (12.0–12.7) | 2.2 (1.8–2.8) |
| Former | 27.3 (25.8–28.8) | 12.5 (12.3–12.8) | 3.0 (2.5–3.8) |
| Active | 23.6 (22.0–25.3) | 11.1 (10.6–11.3) | 2.4 (1.7–3.2) |
| Stroke | |||
| No | 97.5 (97.1–97.8) | 12.1 (11.8–12.3) | n/a |
| Yes | 2.5 (2.2–2.9) | 10.8 (10.3–11.3) | n/a |
Analytical sample of 13,214 adult participants from the National Health and Nutrition Examination Survey, 1999–2004.
To convert bilirubin from umol/L to mg/dL, multiply by 0.058.
Figure 1. The distribution of bilirubin levels in the analytical sample (n = 13,214).
To convert bilirubin from umol/L to mg/dL, multiply by 0.058. National Health and Nutrition Examination Survey 1999–2004.
The association of bilirubin with prevalent stroke
Of the 13,214 participants included in this analysis, 453 reported a history of stroke (2.5%, 95% CI: 2.2, 2.9). The mean serum total bilirubin level was significantly lower among participants with (12.1 µmol/L, 95% CI: 11.8, 12.3) (0.63 mg/dL, 95% CI 0.60–0.66) compared to those without (10.8 µmol/L, 95%: CI 10.3,11.3) (0.71 mg/dL, 95% CI: 0.69, 0.72) a history of stroke.
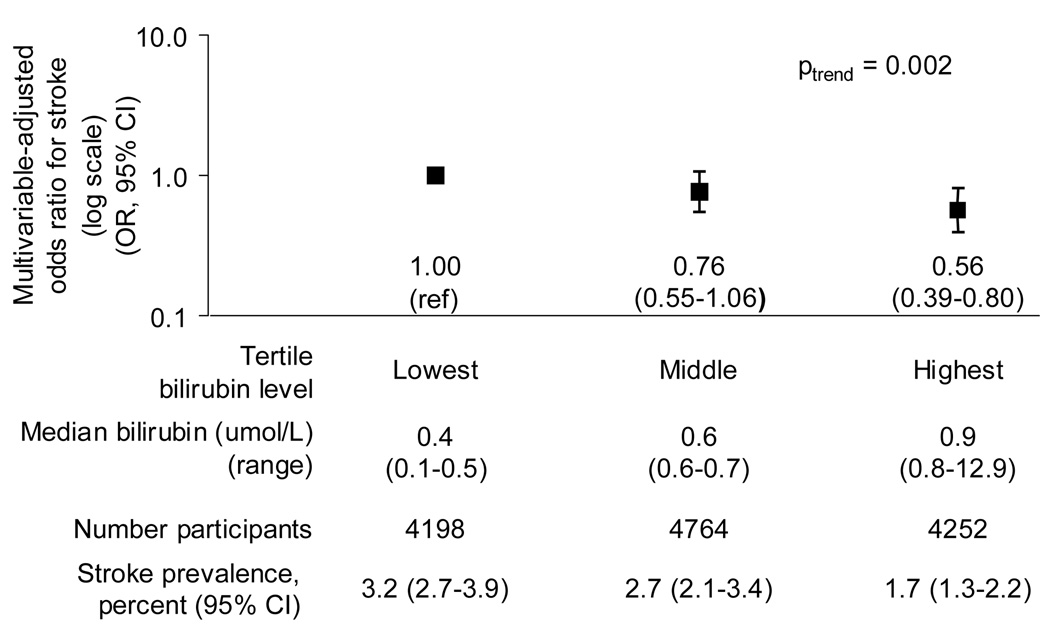
A 1.71 µmol/L (0.1 mg/dL) increment in serum total bilirubin level was associated with a 9% reduced odds of stroke (0.91, 95% CI: 0.86, 0.96) after adjustment for age, gender, race/ethnicity, smoking, hypertension, diabetes, and total:HDL cholesterol ratio. The effect estimate for a 1-standard deviation increment in bilirubin was an OR of 0.72 (95% CI: 0.63,0.82). In addition, we found that the odds of stroke decreased progressively with increasing tertile of bilirubin level; in particular, the second and highest compared with the lowest tertile of bilirubin was associated with a 24 and 44% reduced odds of stroke after multivariable adjustment (OR 0.76, 95% CI: 0.55,1.06, and OR 0.56, 95% CI: 0.39, 0.80, Ptrend = 0.002, respectively) (Figure 2). The inverse association of bilirubin with stroke prevalence remained after excluding participants with a bilirubin level above the reference range (OR 0.90, 95% CI: 0.84, 0.95), indicating that the association was present within the range of clinically normal bilirubin levels.
