Abstract
The androgen receptor crosstalks with transforming growth factor-β (TGF-β) through mechanisms that remain poorly understood. Here we provide strong evidence that 5α-dihydrotestosterone (DHT) intercepts the ability of prostate epithelial cells to undergo TGF-β-induced apoptosis, and present a new model for this androgenic effect. We report that DHT decreases the level of TGF-β receptor II (TβRII) through a transcriptional mechanism, leading to suppression of the ability of TGF-β to down-regulate expression of Bcl-xL and Cyclin Ds, activate caspase-3 and induce apoptosis. Promoter analysis, DNA pulldown and electrophoretic mobility shift assays support that transcriptional downregulation of TβRII by DHT occurs through Sp1/3 response elements, with the binding of Sp1 to the TβRII promoter being suppressed by DHT, largely driven by loss of Sp1 protein and/or activity. These results provide fresh insight on the mechanism of growth control by androgens and progression of prostate cancer to androgen independence.
Keywords: AR, Androgen, TβRII, Prostate, Cancer, Smad, Smad3, tumor suppressor, apoptosis, cyclin D
Introduction
Transforming growth factor-β (TGF-β), which is a multifunctional cytokine with an important role as a potent tumor suppressor in a variety of tissues including the prostate (1–7), propagates signals through two transmembrane serine/threonine receptor kinases, namely TGF-β receptor type I (TβRI) and II (TβRII), which directly activate Smads 2 and 3 through phosphorylating their two C-terminal serine residues (8–10). TGF-β is well recognized to induce growth arrest or/and apoptosis of prostate epithelium, occurring through mechanisms that appear to be intricately controlled by EGF, IGF-I, PI3K/Akt, and androgen receptor (AR) (11–13).
AR is a member of the nuclear receptor superfamily of transcription factors residing predominantly in the cytoplasm as inactive complexes with molecular chaperones, particularly heat shock proteins 70 and 90 (14, 15). Binding of androgen frees AR from its chaperones, thereby promoting the translocation of AR to the nucleus where this receptor functions in transcriptional control of numerous genes involved in development, growth and function of androgen target tissues such as the prostate. Although transcriptional responses of AR occur mainly through the direct binding of AR to DNA at androgen receptor response element (AREs), the function of AR is influenced by its association with numerous co-regulators that also serve as junctions of cross-talk with other signaling pathways (16–19).
One of the important pathways AR cross-talks with is the TGF-β pathway (11, 20–23). In rodents, androgen deprivation leads to rapid elevation in levels of TGF-β, TβRI, TβRII, and activation of Smads 2 and 3, concomitant with the onset of apoptosis (24–27). Further in vivo and in vitro studies support that androgens promote cell survival, in part, through blocking TGF-β-induced responses, although the underlying mechanisms remain poorly investigated (11, 28–30).
AR has been shown to physically interact with Smad3 in the absence of androgen or TGF-β stimulation (11, 21, 22). We previously reported that the physical interaction of AR with Smad3 confers the ability of DHT to suppress TGF-β- or Smad3-induced transcription (11). In our model, AR physically interacts with Smad3 and DHT then blocks the association of Smad3 to SBE (11). Those results suggested that over-activation of AR during prostate tumor progression may lead to loss of TGF-β-induced responses, particularly the ability of TGF-β to function as a tumor suppressor. However, several important issues remained unresolved, principally whether androgens could actually reverse TGF-β-promoted growth arrest or/and apoptosis, the spectrum of TGF-β-regulated genes affected by androgens, and the subset of those gene changes involved in growth control or apoptosis. Here we showed that androgen-bound AR significantly protects cells from TGF-β-induced apoptosis, likely by reversing TGF-β-promoted downregulation of Bcl-xL and cyclin Ds, and activation of caspase-3 in human and rat prostate cell lines. We also provide evidence that androgen broadly influences TGF-β responses through intercepting an additional step in the TGF-β signaling pathway, namely by loss of TβRII expression through a transcriptional mechanism mediated partly by downregulation of Sp1 activity.
Materials and Methods
Materials
Sources were: Recombinant human TGF-β1 (R&D Systems, Minneapolis, MN); PNGase F (New England Biolab, Ipswich, MA); pGL3-basic-luciferase (Promega, Madison, WI); Sp1 consensus (sc-2502) and mutant (sc-2503) oligonucleotides (Santa Cruz Biotechnology, Santa Cruz, CA); characterized Fetal Bovine Serum (FBS) and dextran-charcoal stripped FBS (DC) (HyClone, Logan, UT); CellTiter 96® Non-Radioactive Cell Proliferation Assay Kit (Promega, Madison, WI); Western blot antibodies- see supplemental material.
Cell Culture
NRP-154 and NRP-152 prostatic epithelial cell lines (31, 32) were maintained in GM2.1 culture medium as described previously (33). The DU145 human prostate cancer cell line was maintained in DMEM/F12 medium supplemented with 5 % FBS. LNCaP, C4-2B, and VCaP human prostate cancer cell lines, were maintained in DMEM/F12 containing 10% FBS in poly-D-lysine-coated 75 cm2 culture flask (11). Unless indicated, all experiments in NRP-154 and NRP-152 cells, were performed in 1% DC-GM3 medium, and experiments involving DU145, LNCaP, C4-2B, and VCaP cells, were performed in DMEM/F12 supplemented with 1% or 10% DC-stripped FBS and 15 mM HEPES. Experiments in LNCaP and C4-2B cells transiently infected with Admax-TβRII were performed in DMEM/F12 supplemented with 1% DC-stripped FBS, 15 mM HEPES, and 20 ng/ml EGF.
