Abstract
Connexin43 (Cx43), the most widely expressed and abundant vertebrate gap junction protein, is phosphorylated at multiple different serine residues during its life cycle. Cx43 is phosphorylated soon after synthesis and phosphorylation changes as it traffics through the endoplasmic reticulum and Golgi to the plasma membrane ultimately forming into a gap junction structure. The electrophoretic mobility of Cx43 changes as the protein proceeds through its life cycle with prominent bands often labeled P0, P1 and P2. Many reports have indicated changes in “phosphorylation” based on these mobility shifts and others that occur in response to growth factors or other biological effectors. Here we indicate how phosphospecific and epitope specific antibodies can be utilized to show when and where certain phosphorylation events occur during the Cx43 life cycle. These reagents show that phosphorylation at S364 and or S365 is involved in forming the P1 isoform, an event that apparently regulates trafficking to or within the plasma membrane. Phosphorylation at S325, 328, and/or 330 is necessary to form a P2 isoform and this phosphorylation event is present only in gap junctions. Treatment with protein kinase C activators led to phosphorylation at S368, S279/S282 and S262 with a shift in mobility in CHO cells but not MDCK cells. The shift was dependent on MAPK activity but not phosphorylation at S279/282. However, phosphorylation at S262 could explain the shift. By defining these phosphorylation events, we have begun to be able to sort out the critical signaling pathways that regulate gap junction function.
Keywords: Connexin, Gap Junction, Phosphorylation, Kinase, Cell Signaling
Introduction
Gap junctions are collections of intercellular channels that directly connect the cytoplasmic contents of adjacent cells. They coordinate cell-to-cell communication within tissues by allowing for the transfer of molecules less than 1000 Daltons between cells including ions, amino acids, nucleotides, second messengers (e.g., Ca2+, cAMP, cGMP, IP3) and other metabolites (Loewenstein & Azarnia, 1988; Saez et al., 2003; Simon, Goodenough & Paul, 1998; Willecke et al., 2002). In vertebrates, gap junctions are composed of proteins from the connexin family, which is composed of 21 members in humans (Goodenough & Paul, 2003; Saez et al., 2003; Sohl & Willecke, 2004). Connexins are commonly designated with numerical suffixes referring to the molecular weight of the deduced sequence in kilodaltons (e.g., connexin43 or Cx43) (Saez et al., 2003; Sohl & Willecke, 2004). Connexins are differentially expressed in tissues with some being significantly expressed in only a few tissues and some, like Cx43, being more widespread. Gap junctions play significant regulatory roles in embryonic development, electrical coupling, apoptosis, differentiation, tissue homeostasis and metabolic transport (Goodenough & Paul, 2003; Loewenstein & Azarnia, 1988; Sohl & Willecke, 2004).
Cx43 electrophoreses as multiple isoforms when analyzed by SDS-PAGE, including a faster migrating form that includes non-phosphorylated (P0 or NP) Cx43, and at least two slower migrating forms, commonly termed P1 and P2 (Crow et al., 1990; Musil et al., 1990). Pulse chase analysis indicated that the Cx43 isoforms progress from P0 to P1 to P2 and the P2 isoform is associated with gap junctional structures (Musil & Goodenough, 1991). Cx43 is critical for the synchronous beating of cardiac tissue. Gap junctions composed of Cx43 are localized to intercalated disks in the ventricle where it supports the longitudinal spread of the action potential resulting in coordinated contraction. When cardiac tissue is immunoblotted for Cx43, only slower migrating “phosphorylated” isoforms are observed. Myocardial ischemia leads to Cx43 “dephosphorylation” (i.e., the loss of P1, P2, etc and gain of P0) and loss of localization from the intercalated disk, which likely contributes to contractile failure and arrhythmias (Beardslee et al., 2000; Schulz et al., 2003). We have shown that Cx43 localized to intercalated disks is phosphorylated at S325, S328 and/or S330 and that ischemia leads to loss of this phosphorylation and re-localization of the protein (Lampe et al., 2006).
