Abstract
Background
Omega-3 FA (ω-3 FA) have been demonstrated to have anti-inflammatory properties; postulated to occur through several principal mechanisms, including 1) displacement of arachidonic acid from the cellular membrane, 2) shifting of PGE2 and LTB4 production and 3) molecular level alterations including decreased activation of NF-κB and AP-1. An additional regulator that is likely associated is the production of nitric oxide (NO) by nitric oxide synthetase. NO is a short-lived free radical involved in many biological functions. However, excessive NO production can lead to complications, suggesting that decreased NO production is a potential target for some inflammatory diseases. We hypothesized that pretreating with an ω-3 FA lipid emulsion would decrease the production of NO in macrophages and that this effect would occur through alterations in inducible nitric oxide synthetase (iNOS).
Methods
Greiss reagent was used to assess NO production in RAW 264.7 macrophages following ω-3 or ω-6 FA treatment alone or in combination with LPS-stimulation for 12 hr/24 hr. iNOS levels were determined by western blot. TNF-alpha levels were determined by ELISA.
Results
Following LPS-stimulation, ω-3 FA pretreatment at 12 and 24 hrs produced significantly less NO (p<0.05) compared to ω-6 FA or media-only conditions. ω-3 FA pretreatment at 12 and 24 hrs also had less iNOS protein expression compared to ω-6 FA or media-only conditions. TNF-α production was significantly decreased with ω-3 FA treatment compared to ω-6 FA treatment (p<0.05) after 24 hrs LPS-stimulation.
Conclusion
These experiments demonstrate that in addition to other anti-inflammatory effects, ω-3 FA lipid emulsions also significantly lower NO production in LPS-stimulated macrophages through altered iNOS protein expression.
INTRODUCTION
An increasing emphasis is being placed on the potential for diet and dietary supplements to serve as modulators of the host response to disease, injury and infection. One such supplement under investigation as an anti-inflammatory agent, is eicosapentaenoic acid (EPA) an omega-3 fatty acid (ω-3 FA) which is a primary component of deep water marine fish oils. Clinically these polyunsaturated ω-3 FA are recognized to be essential for normal growth and development and have also been demonstrated to be of benefit in a number of inflammation-associated disease states including artherosclerosis, auto-immune disorders, malignancy and sepsis.1–3 However, in order for widespread clinical use of ω-3 FA to occur, the underlying mechanism for the suggested biological activities of fish oil need to be defined. In these studies a pharmaceutical-grade ω-3 FA lipid emulsion (OmegavenR, Fresinius-Kabi (Bad-Homburg, Germany)) and a formulated dose equivalent ω-6 FA lipid emulsion (LipovenosR, Fresinius-Kabi (Bad-Homburg, Germany)) were used to facilitate experiments in vitro that more adequately represent ω-3 FA effects compared to previously used reagents containing EPA-salts and EPA-albumin complexes that might themselves alter the inflammatory response in vitro.
Previously, in an LPS-stimulated macrophage (MΦ) models treated with this ω-3 FA lipid emulsion has demonstrated an alteration in the inflammatory response at least in part through their ability to decrease TNF-alpha (TNF-α) and prostaglandin E2 (PGE2) production.4–6 However, the mechanism(s) for these alterations appears to occur through a series of complex events including the displacement of arachidonic acid in the cellular membrane leading to the production of less metabolically active compounds including PGE3 instead of PGE2 and leukotriene B5 (LTB5)instead of LTB4.7 Additionally, ω-3 FA lipid emulsion treatment leads to significant alterations in nuclear transcription factor activation, particularly NF-κB and AP-1. 8 Both of which are primary regulators of the inflammatory response in experimental MΦ models.
While TNF-α is significantly decreased by ω-3 FA, this reduction only accounts for approximately 45–50% of the overall anti-inflammatory effects observed experimentally, suggesting that other factors are likely involved. 9 Nitric oxide (NO) may be one such factor that is potentially altered by ω-3 FA subsequently resulting in a decreased pro-inflammatory milieu.