Cell number/Cell viability assays
CellTiter 96® non-radioactive cell proliferation assay kit (Promega, Madison, WI) was used to assess cell viability. In brief, NRP-154/AR4 cells were plated at a density of 5 × 103 cells/100 μl/well in 96 well plates with 1% DC-GM3 medium with ± 2 μg/ml doxycyclin and ± DHT. Cells were incubated for overnight followed by treatment with TGF-β1 for 72 h. Cell viability was expressed as absorbance relative to that of untreated control. For cell number assay, NRP-154 cells in 12-well plates (5 × 104 cells/1ml/well) were infected with AdMax-AR (1:500) for 2h and cultured overnight in 1% DC-GM3 ± DHT. TGF-β1 was added and cells were incubated for an additional 48 h. Cell number was assayed using a Coulter Electronics counter as before (34).
Crystal violet nuclei staining
Cells were fixed with 2% formalin/PBS and incubated with 0.2 mg/ml of crystal violet solution for nuclei staining. Cells were then washed twice with 1X PBS and dye was eluted by adding 1% Triton/PBS. The eluted dye was subjected to spectrophotometry at 550 nm.
Northern blot analysis
Northern blot analysis was performed essentially as described (35). In brief, 10 μg of total RNA was electrophoresed and equal loading and even transfer were assessed by visualization of the 18S rRNAs. mRNA was detected with cDNA probes labeled with 32P-dCTP using Prime-It® RmT Random Primer labeling Kit (Stratagene, La Jolla, CA).
RT-PCR
RT was performed as described (13). Taq Polymerase Master Mix (Promega, Madison, WI) was used for PCR amplification of rat TβRII, using 32 or 28 cycles, respectively, of the following temperature gradients: 95°C for 15 sec, 60°C for 30 sec, and 72 °C for 2 min. β-Actin, amplified as above for 21 cycles, served as an internal control. Refer to Supplementary information for primer sequence.
Transient Transfection and Luciferase Assay
Cells were transfected using either calcium phosphate co-precipitation method (NRP-154) or Invitrogen’s Lipofectamine plus reagent (LNCaP, DU145) as before (11). Luciferase activity was measured using Promega’s Dual Luciferase Assay Kit and a ML3000 Microtiter Plate Luminometer. The Sp1 response element reporter construct, Sp1-luc, was made by inserting 4 copies of a consensus Sp1 response element upstream of the TATA transcription start site (in Hind III and Sal I of MCS) of the basic luciferase cloning vector, pCIS-CK (Stratagene, La Jolla, CA).
Adenoviruses
Adenovirus vectors that direct the expression of HA-TβRII (AdMax-HA- TβRII WT(12)) was constructed using the AdMax system (Microbix Biosystems, Toronto, Canada) as described previously (12). For AdMax-AR, the corresponding region to the coding sequence of AR was subcloned from pCDNA3-AR (11) into the adenovirus shuttle vector pDC515. HEK293 cells in 6 well plates were co-transfected with pDC515-AR (1 μg) and 1 μg of the genomic vector pBHGfrtDE1,3FLP, by calcium phosphate-mediated transfection (11). Virus preparations were performed as previously described (12).
DNA Fragmentation Assay
All of these procedures were performed essentially as described (36, 37). DNA was purified and internucleosomal DNA fragmentation was detected using TACS apoptosis DNA ladder kit (Trevigen, Gaithersburg, MD) according to manufacturer’s instructions.
DNA pull-down assay
Biotin labeled Sp1 oligonucleotides (WT and mutant, 22mer) were dimerized with its complements. For each reaction, 1.5 μg of dimer was incubated for 15 min at room temperature with 50 μl of Dynabeads® M-280 streptavidin (Invitrogen, Carlsbad, CA) washed twice with 2 X B&W buffer (10 mM Tris-Cl (pH 7.5), 1 mM EDTA, 2 M NaCl). After conjugation in 1 X B&W buffer, oligo-conjugated beads were washed three times with 1 X B&W buffer to remove unconjugated oligonucleotides, and resuspended with ice-cold DNAP buffer (10 mM HEPES, pH 7.9, 100 mM KCl, 5 mM MgCl2, 10 % glycerol, 0.5 % NP-40, 1 mM EDTA) containing 1 mM DTT added freshly. 100 μg nuclear protein was incubated with oligo-conjugated beads and reaction volume was adjusted up to 500 μl with 1 X DNAP containing Complete EDTA-free Protease inhibitor Mixture (Roche, Mannheim, Germany), 1mM sodium orthovanadate, 1 mM phenymethylsulfonyl floride, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, and 1 mM DTT. Polydeoxyinosinic-deoxycytidylic acid (5 μg) was added to the reaction tube, which were then incubated for 4 h at 4 °C with gentle mixing on a rotator. Beads were washed three times on ice with DNAP containing 1 mM DTT, eluted with 45 μl of 1 X SDS buffer by treating for 5 min at 85 °C. Eluates were subjected to Western blot analysis.
AR-inducible cell lines, Western blots, Preparation of nuclear and cytosolic extract
See supplemental section.
Results
Androgen protects NRP-154 cells from TGF-β-induced apoptosis
We previously reported that androgens can intercept TGF-β-induced changes in gene expression through a physical interaction of AR with Smad3 in LNCaP and NRP-154 cells transfected with TβRII and AR, respectively. Our EMSA results indicated that AR blocked Smad3 binding to SBE (11). However, the impact of androgens on growth suppression and apoptosis was undefined, due to lack of a suitable prostate carcinoma cell line that expressed AR and responded to TGF-β by growth suppression and/or apoptosis. To resolve this barrier we generated an adenoviral system (AdMax-AR) to efficiently express AR (>90% infection) in the NRP-154 cell line, which is exquisitely sensitive to TGF-β-induced apoptosis (37). The AR or control virus (AdMax-cont) infected cells were treated with DHT (1 or 10 nM) 24 h before the addition of TGF-β and changes in apoptosis and cell morphology were observed 48 h later (Fig. 1A). Fourty-eight h of TGF-β1 treatment killed essentially all cells infected with the control or AR virus, whereas treatment with 1 or 10 nM DHT significantly protected AR expressing cells against killing by TGF-β1 (Fig. 1A, Supplementary Fig. 1S A, and data not shown). Such changes were also reflected at the level of total internucleosomal DNA fragmentation (Fig. 1B), a hallmark of apoptosis. We confirmed that ligand-bound AR can also protect against TGF-β-induced death in a doxycyclin-inducible, AR-expressing NRP-154 clonal cell line (NRP-154/AR4) (Fig. 1C, left), and in non-tumorigenic NRP-152 cells infected with AdMax-AR (Supplementary Fig. 1S B).
