Abstract
DYT1 dystonia is caused by a single GAG deletion in Exon 5 of TOR1A, the gene encoding torsinA, a putative chaperone protein. In this study, central and peripheral nervous system perturbations (transient forebrain ischemia and sciatic nerve transection, respectively) were used to examine the systems biology of torsinA. After forebrain ischemia, quantitative real-time RT-PCR identified increased torsinA transcript levels in hippocampus, cerebral cortex, thalamus, striatum, and cerebellum at 24 h and 7 d. Expression declined toward sham values by 14 d in striatum, thalamus and cortex, and by 21 d in cerebellum and hippocampus. TorsinA transcripts were localized to dentate granule cells and pyramidal neurons in control hippocampus and were moderately elevated in these cell populations at 24 h after ischemia, after which CA1 expression was reduced, consistent with the loss of this vulnerable neuronal population. Increased in situ hybridization signal in CA1 stratum radiatum, stratum lacunosum-moleculare, and stratum oriens at 7 d after ischemia was correlated with the detection of torsinA immunoreactivity in interneurons and reactive astrocytes at 7 and 14 days. Sciatic nerve transection increased torsinA transcript levels between 24 h and 7 days in both ipsilateral and contralateral dorsal root ganglia (DRG). However, increased torsinA immunoreactivity was localized to both ganglion cells and satellite cells in ipsilateral DRG but was restricted to satellite cells contralaterally. These results suggest that torsinA participates in the response of neural tissue to central and peripheral insults and its sustained up-regulation indicates that torsinA may contribute to remodeling of neuronal circuitry. The striking induction of torsinA in astrocytes and satellite cells points to the potential involvement of glial elements in the pathobiology of DYT1 dystonia.
Keywords: DYT1, dystonia, reactive astrocytes, hippocampus, satellite cells, dorsal root ganglia
DYT1 dystonia obeys an autosomal dominant inheritance pattern with reduced penetrance, usually begins in childhood and frequently generalizes. The causal mutation is a GAG deletion in the TOR1A gene that removes a single glutamic acid residue near the carboxy terminus of the encoded protein torsinA (Ozelius et al., 1997; Ozelius et al., 1999). TorsinA belongs to the ATPases associated with a variety of cellular activities (AAA+) superfamily of proteins. TorsinA possesses an N-terminal signal sequence, a single AAA+ module that includes Walker A and Walker B nucleotide binding motifs, sensor 1 and sensor 2 regions, and two biochemically-confirmed glycosylation sites (Ozelius et al., 1997; 1999; Neuwald et al., 1999; Kamm et al., 2004; Callan et al., 2007). Members of the AAA+ family function as molecular chaperones for protein quality control (protein complex assembly, operation, disassembly, protein folding, unfolding, and degradation), membrane fusion and vesicular transport, and cytoskeletal regulation (Neuwald et al., 1999; Vale, 2000; Ogura and Wilkinson, 2001).
In cultured cells, the majority of torsinA is located in lumen of the endoplasmic reticulum (ER) and associates peripherally with the ER membrane, possibly via binding to an integral ER membrane protein (Hewett et al., 2003; Kuner et al., 2003; Liu et al., 2003; Callan et al., 2007). When overexpressed, torsinA is enriched at the nuclear envelope (Goodchild and Dauer, 2004; Naismith et al., 2004). In normal brain, torsinA is present in neuron perikarya and extends distally to the tips of dendrites and axons (Konakova et al., 2001; Konakova and Pulst, 2001; Augood et al., 2003; Kamm et al., 2004).
An assortment of studies has indicated that torsinA may function as a chaperone for unfolded or degraded proteins and may facilitate movement of polytopic proteins to the cell surface (Torres et al., 2004). TorsinA has been found localized to Lewy bodies in Parkinson's disease brain and inclusion bodies in trinucleotide repeat diseases (Shashidharan et al., 2000; Sharma et al., 2001; Walker et al., 2003). Overexpression of torsinA suppresses aggregation of α-synuclein in human H4 neuroglioma cells (McLean et al., 2002), and polyglutamine-induced protein aggregation in C. elegans (Caldwell et al., 2003). TorsinA facilitates clearance of another dystonia-related protein, ε-sarcoglycan, by the ubiquitin proteosome system and protects PC12 cells against a variety of cellular insults including serum deprivation and oxidative stress (Kuner et al., 2003; Shashidharan et al., 2004; Esapa et al., 2007). Similarly, torsinA protects dopaminergic neurons from oxidative stress in mice (Kuner et al., 2004) and C. elegans (Cao et al., 2005).
The chaperone functions of torsinA are critical during developmental processes which seemingly involve interaction with cytoskeletal elements (Ferrari-Toninelli et al., 2004; Kamm et al., 2004; Hewett et al., 2006). The expression of torsinA is developmentally regulated with the highest levels of transcript and protein seen during the prenatal and early postnatal periods (Xiao et al., 2004). The expression of torsinA is particularly intense in cerebellar cortex and striatal cholinergic interneurons at Postnatal Day 14, a period of intense dendritogenesis and synaptogenesis in these regions (Xiao et al., 2004; Vasudevan et al., 2006). A torsinA homologue, OOC-5, present in C. elegans, plays an essential role in Par protein localization. Mutations of ooc-5 result in polarity defects in C. elegans embryos (Basham and Rose, 2001). Attenuated torsinA expression promotes neurite outgrowth in SH-SY5Y human neuroblastoma cells (Ferrari-Toninelli et al., 2004). Conversely, overexpression of mutant torsinA interferes with neurite extension (Hewett et al., 2006). In aggregate, these studies suggest that torsinA may be part of the molecular machinery required for topologically-precise neuritogenesis and/or associated nuclear rotation.
Since penetration of the DYT1 TOR1A ΔGAG deletion is only 30-40% (Ozelius et al., 1997), environmental factors may contribute to the development of dystonia in individuals who harbor this mutation. Trauma to the central (CNS) and peripheral (PNS) nervous systems, hyperthermia, cerebral ischemia and structural disease of the brain and spinal cord are known causes of secondary dystonia (Jankovic and Van der Linden, 1988; LeDoux and Brady, 2003). Similarly, these and other stressors (e.g., intense sensorimotor training) may trigger the onset of dystonia in genetically predisposed individuals (Treves and Korcyzny, 1986). Despite these important clinical associations, understanding of torsinA responses to neural perturbations in vivo is limited. Our study evaluated the temporal and spatial expression of torsinA in response to central and peripheral nervous system injury in models of transient forebrain ischemia and sciatic nerve transection, respectively.
Experimental Procedures
Animals
All experiments were performed in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and with approval of the Institutional Animal Care and Use Committee. Wistar rats (Hilltop Lab Animals, Inc., Scottdale, PA, USA) were used for transient forebrain ischemia since the effects of 4-vessel occlusion (4-VO) have been well characterized in animals of this source and strain (Pulsinelli and Brierley, 1979; Ueda and Nowak, 2005). The sciatic nerve transections were performed in Sprague-Dawley rats (Harlan, Indianapolis, IN, USA).