In this report, we describe how Cx43 phosphorylation changes as the protein proceeds through its life cycle. Specifically, we show that phosphorylation at S364/S365 leads to P1 formation and phosphorylation at S325/S328/S330 is necessary for P2 to form. Activation of specific kinases changes the gating properties of gap junction channels, the extent of gap junction assembly, the half-life of Cx43 and, in some cases/cell types, its electrophoretic mobility. Previously we showed that activation of protein kinase C led to phosphorylation of Cx43 at S368 (Lampe et al., 2000) with a change in electrophoretic mobility in Chinese Hamster Ovary (CHO) but not Normal Rat Kidney (NRK) cells (Solan et al., 2003). Here, we show that the change in electrophoretic mobility was apparently due to different pools of Cx43 being phosphorylated on S262 via MAPK activation. These results support and refute some of the roles specific kinases and signaling pathways have in the regulation of gap junctional communication and help define the roles that particular phosphorylation events play in regulating the life cycle.
Materials and Methods
Antibodies and Reagents
All general chemicals, unless otherwise noted, were purchased from Fisher Scientific. Phorbol 12-Myristate 13-Acetate (PMA) and a rabbit antibody against Cx43 (C6219) were from Sigma (St. Louis, MO). Mouse anti-Cx43 antibodies, Cx43CT1 (referred to as CT) and Cx43IF1 were prepared against amino acids 360-382 of Cx43 and antibody Cx43NT1 against amino acids 1-20 of Cx43 at the Fred Hutchinson Cancer Research Center Hybridoma Development Facility (Seattle, WA). We purchased a phosphospecific antibody to Cx43 at S262 (pS262) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA) and a phosphospecific activated MAPK antibody from Cell Signaling Technology (Beverly, MA). We made rabbit anti-pS262, pS279/282, pS368, pS325/328/330-Cx43 phosphospecific antibodies by custom commercial preparation (ProSci Inc., Poway, CA; 13 week schedule) against synthetic peptides phosphorylated at the specified residues that had been linked via the N-terminal cysteine to maleimide-activated KLH (Pierce Biotechnology, Rockford, IL), and phosphospecific antibodies were affinity purified as we have previously published (Lampe et al., 2006).
Cell Culture
Madin-Darby Canine Kidney (MDCK), Normal Rat Kidney cells E51, HeLa and CHO cells were cultured in Dulbeccos Minimal Essential Medium (Fisher Scientific, Pittsburgh PA) supplemented with 5-10% fetal calf serum and antibiotics (100 U/mL penicillin G and 100μg/mL streptomycin) in a humidified 5% CO2 environment. To make MDCK cells expressing wild type Cx43, pIREShygro Cx43 was electroporated into cells using a Nucleofector apparatus (Amaxa Inc, Gaithersburg, MD). HeLa cells expressing S262A mutant Cx43 were transfected with Lipofectamine (Invitrogen, San Diego, CA). In both cases cells were selected at 500μg/ml Hygromycin B and were dilution cloned in media supplemented with hygromycin.
Immunoblotting and Immunofluorescence
Whole cell preparations were lysed in sample buffer supplemented with 50mM NaF, 1mM Na3VO4, 5% β-mercaptoethanol, 1 mM PMSF and 1x Complete protease inhibitors (Roche Molecular Biochemicals, Alameda, CA). Triton insoluble material was collected by centrifugation of cell lysates using 1% Triton X-100 in PBS with the phosphatase and protease inhibitors listed above. Following sonication in sample buffer, samples were separated by sodium dodecylsulfate - 10% polyacrylamide gel electrophoresis (SDS-PAGE). After immunoblotting, protein was detected with rabbit and mouse primary antibodies. Primary antibodies were simultaneously visualized with fluorescent dye-labeled secondary antibodies [AlexaFluor 680 goat anti-rabbit (Molecular Probes) and IRDye800-conjugated donkey anti-mouse IgG (Rockland Immunochemicals)] and directly quantified using the LI-COR Biosciences Odyssey infrared imaging system and associated software.