Figure 1.
Androgen Protects Cells from TGF-β-induced Cell Death in Prostate Cancer Cell Lines. (A) NRP-154 cells were transiently infected with either AdMax-cont or AdMax-AR (1:500), and cultured with ±DHT (1 or 10 nM) for 24 h prior to 48 h of TGF-β1 (10 ng/ml) treatment. (B) The internucleosomal fragmentation was measured following treatment with ±TGF-β1, ±DHT, and ±AdMax-AR in NRP-154. (C) MTT assay in NRP-154/AR4 cells, stably expressing AR and treated as in B (left). DHT was added to NRP-154 cells transiently overexpressing AR for 24 h prior to TGF-β1 treatment. 5 days later, the live cells were cultured in GM 2.1 medium for additional 10 days and cell number was assayed using a Coulter Electronics counter (right). (D) NRP-154 cells transiently overexpressing AR were cultured in ±DHT for 24 h prior to treatment with TGF-β1. After 5 days of TGF-β1 treatment, medium was replaced with GM 2.1 and cells were cultured for 4 days before crystal violet staining to evaluate cell viability. Cell viability was quantified by spectrophotometry at 550 nm, measuring dye eluted from cells. Data shown are the average (±S.D.) of triplicate independent experiments. Results are representative of two to three independent experiments.
To examine the duration of such protection, we next assessed changes in total viable cells after allowing the TGF-β ± DHT treated cultures (5 days) to recover in their normal growth medium without exogenous TGF-β or DHT for 4 to 10 days. Our data supported that as little as 0.1 nM DHT enormously protected the cells from TGF-β1-induced apoptosis, as no cells survived the effects of TGF-β unless protected by DHT, giving rise to >104-fold increases in total cell number with DHT versus without DHT (Fig. 1C, right, and D). Taken together, these data support that androgen receptor signaling very effectively suppresses TGF-β-induced apoptosis in prostate epithelial cells.
DHT suppresses the ability of TGF-β1 to induce expression of PAI-1, activate caspase-3, and suppress the expression of Bcl-xL and cyclin Ds
The above results suggest that androgen-bound AR may interrupt the ability of TGF-β to control the expression of or activate proteins involved in apoptosis or cell cycle control. We previously reported that ligand-bound AR inhibits TGF-β–induced transcriptional responses, using various response element luciferase reporter constructs and the plasminogen activator inhibitor-1 (PAI-1) promoter construct, 3TP-lux (11). To confirm those results at the level of endogenous PAI-l, effects of DHT on TGF-β-induced expression of PAI-1 mRNA and protein were examined in NRP-154 + AR and in LNCaP + TβRII. TGF-β1 strongly induced PAI-1 mRNA and protein levels, occurring in a manner that was abolished by pretreatment with DHT (Fig. 2A). A bone metastastic derivative of LNCaP, C4-2B, which are androgen refractory likely through having constitutively active AR (38), failed to induce PAI-1 protein expression in response to TGF-β1 despite enforced expression of TβRII (Fig. 2A).
Figure 2.
DHT reverses expression of genes regulated by TGF-β1 in NRP-154 and LNCaP cells. (A) NPR-154 ± AR cells were treated with DHT for 24 h prior to TGF-β1. LNCaP and C4-2B cells were incubated with DHT for 24 h prior to TGF-β1. PAI-1 was determined by either RT-PCR (left) or Western blot analysis (right). (B) NRP-154 ± AR cells were pretreated with DHT for 24 h followed by incubation for additional 48 h (left) or 24 h (right) with TGF-β1. (C) NRP-154 ± AR cells and (D) LNCaP and C4-2B cells were treated with ±DHT, and ± TGF-β1. Whole cell lysates (50 μg protein) were subjected to Western blot analysis (B–D). Data shown (A–D) are representative of two to three different experiments/treatments.
We showed that TGF-β1 induces apoptosis, activates caspase-3, downregulates the anti-apoptotic factor Bcl-xL (39), induces cell cycle arrest at G1 and G2/M, and downregulates cyclin D2 in NRP-154 and NRP-152 (derived from preneoplastic rat prostate). To understand how androgen prevents TGF-βinduced growth suppression and apoptosis, we examined whether DHT could affect the ability of TGF-β to control expression of anti/pro-apoptotic factors and cyclin Ds. Either 1 or 10 nM DHT significantly blocked TGF-β-induced activation of caspase-3 (Fig. 2B), whereas 10 nM DHT was necessary to reverse the TGF-β1-induced loss of Bcl-xL in NRP-154 ± AR (Fig. 2B), suggesting that the reversal of caspase-3 activation was independent of that of Bcl-xL. We next tested the possibility that androgen treatment can reverse the ability of TGF-β1 to reduce cyclin D expression. In the case of NRP-154 + AR cells, TGF-β1 treatment downregulated cyclin D3 levels at 24 h, and D1 and D3 by 48 h. Such down-regulation of cyclin D3 was reversed by DHT (Fig. 2C). Similar experiments were performed in LNCaP and C4-2B cells infected with AdMax-TβRII. DHT slightly increased the expression of all three cyclin Ds in LNCaP cells. In contrast, C4-2B cells expressed high basal levels of all three cyclin Ds, consistent with expression of constitutively active AR in those cells, and showed no induction by DHT (Fig. 2D). As expected, DHT completely reversed the TGF-β1-mediated down-regulation of cyclin Ds 1, 2 and 3 at both 24 h (Fig. 2D) and 48 h (data not shown) in LNCaP cells. However, C4-2B cells having constitutively active AR were refractory to the down-regulation of cyclin Ds by TGF-β1 (Fig. 2D). Taken together, the above data suggest that distinct mechanisms control the ability of TGF-β to down-regulate each of the D cyclins and that such differential regulation is cell-type specific.
