Abstract
The goals of our study were to evaluate the haplotype pattern at the Rho-associated kinase 2 (ROCK2) locus and prospectively test the association between the ROCK2 tagSNPs with hypertension for verified incident hypertensive patients (N=607) and healthy, normotensive controls (N=586) in a HYPGENE study. Rho-associated kinases (ROCKs) play a central role in signaling pathways that are involved in vascular smooth muscle contraction and endothelial nitric oxide availability. Using a set of stringent criteria (minor allele frequency ≥ 0.05, pairwise r2 ≥ 0.95), we identified 18 haplotype-tagging single-nucleotide polymorphisms (tagSNPs) from the 109 SNPs available in the HapMap Caucasian data set. TagSNPs were genotyped using the Illumina BeadStation platform. The 18 tagSNPs consisted of two linkage disequilibrium (LD) blocks. A haplotype defined by four SNPs (rs965665, rs10178332, rs6755196, rs10929732) in LD block 2 was recessively associated with a lower risk of hypertension (p=0.003). Homozygotes for the minor alleles had an 85% lower risk of hypertension than carriers of the common allele. The associations were independent of baseline age, cardiorespiratory fitness, body mass index, sex, and follow-up time. The LD block 2 spans about 137 kb of genomic DNA at the 5′-end of the ROCK2 locus and covers exons encoding the kinase domain of the protein. Our data strongly suggest that a major haplotype block at the ROCK2 locus is recessively associated with a lower risk of hypertension. Identification of functional mutation(s) could thus help in the development of ROCK2-specific treatments.
Keywords: hypertension, tagSNPs, haplotype block, linkage disequilibrium, ROCK2
Introduction
The roles of normal endothelial function and vascular smooth muscle cell (VSMC) contractility in the regulation of vascular resistance and, consequently, blood pressure are well established. The identities of several vasoactive molecules produced by the endothelial cells and the cellular mechanisms contributing to VSMC contraction have previously been reported. Moreover, recent studies suggest that these mechanisms are regulated by several complex signaling pathways.
Rho-associated kinases 1 and 2 (ROCK1 and ROCK2) are immediate downstream targets of a small GTP-binding protein RhoA 1. The ROCK isoforms are encoded by separate genes on human chromosomes 18q11 (ROCK1) and 2p24 (ROCK2). The ROCKs affect several cellular functions, one of which is cellular contraction, by controlling actin cytoskeletal assembly. The ROCKS enhance actin-myosin association in VSMCs by directly promoting myosin light chain phosphorylation and by inhibiting myosin light chain phosphorylase 2, 3. ROCKs also phosphorylate other molecules that contribute to cellular contraction, such as alpha-adducin, LIM kinases, and smooth muscle-specific basic calponin 1. ROCKs have been reported to affect nitric oxide bioavailability by decreasing the stability of the endothelial nitric oxide synthase (NOS3) messenger RNA and by limiting NOS3 activation via suppression of the PI3K/Akt pathway 1.
Both animal and human studies have shown that treatment with Rho-kinase inhibiting compounds lowers blood pressure 4–6. However, because the available Rho-kinase inhibitors affect both ROCK1 and ROCK2, it has been difficult to evaluate the independent effect of the ROCK isoforms on blood pressure. Seasholtz and co-workers recently reported statistically significant associations between DNA sequence variants at the ROCK2 locus and blood pressure in 168 pairs of mono- and dizygotic twins 7. A C/A transversion in exon 10, which induced a threonine to asparagine substitution in codon 431, accounted for 3% to 5% of the variance in resting and systolic blood pressures, thus suggesting that ROCK2 may contribute to blood pressure levels independently.
The goals of our study were to evaluate the haplotype pattern at the ROCK2 locus by genotyping a dense panel of linkage disequilibrium-tagging SNPs, and to test the associations between the ROCK2 SNPs and hypertension for a large cohort of prospectively verified new incident hypertensive patients and healthy, normotensive controls. Our data strongly suggest that a major haplotype block at the ROCK2 locus is recessively associated with a lower risk of hypertension.