Results
Formation of the P2 Isoform
As indicated above, Cx43 demonstrates multiple electrophoretic isoforms when analyzed by SDS-PAGE, including a faster migrating form that includes non-phosphorylated (P0 or NP) Cx43 and at least two slower migrating forms, commonly termed P1 and P2 as shown in the first lane (i.e., Ab: Total, Prep: WC) of Fig. 1A. Consistent with Musil and Goodenough (1991), the P2 isoform was insoluble after extraction of cells with Triton X-100 as indicated in the middle lane (Ab: Total, Prep: Tx Ins) of Fig 1A. When that same lane is simultaneously probed with an antibody (pS325) that is specific for Cx43 when it is phosphorylated at S325, S328 and/or S330, we found that only the P2 isoform was present (third lane, Ab:pS325, Prep:Tx Ins). We had previously shown that this phosphospecific antibody labeled the intercalated disk region of cardiomyocytes (Lampe et al., 2006) and that S325/328/330 phosphorylation was important in gap junction assembly (Cooper & Lampe, 2002). In Fig. 2B& C, we show that the phosphospecific antibody and one to total Cx43 overlay to a large extent at gap junctional structures but very little at cytoplasmic regions. Close examination of the overlay panel (Fig. 2D) indicates that not all of the apparent junctional material is positive for the pS325 antibody. We conclude that phosphorylation at S325, S328 and/or S330 is specific for gap junctional Cx43 and that these phosphorylation events are likely involved in gap junction assembly.
Fig. 1.
The P2 isoform of Cx43 is phosphorylated at S325/328 and/or 330. (A) Detection of Cx43 present in whole cell lysates via Western immunoblot with a mouse antibody to total Cx43 shows the characteristic 3 isoforms (first lane, note P0, P1 and P2). Triton X-100 insoluble extracts (Tx Ins, lanes 2 and 3) show predominately the P2 isoform while the rabbit antibody to Cx43 phosphorylated at S325/328/330 (pS325) shows exclusively the P2 isoform. Immunofluroescence detection of cells with both Total Cx43 and the p325 antibodies show extensive overlay in gap junctional regions (B-D).
Fig. 2.
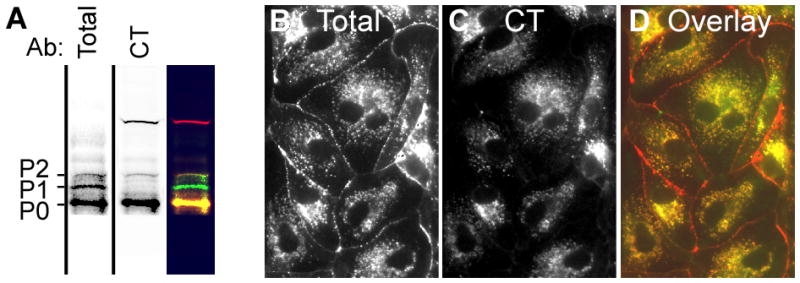
The CT antibody recognizes the P0 isoform and Cx43 present in cytoplasmic membranes. (A) The antibody to total Cx43 recognizes all 3 isoforms of Cx43 (First lane) while probing the same preparation with the CT antibody (second lane) shows predominately the P0 isoform. (B) Immunofluorescence detection of cells with both Total Cx43 and the CT antibodies show extensive overlay in cytoplasmic membrane regions (B-D).
Formation of the P1 Isoform
We produced a monoclonal antibody named CT that recognizes primarily the P0 isoform of Cx43 (Fig. 2A, compare lane 1 & 2). We have epitope mapped this antibody and found that it binds to Cx43 when it is not phosphorylated at S364 or S365 and, in contrast to the pS325 antibody in Fig. 1C, it labels almost exclusively cytoplasmic membranes reminiscent of Golgi staining (Sosinsky et al., 2007). If we compare the staining pattern of MDCK cells expressing wild-type Cx43 using the CT antibody (mouse) and one to total Cx43 (rabbit), we see essentially complete overlay of the punctate CT staining with the antibody for total Cx43 in cytoplasmic membrane structures reminiscent of the Golgi apparatus and essentially no overlay with the plasma membrane/gap junctional staining observed for the total Cx43 antibody (Fig 2A-D). We conclude that the epitope recognized by the CT antibody is lost (i.e., likely due to phosphorylation) when Cx43 is present in gap junction structures.