NO is a short lived free radical and internal messenger that mediates a variety of functions including vascular homeostasis, neurotransmission and host defense. NO is synthesized from L-arginine by nitric-oxide synthase (NOS). Three isoforms of NOS have been identified including: endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). 10 In the MΦ, iNOS is the primary regulator of NO production; a known principal mediator of macrophage bactericidal and tumoricidal activities.11,12 However, when there is excess or prolonged production of NO, the potential for inflammation-associated tissue damage is present13,14; suggesting a potential role for targeted attenuation of iNOS mediated NO production.
Regulation of iNOS gene expression is through transcriptional regulation, particularly focused by NF-κB activation.15,16 In the mouse, iNOS gene promoter contains two NF-κB binding sites, both of which need to be bound in order to get full induction of iNOS by LPS stimulation. However, in addition to NF-κB there are also binding sites for C/EBP, AP-1 and CREB; all are thought to be involved in regulating iNOS production.17,18
ω-3 FA lipid emulsions have been demonstrated to significantly decrease NF-κB activation in the LPS-stimulated MΦ model.8 Additionally, ω-3 FA lipid emulsions have also altered the activation of the mitogen-activated protein kinase (MAPK) pathway components as well as attenuation of AP-1 activation.9 Furthermore, since iNOS/NO production is at least in part regulated by these nuclear transcription factors; in combination, these observations support the potential for ω-3 FA to modify NO production.
In the present studies, we hypothesized that OmegavenR, an ω-3 FA lipid emulsion, pretreatment would decrease the production of NO in LPS-stimulated macrophages and that this effect would occur through alterations in inducible nitric oxide synthase (iNOS).
MATERIALS AND METHODS
Materials
RAW 264.7 cells (murine macrophage cell line) were purchased form American Type Tissue Culture Collection (ATCC, Rockville, MD) Escherichia coli 0111:B4 LPS, β-actin antibody and the Greiss reagent was purchased from Sigma (St. Louis, MO). Omegaven® (ω-3 FA lipid emulsion) and Lipovenos® (ω-6 FA lipid emulsion) were purchased from Fresinius-Kabi (Bad-Homburg, Germany). Endotoxin free serum was purchased from Hyclone (Logan, UT). Western blot detection reagents were purchased from Cell Signaling (Beverly, MA). iNOS antibodies were purchased from Santa Cruz (Santa Cruz, CA). TNF-α ELISA was purchased from R & D Systems (Minneapolis, MN). Tissue culture media and all other cell culture reagents were purchased from Fisher Scientific (Pittsburg, PA).
Cell Culture
RAW 264.7 cells were suspended in complete medium; DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 U/ml streptomycin. Cells were plated in 24-well plates at a density of 5×105 cells/well. All experiments were performed in a humidified atmosphere under 5% CO2 at 37°C.
Experimental Design
Cells were randomly separated into three groups: media alone (DMEM), ω-3 FA emulsion, or ω-6 FA emulsion. Cells were allowed to adhere for 2 hrs and then incubated with each treatment or DMEM alone for 4 hrs. Equivalent FA concentrations for the ω-3 and ω-6 emulsions were prepared by dilution for experiments as previously and optimal concentrations for macrophage TNF-α inhibition were previously determined by dose-response studies. 8,19 Briefly, the ω-3 FA emulsion contains 2.1g/100 ml EPA was added in concentrations of 1.44 ul · ml−1 corresponding to 100 µM EPA. Control experiments were carried out with a lipid emulsion based on soybean oil (ω-6 FA) at the same dilutions to exclude a non-specific effect of lipid emulsion on the cells and cell viability assays were also performed and found no increase in toxicity with these emulsions at these concentrations (4). The cells were then washed twice with DMEM and stimulated with 1ìg/mL LPS for 12 or 24 hrs.
ELISA for TNF-α
Supernatants from treated RAW 264.7 cells were collected and immediately frozen at −70°C. The samples were subsequently analyzed for TNF-α protein following the protocol provided by R&D systems.
Assessment of NO Production
Cells were pretreated with ω-3 FA, ω-6 FA or media alone for 4 hrs and then stimulated with LPS for 12 or 24 hrs. Supernatants were then collected and NO levels were determined using the Greiss reagent. One hundred microliters of supernatant was removed and mixed with assay buffer and 100 µL of Greiss reagent according to manufacturer’s instructions (Sigma, Saint Louis, MO). The absorbance of the mixture was measured at 595 nm using an Elx808 Absorbance Microplate Reader (Bio Tek, Winooski, Vermont).