Figure 2. Multivariable-adjusted odds ratio for stroke among all participants.
Age, sex, race/ethnicity, smoking, hypertension, total:HDL cholesterol ratio, and diabetes were adjusted for. Odds ratios and 95% confidence intervals (CI) estimated by logistic regression. Y-axis is log scale. The stroke prevalence estimates are unadjusted. To convert bilirubin from umol/L to mg/dL, multiply by 0.058. National Health and Nutrition Examination Survey 1999–2004. OR : Odds ratio. CI : Confidence interval.
We did not find evidence for significant confounding by BMI, CRP, homocysteine, or duration of fasting prior to blood draw (known to influence bilirubin levels). Moreover, the association of bilirubin with stroke did not vary by fasting status (≥ 8 hours versus < 8 hours) (Pinteraction = 0.53) or whether the venous blood sample was drawn as part of the morning, afternoon or evening examination session did not modify the results (Pinteraction = 0.17). Excluding participants with diagnosed active liver disease (n = 174) and additionally for laboratory evidence suggestive of possible undiagnosed liver disease (n = 1075), the association of bilirubin with stroke remained (OR 0.90, 95% CI: 0.85, 0.95, and OR 0.89, 95% CI: 0.85, 0.94, respectively). Additional adjustment for alcohol intake did not change the association of bilirubin with stroke (OR 0.91, 95% CI: 0.85, 0.97). Sex did not significantly alter the association of bilirubin with stroke (Pinteraction = 0.06), nor did smoking status (Pinteraction = 0.72).
The association of bilirubin with adverse stroke outcome
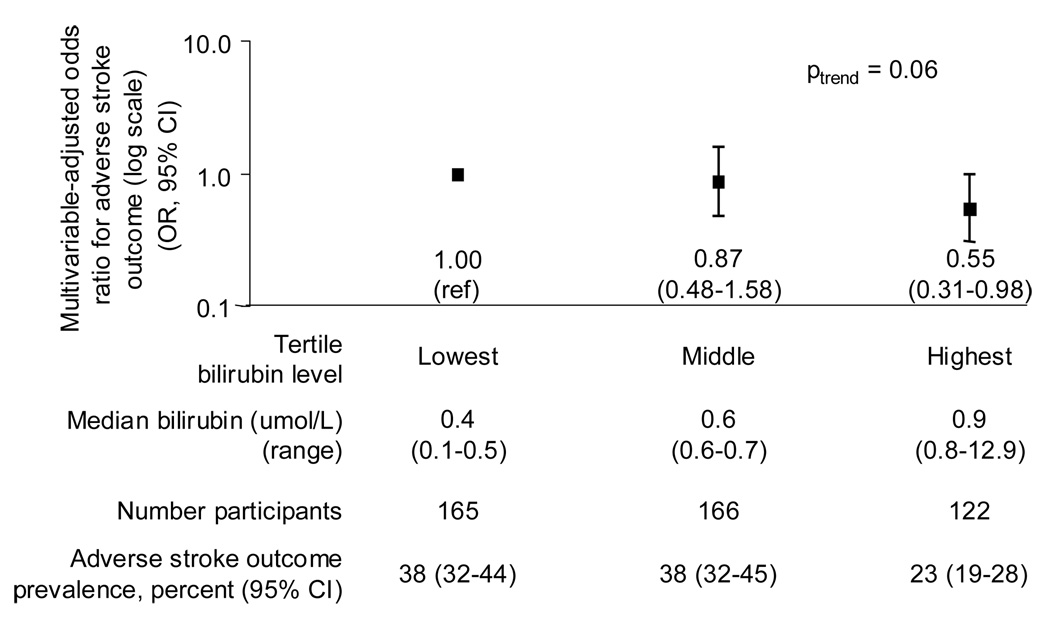
Among the 453 participants who reported a history of stroke, 138 (26.4%, 95% CI: 22.2, 31.1) reported a long-term physical, mental, or emotional problem or illness as a consequence of stroke (defined as an adverse stroke outcome). Participants with an adverse stroke outcome tended to be male, older, and have a greater burden of stroke risk factors, but these differences were not significant (Table 2). Among participants with a history of stroke, a 1.71 µmol/L (0.1 mg/dL) increment in bilirubin level was associated with a 10% reduced odds of an adverse stroke outcome (OR 0.90, 95% CI : 0.80, 1.00) after multivariable adjustment. Log transformation improved the fit of bilirubin in determining the odds of an adverse stroke