Abstract
Microglia are resident macrophage-like antigen presenting cells of the central nervous system (CNS). To avoid escalation of inflammatory processes and bystander damage within the CNS, microglia-driven inflammatory responses need to be tightly regulated and both spatially and temporally restricted. Following traumatic, infectious and autoimmune-mediated brain injury, natural killer (NK) cells have been found in the CNS, but the functional significance of NK cell recruitment and their mechanisms of action during brain inflammation are not well understood. Here, we investigated whether and by which mechanisms human NK cells might edit resting and activated human microglial cells via cytotoxicity. IL-2 activated NK cells efficiently killed both resting allogeneic and autologous microglia in a cell-contact dependent manner. In addition they produced IFN-γ upon microglia recognition. Activated NK cells rapidly formed synapses with human microglial cells, polarizing perforin to the cellular interface. Antibody-mediated NKG2D and NKp46, but not DNAM-1 and NKp30 blockade decreased killing of human microglia by activated NK cells. Up-regulation of MHC class I surface expression by TLR4 stimulation protected microglia from NK cell mediated cytotoxicity These data suggest that brain-infiltrating NK cells might restrict innate and adaptive immune responses within the human CNS via elimination of resting microglia.
Keywords: Human, Cytotoxicity, Microglia, Natural Killer cells
Introduction
Microglia are an integral part of the resident mononuclear phagocyte population in the central nervous system (CNS) and share many phenotypic and functional characteristics with other tissue macrophages and peripheral blood monocytes (1). Any type of injury or pathological process within the CNS leads to activation of these cells from their resting state (2). Subsequent to activation, microglial cells produce proinflammatory cytokines, such as tumor necrosis factor-alpha, interleukin-6 and interleukin-12, and acquire the ability to process antigens for presentation to infiltrating T cells on up-regulated major histocompatibility complex (MHC) class I and class II molecules in conjunction with co-stimulatory molecules (3). To avoid escalation of inflammatory processes and bystander damage within the CNS, microglia-driven inflammatory responses need to be tightly regulated and both spatially and temporally restricted (3) (4) (5).
NK cells have been suggested to edit human myeloid cells during immune responses via cytotoxic activity (6) (7). This NK cell effector function is controlled by germ-line encoded activating and inhibitory receptors on these innate lymphocytes. Only if more activating signals are received due to high expression of activating NK cell receptor ligands or loss of inhibitory NK cell receptor ligands, NK cell killing is unleashed. The main constitutive activating NK cell receptors are NKG2D, the natural cytotoxicity receptors (NCRs) NKp30 and NKp46, and DNAM-1. The ligands for these activating receptors are primarily up-regulated upon cellular stress, like during viral infections or transformation (8) (9). As inhibitory structures, MHC class I molecules are primarily recognized by killer immunoglobulin-like receptors (KIRs) or CD94/NKG2A (8) (9). Therefore, stressed cells with down-regulated MHC class I molecules are preferentially targeted by NK cells.
The immune privilege of the CNS is thought to be maintained by endothelial tight junctions of the blood brain barrier (BBB) and the presence of an immunosuppressive microenvironment (10). BBB disruption leads to recruitment of a large number of leukocytes into the CNS, among which natural killer (NK) cells have been shown to migrate to the CNS following traumatic peripheral axotomy (11) (12) (13), and in the course of infectious as well as autoimmune CNS inflammation (14) (15) (16) (17). Whereas the frequency of CNS-infiltrating NK cells in acute and chronic neuroinflammatory diseases in humans has so far not been thoroughly characterized, it was estimated that NK cells comprise 10-20% of CNS infiltrates in experimental autoimmune encephalomyelitis (EAE) models (15) (16). However, the functional significance of NK cell recruitment and their mechanism of action during brain inflammation are not well understood. In order to address a possible immunoregulatory function of NK cells within the inflamed human CNS, we investigated NK cell interactions with resting and activated human microglia in vitro.
Materials and Methods
Primary human microglia cell culture
Second trimester fetal brain tissue was obtained under institutional IRB approved protocols from the fetal tissue repository (FTR) at the Albert Einstein College of Medicine (New York, NY) or ABR, Inc. (Alameda, CA). Mixed human fetal CNS cell cultures were prepared by enzymatic and mechanical dissociation of the cerebral tissues followed by filtration through nylon meshes of 250- and 130μm pore sizes, as described previously (18). Cells were plated at 106 to 107 cells per ml in Dulbecco’s modified Eagle medium (DMEM) (Cellgro: supplemented with 4.5 g/liter glucose, 4 mM L-glutamine, and 25 mM HEPES; L-tryptophan concentration, 16 μg/ml) plus 5% fetal calf serum (Gemini Bio-products, Woodland, CA), penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (Fungizone; 0.25 μg/ml) for 2 weeks. Briefly, highly enriched cultures of human fetal microglia (>98% CD11b+ cells as determined by flow cytometry, compared to respective isotype control) were generated by gentle collection of floating cells in confluent monolayer cultures consisting of astrocytes, neurons, and microglia at 2 to 3 weeks in vitro. Cells were seeded in low-cell-binding 24-well-plates (Nunc, Tokyo, Japan) at 250,000 cells per well. In some experiments, microglia were preactivated for 48 hours with 100ng/ml or 200ng/ml LPS from Salmonella Minnesota (Sigma Aldrich) and compared to medium alone.
Generation of Macrophages and DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from leukocyte concentrates (New York Blood Center) by density gradient centrifugation (Ficoll-Paque Plus, Amersham Pharmacia Biotech.). Serum was collected, heat inactivated for 30 minutes at 56°C, and filtered. Monocytes were isolated by magnetic CD14+ selection (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany). Monocytes were cultured as described previously (19) in X-vivo media with 1% autologous serum for 10-12 days to generate macrophages. For activation, macrophages were incubated with 100ng/ml LPS from Salmonella Minnesota (Sigma Aldrich) for 48 hours. Immature DCs were generated according to standard protocol (20). Briefly, CD14+ cells were cultured for 5 days in RPMI1640, 1% heat inactivated human Ab serum (Cellgro), gentamicin, IL-4, and GMCSF. Where indicated, DCs were matured with 25μg/ml poly(I:C) (pI:C; Invivogen) or 100ng/ml LPS (Sigma Aldrich) for 48 hours.