Transient forebrain ischemia
Adult male Wistar rats weighing 250 – 300 g were subjected to transient 4-VO ischemia (Pulsinelli and Brierley, 1979) with direct current (DC) potential monitoring as previously described (Ueda and Nowak, 2005). In brief, surgical procedures were carried out under general inhalational anesthesia with 1-2% halothane in 70% N2 and 30% O2. The vertebral arteries were electrocauterized at the first cervical segment and silastic occluding devices were placed around the common carotid arteries. On the following day, rats were re-anesthetized and placed in a stereotaxic frame. Epidural and rectal temperatures were monitored and maintained at 37°C. Hippocampal DC potentials were recorded using glass microelectrodes. The carotid occluding devices were tightened to produce cerebral ischemia, as verified by 10 - 20 mV shifts in DC potential. All hemispheres included in these studies exhibited ischemic depolarizations of 7 - 9 min duration in hippocampus, previously shown to produce consistent loss of CA1 neurons (Ueda and Nowak, 2005). After release of occlusions, scalp incisions were closed, and rats were allowed to recover from anesthesia. Control rats underwent sham surgical procedures that included vertebral artery cauterization and electrode placement without carotid occlusion. Brain tissues for RNA extraction and in situ hybridization were obtained at five post-ischemic survival intervals (6 hr, 24 hr, 7 d, 14 d and 21 d), whereas tissues for immunocytochemistry were collected at three post-ischemic survival intervals (24 hr, 7 d, and 14 d). Preparations from three ischemic and three sham control rats were obtained at each survival interval.
For RNA extraction and in situ hybridization, rats were anesthetized with 5% halothane prior to decapitation. Brains were rapidly removed from the cranial vault and sectioned in the mid-sagittal plane. For in situ hybridization hemispheres were frozen in isopentane (-40 °C), sectioned parasagittally at 16 μm and stored at -80 °C in sealed slide boxes with desiccant capsules. For RNA extraction, cerebellum, striatum, thalamus, hippocampus and cerebral cortex were dissected from the hemisphere and homogenized in RNAwiz™ (Ambion, Austin, TX, USA) on ice within 2-3 min after decapitation.
For immunocytochemistry, rats were overdosed with pentobarbital (100 mg/kg, IP) prior to transcardiac perfusion with heparinized saline and then 4% paraformaldehyde/0.1 M phosphate buffer (PB). Brains were post-fixed for 2 hrs, blocked, and incubated in a cryoprotectant solution (30% sucrose/0.1 M PB, pH 7.4) for at least 48 hrs. Blocks were sectioned at 20 μm and collected on SuperFrost®-Plus glass slides (Fisher Scientific, Pittsburgh, PA, USA).
Sciatic nerve transection
Three-month old male Sprague-Dawley rats (250-300 g) were subjected to left sciatic nerve transection under ketamine/xylazine (87/13 mg/kg, IP) anesthesia. The rat sciatic nerve was readily identified in the posterior thigh between the vastus lateralis and caput vertebralis muscles. In each rat, the left sciatic nerve was completely transected except for the medial epineurium which was left intact to allow for apposition of the cut ends to facilitate axonal regeneration. Wounds were closed and animals were allowed to recover from anesthesia in individual cages. Four age-, weight- and gender-matched non-surgical control rats were only subjected to anesthesia. Four surgical rats were employed at each of five post-transection intervals (24 hr, 3 d, 7 d, 14 d and 28 d). Three rats in each group were utilized for RNA extraction and one was used for immunocytochemical examination of the dorsal root ganglia (DRG).
For RNA extraction, rats were overdosed with pentobarbital (100 mg/kg, IP) prior to transcardiac perfusion with saline and then RNAlater (Ambion) as described in LeDoux et al. (2006). The vertebral column was sharply dissected from the remainder of the carcass and placed in a 15 cc conical tube containing RNAlater. The vertebral column was kept moist with RNAlater while the right and left lumbar (L3-L6) DRG were microsurgically isolated and collected into separate pools for subsequent RNA extraction.
For immunocytochemistry, rats were overdosed with pentobarbital (100 mg/kg, IP) prior to transcardiac perfusion with heparinized saline and then 4% paraformaldehyde/0.1 M PB. The vertebral column was dissected and kept moist with fixative while the right and left lumbar (L4-L5) DRG were isolated and collected into separate vials. DRG were post-fixed for an additional 2 hr and then transferred to a cryoprotectant solution for at least 48 hr. DRGs were sectioned at 15 μm with a cryostat and collected on SuperFrost®-Plus glass slides.
Relative quantitative multiplex real-time RT-PCR (QRT-PCR)
Relative levels of torsinA mRNA were established in all brain and DRG tissues collected for QRT-PCR. In brief, total RNA was extracted with RNAwiz™. RNA purity and concentration were analyzed with a NanoDrop® spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). DNA was removed with DNA-free™ (Ambion). Reverse transcription was performed with Ambion's RETROscript™ kit using 200 ng total RNA as template. The reaction mix was incubated at 44 °C for 1 hr and then at 92 °C for 10 min. QRT-PCR was performed using Taqman® probes (Applied Biosystems, Foster City, CA, USA) for both the target gene (torsinA) and endogenous controls (18S rRNA for brain tissues and GAPDH for DRG). Primer and probe sequences and concentrations along with additional QRT-PCR technical details are provided in Xiao et al. (2004). Of note, technical triplicates were performed on each sample and median values were used for subsequent analyses.
Differential expression of torsinA was determined using the comparative threshold cycle (CT) method. In particular, the expression levels of torsinA transcript in both ischemic and sham brain at each post-ischemic interval were calculated relative to the mean CT value for the sham controls at the corresponding time points. A two-factor (treatment and post-ischemic interval) analysis of variance (ANOVA) was used for statistical analysis with SAS (SAS Institute, Cary, NC, USA).
For relative quantitative analysis of torsinA mRNA in the DRG, expression levels were calculated in reference to the mean CT value for all right and left (L3-L6) DRG from the three non-surgical control rats. One-factor ANOVAs were used for independent analysis of data from the right and left DRG. Single degree-of-freedom post-hoc contrasts were limited to comparisons between individual post-surgical time points and ipsilateral control DRG. Analysis of the ipsilateral (left) DRG at 28 d was limited to two rats since poor quality RNA was obtained from the third animal in this group.
In situ hybridization
Radiolabeled (35S-UTP) complementary RNA (cRNA) probes were used to localize torsinA transcript in cryostat sections of rat brain. Probe and primer pair sequences along with a detailed protocol are provided in Xiao et al. (2004). In brief, probes were made by in vitro transcription with T7 RNA polymerase (Ambion). After fixation, acetylation and dehydration through a graded series of ethanol, slides were incubated overnight in the hybridization buffer with radioactive probes (final concentration - 3 × 104 cpm/μl). After treatment with RNase A (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C for 30 min followed by a high stringency wash at 60 °C for 1 h, slides were dehydrated and then exposed to Kodak Biomax MR Film along with 14C Microscale™ autoradiography standards (RPA504, Amersham Biosciences, Piscataway, NJ, USA) for 5 days. Each sheet of film was exposed to paired ischemia and sham microscopic slides. Autoradiographic images were acquired in transmission mode (ScanMaker 9800 XL, Microtek, Carson, CA, USA) and imported into ImageJ (Java version of NIH Image, http://rsb.info.nih.gov/ij/) for region of interest (ROI) quantification of radioactivity. Optical density was calibrated to the set of autoradiography standards to generate measures of radioactivity (nCi/g tissue) for each ROI. Hippocampal ROIs included internal and external blades of the dentate granule cell layer (iDG, eDG), four regions of stratum pyramidale encompassing CA1 (Z1C, Z2C) and CA3 (Z3C, Z4C), and stratum oriens (SO), stratum radiatum (SR) and stratum lacunosum-moleculare (SLM) of the CA1 region. Signal was acquired from a total of 15 pixels (3 × 5 rectangle) in the center of each ROI, from seven sections per animal and the average was expressed relative to the mean obtained from sham sections hybridized in each of the experimental runs. Two-factor (treatment and post-ischemic interval) ANOVA was used for statistical analysis of relative radioactivity within each ROI with SAS.