DHT downregulates expression of TβRII, but not TβRI
We previously reported that androgens disrupt the binding of Smad3 to SBE through a direct association of AR with Smad3 (11). However, further investigation in our group suggested additional mechanisms may be involved in disruption of TGF-β responses by androgens. Our study suggested that TGF-β1-activated Smad3 was markedly repressed by androgen (unpublished data). Thus, to better understand the mechanism by which AR suppresses TGF-β1 responses, we examined whether DHT may also alter levels of TGF-β receptors. NRP-154 + AR cells were preincubated with DHT for 48 h prior to TGF-β1 treatment, and protein levels of TβRI/II were determined. DHT substantially downregulated levels of TβRII, but not TβRI, irrespective of TGF-β1 treatment (Fig. 3A). We further performed time-course experiments of DHT on TβRII expression. TβRII protein levels were substantially downregulated as early as 3 h of DHT addition and persisted at the same level up to 48 h, whereas no change in TβRI protein levels was observed at any of the time points tested (Fig. 3B). Similar to NRP-154, in DU145 ligand-stimulated AR abolished TβRII protein expression, and neither DHT nor AR alone inhibited TβRII expression (Fig. 3C). We were not able to assess the effect of DHT on regulation of TβRII in LNCaP and C4-2B as their level of endogenous TβRII was undetectable. We therefore used another androgen receptor positive prostate cancer cell line, VCaP, to assess the role of endogenous AR on endogenous TβRII levels. Consistently, 48 h treatment with DHT repressed the level of TβRII in VCaP (Fig. 3D). Taken together, our data suggest that androgen suppresses TGF-β signaling partly by decreasing TβRII.
Figure 3.
Suppression by androgen of level of TβRII, not TβRI. (A and B) Effect of androgen on protein levels of TGF-β receptors. NRP-154 ± AR cells were pretreated ±DHT for 48 h prior to TGF-β1 at the indicted concentrations (A) or for the indicated times before TGF-β1 (10 ng/ml, 1 h) (B). (C) Effect of DHT on level of TβRII in DU145 ± AR (C) and VCaP cells (D), stimulated with DHT for 48 h. Samples deglycosylated were subjected to western blotting. Results are representative of two to three different experiments (A–D).
Transcriptional repression of TβRII by DHT
The relatively rapid (3 h) down-regulation of TβRII protein by DHT (Fig. 3B) suggested a transcriptional mechanism. We therefore examined whether DHT also suppressed the levels of TβRII mRNA. Consistent with the above results, a time-course experiment revealed that DHT significantly decreased the mRNA levels of TβRII, with noticeable changes occurring between 4 to 12 h of treatment (Fig. 4A, left). DU145 + AR also showed robust loss of TβRII expression following 24 h DHT treatment (Fig. 4A, right). Together, these data suggest that DHT controls TβRII protein levels through mRNA stability or transcriptional control rather than protein stability or translational control.
Figure 4.
Transcriptional regulation of TβRII by DHT in NRP-154, LNCaP, DU-145 and VCap cells. (A) RT-PCR for TβRII in NRP-154 cells and effect of DHT on level of TβRII mRNA in DU145 cells, by Northern Blot analysis. (B and C) Cells were transfected with total 1 μg of DNA including TβRII promoter-luciferase (pTβRII -luc) constructs and cmv-renilla, followed by DHT treatment for 48 h or the indicated time. For LNCaP cells (B) with pTβRII(−216/+35)-luc, cells were incubated with 1 μM of MS275 for an additional 24 h, following DHT treatment. Data shown are relative values of firefly luciferase normalized to renilla luciferase. Each bar represents the average of triplicate determinations ± S.E.
To determine if DHT can inhibit expression of TβRII through a transcriptional mechanism, we co-transfected various prostate cell lines with ± pCDNA3-AR, TβRII promoter constructs (pTβRII-luc) along with CMV-renilla, followed by 24 h ±10 nM DHT and monitored changes in relative luciferase activity (Fig. 4B). The pTβRII(−216/+35)-luc construct, which has two positive response elements (PRE1 and 2) shows optimal promoter activity (40). DHT significantly inhibited this promoter activity by about 85% in NRP-154 + AR and by >90% in DU145 + AR cells (Fig. 4B), consistent with protein and mRNA levels. The full-length TβRII promoter construct, pTβRII(−1670/+38)-luc, although less active, was almost completely (> 90%) suppressed by DHT under the same conditions in DU145 + AR cells (Supplementary Fig. 2S A). As LNCaP cells express low to undetectable levels of TβRII and have very low pTβRII-luc activity, we used the histone deacetylase inhibitor MS-275 to enhance this promoter activity, as before (13). DHT (24 h) effectively suppressed expression of this promoter in LNCaP treated with 1 μM MS-275. About 50% inhibition of this promoter activity was observed by DHT in VCaP (Fig. 4B).