Isolation of Natural Killer cells
NK cells were isolated by negative selection using the NK cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer’s instructions. The purity of the isolated B cells and NK cells was higher than 90% and contained less than 5% contaminating T cells as determined by flow cytometry. Separated NK cells were activated with 100U/ml IL-2 for 5 days before co-culture.
IFN-γ production assay
For intracellular staining of IFN-γ, activated NK cells were incubated with PKH26-labeled microglia at a ratio of 10:1 at 37°C in RPMI1640 + 5% human serum + gentamycin. Brefeldin A was added after 1 hour of co-culture and additional 5 hours later cells were harvested and stained with Aqua Live/Dead Cell stain (Invitrogen) according to manufacturers protocol. After fixing the cells with 2% paraformaldehyde, they were permeabilized and stained with fluorochrome labeled anti-IFN-γ. PKH26-, live, small cells were analyzed using Flowjo software (Treestar, Ashland, OR).
Flow Cytometry Analysis
Microglia were stained with fluorochrome-labeled antibodies specific for CD11b (M1/70; BD), MHC class I HLA-A,B,C (W6/32, Biolegends), PVR (CD155, MBL) and their respective isotype controls for 30 minutes at 4°C. Staining with the unlabeled Nectin-2 antibody (Santa Cruz Biotech.) was followed by incubation with a fluorochrome labeled secondary anti-mouse antibody (BD biosciences) for 30 minutes at 4°C. For NKG2D ligand staining, biotinylated NKG2D monomers were kindly provided by Alexander Steinle, Tübingen, Germany, and tetramerized as previously described for MHC class I tetramers (21). HLA-A2/HIV gag peptide tetramers served as a control for NKG2D tetramer staining of NKG2D ligands. After two washes, cells were re-suspended in 200 μl of PBS. At least 30,000 events were collected on a BD LSR II flow cytometer (BD Biosciences, San Jose, CA). Frequencies were calculated using FlowJo software (Tree Star, Ashland, OR).
TO-PRO-3 cytotoxicity assay
Cytotoxicity of NK cells was quantified using the previously described flow cytometry-based TO-PRO-3 assay (22). It has to be noted that this assay might underestimate the total number of dead cells, since the frequency of dying cells in this assay is based on the forward-scatter/sideward-scatter live cell gate rather than on release of chromium-51. A number of previous studies have, however, shown that cytotoxicity measured by the flow cytometric cell-mediated cytotoxicity assay correlates well with the conventional chromium-51 release assay and the PKH-26/TO-PRO-3 iodide-based assay is now widely used as a reproducible experimental system for NK cell and antibody-dependent cell-mediated cytotoxicity (22) (23) (24) (25). Briefly, human microglial cells, macrophages or DCs were labeled with a membrane dye, PKH-26, to allow discrimination of target cells after incubation with effector cells. Post-incubation, cell death within the PKH-26+ target cell population was assessed by the addition of the viability probe TO-PRO-3 iodide (Invitrogen). Flow cytometry data were acquired on a BD LSRII flow cytometer and analyzed with FlowJo software (Tree Star). This method allows analysis to be conducted on a single cell basis and overcomes the need for radioactive chemicals. For neutralization experiments NK cells were preincubated for one hour at 37°C with a 10μg/ml antibody concentration. The blocking antibodies, NKG2D (BAT221), NKp30 (F252) and NKp46 (KL247) were kind gifts of Drs. Alessandro Moretta, Genoa, Italy and Guido Ferlazzo, Messina, Italy. In addition, MHC class I antibody (W6/32, Biolegends), DNAM-1 (MAB 666, R&D), LFA-1 (CD11a, Biolegend), and 2B4 (CD244, Beckman Coulter) were used.
Conjugation of Human microglia with NK cells
For conjugation assays, resting or IL-2 activated NK cells were cocultured with microglia. Cells were mixed at an NK cell to microglia ratio of 1:1 in 20μl of RPMI1640 without serum, prior to being pelleted at 10,000g. The pellets were allowed to conjugate for 1min at 37°C. After initial conjugation, cells were gently resuspended and centrifuged onto poly-lysine-coated 1.5mm coverslips for immunofluorescence analysis. Cells on slides were fixed in 3% paraformaldehyde for 20min at 4°C. Cells were permeabilized with 0.01% Triton-X for 1min at room temperature, and stained with anti—talin (TD77) or anti-perforin (δG9) Abs (Chemicon International), followed by Alexa Fluor 555-conjugated rabbit anti-mouse IgG (Invitrogen). Control Abs used were mouse IgG1 (MOPC-21, BioLegend) and mouse IgG2b,κ (MOPC-141, Sigma). All washes were performed in PBS supplemented with 1% fish skin gelatin (Sigma-Aldrich) and 0.02% saponin (Sigma-Aldrich). Slides were counterstained with DAPI and mounted with Prolong gold antifade reagent (Invitrogen) and visualized with an inverted Olympus IX-70 microscope (DeltaVision Microscope; Applied Precision/Olympus) and a Photometrics CoolSnap QE camera. Serial optical sections (0.2 μm; 20 sections) were acquired for all labelings. Images were then deconvoluted using DeltaVision SoftWoRx software version 3.4.4, and pictures were overlaid using Metamorph software version 6.3r6 (Universal Imaging Corporation). Molecules at the synapse were quantified with ImageJ software. The enrichment of talin at the contact site of each conjugate was measured and compared with the distribution of the same molecule in a similar area opposite to the contact site within the same cell. The relative enrichment (RE) was calculated as the average intensity per volume unit at the contact site or in an equivalent volume opposite to the contact site divided by the average intensity per unit volume of the entire cell. The area of the contact site was defined manually and represented 16.8% ± 4.2% of the total cell volume. The area across the contact site was defined manually and represented 17.2% ± 4.4% of the total cell volume. The values obtained in unconjugated cells were considered as baseline, and data represent the ratio of RE at the contact site to RE at an equivalent volume opposite from the contact site normalized to the values in single cells, assigned as 100%. The percentage of conjugates showing perforin polarization was assessed in randomly selected fields after the analysis of at least 500 conjugates per experiment.
Statistics
Statistical analyses were performed with the student t test using Prism software (Graphpad). A p value of less than 0.05 was considered significant. Plotted data are displayed as mean with standard error of the mean.