Immunocytochemistry
The primary and secondary antibodies used for immunocytochemical studies are listed in Table 1. Cryostat brain and DRG sections were collected in eight and six series, respectively. One series of slides was air dried overnight and stained with cresyl violet (brain) or hematoxylin and eosin (DRG). The other series were processed for immunohistochemical detection of torsinA and biomarkers for hippocampal interneurons (somatostatin and parvalbumin), astrocytes (glial fibrillary acidic protein [GFAP]), microglia (OX-42), intermediate filaments (vimentin) and synaptic vesicles (synaptophysin). Anatomically-equivalent sections from animals representing each of the three post-ischemic survival intervals and corresponding sham controls were processed simultaneously. Ipsilateral and contralateral DRG sections from all post-surgical intervals were processed as a group.
Table 1.
Primary and secondary antibodies
| Antibody name | Type | Target | Concentration | Source |
|---|---|---|---|---|
| TA1 | rabbit polyclonal | torsinA | 1:500 | Dr. Vijaya Ramesh, Harvard Medical School |
| D-M2A8 | mouse monoclonal | torsinA | 1:300 | Dr. Vijaya Ramesh, Harvard Medical School |
| Anti-somatostatin | rabbit polyclonal | somatostatin | 1:250 | Chemicon International, Temecula, CA, USA |
| Parv-19 | mouse monoclonal | parvalbumin | 1:1000 | Sigma, St. Louis, MO, USA |
| GA5 | mouse monoclonal | GFAP | 1:500 | Chemicon |
| OX-42 | mouse monoclonal | microglia | 1:500 | Chemicon |
| V9 | mouse monoclonal | vimentin | 1:10 | Abcam, Cambridge, MA, USA |
| SY38 | mouse monoclonal | synaptophysin | 1:100 | Abcam |
| biotinylated horse anti-mouse | horse polyclonal | mouse IgG (H+L) | 1:500 | Vector, Burlingame, CA, USA |
| biotinylated goat anti-rabbit | goat polyclonal | rabbit IgG (H+L) | 1:500 | Vector |
| Cy2-tagged donkey anti-mouse | donkey polyclonal | mouse IgG (H+L) | 1:250 | Jackson ImmunoResearch Laboratories |
| Cy2-tagged donkey anti-rabbit | donkey polyclonal | rabbit IgG (H+L) | 1:250 | Jackson ImmunoResearch Laboratories |
| rhodamine red-X-tagged donkey anti-mouse | donkey polyclonal | mouse IgG (H+L) | 1:250 | Jackson ImmunoResearch Laboratories |
| rhodamine red-X-tagged donkey anti-rabbit | donkey polyclonal | rabbit IgG (H+L) | 1:250 | Jackson ImmunoResearch Laboratories |
For peroxidase-based detection of torsinA, sections were first incubated in the quenching buffer (10% methanol and 3% H2O2 in 0.02 M phosphate buffered saline [PBS]) for 5 min. Sections were then blocked and permeabilized in PBS containing 2% nonfat dry milk and 0.3% Triton X-100 (Sigma). Next, sections were incubated overnight with primary antibody diluted in PBS with 3% normal serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and 0.1% Triton X-100. Sections were then incubated with biotinylated secondary antibody diluted in PBS with 2% normal serum and 0.1% Triton X-100 for 4 hrs, peroxidase-labeled streptavidin (Vector Laboratories, Burlingame, CA, USA) for 1 hr and then nickel-intensified diaminobenzidine (Vector Laboratories) for visualization. Between each step, slides were thoroughly rinsed with PBS. After the final rinse, slides were air dried overnight, dehydrated, cleared and coverslipped with Permount (Fisher Scientific).
Double-label fluorescent immunocytochemistry was used to examine the relationships between torsinA and cell-type specific biomarkers (Table 1). Two primary antibodies recognizing torsinA were used in these studies, a rabbit polyclonal, TA1, and a mouse monoclonal, D-M2AB. When TA1 was employed, Cy2- or rhodamine red-X-tagged donkey anti-rabbit secondary antibodies (Jackson ImmunoResearch Laboratories) were used to visualize torsinA-immunoreactivity (IR). With D-M2A8, biotinylated horse anti-mouse was used as a secondary antibody followed by Cy2-tagged streptavidin (Jackson ImmunoResearch Laboratories). Slides were thoroughly rinsed, dehydrated, cleared and coverslipped with 1,3-diethyl-8-phenylxanthine mounting medium (Sigma-Aldrich). Sections were visualized with both epifluorescence (Leica DM6000, Leica Microsystems Inc, Bannockburn, IL, USA) and confocal laser-scanning (Bio-Rad Laboratories, Hercules, CA, USA) microscopes. For consistency of illustration, all torsinA-IR was transferred to the RGB green channel and other biomarker-IR to the RGB red channel within Adobe Photoshop (San Jose, CA, USA).
Results
TorsinA transcript is up-regulated after transient forebrain ischemia
QRT-PCR was performed to evaluate the spatial and temporal expression of torsinA mRNA after transient forebrain ischemia, using 18S rRNA as an endogenous control. The efficiencies of torsinA (1.74) and 18S rRNA (1.75) amplification were practically identical; the slope of ΔCT versus log10 ng total RNA was 0.054. The CT values associated with both torsinA and 18S rRNA amplification showed strong linear relationships with log10 ng total RNA (torsinA: R2=0.978; 18S rRNA: R2=0.990).
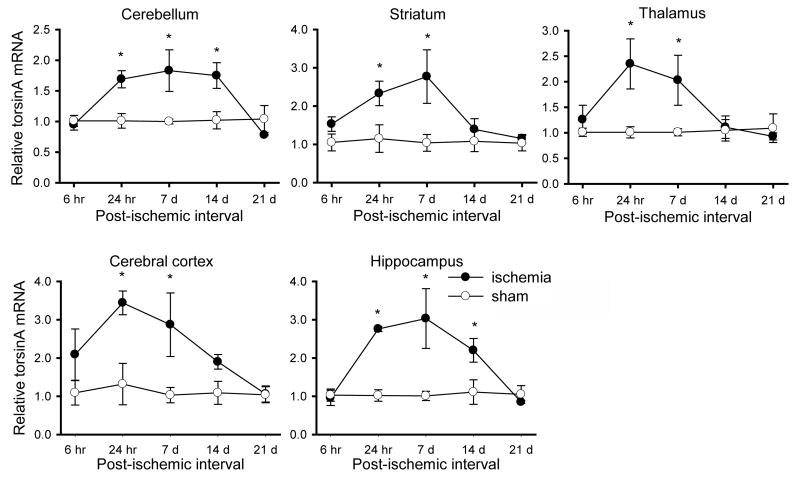
Up-regulation of torsinA mRNA was not detected at the 6 hr post-ischemic interval. However, torsinA mRNA levels were significantly elevated within 24 hr after ischemia in all regions tested (Fig. 1). Transcript levels remained elevated at 7 days in all regions, and then declined toward sham values by 14 d in striatum, thalamus and cortex, and by 21 d in cerebellum and hippocampus.
Fig. 1.