To identify the specific promoter region responsible for suppression by DHT, cells were transiently transfected with various 5′ end truncations of TβRII promoter-luciferase constructs (41) (Fig. 4C). Surprisingly, the effect of inhibition by androgen was observed in all the truncated TβRII-promoter constructs despite differences in the magnitude of repression between cell lines (Fig. 4C). This unexpected observation led us to examine the involvement of transcription factors essential for transcription or initiation of transcription by TATA-less promoters. In the case of TβRII promoter, Sp1 has been shown to be critical in transcriptional initiation of TβRII, as it is TATA-less. All deletion constructs of this promoter tested (Fig. 4C) contain either one or two Sp1 sites at −143 and −25. We introduced mutations between −63 and +2 at sites corresponding to putative transcription factor binding elements including Sp1 site at −25 (Supplementary Fig. 2S B) and tested whether any of those mutants would ablate suppression by DHT. As expected, mutation of the −25 Sp1 site abolished promoter activity in both DU145 and NRP-154 cells, suggesting this Sp1 site functions as a transcriptional start site. Other mutations substantially lowered the basal activity of this promoter, but failed to reverse the inhibitory effect of DHT (Supplementary Fig. 2S C). Taken together, these data strongly implicate Sp1 as a target of androgen-mediated suppression of the TβRII promoter.
DHT inhibits Sp1 expression
To study the potential role of Sp1 in mediating the suppression of the TβRII promoter by DHT, we measured protein levels of Sp1 in NRP-154 + AR cells following treatment with DHT for various times. DHT significantly lowered the level of Sp1 by 6 h of treatment and continued through 48 h (Fig. 5A), correlating with levels of TβRII (Fig. 3). Sp1 levels were similarly decreased by DHT in DU145 + AR cells (Fig. 5A).
Figure 5.
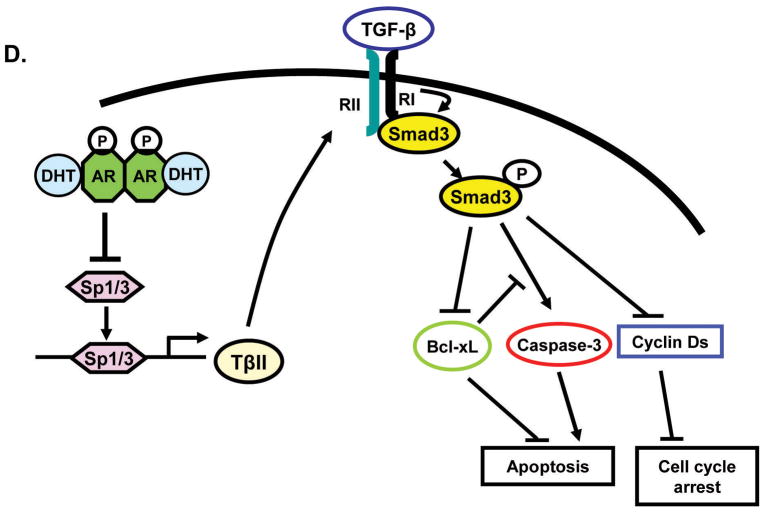
Sp1 is a mediator for transcriptional regulation of TβRII by DHT. (A) Effect of DHT pretreatment (48 h) on Sp1 and Sp3 levels in NRP-154 + AR. Time-course experiments of DHT in NRP-154 + AR. Levels of Sp1 and Sp3 in DU145 ± AR cells treated with DHT. Sp1 protein levels in whole lysates, nuclei, and cytosol after DHT treatment (1 nM) in NRP-154 ± AR cells. (B) NRP154 + AR, DU145, and VCaP cells were transiently transfected with cmv-renilla and either control vector (pCIS-CK) or Sp1 response element-reporter (Sp1-luc). (C) DNA pull-down assay; Biotin-labeled consensus Sp1 oligonucleotides (WT and mutant) were incubated with nuclear lysates from NRP-154 ± AR cells (IP: top, Input; bottom) (left). Effect of overexpressed WT-Sp1 on Sp1-luc activity inhibited by DHT in NRP-154 transiently expressing AR (right). Data shown are relative values of firefly luciferase normalized to renilla luciferase. Each bar represents the average of triplicate determinations ± S.E. (D) Schematic representation of our proposed model.
Although Sp3 is known to function mainly as a transcriptional suppressor, it may also function as a transcriptional activator. Sp3 binds to the same Sp1 response elements, but may either activate or repress transcription, depending of context of other response elements or transcription regulators. Therefore, we also examined changes in Sp3 expression on the same blots. In contrast to Sp1, DHT did not affect Sp3 expression in NRP-154 + AR; however, DHT downregulated Sp3, especially the 60 kDa Sp3 isoform in DU145 + AR (Fig. 5A). Collectively, these data suggest that the transcriptional suppression of TβRII by androgen may occur through down-regulation of Sp1/Sp3.
Sp1 is activated by post-translational modifications that promote its rapid nuclear translocation. We further studied whether the loss of Sp1 protein by androgen in whole cell lysates reflects its level in the nuclear compartment and influences the activity of Sp1. As in Fig. 5A and Supplementary Fig. 3S A, no Sp1 was detected in the cytosolic fraction and a markedly low level of Sp1 was measured in the nuclear fraction of DHT-treated cells, showing similar Sp1 levels in parallel whole cell lysates. We next tested the effect of DHT on the transcriptional activity of Sp1 alone by using an Sp1 reporter construct (Sp1-luc), composed of four tandem consensus Sp1 binding elements inserted upstream of the TATA transcription start site of the promoter-less luciferase reporter pCIS-CK. As expected, DHT strikingly inhibited the activity of Sp1-luc (> 90%) in DU145, supporting loss of Sp1 activity caused by DHT (Fig. 5B), reflecting loss of Sp1 levels in the nucleus (Fig. 5A). Similar results were obtained with NRP-154 + AR and VCaP cells (Fig. 5B). Loss of Sp1 activity was next tested by measuring the physical association of endogenous Sp1 from NRP-154+AR cells to biotinylated WT or mutant Sp1 consensus oligonucleotides that were pull-down by strepavidin-agarose resin (Fig. 5C). Our results showed that Sp1 constitutively binds consensus WT but not mutant Sp1 oligos, and that DHT abolished binding of Sp1 to Sp1 oligo only in cells expressing AR (Fig. 5C). We also performed EMSA using Sp1 consensus oligonucleotides (WT and mutant) and Sp1 binding site in the TβRII promoter region −25 (Supplementary Fig. 3S B and C). Androgen-stimulated AR significantly diminished DNA binding to WT but not mutant Sp1-oligonucleotide (Supplementary Fig. 3S B). Furthermore, when a 32 mer-oligonucleotide designed from TβRII (−25p) was subjected to EMSA, DHT clearly suppressed DNA binding of Sp1 (Supplementary Fig. 3S C).