Assessment of iNOS Levels
Treated cells were lysed with passive lysis buffer (Promega, Madison, WI) and whole cell extracts were isolated. The total protein concentrations were determined using DC protein assay (Bio-Rad, Hercules, CA). For each sample, 30 µg of lysate protein was boiled for 5 minutes and loaded onto SDS-PAGE and transferred to a nitrocellulose membrane. After soaking in a blocking solution containing 1× TBS, 0.1% Tween 20, and 5% w/v nonfat dry milk for an hour; the membranes were incubated with anti-iNOS rabbit polyclonal IgG antibody or anti-β-actin mouse antibody overnight at 4°C. They were then washed three times with TBS/Tween and incubated with HRP-conjugated secondary antibody for 2 hrs at room temperature. After washing, the membrane was incubated with 10 mL of ECL detection agent for 1 minute and exposed to X-ray film for 30 seconds.
Statistics
NO and TNF-α values are presented as means ± SEM. Data were analyzed using Student’s t test or one-way ANOVA with Tukey and Sheffe post hoc tests as appropriate with significance defined at p≤ 0.05.
RESULTS
Effects of ω-3 lipid on TNF-α production
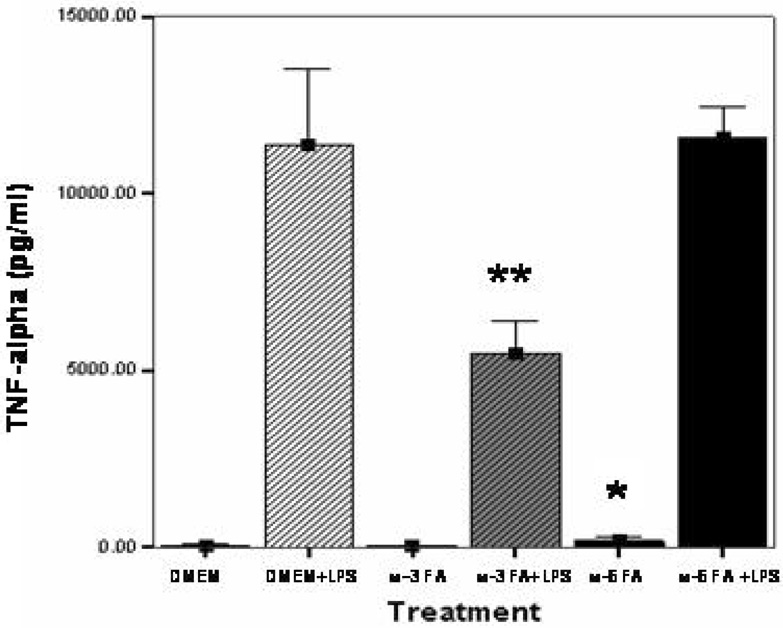
In non-LPS stimulated MΦ’s there is minimal baseline TNF-α production, control (DMEM, 62.1 ± 1.37 pg/m), ω-3 FA (59.6 ± 13.6 pg/ml) and ω-6 FA (182.4 ± 32.3 pg/mL) with a significant baseline alteration observed for ω-6 FA without LPS-stimulation, p<0.05. In the presence of 24 hrs LPS-stimulation there is a significant increase in TNF-α production. DMEM+LPS (11,390 ± 496.4 pg/mL) and ω-6 FA (11,595 ± 203.6 pg/mL) pretreated cells demonstrated similar amounts of TNF-α. However, ω-3 FA pretreatment (5476 ± 209.4 pg/mL) in LPS stimulated macrophages demonstrated a significant decrease in TNF-α compared to DMEM+LPS and ω-6 FA+LPS, p<0.05. (Fig. 1).
Figure 1.
Effect of ω-3 FA and ω-6 FA pretreatment on TNF-α production in LPS-stimulated MΦ. MΦ were pretreated with media-only (DMEM), Φ-3 FA (Omegaven®), or ω-6 FA (Lipovenos®) with or without LPS-stimulation for 24 hrs. Supernatants were collected and analyzed for TNF-α production by ELISA. The data represents mean ± SEM (n=3). * significant (p<0.05) increase from baseline DMEM and w-3 FA alone, ** significant (p<0.05) decrease from DMEM+LPS and ω-6 FA+LPS.