Methods
Subjects
The HYPGENE cohort is based on the Aerobics Center Longitudinal Study (ACLS) database and has been described in detail elsewhere 8. All eligible ACLS subjects for the HYPGENE study reported no history of hypertension and had a resting blood pressure of less than 140/90 mmHg at their first clinic visit. Furthermore, they were required to have at least two clinic visits with a minimum of one year apart. Hypertension in this study was defined as physician-diagnosed hypertension with prescribed medication to lower blood pressure or resting systolic blood pressure of 140 mmHg or more and/or diastolic blood pressure 90 mmHg or more on a follow-up clinic visit. Controls were required to remain normotensive (blood pressure < 140/90 mmHg) and to not take blood pressure–lowering medications throughout the follow-up period. The controls were selected randomly using a sex-specific frequency matching protocol within the 5-year baseline age strata (age at first clinic visit) that allowed up to 20% over-sampling within the sex-by-age strata. Clinical data from both the case and control subjects were confirmed manually by reviewing medical records. The entire HYPGENE cohort includes 629 cases and 605 controls. The current analyses are based on 607 cases and 586 controls, and all were Caucasian subjects. The HYPGENE Study protocol has been approved annually by the Institutional Review Boards of the Pennington Biomedical Research Center and the Cooper Institute. Written informed consent was obtained from all participants.
Phenotype measurements
Resting blood pressure was auscultated as the first and fifth Korotkoff sounds according to a standard sphygmomanometer protocol 9. Cardiorespiratory fitness was assessed by a maximal exercise test following a modified Balke protocol 10. Height and body mass were measured using standardized protocols, and body mass index (BMI) was calculated by dividing body mass (kg) by height squared (m2).
Genotyping
Genomic DNA was prepared from EDTA whole blood with a commercial DNA extraction kit (Gentra Systems, Inc., Minneapolis, MN). The ROCK2 single-nucleotide polymorphisms (SNPs) were selected from the Caucasian data set of the International HapMap consortium (data release 20, January 2006). The HapMap data set contained 160 SNP within 10 kb of the ROCK2 locus (~180 kb of genomic DNA). Of these, 109 SNPs had minor allele frequency greater than 5% and were used for linkage disequilibrium (LD) cluster-tagging SNP (tagSNP) selection. TagSNPs were selected using the pairwise algorithm of the Tagger program 11. The pairwise linkage disequilibrium threshold for the LD clusters was set to r2 ≥ 0.95. Tagger identified 18 tagSNPs, and the number of SNPs in each LD cluster ranged from 1 to 24 (see Supplemental Table I for details).
Genotyping of the ROCK2 SNPs was done using the Illumina (San Diego, CA) GoldenGate chemistry and Sentrix Array Matrix technology on the BeadStation 500GX. Genotype calling was done with the Illumina BeadStudio software, and each call was confirmed manually. For quality control purposes, five CEPH control DNA samples (NA10851, NA10854, NA10857, NA10860, NA10861; all samples included in the HapMap Caucasian panel) were genotyped in triplicate (distributed across 14 arrays). Concordance between the replicates and the ROCK2 genotypes from the HapMap database was 100%.
Statistical analyses
Tests of Hardy-Weinberg equilibrium for each ROCK2 SNP were conducted using the exact test implemented in the PEDSTATS software 12. Pairwise linkage disequilibrium estimates were calculated using the ldmax program, which is available in the GOLD software package 13. Haplotype blocks were generated with the confidence interval method 14 available in the Haploview program 15. Haplotypes were reconstructed using Phase (version 2.1) 16, 17. The initial tests of association between ROCK2 haplotypes and hypertension were carried out using the LAMP software program. These analyses assumed either an unconstrained (--free) or a recessive genetic model, as well as a trait prevalence of 25% 18. Positive associations between LD block haplotypes and hypertension risk were investigated further using logistic regression modeling with individual ROCK2 SNPs. The common allele homozygotes were used as a reference group in the general models; the aggregate group composed of common allele homozygotes and heterozygotes was used as the reference group in the recessive models. All logistic regression models included baseline age, sex, cardiorespiratory fitness, BMI, and follow-up time as covariates.