Results
1. Activated NK cells kill allogeneic and autologous human microglia in a cell-contact dependent manner
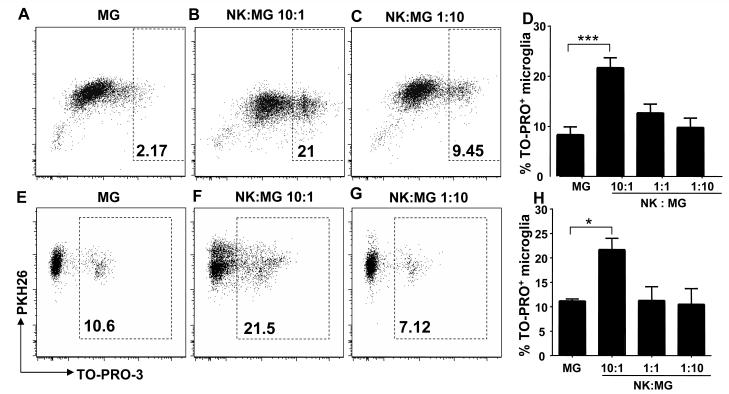
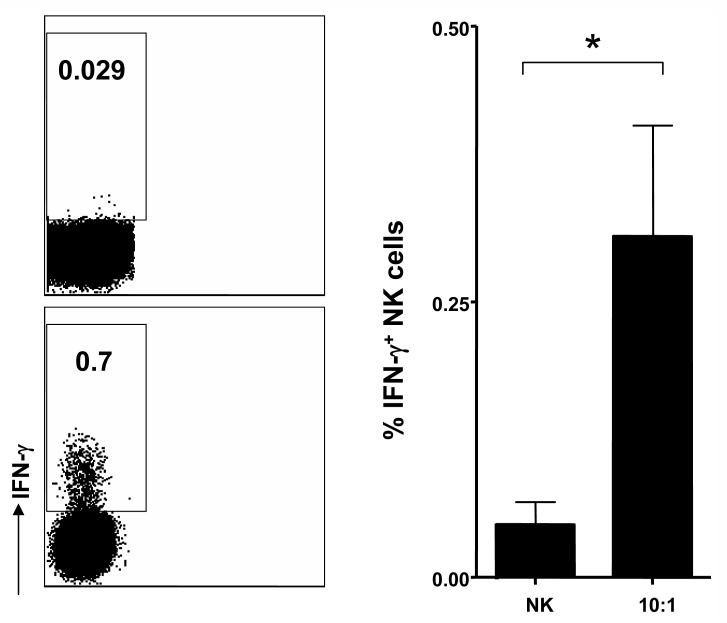
To determine whether human microglial cells are susceptible to NK cell-mediated cell editing by killing, we performed a flow cytometry-based cytotoxicity assay using the fluorophores PKH-26 to label microglia and the DNA intercalating dye TO-PRO-3 iodide (TP3) to determine the viability of PHK-26+ target cells. The extent of cytotoxicity was quantified by the relative number of live human microglial cells labeled with PKH-26 and dead, permeabilized cells labeled with both PKH-26 and TP3 (22). As shown in Figure 1, IL-2 activated NK cells efficiently killed microglial cells at effector to target (E:T) ratios of 10:1 after 4 hours of incubation. Such an E:T ratio might be specifically relevant during acute CNS inflammation during which NK cells comprise 10-20% of CNS cellular infiltrates (15) (16). In addition, microglia cell recognition was associated with induction of IFN-γ production (Fig. 2) by activated NK cells indicating that microglia trigger both NK cell cytokine production and cytototoxic activity. Autologous microglial cells, cocultured with autologous NK cells isolated from fetal liver tissue, were killed at similar levels as allogeneic target cells (Fig. 1). These results suggest that resting microglia can be killed by activated NK cells.
Figure 1. Natural Killer cells kill human microglia.
Fetal human microglial cells were cocultured with allogeneic (upper panel) or autologous (lower panel) IL-2 activated NK cells for 4 hr. The extent of cytotoxicity was quantified by the relative number of live human microglial cells labeled with PKH-26 and dead, permeabilized cells labeled with both PKH-26 and TO-PRO-3 iodide. Fig. 1 A, E: spontaneous permeabilization of primary human microglia. Fig. 1 B, F and C, G: cellular permeabilization at different E:T ratios. Fig. 1 D, H: diagrams summarize at least three experiments and display means and SEM of TO-PRO-3+ microglia cells compared to PKH26+ microglial cells after coculture with increasing E:T ratios. D: MG vs 10:1, *** p<0.0001 for E:T ratio of 10:1. H: MG vs 10:1, * p=0.0492 for E:T ratio of 10:1, student t test. MG: Microglia, NK: Natural Killer cells.
Figure 2. Natural Killer cells produce IFN-γ upon recognition of microglia.
IL-2 activated Natural Killer cells were cocultured with microglia in an E:T ratio of 10:1, as for the cytotoxicity assay. Intracellular staining for IFN-γ was performed after 6 hours of coculture. The frequency of IFN-γ producing activated NK cells was 6.5-fold higher following microglia compared to non target cell recognition (mean frequency ± SEM of IFN-γ producing IL-2 activated NK cells in microglia cocultures vs. IL-2 activated NK cells alone: 0.04 ± 0.01 vs. 0.01 ± 0.002; p = 0.02). Representative and summarized data of four experiments are shown.