outcome (log-transformed bilirubin OR 0.44, 95% CI: 0.22, 0.89). In addition, we found that the odds of an adverse stroke outcome tended to decrease progressively with increasing tertile of bilirubin level; in particular, the second and highest compared with the lowest tertile of bilirubin were associated with a 13 and 44% reduced odds of an adverse stroke outcome after multivariable adjustment (OR 0.87, 95% CI: 0.48, 1.58, and OR 0.55, 95% CI: 0.31, 0.98, respectively, Ptrend = 0.06) (Figure 3).
Table 2.
Characteristics of stroke survivors by adverse stroke outcome.
| Characteristic | Participants with stroke history, without an adverse stroke outcome (n = 315) mean (95% CI) | Participants with stroke history, with an adverse stroke outcome (n = 138) mean (95% CI) |
|---|---|---|
| Age group (years) | ||
| 20–39 | 8.4 (2.1–5.0) | 7.4 (2.9–17.7) |
| 40–59 | 29.0 (22.9–35.9) | 19.0 (11.5–29.8) |
| 60 or over | 62.6 (55.7–69.0) | 73.6 (60.4–83.7) |
| Gender | ||
| Male | 37.7 (31.9–44.0) | 49.7 (37.3–62.0) |
| Female | 62.3 (56.0–68.2) | 50.4 (38.0–62.7) |
| Race/ethnicity | ||
| Non-Hispanic White | 78.3 (71.2–84.1) | 72.0 (61.5–80.5) |
| Non-Hispanic Black | 10.1 (6.8–14.5) | 19.4 (13.3–27.4) |
| Mexican American | 3.3 (1.8–6.1) | 3.9 (2.0–7.6) |
| Other | 8.3 (4.3–15.6) | 4.8 (1.2–16.9) |
| Hypertension | ||
| No | 25.3 (19.8–31.9) | 16.9 (9.9–27.4) |
| Yes | 74.7 (68.2–80.2) | 83.1 (72.6–90.1) |
| Diabetes mellitus | ||
| No | 76.8 (69.5–82.7) | 67.3 (56.9–76.3) |
| Yes | 23.2 (17.3–30.5) | 32.7 (23.7–43.1) |
| Hypercholesterolemia | ||
| No | 45.2 (37.8–52.8) | 42.8 (33.6–52.6) |
| Yes | 54.8 (47.2–62.2) | 57.2 (47.4–66.4) |
| Smoking status | ||
| Never | 43.2 (34.4–52.4) | 46.7 (36.7–56.9) |
| Former | 33.5 (26.2–41.8) | 33.5 (24.2–44.2) |
| Active | 23.3 (17.3–30.7) | 19.9 (11.9–31.3) |
Figure 3. Multivariable-adjusted odds of an adverse stroke outcome among stroke survivors.
Age, sex, race/ethnicity, smoking, hypertension, total:HDL cholesterol ratio, and diabetes were adjusted for. Odds ratios and 95% confidence intervals (CI) estimated by logistic regression. Y-axis is log scale. The adverse stroke outcome prevalence estimates are unadjusted. To convert bilirubin from umol/L to mg/dL, multiply by 0.058. National Health and Nutrition Examination Survey 1999–2004. OR : Odds ratio. CI : Confidence interval.