Since multiple SNPs were used for the association studies, we applied the multiple testing correction as proposed by Nyholt 19. After taking into account pairwise LD between ROCK2 SNPs, the effective number of independent SNPs was found to be 8.2, which translated into an experiment-wise significance threshold of p=0.0062 to keep type I error rate at 5%. Furthermore, a false discovery rate (FDR) was calculated for each set of tests using PROC MULTTEST of the SAS software package (version 9.1; SAS Institute Inc., Cary, NC).
Results
Baseline characteristics between case and control subjects are summarized in Table 1. The mean age of both the case and control subjects at baseline was 43 years. The average follow-up time was 8.7 (SD 6.4) years for the case subjects and 10.1 (SD 7.0) years for the controls. Mean resting blood pressure in all HYPGENE subjects was 114/76 mmHg at baseline. However, both systolic and diastolic blood pressure levels were lower in controls than in cases. At the follow-up visit, the mean resting blood pressure in the controls was 113/75 mmHg, and all controls had resting blood pressure level of 128/84 mmHg or less.
Table 1.
Baseline characteristics of the HYPGENE Study subjects.
| Cases | Controls | |
|---|---|---|
| Number of subjects | 607 | 586 |
| Sex, male/female | 501/106 | 440/146 |
| Age, years | 43.3 (9.2) | 42.6 (8.9) |
| BMI, kg/m2 | 25.1 (3.2) | 24.1 (3.1) |
| Maximal METs | 11.7 (2.1) | 12.3 (2.0) |
| SBP, mmHg | 117.3 (8.7) | 110.6 (9.3) |
| DBP, mmHg | 77.6 (6.0) | 73.8 (6.9) |
| Follow-up, years | 8.7 (6.4) | 10.2 (7.0) |
METs = metabolic equivalents, calculated from treadmill time (see methods for details); SBP = systolic blood pressure; DBP = diastolic blood pressure
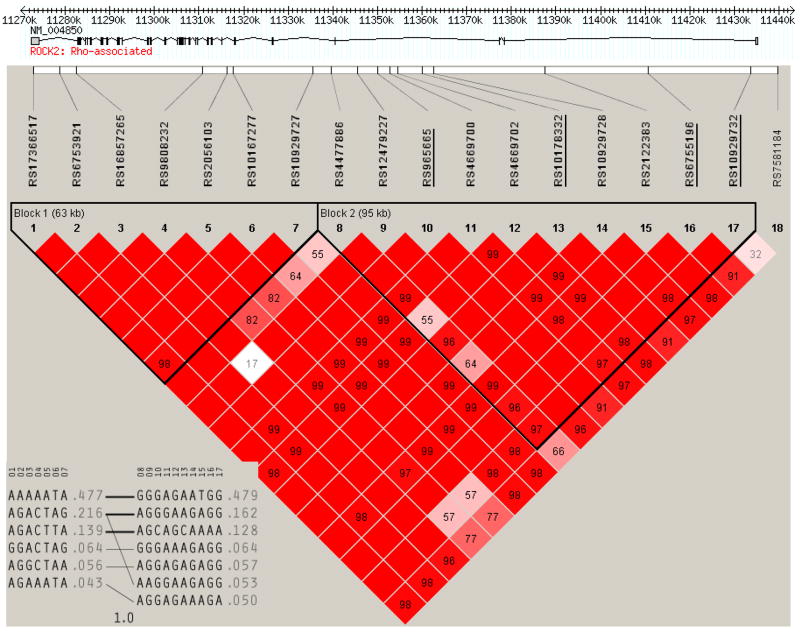
The allele frequencies and pairwise linkage disequilibria among SNPs are summarized in Supplement Tables II and III (will be available online). Genotype call rates were 100% for 13 SNPs, 99.8% for 4 SNPs and 99.7% for 1 SNP. All 18 SNPs were in Hardy-Weinberg equilibrium. The haploview program identified two LD blocks among the 18 ROCK2 tagSNPs in the HYPGENE data (Figure 1). The first block consisted of seven SNPs in the 3′ end of the gene and contained six common (frequency > 5%) haplotypes (Table 2). The second block included 10 SNPs in the 5′ end of the gene and gave rise to seven haplotypes (Table 2). An almost identical LD block structure was confirmed among the 109 HapMap SNPs used for the tagSNP selection (Supplement Figure I).