To confirm our model that loss Sp1 activity by DHT plays a role in the ability of DHT to suppress Sp1-dependent promoter activity, we attempted to reverse the DHT-dependent loss of Sp1 activity by overexpressing WT-Sp1 in NRP-154+AR cells. As shown in Fig. 5C, DNA binding activity of Sp1 inhibited by ligand-activated AR was completely restored by exogenously expressed WT-Sp1. Moreover, expression of exogenous Sp1 partially reversed the ability of DHT to diminish TβRII promoter activity in NRP-154 + AR (P<0.001, Supplementary Fig. 3S D). Taken together, the above results strongly support that DHT blocks transcription of TβRII at least partly through downregulation of Sp1 protein expression and inhibition of total DNA binding resulting from its reduced expression (Fig. 5D).
Discussion
Here we provide the first evidence supporting that DHT, functioning through AR, suppresses the TGF-β signaling pathway controlling apoptosis and growth arrest. Moreover, we show that DHT stimulation interrupts TGF-β signaling through shutting down the production of newly made TβRII through a transcriptional mechanism. This mechanism is likely to function cooperatively with another mechanism we previously described (11), involving the direct binding of active Smad3 to AR which blocks the interaction of Smad3 with SBE on target genes. Here we report the first observation that DHT down-regulates the expression and activity of Sp1/Sp3, and provide evidence that DHT-induced transcriptional repression of TβRII is at least partly mediated by down-regulation of Sp1 levels, leading to reduced association of nuclear Sp1 to Sp1 response elements in the TβRII promoter. Previous studies have clearly established the importance of Sp1 and Sp3 in transcriptional controls of TβRII (42). Further efforts in our laboratory are underway to understand the underlying implications of the DHT-mediated loss of Sp1 activity in the regulation of other androgenic responses, and to delineate the mechanism by which androgens down-regulate Sp1 protein levels or its biological activity. These issues are likely to be of fundamental importance in the regulation of androgenic responses, considering the broad range of TATA-less genes that may be influenced by such changes in Sp1 activity. Preliminary RT-PCR data from NRP-154+AR cells show levels of Sp1 mRNA were not significantly altered by DHT (Supplementary Fig. 4S), suggesting that downregulation of Sp1 by DHT occurs at the level of protein stability or translational control, rather than mRNA stability or transcriptional control. Although our data supports a role for Sp1 as a mediator of transcriptional control of TβRII by DHT, we believe that other transcriptional factors such as CBF-A and YY-1, which are slightly suppressed by ligand-bound AR (preliminary study and data not shown), may also play a role in such regulation. Therefore, we speculate that androgen regulation of TβRII is more complex involving multiple transcriptional factors.
Our data here are consistent with in vivo studies on the rat prostate where androgen ablation induced by castration was shown to activate the TGF-β signaling not only through inducing the expression of TGF-βs but also through elevating the levels of TβRII and Smad3 activation (24, 26, 27). We thus suggest that androgens may protect against apoptosis of prostate epithelial cells through multiple mechanisms, involving loss of TGF-β1 and TβRII, and the direct association of Smad3 with AR. The latter mechanism is more likely to account for suppression of TGF-β responses in LNCaP-TβRII, where TβRII expression is under control of CMV promoter (not controlled by androgen).
It is well known that prostate cancers ultimately fail anti-androgen therapy as they progress towards a state of androgen independence. Although the mechanisms behind this are yet to be resolved, growing evidence support that the AR signaling pathway is constitutively activated rather than suppressed, most likely through AR mimicking the conformation of ligand-activated AR but without androgen (18). AR silencing studies demonstrate that androgen-independent prostate cancer cells require AR for survival, parallel to the requirement of androgen in androgen-dependent epithelial cells (43). Regardless of the mechanisms involved, our study suggests that constitutive activation of the AR pathway may suppress TGF-β signaling through down-regulating TβRII expression. Consistent with our observation is the C4-2B cell line bearing constitutively active AR; these cells are resistant to TGF-β even when TβRII is over-expressed. In fact, even exogenous TβRII was difficult to detect in C4-2 cells. Moreover, EGF, which enhances expression of TβRII in LNCaP cells (13), failed to elevate TβRII levels in C4-2B cells or sensitize them to TGF-β. Our preliminary data showed no differences between LNCaP and C4-2B cells in the efficiency of transfection or infection. This result therefore indicates that constitutively activated AR in C4-2B cells may promote loss of exogenous TβRII, suggesting that an additional mechanism may be operating in androgen refractory prostate cancer that leads to loss of TβRII. Such a mechanism may contribute to the overall loss of TβRII expression found in human prostate cancer and correlate with poor clinical outcome. Based on these observations and ideas, we propose that constitutive activation of AR during prostate cancer progression may cause loss of the tumor suppressor function of TGF-β in prostate carcinoma cells, thus aiding in tumor progression.