DISCUSSION
Experimental data suggests that bilirubin may limit stroke events by inhibiting atherosclerosis, thrombosis and vascular injury, and that bilirubin may limit neuronal damage once a stroke has occurred. We found that bilirubin level was inversely associated with stroke prevalence in over 13,000 participants in a nationally representative community-based cohort of United States adults. Specifically, the highest tertile of bilirubin level was associated with a 44% reduced odds of stroke. Additionally, among those participants with a history of stroke, those with higher bilirubin levels were less likely to report a long term health problem or disability due to stroke, suggesting that higher bilirubin levels are associated with more favorable stroke outcomes. These findings support the hypothesis that bilirubin may be an important endogenous defense mechanism against stroke events and may protect neurologic injury in the setting of stroke.
Bilirubin, once considered simply the metabolic end product of heme degradation by heme-oxygenase (HO), has emerged as an important endogenous anti-inflammatory and antioxidant molecule10. Bilirubin may, in fact, be the most abundant endogenous antioxidant in mammalian tissues4, 12.
The antioxidant and anti-inflammatory qualities of bilirubin may protect from stroke events by limiting the development of atherosclerotic vascular disease. Bilirubin inhibits several aspects of atherogenesis, including LDL oxidation, monocyte migration, vascular smooth muscle cell proliferation, vascular inflammation, vascular reactive oxygen species generation and endothelial dysfunction2, 13, 14. That the antiatherogenic properties of bilirubin may be relevant to stroke is demonstrated by the strong inverse association of bilirubin level with carotid atherosclerotic plaque7. In addition to inhibiting atherosclerosis, bilirubin may also prevent stroke by inhibiting thrombus formation6, 15. It is important to note that carbon monoxide, another product of heme catabolism by HO, also has important roles in cardiovascular homeostasis13.
Beyond possibly preventing stroke events, bilirubin may limit neurologic injury in once a stroke has occurred. In experimental acute ischemic stroke, overexpression of HO attenuates injury, and knockout of HO substantially worsens neuronal damage8, 16. Bilirubin appears to account for the neuroprotection offered by HO, as the neurotoxic effect of HO deletion is reversed by restoring even low concentrations of bilirubin8. The cytoprotection offered by bilirubin greatly exceeds that of glutathione and exceeds that which would be expected based on its intracellular concentration because of rapid recycling by biliverdin reductase: bilirubin can protect from a 10,000-fold excess of oxidants3. The increased expression of biliverdin reductase in experimental stroke further supports a role neuroprotective role for bilirubin in this setting17. Available human data indirectly supports a role for bilirubin limiting neuronal damage in stroke, as low plasma antioxidant capacity is associated with high lesion volume and neurological impairment in stroke18.
Bilirubin may protect not only from cerebrovascular disease but also more broadly from cardiovascular disease. Several investigators have found a strong inverse correlation between bilirubin level and coronary artery disease in men19. That bilirubin may broadly protect from cardiovascular disease is further supported by a prospective study of 1780 individuals followed up for 24 years. A 1.71 µmol/L (0.1 mg/dL) increase in bilirubin decreased cardiovascular disease risk by 10%, though the number of non-coronary events was relatively small20. Additionally, bilirubin is strongly inversely associated with lower extremity peripheral arterial disease10.
The NHANES sampling methodology makes these results generalizable to the United States adult population. Participants are carefully characterized, allowing adjustment for several important covariates. Several limitations do warrant consideration. The diagnosis of stroke was made by self-report, a potential source of misclassification. We are not aware that the self-report of stroke has been validated within the NHANES cohort, but others have found that self-report of a physician diagnosis of stroke can be accurate21. It has also been shown that self-report of outcomes following stroke can be accurate22, though the NHANES interview was not specifically designed to address stroke outcomes. Another potential source of error is that the bilirubin level was determined only once in each participant, and bilirubin exhibits significant within-subject variation23. Additionally, the antioxidant properties of bilirubin may differ depending upon whether it is conjugated or unconjugated; in NHANES fractionation of bilirubin was not performed24. The cross-sectional nature of the NHANES cohort does not allow for determination of the temporal association of bilirubin with stroke or allow any conclusion regarding causation. That the prospective association of bilirubin with cardiovascular disease may be U-shaped supports the need for prospective studies of bilirubin and stroke25, 26. NHANES does not have information regarding the type of stroke; it is possible that bilirubin is most strongly associated with a particular stroke subtype. Experimental data, as reviewed above, suggests that bilirubin might specifically protect from atherothrombotic stroke events, and might specifically limit neurologic damage in acute ischemic stroke, but this is speculative.