Figure 1.
Linkage disequilibrium (LD) block structure of the ROCK2 tagSNPs in the HYPGENE cohort. SNPs defining haplotype 3 in block 2 (#10, 13, 16, 17) that were associated with lower risk of hypertension are underlined. The 5′-end of the gene is located at 11,435 kb and the 3′-end at 11,273 kb.
Table 2.
Associations between the ROCK2 haplotypes from LD blocks 1 and 2 and the risk of hypertension. LD blocks were identified with Haploview program and sequences indicate the nucleotides for SNP 1 – 7 in block 1 and SNPs 8 – 17 in block 2 (see Figure 1 and Supplemental Table II for details). ‘LAMP free’ and ‘LAMP rec.’ indicate p-values from unrestricted and recessive models of the LAMP program, respectively. Column “freq.” indicates the frequency of a given haplotype allele in the HYPGENE cohort.
| Block 1
|
Block 2
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Haplo | Sequence | freq. | LAMP free | LAMP rec. | Haplo | Sequence | freq. | LAMP free | LAMP rec. |
| 1_1 | AAAAATA | 0.477 | 0.7 | 0.4 | 2_1 | GGGAGAATGG | 0.479 | 0.4 | 0.3 |
| 1_2 | AGACTAG | 0.216 | 0.4 | 0.2 | 2_2 | AGGGAAGAGG | 0.162 | 0.4 | 0.2 |
| 1_3 | AGACTTA | 0.139 | 0.012 | 0.003* | 2_3 | AGCAGCAAAA | 0.128 | 0.008 | 0.002* |
| 1_4 | GGACTAG | 0.064 | 0.5 | 0.7 | 2_4 | GGGAAAGAGG | 0.064 | 0.5 | 0.7 |
| 1_5 | AGGCTAA | 0.056 | 0.3 | 0.13 | 2_5 | AGGAGAGAGG | 0.057 | 0.3 | 0.14 |
| 1_6 | AGAAATA | 0.043 | 0.5 | 0.2 | 2_6 | AAGGAAGAGG | 0.053 | 0.9 | 0.7 |
| 2_7 | AGGAGAAAGA | 0.05 | 0.3 | 0.14 | |||||
False discovery rate: q = 0.02
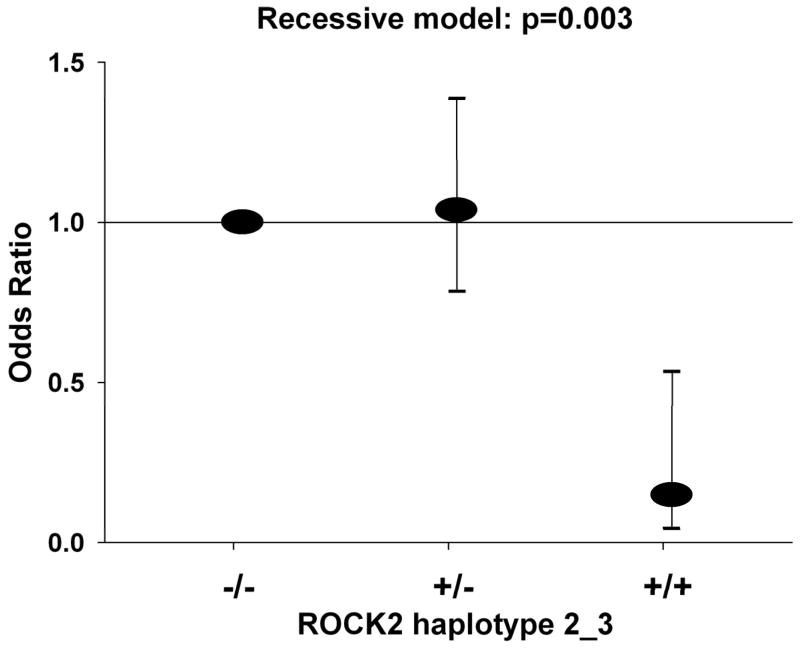
Haplotype 1_3 in block 1 (p=0.003) and haplotype 2_3 in block 2 (p=0.002) were significantly associated with hypertension under the recessive models (Table 2, Figure 2). Haplotype 2_3 in block 2 contained the minor alleles of rs965665, rs10178332, and rs6755196, as well as 72% of the rs10929732 minor alleles. Haplotype 2_3 genotype frequencies were in Hardy-Weinberg equilibrium for all HYPGENE subjects. However, 86% of the haplotype 2_3 homozygotes were found among the controls whereas only 14% were found among the case subjects. The associations were confirmed in single SNP analyses: homozygotes for the minor alleles at loci rs965665, rs10178332, and rs6755196 had an 85% lower risk of hypertension than carriers of the common allele (p<0.0048 in a recessive model; Supplemental Table IV). A closer inspection of haplotype 1_3 in block 1 revealed that the association identified by LAMP was due to complete linkage disequilibrium among the alleles of haplotype 2_3. Likewise, none of the SNPs in block 1 were associated with risk of hypertension in the single SNP analyses (Table 3).
Figure 2.
Haplotype 2_3 of the ROCK2 locus and risk of hypertension.
Table 3.