Phosphorylation on S262 Creates a Distinct P2 Isoform of Cx43
PMA treatment leads to downregulation of gap junctional communication in many cell types. In some cell types it also results in a mobility shift of Cx43 to slower migrating forms. Utilizing several specific Cx43 phosphoantibodies and site-directed mutants with cell lines which do or do not shift, we explored which specific phosphorylation sites might be associated with these events. We examined 2 cell lines: MDCK cells stably transfected with Cx43, which do not shift in response to PMA and CHO cells, which do shift. Cells were treated with PMA for 30 minutes and immunoblotting was performed using antibodies specific for Cx43 phosphorylated at S368, S262, S279/282 and S325/328/330 (Fig. 3). Using an antibody to the N-terminus of Cx43, which does not discriminate between phosphoforms, we show that MDCK cells do not shift in response to PMA whereas CHO cells show a dramatic increase in the apparent P2 isoform (Total panel). This was not due to phosphorylation on S325/328/330 as this signal was not apparent in CHO cells at all (probably because most of their Cx43 is in cytoplasmic membranes), nor did it increase in MDCK cells upon PMA treatment (data not shown). In MDCK cells, phosphorylation on S368 can occur on essentially any of the isoforms, including P0, while in CHO cells pS368 was found exclusively on the P2 form (Fig. 3, compare Total and pS368 panel). In both cell types, phosphorylation on S262 (Fig. 3, pS262 panel) and S279/282 (pS279 panel) was predominantly on the P2 isoform. This could indicate that one or both of these events affects a conformational change resulting in a P2 isoform. To look at this further, we examined the ability of Cx43 mutated at these sites to shift in response to stimuli, both experimentally and in the literature. We have shown previously that HeLa cells expressing wild type Cx43 or Cx43 with a S368A mutation exhibit a migration shift in response to PMA (Solan et al., 2003). Here, we show that in HeLa cells expressing a S262A mutant, Cx43 does not shift to the P2 form in response to PMA treatment, although we did observe an apparent shift to a position just above the P0 form (Fig. 3, denoted by asterisks in the lower two panels). Note that the S262A mutants were able to make P2, but since this was present in unstimulated cells it is likely to represent P2 formed by phosphorylation on S325/328/330. When we blotted with the pS279 antibody, we found that phosphorylation on S279/S282 did occur upon PMA treatment and was found on all isoforms except P0. Since p279/282 phosphorylation alone did not lead to formation of the P2 isoform and S262A mutants, which cannot be phosphorylated on this site, did not shift to P2, we conclude that phosphorylation on S262 can lead to a Cx43 isoform which migrates in the P2 position. Furthermore, this P2 species is distinct from P2 phosphorylated on S325/328/330 since CHO cells were not phosphorylated on these latter sites.
Fig. 3.

S262 phosphorylation appears to be involved in a shift to a P2 isoform position upon TPA treatment. MDCK cells expressing wild type Cx43 (MDCK), CHO cells, or HeLa cells expressing Cx43 with a serine to alanine mutation (HeLa-262A) were either treated (+) with PMA or not (-) and probed with the NT antibody to total Cx43 (Total), to Cx43 with S279/S282 phosphorylated (pS279), Cx43 with S368 phosphorylated (pS368) and Cx43 with S262 phosphorylated (pS262).
We feel this provides direct evidence that P2 can be a heterogenous mixture of phosphoforms, some of which is Cx43 phosphorylated at S325/328/330 representing the ‘classic’ gap junction associated, Triton X-100 insoluble form of P2 (Musil & Goodenough, 1991), but some of which are, instead, formed by phosphorylation at S262 and associated with phosphorylation at S279/282, S262 and S368 which have been linked to decreases in gap junction communication (e.g., Doble et al., 2004; Lampe et al., 2000; Warn-Cramer et al., 1996).
Distinct Pools of Cx43 are Targeted for Phosphorylation in Different Cell Types
In MDCK cells that make P2 that is phosphorylated at S325/328/330 but do not shift in response to PMA, it appears that PMA induced phosphorylation on S262 and S279/282 specifically on the P2 isoform and not on the P0 form. The rationale behind this reasoning is that phosphorylation on S262 is not adding to the total amount of P2. However, in the CHO cells, which do not assemble junctions very well and make little gap junctional P2, the P0 form seems to become phosphorylated on these sites resulting in the migration shift and labeling exclusively on the P2 isoform. Note that, similar to the MDCK cells, there is a fraction of the CHO P0 form that does not shift nor become phosphorylated on these sites (possibly protected in the endoplasmic reticulum), indicating that a pool of Cx43 is refractory to these phosphorylation events. Phosphorylation on S368 in response to PMA is also differentially regulated. In MDCK cells, essentially all isoforms can become phosphorylated on S368, indicating that this event is independent of S262 or S279/282 phosphorylation. In the CHO cells, pS368 is found only in the P2 form of Cx43, not in the remaining “unshifted” P0 isoform. We hypothesize that this ‘unshifted’ isoform is the same pool that was refractory to S262 and S279/282 phosphorylation.