Effects of ω-3 lipid on NO production
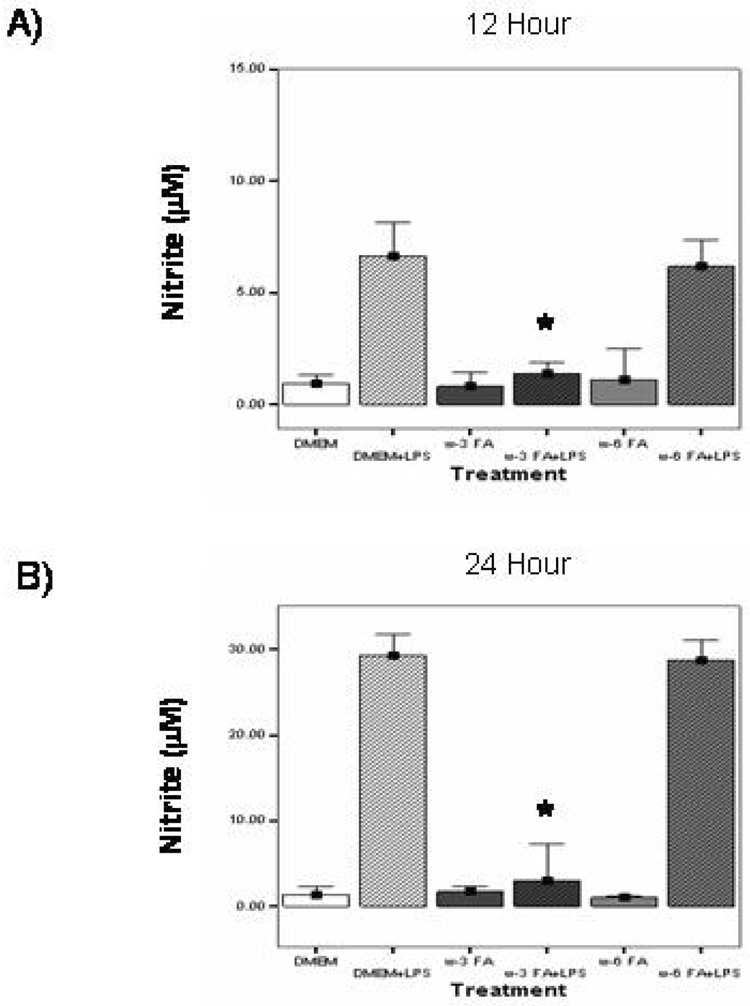
At both 12 and 24 hrs, NO production by unstimulated macrophages is low. At 12 hrs, NO concentrations were similar between treatment groups with DMEM (0.967 ± 0.14 µM), ω-3 FA (0.818 ± 0.24 µM) , and ω-6 FA (0.818 ± 0.24 µM). Similar findings were also observed at 24 hrs, with unstimulated NO concentrations for DMEM (1.35 ± 0.22 µM), ω-3 FA (1.71 ± 0.14 mM) and ω-6 FA (0.982 ± 0.05 mM) demonstrating no significant alterations between treatment groups. When stimulated with LPS, there was a large increase in NO concentrations for both 12 and 24hours.
-
12 hour results: DMEM (7.03 ± 0.56 µM) and ω-6 FA (5.18 ± 0.49 µM);
24 hour results: DMEM (29.31 ± 0.57 µM) and ω-6 FA (28.80 ± 0.52 µM).
This effect is significantly decreased by ω-3 FA pretreatment at both time points (1.38 ± 0.21 µM, p<0.05 at 12 hours and 3.00 ± 0.99 µM at 24 hours, p<0.05),. (Fig. 2A. and 2B).
Figure 2.
A and B Effects of ω-3 FA and ω-6 FA pretreatment on NO production in LPS-stimulated MΦ. In both experiments, MΦ were pretreated with media-only (DMEM), ω-3 FA (Omegaven®) or ω-6 FA (Lipovenos®) for 4 hrs. A) cells were washed and stimulated with or without LPS for 12 hrs. B) cells were washed and stimulated with or without LPS for 24 hrs. Supernatants were then collected and NO levels were measured by the Griess reagent. Each value represents the mean ±SEM of 3 assays. * significant (p<0.05) decrease in NO production compared to DMEM+LPS and ω-6 FA+LPS.