Associations between the individual ROCK2 SNPs and the risk of hypertension in the HYPGENE subjects. P-values are from additive and recessive logistic regression models that included baseline age, cardiorespiratory fitness, body mass index, sex, and follow-up time as covariates. SNPs 1 – 7 are in LD block 1 and SNPs 8 – 17 in LD block 2.
| # | Name | Position | MAF | Additive | Recessive |
|---|---|---|---|---|---|
| 1 | rs17366517 | 11,271,266 | 0.064 | 0.6794 | 0.6607 |
| 2 | rs6753921 | 11,277,353 | 0.477 | 0.513 | 0.4536 |
| 3 | rs16857265 | 11,281,010 | 0.056 | 0.5329 | 0.2754 |
| 4 | rs9808232 | 11,309,718 | 0.48 | 0.7532 | 0.6852 |
| 5 | rs2056103 | 11,315,354 | 0.475 | 0.6864 | 0.8013 |
| 6 | rs10167277 | 11,316,598 | 0.336 | 0.5371 | 0.2656 |
| 7 | rs10929727 | 11,334,812 | 0.279 | 0.7612 | 0.5152 |
|
| |||||
| 8 | rs4477886 | 11,338,892 | 0.451 | 0.2038 | 0.2805 |
| 9 | rs12479227 | 11,344,951 | 0.053 | 0.9794 | 0.9629 |
| 10 | rs965665 | 11,349,473 | 0.128 | 0.0121 | 0.0031* |
| 11 | rs4669700 | 11,352,136 | 0.216 | 0.2746 | 0.1151 |
| 12 | rs4669702 | 11,354,050 | 0.279 | 0.779 | 0.5273 |
| 13 | rs10178332 | 11,359,615 | 0.131 | 0.0173 | 0.0047* |
| 14 | rs10929728 | 11,362,081 | 0.336 | 0.5471 | 0.2722 |
| 15 | rs2122383 | 11,387,366 | 0.48 | 0.3473 | 0.4236 |
| 16 | rs6755196 | 11,410,923 | 0.131 | 0.0171 | 0.0046* |
| 17 | rs10929732 | 11,434,090 | 0.182 | 0.0483 | 0.0138 |
|
| |||||
| 18 | rs7581184 | 11,440,129 | 0.381 | 0.1001 | 0.0504 |
False discovery rate: q < 0.05
Discussion
The present study identified a major haplotype block in the ROCK2 gene locus as being associated with a lower risk of hypertension in a recessive manner. The homozygotes of a haplotype that was defined by four SNPs tagging an LD block that covered ~137 kb of the gene had an 85% lower risk of hypertension than those who carried no copy or only one copy of the haplotype. Although two previous studies have tested the associations between ROCK2 gene polymorphisms and blood pressure 7, 20, our study is the first to report a systematic dense tagSNP screening of the ROCK2 locus in terms of hypertension risk. In a cohort of mono- and dizygotic twins with a median age of 41 years, the Thr431Asn (rs9808232) variant in exon 10 was reported to be associated with resting systolic and diastolic blood pressures 7. However, in a population-based sample of men and women selected from the top and bottom 5th percentiles of blood pressure distribution, three SNPs located in the ROCK2 region were not associated with blood pressure levels 20. All four SNPs reported in these previous studies were covered by our tagSNP panel (LD clusters 2, 4, and 6 in Supplemental Figure III), but none of them was associated with a higher risk of hypertension. None of the SNPs associated with a lower risk of hypertension in our study (LD clusters 1, 5, and 7) was tested in the previous reports. The locus heterogeneity in associated ROCK2 markers between the previous reports 7 and our current study may be related to differences in study design (cross-sectional twin study vs. longitudinal cohort study), primary traits (resting blood pressure vs. incidence of hypertension), gender distribution (22% vs. 79% males), and sample size (344 vs. 1193).