Discussion
There have been many attempts to correlate gap junction function with changes in Cx43 mobility by SDS-PAGE. The reasons for this interest have been multifold including the fact that mobility changes have been associated with many important disease processes such as hypoxia in cardiac tissue and changes in gap junction function in response to specific stimuli including tumor promoting and many other drugs. Since different cell types often respond differently to these stimuli, in many cases conflicting data on Cx43 mobility changes made it difficult to draw clear conclusions. This was due both to the fact that different cell lines vary in their ability to assemble and regulate gap junctions and to a lack of understanding of what conformational information was being conveyed by the migration shift. It is important to remember that, though phosphorylation drives the migration change, it presumably is not a molecular weight change that is being detected via SDS-PAGE since addition of a phosphate would only add 80 Da to the molecular mass, but rather a conformational change in the protein triggered by these phosphorylation events. The development of phosphospecific antibodies is allowing us to more accurately dissect and understand which phosphorylation events and signaling pathways are important in gap junction regulation. Using these tools we have found several steps in the Cx43 lifecycle that can be regulated by phosphorylation in at least some cell types.
Inclusion in the Gap Junction Plaque and Formation of P2
Using a phospho-antibody specific for phosphorylation at S325, S328 and S330 we have shown that these sites are phosphorylated in the gap junction plaque associated, Triton X-100 insoluble, P2 isoform of Cx43. Cells expressing site directed mutants, in which these serines were converted to alanines, do not assemble gap junctions efficiently nor did they make the P2 isoform of Cx43 (Lampe et al., 2006). These data indicate that phosphorylation on S325, S328 and/or S330 are required for formation of the gap junction plaque associated P2 isoform of Cx43 (see model in Fig. 4). In addition, previous work from our lab has shown that Casein Kinase 1 is important for plaque formation, as inhibition of CK1 led to a decrease in gap junction plaques and an increase in hemichannels in the plasma membrane (Cooper & Lampe, 2002). Taken together, these data are consistent with the idea that phosphorylation on some combination of S325/328/330 by CK1 results in a conformational change resulting in the P2 isoform and inclusion in a gap junction plaque.
Fig. 4.

Model of how Cx43 phosphorylation at S364/S365 and S325/S328/S330 could affect the gap junction life cycle.
Transport to the Plasma Membrane and Formation of P1
Use of a monoclonal antibody specific for Cx43 not phosphorylated on S364 or S365, termed “CT”, showed that these residues appear to be important for trafficking to the plasma membrane. Immunofluorescence staining showed that this antibody recognized Cx43 in the cytoplasm only and not in the plasma membrane (Fig. 2B-D) while immunoblots showed that, in resting cells, this antibody recognized primarily the P0 form of Cx43. Cell-surface biotinylation assays showed that essentially all isoforms, including P0, could reach the plasma membrane, while acquisition of Triton X-100 insolubility and inclusion in plaques was correlated with phosphorylation to the P2 form (Musil & Goodenough, 1991). The functional relevance of the P1 form, however, has not been shown. Interestingly, one feature of the “CT” antibody is that it never recognizes the P1 form (Fig. 2 and Sosinsky et al., 2007). Since “CT” recognizes non-phosphorylated S364 and S365 and does not recognize P1, it is likely that phosphorylation on one or both of these residues leads to the P1 isoform.
While the cell-surface biotinylation data indicates that the phosphorylation event leading the P1 isoform may occur in the plasma membrane (Musil & Goodenough, 1991), the immunofluorescence data (Fig. 2) indicates that this event is required for trafficking from the cytoplasm to the plasma membrane (Fig. 4). Since hemichannels are made up of 6 connexins, it may be that only a fraction of these need be phosphorylated to propel forward trafficking. This would result in the cytoplasmic Cx43 being “CT” reactive, i.e., not phosphorylated on S364 or S365. The P0 or “CT” isoform in the plasma membrane could be diffuse and therefore undetectable by immunofluorescence, until entering a gap junction plaque where it would become more concentrated and eventually phosphorylated to the P2 isoform.