Effects of N-3 lipid on iNOS levels
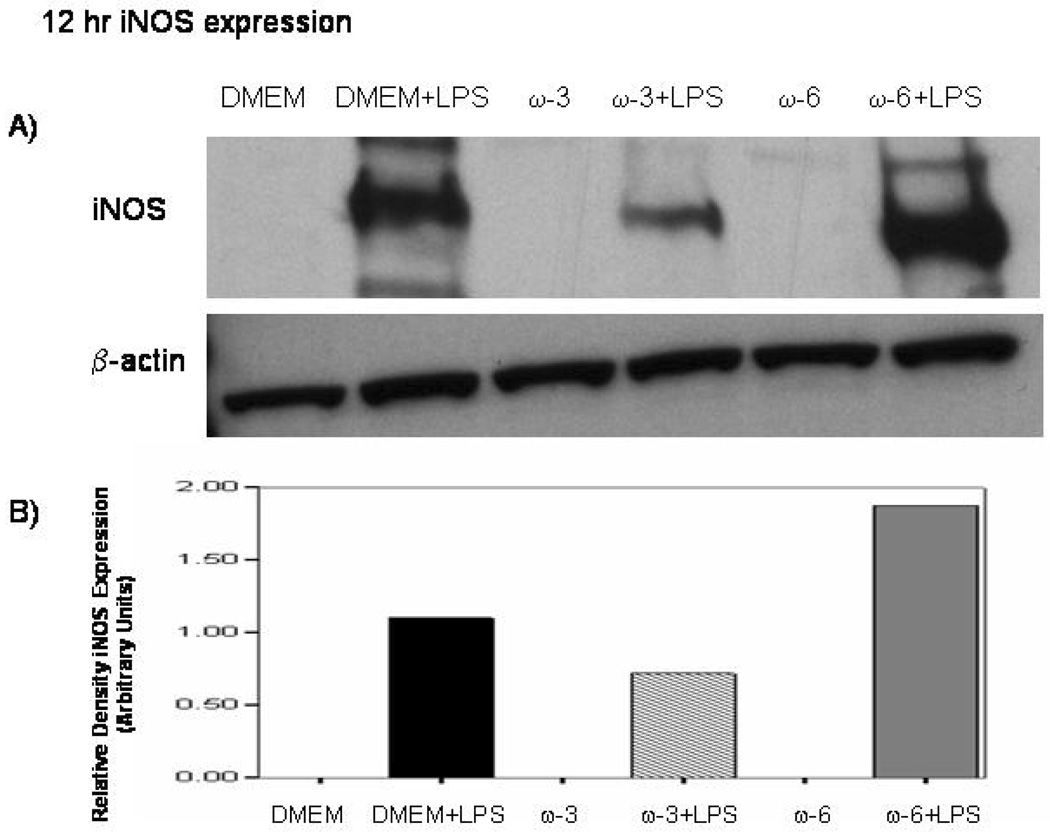
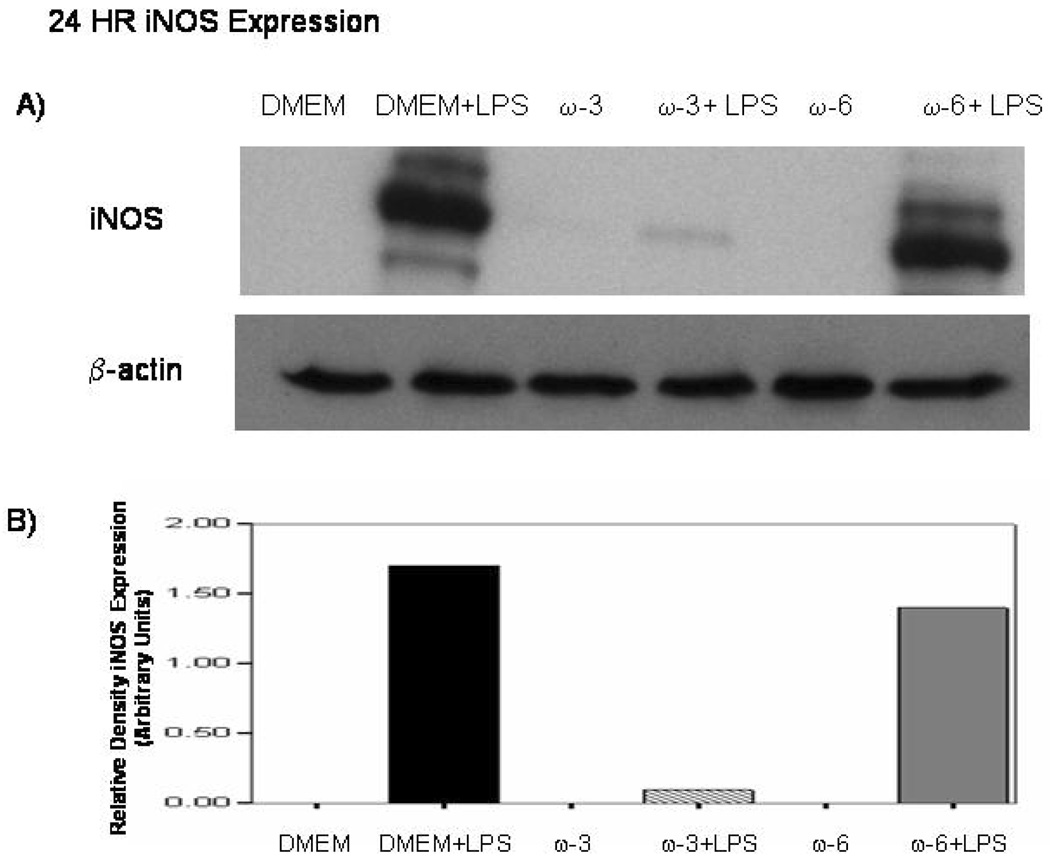
Western blots reveal no detectable iNOS presence in unstimulated macrophages at 12 and 24 hrs. With LPS there is a increase in iNOS protein expression. However, pretreatment with ω-3 FA prior to LPS-stimulation decreased iNOS expression at 12 hrs while ω-6 FA increased iNOS expression (Figure 3A and demonstrated with densitometry in Figure 3B) while at 24 hr ω-3 FA almost completely eliminates the LPS-induced iNOS protein expression and ω-6 FA pretreatment did not alter iNOS protein expression from that of media-alone controls. (Fig. 4A. and 4B.)
Figure 3.
A and B Effect of ω-3 FA and ω-6 FA pretreatment on LPS-induced iNOS protein expression. MΦ were pretreated with media-only, ω-3 FA (Omegaven®) or ω-6 FA (Lipovenos®) for 4 hrs. A) cells were then stimulated with or without LPS for 12 hrs and β-actin was the internal loading control for this experiment. ω-3 FA were found to decrease iNOS protein expression after LPS-stimulation compared to DMEM+LPS and ω-6 FA+LPS. B) Ban intensity was measured using densitometry in which iNOS protein expression was normalized relative to the β-actin expression levels.
Figure 4.