Our data suggest that the haplotype block tagged by SNPs rs965665, rs10178332, and rs6755196 may contain functional sequence variant(s) that reduce the level of ROCK2 expression and/or its activity. However, this hypothesis must be experimentally confirmed. Identification of the underlying variant(s) will be a major challenge because of the large size of the LD block and high number of sequence variants in the block. Based on the HYPGENE tagSNP data, the block spans about 98 kb of genomic DNA and includes most of the sequences of intron 1 and exons 2, 3 and 4. However, after considering all 109 HapMap SNPs that were used for the tagSNP selection, the LD block expanded to 137 kb and covered about 84% of the ROCK2 locus, including exons 2 to 21. In fact, 24 SNPs in the LD cluster (r2 ≥ 0.95) tagged by rs6755196 in the HapMap data were distributed over 162 kb of genomic DNA (see Supplemental Figure II and Table I for details). Interestingly, the block included the exons encoding the kinase domain (including the ATP binding domain) of ROCK2, whereas the exons encoding the RhoA binding domain, granzyme B cleavage site, and C-terminal autoinhibitory domains (such as the pleckstrin-homology domain) all fell outside the block 21, 22. Thus, the LD block pattern would suggest that functional mutation(s) would reduce the kinase domain activity directly rather than modifying the carboxy-terminal regulatory elements of the protein. Interestingly, currently available ROCK inhibitors act on the kinase domain of the molecule. It is also possible that these SNPs located within the ROCK2 locus modify regulatory elements affecting expression of other genes in the same chromosomal region. In addition, we can not rule out possible effects of the SNPs on microRNA production. However, these hypotheses need to be investigated in future studies.
Our data on the recessive protective effect of the ROCK2 SNPs on hypertension risk fit well with the theory of dominance first proposed by Wright and later elaborated by Kacser and Burns 23. The Kacser and Burns theory posited a hyperbolic relationship between the activity of a single component (gene product) of a multi-enzyme system and the overall flux rate of the system. The activity of the gene products yielded by the “wild-type” (homozygote) and mutant (heterozygote, homozygote) genotypes---the latter resulting from “upward” mutations that increase enzyme activity---are usually located on the plateau of a hyperbolic curve. In contrast, the enzymatic activity of mutant genotypes, as created by “downward” mutations that decrease enzymatic activity, are likely to fall on the steep downward slope of the curve, thereby resulting in recessivity.