Induced Phosphorylation Can Lead to a distinct P2
Treatment of cells with various stimuli can result in a shift of Cx43 to slower migrating forms and is often associated with downregulation of gap junctional communication. Several studies have focused on using growth factors and PMA in combination with MAPK and PKC inhibitors to correlate changes in Cx43 isoform migration with shutdown of gap junctional communication. In one study, IAR6.1 cells, which endogenously express Cx43 and make P2 in resting cells, exhibited a decrease in gap junctional communication and a migration shift in response to both PMA and EGF (Rivedal & Opsahl, 2001). In these cells, inhibition of ERK1/2 but not PKC inhibition could inhibit the migration shift in response to PMA and EGF, although it did not reverse PMA induced inhibition of gap junctional communication. This led the authors to conclude that the migration shift was due to phosphorylation on Cx43 via ERK1/2. However, which sites might be responsible was not determined. The sites where ERK1/2 phosphorylates Cx43 have been determined to be S255, S279 and S282 and when wild type Cx43 or S279/S282/S255A mutant Cx43 were expressed in HeLa cells, EGF treatment led to a migration shift in both wild type and mutant expressing cells, although inhibition of communication was only observed in wild type Cx43 expressing cells (Warn-Cramer et al., 1998; Warn-Cramer et al., 1996). Inhibition of ERK1/2 reversed both of these effects. Both of these studies are consistent with the idea that ERK1/2 activation can lead to P2 formation, although not through phosphorylation on S279 or S282. Interestingly, when we used PD98059, the same ERK1/2 inhibitor used in the studies described above, TPA was able to activate ERK1/2 regardless of the presence of inhibitor, even though ERK1/2 were inhibited in resting cells (data not shown). Thus, it seems consistent with the data that S262 is phosphorylated in an ERK1/2 dependent manner and that this event is responsible for the TPA and EGF induced mobility shift. While the functional consequences of S262 phosphorylation are not yet clear, it does seem apparent that identification of specific phosphorylation sites and the specific signaling pathways involved will allow us to design pertinent experiments that will allow a greater understanding of gap junction regulation.
Acknowledgments
These studies were supported by Grants from the National Institutes of Health: GM055632 (PDL).
Abbreviations
- Cx
connexin
- PKA
cAMP-dependent protein kinase
- MAPK
Mitogen-activated protein kinase
- PKC
protein kinase C
- CK1
casein kinase 1
- PMA
phorbol 12-myristate 13-acetate
- SDS-PAGE
sodium dodecylsulfate-polyacrylamide gel electrophoresis
References
- Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- Cooper CD, Lampe PD. Casein kinase 1 regulates connexin43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol. 1990;10:1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Dang X, Ping P, Fandrich RR, Nickel BE, Jin Y, Cattini PA, Kardami E. Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J Cell Sci. 2004;117:507–14. doi: 10.1242/jcs.00889. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–94. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Cooper CD, King TJ, Burt JM. Analysis of Connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J Cell Sci. 2006;119:3435–42. doi: 10.1242/jcs.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;126:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein WR, Azarnia R. Regulation of intercellular communication and growth by the cellular src gene. Ann New York Acad Sci. 1988;551:337–346. doi: 10.1111/j.1749-6632.1988.tb22359.x. [DOI] [PubMed] [Google Scholar]
- Musil LS, Cunningham BA, Edelman GM, Goodenough DA. Differential phosphorylation of gap junction protein connexin43 in junctional communication-competent and deficient cell lines. J Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivedal E, Opsahl Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis. 2001;22:1543–1550. doi: 10.1093/carcin/22.9.1543. [DOI] [PubMed] [Google Scholar]
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, Konietzka I, Heusch G. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. Faseb J. 2003;17:1355–1357. doi: 10.1096/fj.02-0975fje. [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol. 1998;8:295–298. doi: 10.1016/s0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Solan JL, Fry MD, TenBroek EM, Lampe PD. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci. 2003;116:2203–2211. doi: 10.1242/jcs.00428. [DOI] [PubMed] [Google Scholar]
- Sosinsky GE, Solan JL, Gaietta GM, Ngan L, Mackey M, Lampe PD. The C-terminus of Connexin43 present in the Golgi and in gap junctions is conformationally distinct: Specific serines and prolines influence subcellular localization and ZO-1 interaction 2007 [Google Scholar]
- Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J Biol Chem. 1998;273:9188–9196. doi: 10.1074/jbc.273.15.9188. [DOI] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LWM, Eckhart W, Lau AF. Characterization of the MAP kinase phosphorylation sites on the connexin43 gap junction protein. J Biol Chem. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]