A and B Effect of ω-3 FA and ω-6 FA pretreatment on LPS-induced iNOS protein expression. MΦ were pretreated with media-only, ω-3 FA (Omegaven®) or ω-6 FA (Lipovenos®) for 4 hrs. A) cells were then stimulated with or without LPS for 24 hrs and β-actin was the internal loading control for this experiment. ω-3 FA were found to decrease iNOS protein expression after LPS-stimulation compared to DMEM+LPS and ω-6 FA+LPS. B) Ban intensity was measured using densitometry in which iNOS protein expression was normalized relative to the β-actin expression levels.
DISCUSSION
NO is a ubiquitous messenger in biological systems, participating in the regulation of a variety of biologic processes when it is released by MΦ in response to noxious stimuli (endotoxin, pro-inflammatory cytokines, etc.). A significant portion of NO is released several hours after an initial stimulation, the magnitude of this response is determined by the upregulation of iNOS gene transcription. Under homeostatic conditions, NO participates in a host-beneficial system to destroy pathogens, cause vasodilation and decrease thrombosis. 12,20,21 While these are useful actions when local infection is present, NO is also a major factor in the elaboration and propagation of tissue damage in conditions such as sepsis, abdominal malignancies and autoimmune diseases. 22–24 In the presence of these pathologic pro-inflammatory circumstances, altering MΦ NO production could potentially attenuate a variety of pro-inflammatory mediators; specifically, NO-associated pathways.