Quantitative RT-PCR analysis of regional torsinA expression after transient forebrain ischemia. TorsinA transcript levels in each brain region are expressed relative to the mean CT value for the sham controls at each time point. A significant increase in torsinA transcript was observed in all regions at 24 hr and 7 d post-ischemic intervals, persisting in cerebellum and hippocampus at 14 d. Results are expressed as the mean ± SEM (*, P < 0.05 for the difference between ischemic and sham groups at each interval).
In hippocampus and cerebellum, there were strong overall effects of ischemia (hippocampus [F1, 20 = 21.63, P = 0.0002], cerebellum [F1, 20 = 13.12, P = 0.0017]), post-ischemic interval (hippocampus [F4, 20 = 5.24, P = 0.0047], cerebellum [F4, 20 = 4.17, P = 0.0129]) and their interaction (hippocampus [F4, 20 = 5.34, P = 0.0043], cerebellum [F4, 20 = 4.48, P = 0.0095]) on torsinA mRNA levels (Fig. 1); in these two regions, increased expression of torsinA transcript were present at 24 hr, 7 d, and 14 d but not at 6 hr or 21 d. In the other three regions examined, the effects of ischemia were also considerable (striatum [F1, 20 = 13.96, P = 0.0013], thalamus [F1, 20 = 8.43, P = 0.0088], cerebral cortex [F1, 20 = 17.72, P = 0.0004]), although there were no significant effects of post-ischemic interval and the ischemia × post-ischemic interval interaction on the relative levels of torsinA transcript. As seen in Fig. 1, individual contrasts between the ischemia and sham groups were significant (P < 0.05) in all regions tested at the 24 hr and 7 d post-ischemic intervals and, additionally, in cerebellum and hippocampus at the 14 d post-ischemic interval. The degree of torsinA mRNA up-regulation was greatest in ischemic cerebral cortex and hippocampus (>3 fold). TorsinA mRNA up-regulation in thalamus and striatum was also substantial (>2 fold compared to control).
The distribution of torsinA mRNA expression determined by in situ hybridization was consistent with its generalized expression in major neuron populations, and this was not grossly impacted following ischemia (Fig. 2). A decline in signal was evident in the hippocampal CA1 pyramidal layer at longer post-ischemic intervals, consistent with the loss of these neurons. In addition, a more diffuse increase in torsinA hybridization was apparent in hippocampal neuropil at 7 and 14 days, as further supported by quantitative analyses. Among the 9 hippocampal ROIs analyzed, SLM (F1, 16 = 18.13, P < 0.0006) and iDG (F1, 16 = 6.88, P < 0.0185) showed the largest overall effects of ischemia on relative torsinA radioactivity (Fig. 3). The ischemia × post-ischemic interval interaction was also significant in iDG (F3, 16 = 4.89, P < 0.0134) and SLM (F3, 16 = 5.71, P < 0.0074). In SLM, for example, relative torsinA radioactivity was only increased at the 7 d post-ischemic interval. In Z1C and Z2C, the effects of post-ischemic interval (F1, 16 = 13.03, P < 0.0001 and F1, 16 = 16.86, P < 0.0001, respectively) and the ischemia × post-ischemic interval interaction (F3, 16 = 10.32, P < 0.0005; F3, 16 = 17.33, P < 0.0001) were highly significant due to increased signal in post-ischemic tissue at 24 hr and decreased signal at 7 d. Other noteworthy findings were the effects of ischemia in SR (F1, 16 = 9.26, P < 0.0077) and the ischemia × post-ischemic interval interaction in eDG (F1, 16 = 8.37, P < 0.0014). Individual contrasts between the ischemia and sham groups showed that relative torsinA radioactivity first became elevated at the 24 hr post-ischemic interval in iDG, eDG, Z2C, and Z1C (P< 0.05, for all). Then, at the 7 d post-ischemic interval, relative torsinA radioactivity was significantly up-regulated in SO, SR and SLM (P < 0.05, for all) and down-regulated in Z2C and Z1C (P < 0.05, for both). By 21 d post-ischemia, torsinA hybridization returned toward sham values in all regions except Z2C.
Fig. 2.
In situ hybridization for torsinA. Representative parasagittal sections are illustrated for a sham control and at indicated post-ischemic intervals, together with higher magnification images of the corresponding hippocampus. Evident changes included a decrease in torsinA expression in the CA1 pyramidal cell layer at late post-ischemic intervals, accompanied by elevated signal in neighboring neuropil. Scale bars indicate 5 mm for whole-brain images and 500 μm for hippocampal images. Upper panel identifies ROIs subjected to further quantitative analysis (see Fig. 3).
Fig. 3.
Quantitative in situ hybridization analysis of hippocampal torsinA transcript levels. TorsinA hybridization was determined in the ROIs indicated in Fig. 2 and expressed relative to the mean value for sham sections hybridized in each experimental run. A slight but significant increase in torsinA transcript was observed in dentate granule cells (iDG and eDG) and CA1 neurons (Z1C and Z2C) at 24 hr after ischemia. Expression in the CA1 pyramidal layer decreased at later intervals, accompanied by increased expression in SR, SLM, and SO at 7 d. Results are expressed as the mean ± SEM (*, P < 0.05 for the difference between ischemic and sham groups at each post-ischemic interval).
TorsinA is up-regulated in hippocampal astroctyes and interneurons
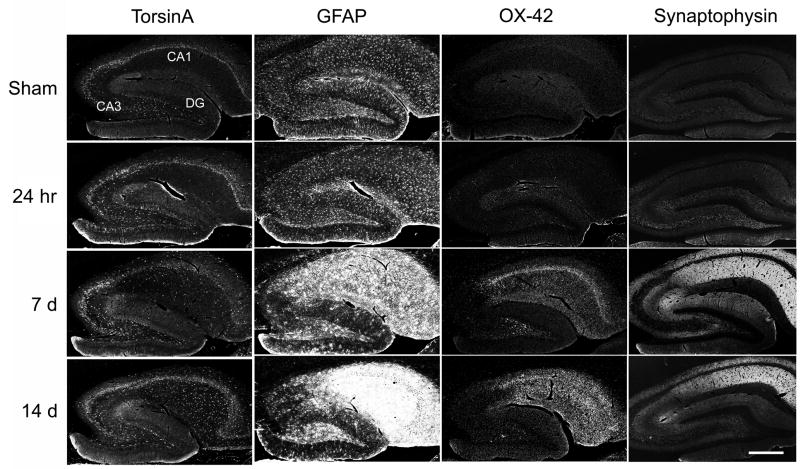
Changes in torsinA immunocytochemistry were consistent with the hybridization results and permitted signal localization to specific cell types. The initial increase in torsinA–IR at 24 hr occurred in the major hippocampal neuron populations that exhibited endogenous expression, whereas later diffuse increases overlapped the distributions of glial activation and synaptophysin up-regulation that took place in regions of CA1 neuron loss (Fig. 4).
Fig. 4.
Distribution of torsinA immunoreactivity and comparison with other markers. Torsin A-IR was moderately increased in the major hippocampal neuron populations at the 24 hr post-ischemic interval, and overlapped the distributions of astrocyte (GFAP) and microglial (OX-42) activation and synaptophysin upregulation at later intervals. Confocal gray scale images of fluorescent immunocytochemistry are shown for torsinA (antibody TA1), GFAP, and OX-42, and inverted gray-scale images of peroxidase detection are shown for synaptophysin. Scale bar=500 μm.