2. Rapid synapse formation and perforin polarization in NK cell-microglia conjugates
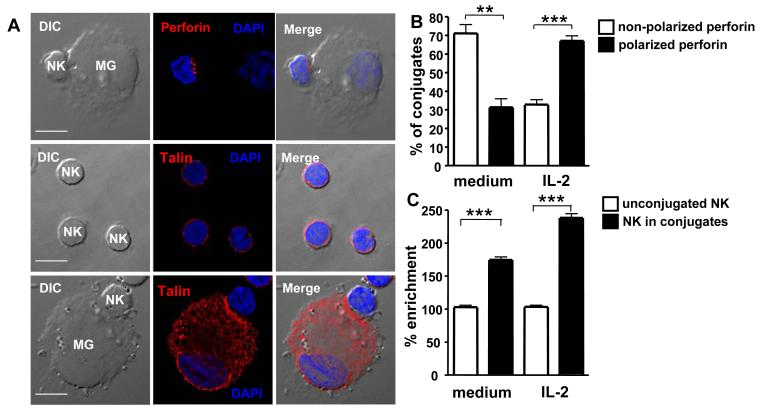
To further analyze microglia killing by NK cells, we determined the formation and kinetics of cytotoxic cell-to-cell contact interactions between NK cells and microglial cells in vitro. For this purpose, we analyzed the polarization of the cytoskeletal protein talin, indicative of synapse formation, and of perforin, a pore-forming molecule of cytotoxic lymphocytes, in NK cells by 2-dimensional immunofluorescence microscopy after 1 min. As shown in Figure 3, talin was evenly distributed at the cell surface in unconjugated, resting and activated NK cells. In microglia/NK cell conjugates, talin was enriched at the cell contact area compared to the opposite side from the interface between microglia and NK cells (relative enrichment [RE] mean ± SEM: 169.2% ± 4.5% in resting NK cells and 230.2% ± 5.9% in activated NK cells, respectively). The percentage of conjugates showing perforin polarization was assessed after the analysis of at least 500 conjugates per experiment, in randomly selected fields. When resting NK cells formed conjugates with microglia, 31.4% ± 4.7% (mean ± SEM of conjugates recruited perforin to the contact area within 1 min of coculture (Figure 3B) compared to 67.2% ± 2.7% (mean ± SEM) when activated NK cells formed conjugates with microglia. These data indicated that NK cells rapidly formed immunological synapses with human microglial cells and that the cytotoxic molecule perforin was rapidly polarized to the interface between microglia and NK cells.
Figure 3. Synapse formation and perforin polarization in NK cell-microglia conjugates.
Polarization of the cytoskeletal protein talin, indicative of synapse formation, and of the cytotoxic molecule perforin in NK cells was determined by 2-dimensional immunofluorescence microscopy after 1 min. Fig. 3A: Perforin and talin polarization in IL-2 activated NK cells conjugated to microglia. Fig. 3B: Perforin is polarized in conjugates of microglia with IL-2 activated NK cells. Non-polarized vs. polarized perforin in conjugates with resting NK cells, ** p=0.001; Non-polarized vs. polarized perforin in conjugates with activated NK cells, *** p<0.0001; student t test. Fig. 3C: relative enrichment (RE) of talin to cell contact areas in NK cell conjugates with microglia compared to NK cells alone. Resting NK cells alone vs. conjugated, *** p<0.0001; Activated NK cells alone vs. conjugated, *** p<0.0001; student t test. Representative and summarized data for three experiments are shown.
3. NK cell-mediated cytotoxicity towards human microglia is NKG2D- and NKp46-dependent and enhanced by MHC class I blockade
In order to address the functional relevance of the observed cytotoxic conjugate formation, we analyzed whether interruption of cell-contact and mAb-mediated masking of activating NK cell receptors could affect NK cell cytotoxicity against microglial cells. To assess the dependency on soluble factors as opposed to direct cell-to-cell contact on microglial cell cytotoxicity, we quantified NK cell cytotoxic activity in transwell vs. non-transwell cocultures. As shown in Fig. 4A, the frequency of TP3+ microglial cells in transwell cultures at E:T rations of 10:1 was similar to spontaneous microglial cytotoxicity, demonstrating that NK cell killing of human microglia was primarily cell-contact dependent. Furthermore, a marked inhibition was detected upon addition of anti-NKp46 mAb and NK-mediated cytotoxicity was virtually abrogated following blockade of the activating NKG2D receptor. In contrast, the addition of anti-NKp30 mAb as well as the isotype control antibody had no effect on cytotoxicity (Fig. 4B). In addition, DNAM-1-, LFA-1-, and 2B4-specific monoclonal blocking antibodies did also not inhibit NK cell-mediated lysis of human microglial cells (Fig. 4C). The observation that cell-contact dependent NK cell killing of microglia is not inhibited by LFA-1 blockade indicates a certain level of redundancy of adhesion molecules in mediating synapse formation between the two cell types (26). Moreover, in contrast to immature DCs, which are killed via NKp30, NKp46 and DNAM-1 mediated recognition (27) (28) (29), but similar to macrophages which have been found to be susceptible to NKG2D dependent cytotoxicity by NK cells (19), our data indicate that microglia cell lysis by NK cells is specifically dependent on NKG2D- and NKp46-mediated recognition.
Figure 4. Killing of human microglia by NK cells is dependent on cell-contact and NKG2D and NKp46.
Microglia cells were co-cultured with purified and IL-2 activated NK cells for 4 hr at an E;T ratio of 10:1. Specific cell lysis was quantified by TO-PRO-3 staining. Shown are means and SEM of TO-PRO-3+ microglia cells compared to PKH26+ microglia cells. Fig. 4A: Co-culture was performed using transwell (TR) and non-transwell plates. 10:1 vs. TR 10:1, *p=0.0492. TR: transwell. Fig. 4B: NK cells were preincubated for one hour w/o antibodies, with IgM and IgG Isotype control antibodies, anti-NKp30, anti-NKp46 or anti-NKG2D antibody. NK:MG 10:1 vs NKG2D, *** P<0.0009, NK:MG 10:1 vs NKp46, *p = 0.03. Fig. 4C: NK cells were preincubated for one hour with IgG Isotype control antibodies, anti-DNAM-1, anti-LFA-1 or anti-2B4 antibody, ** p = 0.0073. Three experiments are summarized.
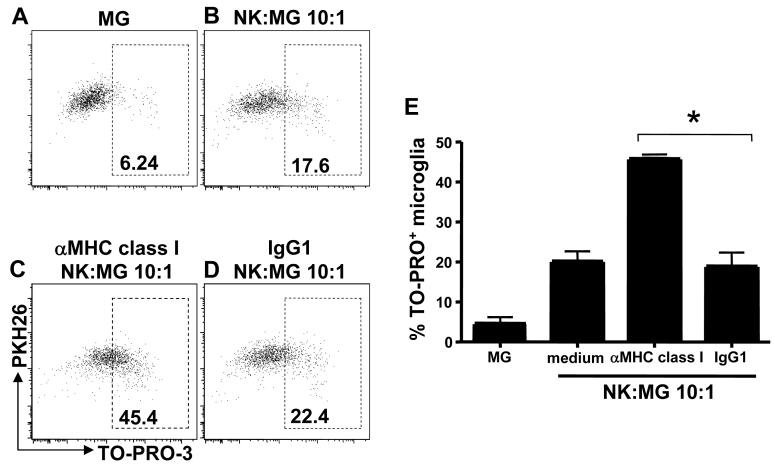
With respect to stimulation of inhibitory NK cell receptors by microglia cells, blockade of the MHC class I molecules, HLA-A,B,C, enhanced NK cell-mediated cell cytotoxicity, indicating that the modest MHC class I expression of microglia, nevertheless, inhibited NK cell killing to some extent (Fig. 5). These data indicated that the opposing activating and inhibitory signals, involved in NK cell recognition of microglia, were mediated by the NK cell receptors NKG2D and NKp46, and MHC class I binding receptors, respectively.