A recent report demonstrates that loss of the tumor suppressor PTEN, occurring at high incidence in late-stage prostate cancers and leading to constitutive activation of Akt and mTOR, promotes androgen independence (44). We previously showed the PI3K/Akt/mTOR pathway can suppress TGF-β signaling through suppressing the activation of Smad3 (33). Thus, loss of PTEN may promote androgen independence at least partly through suppressing TGF-β signaling by an AR-independent mechanism or/and through a mechanism involving the activation of AR by the PI3K/Akt/mTOR pathway. Regardless of the specific mechanisms involved, loss of growth suppression/apoptosis by TGF-β is likely to enhance tumor growth. Thus, restoring TGF-β responses in androgen-independent epithelial cells, perhaps by intercepting the ability of AR to disrupt TGF-β signaling, is likely to have important therapeutic implications.
Supplementary Material
Acknowledgments
National Cancer Institute Grants R01CA092102 and R01CA102074 (D. Danielpour).
We thank Drs. Joan Massagué for 3TP-lux plasmid, and Harvey Lodish for pCMV5-TβRII.
Footnotes
This study was supported by the NCI Grants R01CA092102 and R01CA102074
References
- 1.Roberts AB, Sporn MB. The transforming growth factor beta. New York: Springer-Verlag; 1990. [Google Scholar]
- 2.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:8621–3. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y, Kyprianou N. Restoration of transforming growth factor beta signaling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Res. 1999;59:1366–71. [PubMed] [Google Scholar]
- 4.Tang B, de Castro K, Barnes HE, et al. Loss of responsiveness to transforming growth factor beta induces malignant transformation of nontumorigenic rat prostate epithelial cells. Cancer Res. 1999;59:4834–42. [PubMed] [Google Scholar]
- 5.Song K, Cornelius SC, Danielpour D. Development and characterization of DP-153, a nontumorigenic prostatic cell line that undergoes malignant transformation by expression of dominant-negative transforming growth factor beta receptor type II. Cancer Res. 2003;63:4358–67. [PubMed] [Google Scholar]
- 6.Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 7.Tu WH, Thomas TZ, Masumori N, et al. The loss of TGF-beta signaling promotes prostate cancer metastasis. Neoplasia. 2003;5:267–77. doi: 10.1016/S1476-5586(03)80058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrana JL, Attisano L. The Smad pathway. Cytokine Growth Factor Rev. 2000;11:5–13. doi: 10.1016/s1359-6101(99)00024-6. [DOI] [PubMed] [Google Scholar]
- 9.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–44. [PubMed] [Google Scholar]
- 10.Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19:176–84. doi: 10.1016/j.ceb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Chipuk JE, Cornelius SC, Pultz NJ, et al. The androgen receptor represses transforming growth factor-beta signaling through interaction with Smad3. J Biol Chem. 2002;277:1240–8. doi: 10.1074/jbc.M108855200. [DOI] [PubMed] [Google Scholar]
- 12.Danielpour D, Song K. Cross-talk between IGF-I and TGF-beta signaling pathways. Cytokine Growth Factor Rev. 2006;17:59–74. doi: 10.1016/j.cytogfr.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Song K, Krebs TL, Danielpour D. Novel permissive role of epidermal growth factor in transforming growth factor beta (TGF-beta) signaling and growth suppression. Mediation by stabilization of TGF-beta receptor type II. J Biol Chem. 2006;281:7765–74. doi: 10.1074/jbc.M511781200. [DOI] [PubMed] [Google Scholar]
- 14.Veldscholte J, Berrevoets CA, Zegers ND, van der Kwast TH, Grootegoed JA, Mulder E. Hormone-induced dissociation of the androgen receptor-heat-shock protein complex: use of a new monoclonal antibody to distinguish transformed from nontransformed receptors. Biochemistry. 1992;31:7422–30. doi: 10.1021/bi00147a029. [DOI] [PubMed] [Google Scholar]
- 15.Ohara-Nemoto Y, Nemoto T, Sato N, Ota M. Characterization of the nontransformed and transformed androgen receptor and heat shock protein 90 with high-performance hydrophobic-interaction chromatography. J Steroid Biochem. 1988;31:295–304. doi: 10.1016/0022-4731(88)90353-6. [DOI] [PubMed] [Google Scholar]
- 16.Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 17.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 18.Burd CJ, Morey LM, Knudsen KE. Androgen receptor corepressors and prostate cancer. Endocr Relat Cancer. 2006;13:979–94. doi: 10.1677/erc.1.01115. [DOI] [PubMed] [Google Scholar]
- 19.Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruckheimer EM, Kyprianou N. Dihydrotestosterone enhances transforming growth factor-beta-induced apoptosis in hormone-sensitive prostate cancer cells. Endocrinology. 2001;142:2419–26. doi: 10.1210/endo.142.6.8218. [DOI] [PubMed] [Google Scholar]
- 21.Hayes SA, Zarnegar M, Sharma M, et al. SMAD3 represses androgen receptor-mediated transcription. Cancer Res. 2001;61:2112–8. [PubMed] [Google Scholar]
- 22.Kang HY, Huang KE, Chang SY, Ma WL, Lin WJ, Chang C. Differential modulation of androgen receptor-mediated transactivation by Smad3 and tumor suppressor Smad4. J Biol Chem. 2002;277:43749–56. doi: 10.1074/jbc.M205603200. [DOI] [PubMed] [Google Scholar]
- 23.Gerdes MJ, Dang TD, Larsen M, Rowley DR. Transforming growth factor-beta1 induces nuclear to cytoplasmic distribution of androgen receptor and inhibits androgen response in prostate smooth muscle cells. Endocrinology. 1998;139:3569–77. doi: 10.1210/endo.139.8.6138. [DOI] [PubMed] [Google Scholar]