Double labeling (Fig. 5) identified torsinA co-localization in GFAP-positive astrocytes but not OX-42-labeled microglia. Additional cells strongly-IR for torsinA included parvalbumin and somatostatin-IR interneurons. As seen in Fig. 6, hippocampal somatostatin and parvalbumin interneurons were torsinA-IR in both post-ischemic and sham tissue. However, after ischemia, the relative intensity of torsinA-IR was higher in these cell types than in adjacent neurons. Many of the parvalbumin-IR interneurons were scattered among pyramidal cells in the hippocampal pyramidal cell layer and granule cells in the dentate gyrus whereas others were localized to the dentate hilus and near the edge of the SO. Most of the somatostatin-IR interneurons were found in the dentate subgranular proliferative zone, dentate hilus, SO and the boundary region between the SR and SLM.
Fig. 5.
Cellular localization of torsinA immunoreactivity. High-magnification confocal images of double-label fluorescent immunocytochemistry for simultaneous detection of torsinA (green, right column gray scale) and cell markers GFAP, OX-42 (red, middle column gray scale) in post-ischemic (7 d) hippocampus. TorsinA co-localizes with GFAP-positive astrocytes but not OX-42-labeled microglia. Double-labeled cells appear yellow in the merged images. Scale bar=50 μm.
Fig. 6.
Cellular localization of torsinA immunoreactivity. High-magnification confocal images of double-label fluorescent immunocytochemistry for simultaneous detection of torsinA (green, right column gray scale) and cell markers parvalbumin and somatostatin (red, middle column gray scale) in sham and post-ischemic (7 d) hippocampus. TorsinA-IR is prominent in neurons expressing parvalbumin and somatostatin (arrows). Double-labeled cells appear yellow in the merged images. Scale bar=50 μm.
As seen in Fig 7, torsinA-IR was increased in neurons in the subgranular proliferative zone and hilus of the dentate gyrus and pyramidal cells of the CA2 and CA1 regions at 24 hr post-ischemia. At 7 d post-ischemia, torsinA-IR was mildly decreased in CA1 pyramidal cells and increased in neurons in the subgranular proliferative zone and hilus of the dentate gyrus, CA3/CA2 pyramidal cells and the cells in the SR. In the 14 d post-ischemic hippocampus, robust torsinA-IR was apparent in cells scattered within the SR and hilus of the dentate gyrus and moderately-IR cells were sparsely distributed within the SO.
Fig. 7.
Immunoperoxidase detection of torsinA in post-ischemic hippocampus. Using the TA1 polyclonal antibody, increased torsinA-IR was detected in the cells in the subgranular proliferative zone (arrow) and hilus of the dentate gyrus, and pyramidal cells in CA3 and CA2 region at all time points after ischemia, and in cells scattered within the SR and SO of CA1 at 14 d after ischemia. M, molecular layer; G, granule cell layer; and H, hilus of the dentate gyrus. Scale bar = 200 μm.
TorsinA transcript and protein are up-regulated in bilateral DRG after unilateral sciatic nerve transection
TorsinA mRNA expression was increased after sciatic nerve transection (Fig. 8), as assessed by QRT-PCR. The amplification efficiencies for torsinA and GAPDH were 0.95 and 1.01 respectively and the slope of ΔCT versus log10 ng total RNA was -0.043. The CT values associated with torsinA and GAPDH amplification showed strong linear relationships with log10 ng total RNA (torsinA: R2 = 0.94; GAPDH: R2 = 0.99). The overall effect of sciatic nerve transection was robust in contralateral DRG (F5, 12 = 8.31, P < 0.0015) but did not reach statistical significance in ipsilateral DRG (F5, 11 = 2.47, P < 0.098). Post-hoc contrasts showed that torsinA expression differed between the control and surgical groups in both ipsilateral and contralateral DRG at 24 hr and 3 d (P < 0.05, for all), with a significant increase persisting in the contralateral DRG at 7 d (P < 0.05).
Fig. 8.
Temporal profiles of torsinA mRNA expression in DRG after unilateral sciatic nerve transection. TorsinA mRNA expression levels in ipsilateral and contralateral DRGs were calculated in reference to the mean CT value for all DRG from control rats (C). Prominent bilateral increases in torsinA transcript were observed bilaterally at 1 and 3 days. Symbols indicate the mean ± SEM (*, P < 0.05 for the difference between lesioned and control animals).
In control DRG, torsinA-IR was detected in satellite cells, the somas of ganglion cells, and axons (Fig. 9). After sciatic nerve transection, torsinA-IR increased in both ganglion and satellite cells ipsilateral to the lesion, but only in satellite cells contralaterally. In ipsilateral ganglion cells, torsinA-IR was most prominent at 3 and 7 d after sciatic nerve transection. GFAP-IR co-labeled torsinA-positive satellite cells (Fig. 10).
Fig. 9.
Immunocytochemical localization of torsinA expression in DRG after unilateral sciatic nerve transection. TorsinA-IR increases were detected ipsilaterally in both ganglion cells (G) and satellite cells (arrow), but only in satellite cells contralaterally. C, non-surgical control. Scale bar = 100 μm.
Fig. 10.
Co-localization of torsinA and GFAP in satellite cells. Confocal images of fluorescent immunocytochemistry illustrate co-localization in satellite cells (arrow) of ipsilateral DRG 14 d after sciatic nerve transaction. Co-localization appears yellow in the merged images. G, ganglion cell. Scale bar=50 μm.
TorsinA and vimentin co-localize in neuronal and glial elements in the CNS and PNS
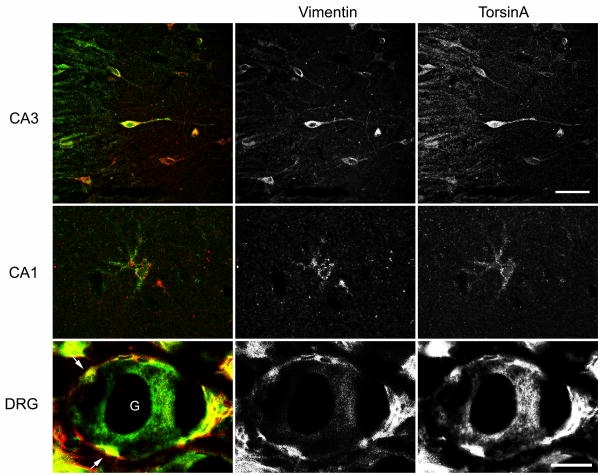
Given the apparent up-regulation of GFAP after both CNS and PNS insults and prominent expression of torsinA in GFAP-IR reactive astrocytes and satellite cells, we elected to localize a closely related intermediate filament protein, vimentin, in hippocampus and DRG (Fig. 11). Vimentin-IR interneurons and reactive astrocytes were readily apparent in post-ischemic hippocampus. In interneurons, vimentin-IR was diffuse and co-localized with torsinA-IR. Although many reactive astrocytes expressed both vimentin and torsinA, co-localization of vimentin- and torsinA-IR was weak in these cells. After sciatic nerve transection, vimentin-IR was readily visualized in satellite cells of the ipsilateral and contralateral DRG.
Fig. 11.
Co-localization of torsinA and vimentin expression after ischemia and sciatic nerve transection. Confocal images of fluorescent immunocytochemistry illustrate co-localization in a subset of CA3 interneurons and CA1 astrocytes in 7 d post-ischemic hippocampus. Vimentin also co-localizes with the component of torsinA expression in satellite cells (arrow) of ipsilateral DRG 14 d after sciatic nerve transection. Co-localization appears yellow in the merged images. G, ganglion cell. Scale bars=50 μm (top three panels) and 25 μm (other panels).