In the present study, initial experiments evaluated the effects of ω-3 FA pretreatment on TNF-α inhibition in 24 hr LPS-stimulated RAW 264.7 MΦ. In this well described model4,6, TNF-α̣ is used as a surrogate marker of inflammation to evaluate consistency across experiments. In this model, the TNF-α effects of the lipid emulsion following 3 hrs of LPS stimulation have been defined; as such, the present experiments were necessary to define the RAW 264.7 MΦ response following prolonged LPS stimulation (24 hrs) with ω-3 FA pretreatment. The experiments demonstrated that using the standard 4 hour MΦ pretreatment with ω-3 FA significantly decreases TNF-α production even after 24 hrs of LPS stimulation. In contrast, ω-6 FA pretreatment did not significantly decrease TNF-α production under similar conditions.
Previous studies with ω-3 FA suggested that the alterations in TNF-α production were occurring primarily through modulation of PGE2 output, leading to decreased inflammation. However, experiments with selective COX-2 inhibitors have demonstrated this not to be the case. In this LPS-stimulated MΦ model, pretreatment with COX-2 inhibitors in combination with ω-3 FA can lead to even further reductions in TNF-α production. This suggests that in the presence of ω-3 FA, other mechanisms are involved in altering the inflammatory response. These observations are also supported by data demonstrating that ω-3 FA pretreatment can significantly alter transcription factor activation in LPS-stimulated MΦ leading to decreased NF-κB and AP-1 activation; two primary regulators of the inflammatory response in LPS-stimulated MΦ. However, with all these alterations only an approximate 50% reduction in total TNF-α levels is observed compared to controls, suggesting that other factors are involved in modulating the inflammatory response subsequent to ω-3 FA pretreatment.
Since NO is primarily regulated through NF-κB, ω-3 FA pretreatment was hypothesized to significantly alter NO production in this model. Initial experiments demonstrated a significant decrease in NO production in LPS-stimulated macrophages pretreated with ω-3 FA compared to ω-6 FA and media-only cells at both 12 and 24 hrs. This decreased NO production suggested regulation at the level of iNOS protein expression, the principal modulator of NO production; which was confirmed by western blot analysis.
The present experiments support the work of Ohata et al.25 and Khari-El-Din et al.26 however in these previous studies, DHA-complexes and ethyl-ester forms of the ω-3 FA were used, which are not clinically useful. In these experiments, we were able to demonstrate that a clinically used and available ω-3 FA lipid emulsion could significantly decrease NO production in LPS-stimulated MΦ and that this inhibition is associated with altered iNOS protein expression. These observations support further studies to define molecular level regulation of inflammation by ω-3 FA; potentially validating the potential role of ω-3 FA as clinically useful components of treatment strategies.
Acknowledgments
Supported in part by: NIDDK-1 K08 DK DK60778 (Espat)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher Aldridge, Department of Surgery, University of Illinois at Chicago..
Anthony Razzak, Department of Surgery, University of Illinois at Chicago..
Tricia A. Babcock, Department of Surgery, University of Illinois at Chicago..