Figure 5. MHC class I blockade enhances NK cell cytotoxicity towards human microglia.
Microglia cells were co-cultured with purified and IL-2 activated NK cells for 4 hr in the presence and absence of a monoclonal blocking MHC class I-specific antibody or an isotype control. Specific cell lysis was quantified by TO-PRO-3 staining. The diagram displays means and SEM of TO-PRO-3+ microglia cells compared to PKH26+ microglia cells after coculture. MHC class I vs. IgG1, * P=0.0171, student t test. Representative data for three experiments are shown.
4. Activated microglia are protected from NK cell-mediated cytotoxicity
Microglia are resident macrophage-like cells of the CNS, and recognize the presence of pathogens in part via Toll-like receptors (TLR) that detect ligands on bacterial, viral, and fungal pathogens. Since TLR signaling in macrophages has been shown to induce NKG2D ligand expression (30), and since TLR-mediated activation of monocyte-derived macrophages is reported to be associated with an increased susceptibility towards NK cell-mediated cytotoxicity (19), we investigated whether TLR ligation interfered with the expression of ligands for activating NK cell receptors, i.e. NKG2D and DNAM-1, and the susceptibility of microglial cells towards NK cell cytotoxicity.
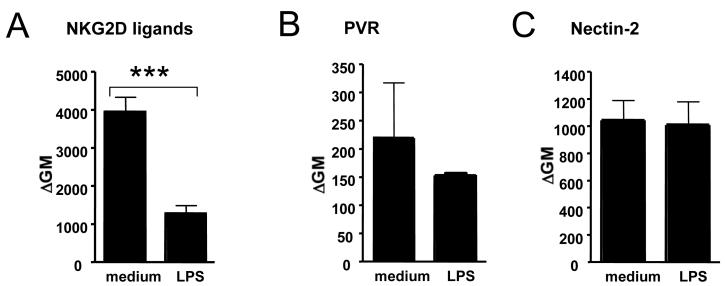
Using NKG2D tetramers, we found that NKG2D ligands are constitutively expressed on human microglial cells in vitro and were downregulated following LPS stimulation (Fig. 6A). Microglia also constitutively expressed low levels of DNAM-1 ligands, i.e. poliovirus receptor and Nectin-2, whose expression remained, however, unchanged following LPS stimulation (Fig. 6B and C).
Figure 6. Surface expression of ligands for activating NK cell receptors, i.e. NKG2D and DNAM-1, on resting and activated microglial cells.
NKG2D ligands were constitutively expressed on human microglial cells in vitro and downregulated following LPS stimulation (Fig. 6A). Microglia also constitutively expressed DNAM-1 ligands, poliovirus receptor (PVR) (Fig. 6B) and Nectin-2 (Fig. 6C), whose low expression remained, however, unchanged following LPS stimulation. Summarized data from three experiments are shown.
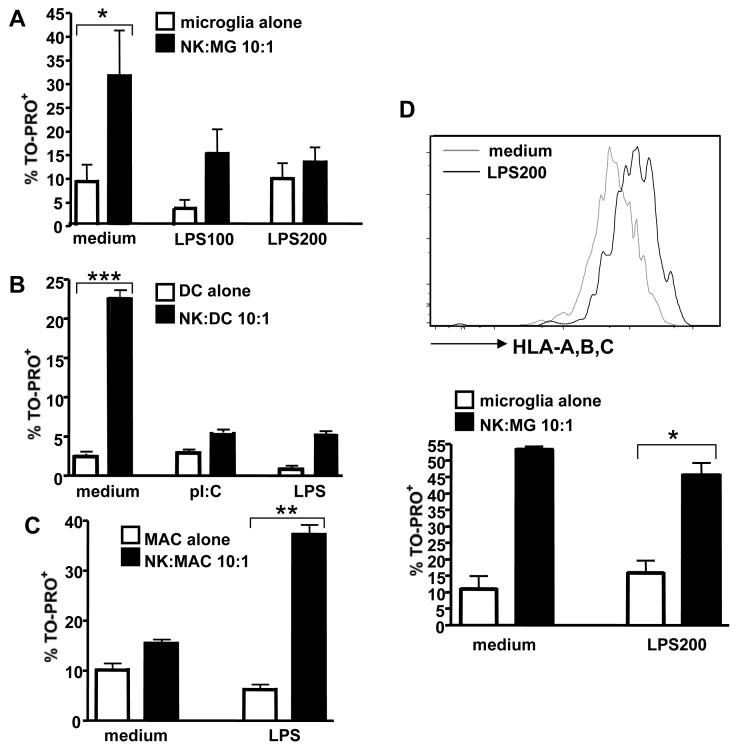
TLR-ligation on human microglia by LPS protected microglial cells from NK cell-mediated cytotoxicity (Fig. 7A). Matured DCs were even more resistant (Fig. 7B). In contrast, monocyte-derived macrophages were more susceptible to NK cell mediated cell lysis following LPS stimulation (Fig. 7C), indicating a functional difference of resting and activated primary microglia compared to monocyte-derived macrophages. LPS stimulation resulted in an up-regulation of MHC class I surface expression on microglial cells and the protective effect of microglia activation could be reversed by MHC class I blockade, indicating that this effect was primarily MHC class I-mediated (Fig. 7D). Altogether, these data suggested that activated microglia, in contrast to macrophages, were resistant to NK cell-mediated cytotoxicity and that activation of microglia was associated with expression of more inhibitory than activating ligands for NK cell receptors.
Figure 7. Activated microglia are protected from NK cell mediated killing.