Discussion
In normal brain, expression of torsinA is high in neurons and low in glia, and its developmental regulation indicates that torsinA may play a role in postnatal maturational events in the CNS (Xiao et al., 2004). The present results indicate an altered pattern of torsinA expression, notably involving astroglial cell types, after challenges to both the central and peripheral nervous systems. The similar quantitative and temporal features of torsinA up-regulation after sciatic nerve transection and 4-VO suggest the presence of common transcriptional control mechanisms, as well as a common role for this protein in the reparative and/or adaptive responses to such perturbations.
Post-ischemic torsinA expression in the CNS
Brief durations of global ischemia such as used in these studies result in selective loss of hippocampal CA1 pyramidal cells and interneurons of dentate hilus (Pulsinelli et al., 1982a; Nishino and Nowak, 2004; Ueda and Nowak, 2005). However, 4-vessel occlusion produces severe reductions in blood flow throughout forebrain structures, as well as appreciable perfusion deficits in cerebellum and brainstem (Pulsinelli et al., 1982b). The rapid, generalized increase in torsinA transcript levels throughout the brain (Fig. 1), maintaining the distribution in major neuron populations seen in control brain (Fig. 2), appears to reflect a relatively homogeneous response to this initial ischemic insult. This parallels in many respects the acute post-ischemic induction of many ischemia-responsive genes including many heat shock proteins (Nowak, 1991; Kawagoe et al., 1992; Xue et al., 1998; Nowak and Kiessling, 1999; Yagita et al., 2001; Tanaka et al., 2002). Studies in other in vivo and in vitro models also suggest a role for torsinA in response to oxidative stress (Hewett et al., 2003; Kuner et al., 2004; Cao et al., 2005). In contrast, the delayed component of torsinA increase includes prominent expression in reactive astrocytes in regions of hippocampus (e.g., CA1) known to undergo neuron loss and synaptic reorganization after ischemia (Arabadzisz and Freund, 1999; Briones et al., 2004). In agreement with previous work, astrocytic and microglial markers showed the most prominent post-ischemic response in the CA1 region (Schmidt-Kastner et al., 1990; Morioka et al., 1991; Gottlieb and Matute, 1999). Although not specifically evaluated in this study, the persistent modest increase in torsinA expression noted in cerebellum (Fig. 1) may be a correlate of glial activation in response to Purkinje cell vulnerability that also has been described after global ischemia (Diemer and Siemkovicz, 1981).
Astrocytes are believed to carry out protective functions such as maintenance of ionic homeostasis, prevention of excitotoxicity (via glutamate uptake), scavenging free radicals, provision of nutrients and growth factors, and support of synaptogenesis and neurogenesis (Panickar and Norenberg, 2005). Astrocytes are associated with the synapses, enwrapping many pre- and post-synaptic terminals, facilitating synaptic formation and synaptic neurotransmitter release (Araque et al., 1999; Grosche et al., 1999; Ventura and Harris, 1999; Riquelme et al., 2002; Liu et al., 2004; Sobkowicz et al., 2006). The up-regulation of torsinA in reactive astrocytes may facilitate one or more protective functions of astrocytes such as glutamate uptake via movement of the polytopic glutamate transporter to the cell surface. Alternatively, torsinA may contribute to the morphological and topological changes that reactive astrocytes must undergo in order to facilitate synaptogenesis and network reorganization (Lepekhin et al., 2001; Witcher et al., 2007).
These results also demonstrate up-regulation of torsinA expression in surviving somatostatin- and parvalbumin-IR interneurons (Fig. 6), which likely contribute to the partial maintenance of torsinA transcript levels in the CA1 stratum pyramidale at late post-ischemic intervals despite the death of most pyramidal neurons (Fig. 3). It is well established that parvalbumin-IR interneurons in the hippocampus are resistant to ischemic stress (Nitsch et al., 1989; Ferrer et al., 1995). Similarly, somatostatin-IR interneurons in the CA1 region are also relatively resistant to ischemia (Bering and Johansen, 1993; Bering et al., 1997). Surviving interneurons in the CA1 region undergo significant morphological changes and may serve as substitute targets for Schaffer collateral and other excitatory synaptic input after hippocampal ischemia (Arabadzisz and Freund, 1999). Accordingly, it is rational to postulate that torsinA may participate in structural changes and/or transport of cell surface receptors in these hippocampal interneurons.
Transient global ischemia is followed by neurogenesis in the subgranular proliferative zone (Sharp et al., 2002; Kokaia and Lindvall, 2003) and there is some evidence to suggest a parallel response in the posterior periventricular zone adjacent to the hippocampus (Nakatomi et al., 2002). Neuroblasts originating from the subgranular proliferative zone give rise to neurons within the granule cell layer and GFAP-IR astrocytes in the dentate hilus. Although BrdU labeling was not examined in this study, the numerous cells robustly-IR for torsinA in the subgranular proliferative zone (Fig. 7) may be such neuroblasts. Developmental profiles of torsinA transcript and protein expression are also consistent with a role for torsinA during neurogenesis in brain (Xiao et al., 2004).
TorsinA in the injured PNS
TorsinA is constitutively expressed in the PNS, where it is present in both neuronal and glia elements (ganglion and satellite cells, respectively) in the DRG. After unilateral sciatic nerve transection torsinA transcript increased bilaterally (Fig. 8). TorsinA immmunoreactivity was detected in both cell types in the DRG ipsilateral to the lesion, but was more prominently increased in satellite cells contralaterally (Figs. 8-10). Bilateral effects of sciatic nerve transection are consistent with work from other laboratories. Ryoke and colleagues (2000) reported that a conditioning lesion (i.e., left sciatic nerve transection) enhanced expression of IL-1β and TGF-β in the contralateral DRG and promoted nerve regeneration. Similarly, increased expression of four cytokines (IL-1β, TGF-β1, TNF-α, and IL-10) was apparent in contralateral DRG for up to 35 days after sciatic nerve transection (Ruohonen et al., 2002). Up-regulation of torsinA in ipsilateral ganglion cells is consistent with its potential role in the response to cellular stress as noted above in the context of cerebral ischemia. However, the mechanism for up-regulation of torsinA in contralateral satellite cells is less obvious. Satellite cells appear to play important roles in the physiology of their contiguous ganglion cells (Dublin and Hanani, 2007; Kuo et al., 2007). Satellite cells exhibit increased gap-junction coupling in response to the ipsilateral application of noxious stimuli (Dublin and Hanani, 2007). Up-regulation of p75 in satellite cells appears to be responsible for sympathetic sprouting in the DRG after peripheral nerve lesions (Hu and McLachlan, 2000). Since unilateral sciatic nerve transection is associated with compensatory changes in gait and appendicular loads, up-regulation of torsinA in contralateral satellite cells could be related to increased signaling demands on the adjacent ganglion cells. Alternatively, up-regulation of torsinA in satellite cells could be part of a generalized response to the surgical intervention, possible due to plasma TGF-β. Interestingly, work in Drosophila suggests that TGF-β signaling may be defective in DYT1 dystonia (Koh et al., 2004). Over-expression of Smad2, a downstream effector of TGF- β, suppressed ultrastructural defects at the neuromuscular junction in ΔGAG human torsinA flies.
TorsinA structure and function
TorsinA protein is structurally related to the ClpB/Hsp100 family of proteins which are required for thermotolerance in yeast and plants (Hong and Vierling, 2000; Ung et al., 2007). Yeast and plant Hsp104, which are members of the same ClpB/Hsp100 subfamily of AAA+ proteins, have been shown to interact with Hsp70 and Hsp40 co-chaperones in the process of returning aggregated proteins to their active states (Weibezahn et al., 2004). There are no legitimate Hsp104 orthologs in mammals. Accordingly, torsinA may play a similar role in mammalian systems (McLean et al., 2002; Kuner et al., 2003).