W. Scott Helton, Departments of Surgery and Pharmacology, University of Illinois at Chicago.
N. Joseph Espat, Surgical Oncology, Department of Surgery, Roger Williams Medical Center.
REFERENCES
- 1.Kromhout D, Bosschieter EB, de Lezenne CC. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 2.Kremer JM, Robinson DR. Studies of Dietary Supplementation with Omega-3 Fatty Acids in Patients with Rheumatoid Arthritis. World Review of Nutritional Diet. 1991;66:367–382. doi: 10.1159/000419305. [DOI] [PubMed] [Google Scholar]
- 3.Beck SA, Smith KL, Tisdale MJ. Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res. 1991;51:6089–6093. [PubMed] [Google Scholar]
- 4.Babcock TA, Helton WS, Hong D, Espat NJ. Omega-3 fatty acid lipid emulsion reduces LPS-stimulated macrophage TNF-alpha production. Surg Infect (Larchmt) 2002;3:145–149. doi: 10.1089/109629602760105817. [DOI] [PubMed] [Google Scholar]
- 5.Lo CJ, Chiu KC, Fu M, Lo R, Helton S. Fish Oil Decreases Macrophage Tumor Necrosis Factor Gene Transcription by Altering the NFkappaB Activity. J Surg Res. 1999;82:216–221. doi: 10.1006/jsre.1998.5524. [DOI] [PubMed] [Google Scholar]
- 6.Babcock TA, Novak T, Ong E, Jho DH, Helton WS, Espat NJ. Modulation of lipopolysaccharide-stimulated macrophage tumor necrosis factor-alpha production by omega-3 fatty acid is associated with differential cyclooxygenase-2 protein expression and is independent of interleukin-10. J Surg Res. 2002;107:135–139. doi: 10.1006/jsre.2002.6498. [DOI] [PubMed] [Google Scholar]
- 7.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci U S A. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-kappa B inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2003;284:L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 9.Babcock TA, Kurland A, Helton WS, Rahman A, Anwar KN, Espat NJ. Inhibition of activator protein-1 transcription factor activation by omega-3 fatty acid modulation of mitogen-activated protein kinase signaling kinases. JPEN J Parenter Enteral Nutr. 2003;27:176–180. doi: 10.1177/0148607103027003176. [DOI] [PubMed] [Google Scholar]
- 10.Marletta MA. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993;268:12231–12234. [PubMed] [Google Scholar]
- 11.Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan CF, Hibbs JB., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 13.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 14.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 15.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 16.Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, Murphy WJ. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci U S A. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eberhardt W, Pluss C, Hummel R, Pfeilschifter J. Molecular mechanisms of inducible nitric oxide synthase gene expression by IL-1beta and cAMP in rat mesangial cells. J Immunol. 1998;160:4961–4969. [PubMed] [Google Scholar]
- 18.Guan Z, Baier LD, Morrison AR. p38 mitogen-activated protein kinase down-regulates nitric oxide and up-regulates prostaglandin E2 biosynthesis stimulated by interleukin-1beta. J Biol Chem. 1997;272:8083–8089. doi: 10.1074/jbc.272.12.8083. [DOI] [PubMed] [Google Scholar]
- 19.Babcock TA, Helton WS, Hong D, Espat NJ. Omega-3 fatty acid lipid emulsion reduces LPS-stimulated macrophage TNF-alpha production. Surg Infect (Larchmt) 2002;3:145–149. doi: 10.1089/109629602760105817. [DOI] [PubMed] [Google Scholar]
- 20.Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993;54:171–178. [PubMed] [Google Scholar]
- 21.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147 Suppl 1:S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCartney-Francis N, Allen JB, Mizel DE, Albina JE, Xie QW, Nathan CF, Wahl SM. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993;178:749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petros A, Bennett D, Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991;338:1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- 24.Vodovotz Y, Lucia MS, Flanders KC, Chesler L, Xie QW, Smith TW, Weidner J, Mumford R, Webber R, Nathan C, Roberts AB, Lippa CF, Sporn MB. Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer's disease. J Exp Med. 1996;184:1425–1433. doi: 10.1084/jem.184.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohata T, Fukuda K, Takahashi M, Sugimura T, Wakabayashi K. Suppression of nitric oxide production in lipopolysaccharide-stimulated macrophage cells by omega 3 polyunsaturated fatty acids. Jpn J Cancer Res. 1997 Mar;88(3):234–237. doi: 10.1111/j.1349-7006.1997.tb00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu CY, Penfield JG, Khair-el-Din TA, Sicher SC, Kielar ML, Vazquez MA, Che L. Docosahexaenoic acid, a constituent of fetal and neonatal serum, inhibits nitric oxide production by murine macrophages stimulated by IFN gamma plus LPS, or by IFN gamma plus Listeria monocytogenes. J Reprod Immunol. 1998 Apr;38(1):31–53. doi: 10.1016/s0165-0378(98)00005-9. [DOI] [PubMed] [Google Scholar]