TLR-ligation on human microglia by LPS protected microglial cells from NK cell-mediated cytotoxicity (Fig. 7A), *p=0.02. In contrast, monocyte-derived macrophages were more susceptible to NK cell mediated cell lysis following LPS stimulation (Fig. 7C), **p=0.004, whereas matured DCs were less susceptible (Fig. 7B), ***p<0.001. In addition, LPS stimulation resulted in an up-regulation of MHC class I surface expression on microglial cells and the protective effect of microglia activation could be reversed by MHC class I blockade, *p=0.002, indicating that this effect was primarily MHC class I-mediated (Fig. 7D). At least 3 experiments for each cell-type are summarized.
Discussion
Our study has shown that human microglial cells were susceptible to allogeneic and autologous NK cell editing by cytotoxicity. NK cells rapidly formed synapses with human microglial cells, in which the cytotoxic molecule perforin was polarized to the cell contact area. Killing of microglia was dependent on NKG2D and NKp46 engagement and counterbalanced by MHC class I binding inhibitory NK cell receptors. Microglial activation by TLR ligation was associated with up-regulation of MHC class I molecules, and thereby inhibited NK cell-mediated killing of human microglia. These data suggest that brain-infiltrating NK cells might have the capacity, via elimination of resting microglial cells, to negatively regulate the afferent limb of innate and adaptive immune responses within the human CNS.
Because of the lack of mouse strains that are selectively deficient in NK cells, the study of NK cell function in vivo has been challenging in the past (31) and animal models of acute inflammatory CNS diseases provided evidence for both disease accelerating and protective effects of NK cells (32) (33) (34) (17) (35). It has been suggested that NK cells could be pathogenic by shaping Th1-polarized adaptive immune responses, by activating CNS-infiltrating dendritic cells (DCs) and/or via direct recognition and lysis of inflammation-activated brain cells (36) (35). However, most EAE studies reported that NK cells protect from autoimmune-mediated tissue damage, presumably by editing initiator and effector cell populations (33) (15) (16) (37).
Several cell types of the human lymphoid and myeloid lineages have been suggested as targets of autologous NK cell cytotoxicity. T cells have been shown to up-regulate NKG2D ligands following activation and to become susceptible to autologous NK cell mediated cell lysis in vitro (38) (39) (25). Lu and colleagues (37) demonstrated that the interaction between the mouse homologue of the human MHC class Ib molecule HLA-E, Qa-1-Qdm, on activated T cells and CD94-NKG2A inhibitory NK cell receptors, protects activated CD4+ T cells from perforin-mediated NK cell cytotoxicity. Antibody-dependent blockade of this Qa-1-CD94/NKG2A interaction resulted in potent NK-dependent elimination of activated autoreactive T cells and amelioration of EAE in MOG35-55 immunized C57BL/6 mice (37). HLA-E-CD94/NKG2A as well as classical MHC class I ligand-KIR interactions have also been proposed to be involved in the protection of mature DCs from autologous NK cell-mediated killing (40). In addition, activated NK cells can kill autologous immature myeloid DCs via NKp30, NKp46 and DNAM-1 mediated recognition (27) (28) (29) (41) (42) (43) (44) (45) and, similar to microglia, pathogen infection, TLR ligation or stimulation by proinflammatory cytokines, protect DCs from NK cell-mediated cytotoxicity, presumably via increased surface expression of MHC class I molecules (41) (42) (46) (45) (44) (40).
In contrast to DCs, monocyte-derived macrophages are resistant to autologous NK cell cytotoxicity unless they become activated and express NKG2D ligands (NKG2DL) by high doses of LPS (200 ng/ml) (19). Consequently, NK cell—mediated cytotoxicity of LPS-stimulated macrophages was abrogated by an anti-NKG2D specific mAb (19). While these data indicate that the stimulatory signal resulting from NKG2D-NKG2DL interactions overcomes the inhibitory signal provided by MHC class I ligands on activated macrophages, we observed that microglia stimulated with a high dose of LPS (200 ng/ml) were less susceptible to NK cell-mediated cytotoxicity. Thus, similar to immature and mature DCs, resting microglia were susceptible to NK-cell-mediated cytotoxicity while microglial activation protected these CNS resident antigen presenting cells from being killed by NK cells. The decrease of NK cell susceptibility upon immune stimulation suggested a role for CNS-infiltrating NK cells in limiting antigen presenting function by microglial cells during neuroinflammation. By this mechanism, NK cells might reduce the microglial pool, but allow fully activated microglial cells present antigens to infiltrating T cells and to initiate a limited immune response in the brain.
Investigating the interface between CD8+ T cells, NK cells and oligodendrocytes as a potential mechanisms of immune—mediated tissue injury in multiple sclerosis, Saikali et al. (47) also addressed the susceptibility of fetal brain-derived microglia cells towards cell lysis by NK cells. Studying 2 CNS cell samples, they reported that IL-2 activated NK cells efficiently lyse non-activated allogeneic microglia, but did not find any differences between samples treated with and without NKG2D specific blocking antibodies. In contrast, we found that microglia killing by NK cells is substantially and reproducibly inhibited by NKG2D blockade. These differences might be explained by using different clones for antibody-mediated blockade of NKG2D recognition.
While our findings are compatible with the aggravating effect of NK cell depletion in autoimmune CNS inflammation (33) (15) (48) (37), they do not exclude the possibility that CNS-invading NK cells might display unwanted cytotoxic activity against neuronal cells, oligodendrocytes, or astrocytes. However, experimental data on the interactions between NK cells and CNS resident cells are both scarce and controversial. Oligodendrocytes have been reported to be either susceptible (36) (47) or not (49) (50) towards IL-2 activated NK cell killing. CNS neurons appear to be resistant to NK cell cytotoxicity (51) (52), and Hammarberg and colleagues (12) reported that CNS-infiltrating NK cells produce high levels of neurotrophic factors, such as brain-derived neurotophic factor (BDNF)- and neurotrophin-3 (NT-3) which protected embryonic motoneuron cultures from TNF-α- or IFN-γ-mediated neuronal cell injury.