In addition to its role in thermotolerance, plant Hsp104 also plays a critical role in the development of chloroplasts (Ung et al., 2007). In similar fashion, torsinA may be essential for postnatal maturation of the CNS (Xiao et al., 2004; Goodchild et al., 2005). Hence, the appearance of dystonia in carriers of the DYT1 ΔGAG TOR1A mutation could be due to defects in neurodevelopment, the stress response, or a combination thereof. Ultimately, these defects may be the consequence of anomalies in CNS intercellular signaling since torsinA appears to play an important role in synaptic vesicle recycling (Granata et al., 2007). In transgenic mice, for example, overexpression of mutant torsinA is associated with increased striatal dopamine turnover (Zhao et al., 2008). Theoretically, torsinA may contribute to vesicle turnover in both neurons and glia.
Several, largely-independent lines of evidence indicate that cytoskeletal interactions at the nuclear envelope and endoplasmic reticulum are an important aspect of torsinA biology. TorsinA knock-out and homozygous ΔGAG knock-in mice develop morphological abnormalities of the nuclear envelope (Goodchild et al., 2005). TorsinA interacts with LAP1 in the nuclear envelope and Lull1 in the endoplasmic reticulum (Goodchild and Dauer, 2005). In a yeast two-hybrid study, torsinA was shown to interact with kinesin light chain (Kamm et al., 2004). In more recent work, torsinA co-immunoprecipitated with a multi-molecular complex that included vimentin, tubulin, actin, kinesin light chain, LAP1, LULL1 and nesprin (Hewett et al., 2006; Nery et al., 2007). In vitro studies have shown that mutant torsinA interferes with cytoskeletal events which involve vimentin (Hewett et al., 2006). Vimentin, a member of the intermediate filament family of proteins, is expressed in developing brain (Sancho-Tello et al., 1995; Hutchins and Casagrande, 1989) and is also induced in reactive glia (Kindy et al., 1992; Braun et al., 1998). Vimentin plays an important role in cellular morphology and organelle positioning (Goldman et al., 1996). As shown here (Fig. 11), the increased detection of vimentin in reactive astrocytes and surviving interneurons after 4-VO is compatible with previous studies.
Conceptually, cellular polarity is of obvious importance to neural and neuronal architecture (Arimura and Kaibuchi, 2005). Theoretically, mutations in torsinA could interfere with the asymmetric localization of Par proteins (Basham and Rose, 1999). The polarity protein Par-3 interacts with the p75 neurotrophin receptor at the axon-glial junction during myelination in the PNS (Chan et al., 2006). It may be speculated that defective or deficient torsinA could disrupt normal structural relationships between neurons and glia. Microstructural changes have been identified in white matter in DYT1 mutation carriers with diffusion tensor magnetic resonance imaging (DT-MRI) (Carbon et al., 2004; Blood et al., 2006). Fractional anisotropy (FA), a measure of axonal integrity and coherence, was significantly reduced in the white matter underlying sensorimotor cortex in DYT1 mutant carriers (Carbon et al., 2004).
Conclusions
Neurodevelopmental abnormalities may be central to the pathobiology of DYT1 dystonia given that generalized dystonia rarely develops during adulthood in ΔGAG mutation carriers (Bressman et al., 2000; Carbon et al., 2008). On the other hand, the reduced penetrance of the ΔGAG TOR1A mutation suggests that a perturbation (e.g., trauma, hypoxia, severe febrile illness, intense sensorimotor training) to the CNS or PNS may be required for the appearance of a dystonic phenotype. Hypoxic-ischemic insults are believed to be pathogenic in cases of dystonic cerebral palsy (Kyllerman, 1982; Treves and Korcyzyn, 1986; Cerovac et al., 2007), an example of secondary dystonia, and it is conceivable that perinatal factors such as hypoxia-ischemia may increase expressivity of dystonia in carriers of the DYT1 mutation. Its well-demarcated gross anatomy and precise intrinsic connections make the post-ischemic rat hippocampus an ideal model system in which to study the reactive synaptogenesis, astrocytosis, and neurogenesis that follow loss of CNS neurons, and sciatic nerve transection offers comparable advantages in the PNS. The induction of torsinA in glia suggests a novel avenue through which to explore the cellular and systems pathopathophysiology of DYT1 dystonia.
Acknowledgments
This work was supported by the Dystonia Medical Research Foundation and the National Institute of Neurological Disorders and Stroke (R01-NS048458 and R03-NS050185 to MSL, and R01-NS032344 to TSN). TorsinA antibodies were generous gifts from Dr. Vijaya Ramesh, Department of Neurology, Harvard Medical School
Abbreviations
- 4-VO
4-vessel occlusion
- AAA+
ATPases associated with a variety of cellular activities
- ANOVA
analysis of variance
- CNS
central nervous system
- cRNA
complementary RNA
- CT
threshold cycle
- DC
direct current
- DG
dentate gyrus
- DRG
dorsal root ganglia
- ER
endoplasmic reticulum
- GFAP
glial fibrillary acidic protein
- IR
immunoreactivity
- PB
phosphate buffer
- PBS
phosphate buffered saline
- PNS
peripheral nervous systems
- QRT-PCR
relative quantitative multiplex real-time reverse transcriptase polymerase chain reaction
- ROI
region of interest
- SLM
stratum lacunosum-moleculare
- SO
stratum oriens
- SR
stratum radiatum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arabadzisz D, Freund TF. Changes in excitatory and inhibitory circuits of the rat hippocampus 12-14 months after complete forebrain ischemia. Neuroscience. 1999;92:27–45. doi: 10.1016/s0306-4522(98)00736-2. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Arimura N, Kaibuchi K. Key regulators in neuronal polarity. Neuron. 2005;48:881–884. doi: 10.1016/j.neuron.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Keller-McGandy CE, Siriani A, Hewett J, Ramesh V, Sapp E, DiFiglia M, Breakefield XO, Standaert DG. Distribution and ultrastructural localization of torsinA immunoreactivity in the human brain. Brain Res. 2003;986:12–21. doi: 10.1016/s0006-8993(03)03164-0. [DOI] [PubMed] [Google Scholar]
- Basham SE, Rose LS. Mutations in ooc-5 and ooc-3 disrupt oocyte formation and the reestablishment of asymmetric PAR protein localization in two-cell Caenorhabditis elegans embryos. Dev Biol. 1999;215:253–263. doi: 10.1006/dbio.1999.9447. [DOI] [PubMed] [Google Scholar]
- Basham SE, Rose LS. The Caenorhabditis elegans polarity gene ooc-5 encodes a Torsin-related protein of the AAA ATPase superfamily. Development. 2001;128:4645–4656. doi: 10.1242/dev.128.22.4645. [DOI] [PubMed] [Google Scholar]
- Bering R, Draguhn A, Diemer NH, Johansen FF. Ischemia changes the coexpression of somatostatin and neuropeptide Y in hippocampal interneurons. Exp Brain Res. 1997;115:423–429. doi: 10.1007/pl00005712. [DOI] [PubMed] [Google Scholar]