Should future work confirm that higher bilirubin level is associated with lessened stroke risk and improved stroke outcomes, interventions that increase bilirubin levels, perhaps by the induction of heme-oxygenase or by the inhibition of UDP-glucuronosyltransferase (coined ‘iatrogenic Gilbert syndrome’), may be appropriate investigational strategies for the prevention and/or treatment of stroke27, 28.
Summary
In this nationally representative cross-sectional survey of United States adults, serum total bilirubin level was inversely associated with prevalent stroke, and individuals with a history of stroke and higher bilirubin levels were less likely to have suffered an adverse stroke outcome. These results are consistent with the hypotheses that the vasculoprotective properties of bilirubin may provide protection from stroke events, and that the neuroprotective capacity of bilirubin may limit neurologic injury in the setting of stroke. Therapeutic strategies to increase either tissue or circulating bilirubin levels may one day be used in the prevention and/or treatment of stroke.
Supplementary Material
Acknowlegements
Dr. Perlstein was supported by the NHLBI Training Grant T32 HL07604 and an American College of Cardiology Foundation / Merck Research Fellowship Award. Dr. Beckman was supported by American Diabetes Association Career Development Award 1-06-CD-01. Dr. Creager is the Simon C. Fireman Scholar in Cardiovascular Medicine. Dr. Pande was supported by NHLBI training grant T32 HL07604 and by a Research Career Development Award (K12 HL083786) from the NHLBI. Dr. Weuve is partially supported by NIEHS R01ES005257. This work was also supported by NHLBI grant R01 HL075771.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BMI
Body mass index
- CI
Confidence interval
- CRP
C-reactive protein
- HDL
High-density lipoprotein
- HO
Heme oxygenase
- NHANES
National Health and Nutrition Examination Survey
- OR
Odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation at a meeting: This work was presented as an abstract at the Society of Vascular Medicine and Biology annual meeting, Baltimore, Maryland, June 2007.
Contributor Information
Todd S. Perlstein, Department of Medicine, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Reena L. Pande, Department of Medicine, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Mark A. Creager, Department of Medicine, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Jennifer Weuve, Department of Environmental Health, Harvard School of Public Health, Boston, Massachusetts.
Joshua A. Beckman, Department of Medicine, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
References
- 1.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–e449. [PubMed] [Google Scholar]
- 2.Kawamura K, Ishikawa K, Wada Y, et al. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:155–160. doi: 10.1161/01.ATV.0000148405.18071.6a. [DOI] [PubMed] [Google Scholar]
- 3.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa K, Navab M, Leitinger N, et al. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997;100:1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.True AL, Olive M, Boehm M, et al. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res. 2007;101:893–901. doi: 10.1161/CIRCRESAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 7.Ishizaka N, Ishizaka Y, Takahashi E, et al. High serum bilirubin level is inversely associated with the presence of carotid plaque. Stroke. 2001;32:580–583. doi: 10.1161/01.str.32.2.580-b. [DOI] [PubMed] [Google Scholar]
- 8.Dore S, Sampei K, Goto S, et al. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med. 1999;5:656–663. [PMC free article] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics. [Accessed February 15, 2007];Hyattsville, MD: National Center for Health Statistics; National Health and Nutrition Examination Survey 03–04. SP Questionnaire Component: Physical Functioning Questionnaire Data. 2005 ( http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/pfq_c.pdf)