In conclusion, we found that NK cells were capable of targeting human microglia. These findings extend the functional reach of the NK system to include the regulation of CNS intrinsic immune responses. Together with the reported cytotoxic activity of NK cells towards activated T cells, activated macrophages and immature DCs, our study adds to the evidence for NK cells as immunoediting lymphocytes involved in the shaping of adaptive immunity beyond their already described polarizing activity via cytokine secretion (53). The mechanism, identified in our experiments, might be involved in limiting neuroinflammation associated with BBB disruption and NK cells recruitment into the CNS. Although much has yet to be learned about the function of NK cells during infectious and autoimmune CNS diseases, these data suggest that therapeutic targeting of NK cells or NK cell subsets during CNS inflammation (16) might have clinical merit.
Acknowledgments
We thank Dr. Sunhee Lee (Albert Einstein College of Medicine) for sharing her expertise in human microglial cell culture. J.D.L. is a recipient of the Dana Foundation and Irvington Institute’s Human Immunology Fellowship from the Cancer Research Institute and is supported by a Pilot Grant from the National Multiple Sclerosis Society (PP1145) and an Institutional Clinical and Translational Science Pilot and Collaborative Project Grant (to the Rockefeller University Hospital). C.S.R. is the Wollowick Family Professor in Multiple Sclerosis Research. C.M. is supported by the Dana Foundation’s Neuroimmunology program, the Arnold and Mabel Beckman Foundation, the Alexandrine and Alexander Sinsheimer Foundation, the Burroughs Wellcome Fund, the Starr Foundation, the National Cancer Institute (R01CA108609 and R01CA101741), the National Institute of Allergy and Infectious Diseases (RFP-NIH-NIAID-DAIDS-BAA-06-19), the Foundation for the National Institutes of Health (Grand Challenges in Global Health), and an Institutional Clinical and Translational Science Award (to the Rockefeller University Hospital).
References
- 1.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 2.Kettenmann H. Triggering the brain’s pathology sensor. Nat Neurosci. 2006;9:1463. doi: 10.1038/nn1206-1463. [DOI] [PubMed] [Google Scholar]
- 3.Aloisi F. Immune function of microglia. Glia. 2001;36:165. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 4.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 5.Lünemann A, Ullrich O, Diestel A, Jons T, Ninnemann O, Kovac A, Pohl EE, Hass R, Nitsch R, Hendrix S. Macrophage/microglia activation factor expression is restricted to lesion-associated microglial cells after brain trauma. Glia. 2006;53:412. doi: 10.1002/glia.20301. [DOI] [PubMed] [Google Scholar]
- 6.Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008;15:226. doi: 10.1038/sj.cdd.4402170. [DOI] [PubMed] [Google Scholar]
- 7.Strowig T, Brilot F, Münz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008;180:7785. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 9.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 10.Aloisi F, Ria F, Adorini L. Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today. 2000;21:141. doi: 10.1016/s0167-5699(99)01512-1. [DOI] [PubMed] [Google Scholar]
- 11.Raivich G, Liu ZQ, Kloss CU, Labow M, Bluethmann H, Bohatschek M. Cytotoxic potential of proinflammatory cytokines: combined deletion of TNF receptors TNFR1 and TNFR2 prevents motoneuron cell death after facial axotomy in adult mouse. Exp Neurol. 2002;178:186. doi: 10.1006/exnr.2002.8024. [DOI] [PubMed] [Google Scholar]
- 12.Hammarberg H, Lidman O, Lundberg C, Eltayeb SY, Gielen AW, Muhallab S, Svenningsson A, Linda H, Meide P. H. van Der, Cullheim S, Olsson T, Piehl F. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci. 2000;20:5283. doi: 10.1523/JNEUROSCI.20-14-05283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byram SC, Serpe CJ, Pruett SB, Sanders VM, Jones KJ. Natural killer cells do not mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:417. doi: 10.1016/s0889-1591(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 14.Traugott U, Raine CS. Further lymphocyte characterization in the central nervous system in multiple sclerosis. Ann N Y Acad Sci. 1984;436:163. doi: 10.1111/j.1749-6632.1984.tb14788.x. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, Tanuma N. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1681. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, Weaver JT, Ljunggren HG, Biron CA, Littman DR, Ransohoff RM. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- 17.Hansen DS, Bernard NJ, Nie CQ, Schofield L. NK cells stimulate recruitment of CXCR3+ T cells to the brain during Plasmodium berghei-mediated cerebral malaria. J Immunol. 2007;178:5779. doi: 10.4049/jimmunol.178.9.5779. [DOI] [PubMed] [Google Scholar]
- 18.Lee SC, Liu W, Brosnan CF, Dickson DW. Characterization of primary human fetal dissociated central nervous system cultures with an emphasis on microglia. Lab Invest. 1992;67:465. [PubMed] [Google Scholar]
- 19.Nedvetzki S, Sowinski S, Eagle RA, Harris J, Vely F, Pende D, Trowsdale J, Vivier E, Gordon S, Davis DM. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 20.Münz C, Dao T, Ferlazzo G, de Cos MA, Goodman K, Young JW. Mature myeloid dendritic cell subsets have distinct roles for activation and viability of circulating human natural killer cells. Blood. 2005;105:266. doi: 10.1182/blood-2004-06-2492. [DOI] [PubMed] [Google Scholar]
- 21.Busch DH, Pilip IM, Vijh S, Pamer EG. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 22.Lee-MacAry AE, Ross EL, Davies D, Laylor R, Honeychurch J, Glennie MJ, Snary D, Wilkinson RW. Development of a novel flow cytometric cell-mediated cytotoxicity assay using the fluorophores PKH-26 and TO-PRO-3 iodide. J Immunol Methods. 2001;252:83. doi: 10.1016/s0022-1759(01)00336-2. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson RW, Lee-MacAry AE, Davies D, Snary D, Ross EL. Antibody-dependent cell-mediated cytotoxicity: a flow cytometry-based assay using fluorophores. J Immunol Methods. 2001;258:183. doi: 10.1016/s0022-1759(01)00474-4. [DOI] [PubMed] [Google Scholar]