Considering the role of ROCK2 in the regulation of smooth muscle cell contraction and nitric oxide production pathways, one could speculate that a lower ROCK2 activity would translate into lower contractility and greater nitric oxide availability and, consequently, lower blood pressure. Thus, any mutations that decrease ROCK2 expression and/or activity may reduce VSMC contractility, increase NO production, and lower blood pressure (hypertension risk) in a recessive manner.
Our study had several strengths. First, all subjects were healthy and normotensive at baseline. Therefore, all our case subjects were true incident case subjects and all controls were confirmed to remain normotensive throughout the follow-up period. Thus, the risk of additional confounding variables due to undiagnosed hypertension among the controls was minimized. Second, we statistically controlled for two major predictors of hypertension--- cardiorespiratory fitness and body mass index---and confirmed that the observed association of ROCK2 locus with hypertension is independent of fitness level and obesity. Third, we implemented a rigorous tagSNP selection strategy that captured all major ROCK2 haplotypes present in Caucasians. At the same time, the main limitation of the study was that our results were limited to Caucasians.
In summary, data from the HYPGENE study suggested that DNA sequence variation in the 5′ end of the ROCK2 gene protects against hypertension in a recessive manner. Identification of functional mutation(s) could thus potentially help in the development of ROCK2-specific therapies for hypertension and other ROCK2-related disorders.
Supplementary Material
Acknowledgments
Sources of funding: The HYPGENE Study by is supported by NHLBI grant HL-069870 (T. Rankinen, PI). The ACLS was supported for many years by the National Institute of Aging Grant AG-06945 (S.N. Blair, PI). C. Bouchard is partially supported by the George A. Bray Chair in Nutrition and T. Church by the John S. McIlhenny Endowed Chair of Health Wisdom.
We thank the physicians and technicians of the Cooper Clinic for collecting the data, and the Cooper Institute for the collaboration to establish the ACLS DNA bank.
References
- 1.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290:C661–668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kureishi Y, Kobayashi S, Amano MM, et al. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- 3.Kimura K, Ito M, Amano M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 4.Nohria A, Grunert ME, Rikitake Y, et al. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99:1426–1432. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 6.Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 7.Seasholtz TM, Wessel J, Rao F, et al. Rho kinase polymorphism influences blood pressure and systemic vascular resistance in human twins: role of heredity. Hypertension. 2006;47:937–947. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- 8.Rankinen T, Church TS, Rice T, Blair SN, Bouchard C. Cardiorespiratory fitness, BMI, and risk of hypertension: the HYPGENE Study. Med Sci Sports Exerc. 2007;39:1687–1692. doi: 10.1249/mss.0b013e31812e527f. [DOI] [PubMed] [Google Scholar]
- 9.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 10.Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. US Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 11.de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 12.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abecasis GR, Cookson WO. GOLD--graphical overview of linkage disequilibrium. Bioinformatics. 2000;16:182–183. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 15.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 16.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Boehnke M, Abecasis GR. Efficient study designs for test of genetic association using sibship data and unrelated cases and controls. Am J Hum Genet. 2006;78:778–792. doi: 10.1086/503711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rana BK, Insel PA, Payne SH, et al. Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106. doi: 10.1161/01.HYP.0000252029.35106.67. [DOI] [PubMed] [Google Scholar]
- 21.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 22.Sebbagh M, Hamelin J, Bertoglio J, Solary E, Breard J. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J Exp Med. 2005;201:465–471. doi: 10.1084/jem.20031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kacser H, Burns JA. The molecular basis of dominance. Genetics. 1981;97:639–666. doi: 10.1093/genetics/97.3-4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.