- 10.Perlstein TS, Pande RL, Beckman JA, Creager MA. Serum total bilirubin level and prevalent lower-extremity peripheral arterial disease: National Health and Nutrition Examination Survey (NHANES) 1999 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:166–172. doi: 10.1161/ATVBAHA.107.153262. [DOI] [PubMed] [Google Scholar]
- 11.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 12.Gopinathan V, Miller NJ, Milner AD, Rice-Evans CA. Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett. 1994;349:197–200. doi: 10.1016/0014-5793(94)00666-0. [DOI] [PubMed] [Google Scholar]
- 13.Durante W. Carbon monoxide and bile pigments: surprising mediators of vascular function. Vasc Med. 2002;7:195–202. doi: 10.1191/1358863x02vm424ra. [DOI] [PubMed] [Google Scholar]
- 14.Erdogan D, Gullu H, Yildirim E, et al. Low serum bilirubin levels are independently and inversely related to impaired flow-mediated vasodilation and increased carotid intima-media thickness in both men and women. Atherosclerosis. 2006;184:431–437. doi: 10.1016/j.atherosclerosis.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Lindenblatt N, Bordel R, Schareck W, et al. Vascular heme oxygenase-1 induction suppresses microvascular thrombus formation in vivo. Arterioscler Thromb Vasc Biol. 2004;24:601–606. doi: 10.1161/01.ATV.0000118279.74056.8a. [DOI] [PubMed] [Google Scholar]
- 16.Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- 17.Panahian N, Huang T, Maines MD. Enhanced neuronal expression of the oxidoreductase--biliverdin reductase--after permanent focal cerebral ischemia. Brain Res. 1999;850:1–13. doi: 10.1016/s0006-8993(99)01726-6. [DOI] [PubMed] [Google Scholar]
- 18.Leinonen JS, Ahonen JP, Lonnrot K, et al. Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke. 2000;31:33–39. doi: 10.1161/01.str.31.1.33. [DOI] [PubMed] [Google Scholar]
- 19.Novotny L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Exp Biol Med (Maywood) 2003;228:568–571. doi: 10.1177/15353702-0322805-29. [DOI] [PubMed] [Google Scholar]
- 20.Lin JP, O'Donnell CJ, Schwaiger JP, et al. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation. 2006;114:1476–1481. doi: 10.1161/CIRCULATIONAHA.106.633206. [DOI] [PubMed] [Google Scholar]
- 21.Simpson CF, Boyd CM, Carlson MC, et al. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: factors that modify agreement. J Am Geriatr Soc. 2004;52:123–127. doi: 10.1111/j.1532-5415.2004.52021.x. [DOI] [PubMed] [Google Scholar]
- 22.Berge E, Fjaertoft H, Indredavik B, Sandset PM. Validity and reliability of simple questions in assessing short- and long-term outcome in Norwegian stroke patients. Cerebrovasc Dis. 2001;11:305–310. doi: 10.1159/000047658. [DOI] [PubMed] [Google Scholar]
- 23.Van Hoydonck PG, Schouten EG, Temme EH. Reproducibility of blood markers of oxidative status and endothelial function in healthy individuals. Clin Chem. 2003;49:963–965. doi: 10.1373/49.6.963. [DOI] [PubMed] [Google Scholar]
- 24.Wu TW, Fung KP, Wu J, et al. Antioxidation of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol. 1996;51:859–862. doi: 10.1016/0006-2952(95)02395-x. [DOI] [PubMed] [Google Scholar]
- 25.Breimer LH, Wannamethee G, Ebrahim S, Shaper AG. Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem. 1995;41:1504–1508. [PubMed] [Google Scholar]
- 26.Troughton JA, Woodside JV, Young IS, et al. Bilirubin and coronary heart disease risk in the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Eur J Cardiovasc Prev Rehabil. 2007;14:79–84. doi: 10.1097/01.hjr.0000230097.81202.9f. [DOI] [PubMed] [Google Scholar]
- 27.Stocker R, Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114:2178–2189. doi: 10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- 28.McCarty MF. "Iatrogenic Gilbert syndrome"--a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses. 2007;69:974–994. doi: 10.1016/j.mehy.2006.12.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.