- 24.Fischer K, Andreesen R, Mackensen A. An improved flow cytometric assay for the determination of cytotoxic T lymphocyte activity. J Immunol Methods. 2002;259:159. doi: 10.1016/s0022-1759(01)00507-5. [DOI] [PubMed] [Google Scholar]
- 25.Roy S, Barnes PF, Garg A, Wu S, Cosman D, Vankayalapati R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J Immunol. 2008;180:1729. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 26.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol. 2001;19:375. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 27.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Münz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spaggiari GM, Carosio R, Pende D, Marcenaro S, Rivera P, Zocchi MR, Moretta L, Poggi A. NK cell-mediated lysis of autologous antigen-presenting cells is triggered by the engagement of the phosphatidylinositol 3-kinase upon ligation of the natural cytotoxicity receptors NKp30 and NKp46. Eur J Immunol. 2001;31:1656. doi: 10.1002/1521-4141(200106)31:6<1656::aid-immu1656>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 29.Pende D, Castriconi R, Romagnani P, Spaggiari GM, Marcenaro S, Dondero A, Lazzeri E, Lasagni L, Martini S, Rivera P, Capobianco A, Moretta L, Moretta A, Bottino C. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- 30.Hamerman JA, Ogasawara K, Lanier LL. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J Immunol. 2004;172:2001. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- 31.Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, Jaeger S, Andre P, Gauthier L, Daniel L, Chemin K, Morel Y, Dalod M, Imbert J, Pierres M, Moretta A, Romagne F, Vivier E. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lünemann JD, Münz C. Do natural killer cells accelerate or prevent autoimmunity in multiple sclerosis? Brain. 2008;131:1681–1683. doi: 10.1093/brain/awn132. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vollmer TL, Liu R, Price M, Rhodes S, La Cava A, Shi FD. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J Immunol. 2005;174:2696. doi: 10.4049/jimmunol.174.5.2696. [DOI] [PubMed] [Google Scholar]
- 35.Winkler-Pickett R, Young HA, Cherry JM, Diehl J, Wine J, Back T, Bere WE, Mason AT, Ortaldo JR. In vivo regulation of experimental autoimmune encephalomyelitis by NK cells: alteration of primary adaptive responses. J Immunol. 2008;180:4495. doi: 10.4049/jimmunol.180.7.4495. [DOI] [PubMed] [Google Scholar]
- 36.Morse RH, Seguin R, McCrea EL, Antel JP. NK cell-mediated lysis of autologous human oligodendrocytes. J Neuroimmunol. 2001;116:107. doi: 10.1016/s0165-5728(01)00289-2. [DOI] [PubMed] [Google Scholar]
- 37.Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabinovich BA, Li J, Shannon J, Hurren R, Chalupny J, Cosman D, Miller RG. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol. 2003;170:3572. doi: 10.4049/jimmunol.170.7.3572. [DOI] [PubMed] [Google Scholar]
- 39.Cerboni C, Zingoni A, Cippitelli M, Piccoli M, Frati L, Santoni A. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood. 2007;110:606. doi: 10.1182/blood-2006-10-052720. [DOI] [PubMed] [Google Scholar]
- 40.Persson CM, Assarsson E, Vahlne G, Brodin P, Chambers BJ. Critical role of Qa1b in the protection of mature dendritic cells from NK cell-mediated killing. Scand J Immunol. 2008;67:30. doi: 10.1111/j.1365-3083.2007.02034.x. [DOI] [PubMed] [Google Scholar]
- 41.Chiesa M. Della, Vitale M, Carlomagno S, Ferlazzo G, Moretta L, Moretta A. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol. 2003;33:1657. doi: 10.1002/eji.200323986. [DOI] [PubMed] [Google Scholar]
- 42.Wilson JL, Heffler LC, Charo J, Scheynius A, Bejarano MT, Ljunggren HG. Targeting of human dendritic cells by autologous NK cells. J Immunol. 1999;163:6365. [PubMed] [Google Scholar]
- 43.Parajuli P, Nishioka Y, Nishimura N, Singh SM, Hanibuchi M, Nokihara H, Yanagawa H, Sone S. Cytolysis of human dendritic cells by autologous lymphokine-activated killer cells: participation of both T cells and NK cells in the killing. J Leukoc Biol. 1999;65:764. doi: 10.1002/jlb.65.6.764. [DOI] [PubMed] [Google Scholar]
- 44.Carbone E, Terrazzano G, Ruggiero G, Zanzi D, Ottaiano A, Manzo C, Karre K, Zappacosta S. Recognition of autologous dendritic cells by human NK cells. Eur J Immunol. 1999;29:4022. doi: 10.1002/(SICI)1521-4141(199912)29:12<4022::AID-IMMU4022>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 45.Chambers BJ, Salcedo M, Ljunggren HG. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1) Immunity. 1996;5:311. doi: 10.1016/s1074-7613(00)80257-5. [DOI] [PubMed] [Google Scholar]
- 46.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 47.Saikali P, Antel JP, Newcombe J, Chen Z, Freedman M, Blain M, Cayrol R, Prat A, Hall JA, Arbour N. NKG2D-mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis. J Neurosci. 2007;27:1220. doi: 10.1523/JNEUROSCI.4402-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu W, Fazekas G, Hara H, Tabira T. Mechanism of natural killer (NK) cell regulatory role in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;163:24. doi: 10.1016/j.jneuroim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Satoh J, Kim SU, Kastrukoff LF. Absence of natural killer (NK) cell activity against oligodendrocytes in multiple sclerosis. J Neuroimmunol. 1990;26:75. doi: 10.1016/0165-5728(90)90122-4. [DOI] [PubMed] [Google Scholar]
- 50.Satoh J, Kim SU, Kastrukoff LF. Cytolysis of oligodendrocytes is mediated by killer (K) cells but not by natural killer (NK) cells. J Neuroimmunol. 1991;31:199. doi: 10.1016/0165-5728(91)90041-5. [DOI] [PubMed] [Google Scholar]
- 51.Backstrom E, Chambers BJ, Ho EL, Naidenko OV, Mariotti R, Fremont DH, Yokoyama WM, Kristensson K, Ljunggren HG. Natural killer cell-mediated lysis of dorsal root ganglia neurons via RAE1/NKG2D interactions. Eur J Immunol. 2003;33:92. doi: 10.1002/immu.200390012. [DOI] [PubMed] [Google Scholar]
- 52.Backstrom E, Ljunggren HG, Kristensson K. NK cell-mediated destruction of influenza A virus-infected peripheral but not central neurones. Scand J Immunol. 2007;65:353. doi: 10.1111/j.1365-3083.2007.01912.x. [DOI] [PubMed] [Google Scholar]
- 53.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]