- 24.Kyprianou N, Isaacs JT. Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol. 1989;3:1515–22. doi: 10.1210/mend-3-10-1515. [DOI] [PubMed] [Google Scholar]
- 25.Kyprianou N, Isaacs JT. Identification of a cellular receptor for transforming growth factor- beta in rat ventral prostate and its negative regulation by androgens. Endocrinology. 1988;123:2124–31. doi: 10.1210/endo-123-4-2124. [DOI] [PubMed] [Google Scholar]
- 26.Kim IY, Ahn HJ, Zelner DJ, Park L, Sensibar JA, Lee C. Expression and localization of transforming growth factor-beta receptors type I and type II in the rat ventral prostate during regression. Mol Endocrinol. 1996;10:107–15. doi: 10.1210/mend.10.1.8838150. [DOI] [PubMed] [Google Scholar]
- 27.Brodin G, ten Dijke P, Funa K, Heldin CH, Landstrom M. Increased smad expression and activation are associated with apoptosis in normal and malignant prostate after castration. Cancer Res. 1999;59:2731–8. [PubMed] [Google Scholar]
- 28.Danielpour D. Functions and regulation of transforming growth factor-beta (TGF-beta) in the prostate. Eur J Cancer. 2005;41:846–57. doi: 10.1016/j.ejca.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Kundu SD, Kim IY, Yang T, et al. Absence of proximal duct apoptosis in the ventral prostate of transgenic mice carrying the C3(1)-TGF-beta type II dominant negative receptor. Prostate. 2000;43:118–24. doi: 10.1002/(sici)1097-0045(20000501)43:2<118::aid-pros6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.Lucia MS, Sporn MB, Roberts AB, Stewart LV, Danielpour D. The role of transforming growth factor-beta1, -beta2, and -beta3 in androgen-responsive growth of NRP-152 rat prostatic epithelial cells. J Cell Physiol. 1998;175:184–92. doi: 10.1002/(SICI)1097-4652(199805)175:2<184::AID-JCP8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Danielpour D, Kadomatsu K, Anzano MA, Smith JM, Sporn MB. Development and characterization of nontumorigenic and tumorigenic epithelial cell lines from rat dorsal-lateral prostate. Cancer Res. 1994;54:3413–21. [PubMed] [Google Scholar]
- 32.Bonham MJ, Danielpour D. Improved purification and yields of RNA by RNeasy. Biotechniques. 1996;21:57–60. doi: 10.2144/96211bm12. [DOI] [PubMed] [Google Scholar]
- 33.Song K, Wang H, Krebs TL, Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-beta/ALK5-mediated Smad3 activation. Embo J. 2006;25:58–69. doi: 10.1038/sj.emboj.7600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song K, Krebs TL, Danielpour D. Novel Permissive Role of Epidermal Growth Factor in Transforming Growth Factor beta (TGF-beta) Signaling and Growth Suppression: MEDIATION BY STABILIZATION OF TGF-beta RECEPTOR TYPE II. J Biol Chem. 2006;281:7765–74. doi: 10.1074/jbc.M511781200. [DOI] [PubMed] [Google Scholar]
- 35.Stewart LV, Song K, Hsing AY, Danielpour D. Regulation of trespin expression by modulators of cell growth, differentiation, and apoptosis in prostatic epithelial cells. Exp Cell Res. 2003;284:303–15. doi: 10.1016/s0014-4827(02)00037-x. [DOI] [PubMed] [Google Scholar]
- 36.Danielpour D. Transdifferentiation of NRP-152 rat prostatic basal epithelial cells toward a luminal phenotype: regulation by glucocorticoid, insulin-like growth factor-I and transforming growth factor-beta. J Cell Sci. 1999;112(Pt 2):169–79. doi: 10.1242/jcs.112.2.169. [DOI] [PubMed] [Google Scholar]
- 37.Hsing AY, Kadomatsu K, Bonham MJ, Danielpour D. Regulation of apoptosis induced by transforming growth factor-beta1 in nontumorigenic rat prostatic epithelial cell lines. Cancer Res. 1996;56:5146–9. [PubMed] [Google Scholar]
- 38.Bhuiyan MM, Li Y, Banerjee S, et al. Down-regulation of androgen receptor by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66:10064–72. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- 39.Chipuk JE, Bhat M, Hsing AY, Ma J, Danielpour D. Bcl-xL blocks transforming growth factor-beta 1-induced apoptosis by inhibiting cytochrome c release and not by directly antagonizing Apaf-1-dependent caspase activation in prostate epithelial cells. J Biol Chem. 2001;276:26614–21. doi: 10.1074/jbc.M100913200. [DOI] [PubMed] [Google Scholar]
- 40.Choi SG, Yi Y, Kim YS, et al. A novel ets-related transcription factor, ERT/ESX/ESE-1, regulates expression of the transforming growth factor-beta type II receptor. J Biol Chem. 1998;273:110–7. doi: 10.1074/jbc.273.1.110. [DOI] [PubMed] [Google Scholar]
- 41.Bae HW, Geiser AG, Kim DH, et al. Characterization of the promoter region of the human transforming growth factor-beta type II receptor gene. J Biol Chem. 1995;270:29460–8. doi: 10.1074/jbc.270.49.29460. [DOI] [PubMed] [Google Scholar]
- 42.Ko Y, Banerji SS, Liu Y, et al. Expression of transforming growth factor-beta receptor type II and tumorigenicity in human breast adenocarcinoma MCF-7 cells. J Cell Physiol. 1998;176:424–34. doi: 10.1002/(SICI)1097-4652(199808)176:2<424::AID-JCP21>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 43.Litvinov IV, Vander Griend DJ, Antony L, et al. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc Natl Acad Sci U S A. 2006;103:15085–90. doi: 10.1073/pnas.0603057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiao J, Wang S, Qiao R, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–91. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.