- Bering R, Johansen FF. Expression of somatostatin mRNA and peptide in rat hippocampus after cerebral ischemia. Regul Pept. 1993;49:41–48. doi: 10.1016/0167-0115(93)90382-i. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Tuch DS, Makris N, Makhlouf ML, Sudarsky LR, Sharma N. White matter abnormalities in dystonia normalize after botulinum toxin treatment. Neuroreport. 2006;17:1251–1255. doi: 10.1097/01.wnr.0000230500.03330.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressman SB, Sabatti C, Raymond D, de Leon D, Klein C, Kramer PL, Brin MF, Fahn S, Breakefield X, Ozelius LJ, Risch NJ. The DYT1 phenotype and guidelines for diagnostic testing. Neurology. 2000;54:1746–1752. doi: 10.1212/wnl.54.9.1746. [DOI] [PubMed] [Google Scholar]
- Braun N, Zhu Y, Krieglstein J, Culmsee C, Zimmermann H. Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J Neurosci. 1998;18:4891–4900. doi: 10.1523/JNEUROSCI.18-13-04891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones TL, Suh E, Jozsa L, Hattar H, Chai J, Wadowska M. Behaviorally-induced ultrastructural plasticity in the hippocampal region after cerebral ischemia. Brain Res. 2004;997:137–146. doi: 10.1016/j.brainres.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Caldwell GA, Cao S, Sexton EG, Gelwix CC, Bevel JP, Caldwell KA. Suppression of polyglutamine-induced protein aggregation in Caenorhabditis elegans by torsin proteins. Hum Mol Genet. 2003;12:307–319. doi: 10.1093/hmg/ddg027. [DOI] [PubMed] [Google Scholar]
- Callan AC, Bunning S, Jones OT, High S, Swanton E. Biosynthesis of the dystonia-associated AAA+ ATPase torsinA at the endoplasmic reticulum. Biochem J. 2007;401:607–612. doi: 10.1042/BJ20061313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Gelwix CC, Caldwell KA, Caldwell GA. Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. J Neurosci. 2005;25:3801–3812. doi: 10.1523/JNEUROSCI.5157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Kingsley PB, Su S, Smith GS, Spetsieris P, Bressman S, Eidelberg D. Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol. 2004;56:283–286. doi: 10.1002/ana.20177. [DOI] [PubMed] [Google Scholar]
- Carbon M, Ghilardi MF, Argyelan M, Dhawan V, Bressman SB, Eidelberg D. Increased cerebellar activation during sequence learning in DYT1 carriers: an equiperformance study. Brain. 2008;131:146–154. doi: 10.1093/brain/awm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerovac N, Petrović I, Klein C, Kostić VS. Delayed-onset dystonia due to perinatal asphyxia: a prospective study. Mov Disord. 2007;22:2426–2429. doi: 10.1002/mds.21747. [DOI] [PubMed] [Google Scholar]
- Chan JR, Jolicoeur C, Yamauchi J, Elliott J, Fawcett JP, Ng BK, Cayouette M. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–836. doi: 10.1126/science.1134069. [DOI] [PubMed] [Google Scholar]
- Diemer NH, Siemkovicz E. Regional neurone damage after cerebral ischemia in the normo- and hypoglycemic rat. Neuropathol Appl Neurobiol. 1981;7:217–227. doi: 10.1111/j.1365-2990.1981.tb00091.x. [DOI] [PubMed] [Google Scholar]
- Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun. 2007;21:592–598. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Esapa CT, Waite A, Locke M, Benson MA, Kraus M, McIlhinney RA, Sillitoe RV, Beesley PW, Blake DJ. SGCE missense mutations that cause myoclonus-dystonia syndrome impair ε-sarcoglycan trafficking to the plasma membrane: modulation by ubiquitination and torsinA. Hum Mol Genet. 2007;16:327–342. doi: 10.1093/hmg/ddl472. [DOI] [PubMed] [Google Scholar]
- Ferrari-Toninelli G, Paccioretti S, Francisconi S, Uberti D, Memo M. TorsinA negatively controls neurite outgrowth of SH-SY5Y human neuronal cell line. Brain Res. 2004;1012:75–81. doi: 10.1016/j.brainres.2004.02.080. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Soriano MA, Vidal A, Planas AM. Survival of parvalbumin-immunoreactive neurons in the gerbil hippocampus following transient forebrain ischemia does not depend on HSP-70 protein induction. Brain Res. 1995;692:41–46. doi: 10.1016/0006-8993(95)00527-w. [DOI] [PubMed] [Google Scholar]
- Goldman RD, Khuon S, Chou YH, Opal P, Steinert PM. The function of intermediate filaments in cell shape and cytoskeletal integrity. J Cell Biol. 1996;134:971–983. doi: 10.1083/jcb.134.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Dauer WT. Mislocalization to the nuclear envelope: an effect of the dystonia-causing torsinA mutation. Proc Natl Acad Sci U S A. 2004;101:847–852. doi: 10.1073/pnas.0304375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Dauer WT. The AAA+ protein torsinA interacts with a conserved domain present in LAP1 and a novel ER protein. J Cell Biol. 2005;168:855–862. doi: 10.1083/jcb.200411026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–932. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Gottlieb M, Matute C. Expression of nerve growth factor in astrocytes of the hippocampal CA1 area following transient forebrain ischemia. Neuroscience. 1999;91:1027–1034. doi: 10.1016/s0306-4522(98)00612-5. [DOI] [PubMed] [Google Scholar]
- Granata A, Watson R, Collinson LM, Schiavo G, Warner TT. The dystonia-associated protein torsinA modulates synaptic vesicle recycling. J Biol Chem. 2008;283:7568–7579. doi: 10.1074/jbc.M704097200. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Hewett J, Ziefer P, Bergeron D, Naismith T, Boston H, Slater D, Wilbur J, Schuback D, Kamm C, Smith N, Camp S, Ozelius LJ, Ramesh V, Hanson PI, Breakefield XO. TorsinA in PC12 cells: localization in the endoplasmic reticulum and response to stress. J Neurosci Res. 2003;72:158–168. doi: 10.1002/jnr.10567. [DOI] [PubMed] [Google Scholar]
- Hewett JW, Zeng J, Niland BP, Bragg DC, Breakefield XO. Dystonia-causing mutant torsinA inhibits cell adhesion and neurite extension through interference with cytoskeletal dynamics. Neurobiol Dis. 2006;22:98–111. doi: 10.1016/j.nbd.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Hong SW, Vierling E. Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci U S A. 2000;97:4392–4397. doi: 10.1073/pnas.97.8.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, McLachlan EM. Distinct sprouting responses of sympathetic and peptidergic sensory axons proximal to a sciatic nerve transection in guinea pigs and rats. Neurosci Lett. 2000;295:59–63. doi: 10.1016/s0304-3940(00)01583-4. [DOI] [PubMed] [Google Scholar]
- Hutchins JB, Casagrande VA. Vimentin: changes in distribution during brain development. Glia. 1989;2:55–66. doi: 10.1002/glia.440020107. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Van der Linden C. Dystonia and tremor induced by peripheral trauma: predisposing factors. J Neurol Neurosurg Psychiatry. 1988;51:1512–1529. doi: 10.1136/jnnp.51.12.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm C, Boston H, Hewett J, Wilbur J, Corey DP, Hanson PI, Ramesh V, Breakefield XO. The early onset dystonia protein torsinA interacts with kinesin light chain 1. J Biol Chem. 2004;279:19882–19892. doi: 10.1074/jbc.M401332200. [DOI] [PubMed] [Google Scholar]
- Kawagoe J, Abe K, Sato S, Nagano I, Nakamura S, Kogure K. Distributions of heat shock protein-70 mRNAs and heat shock cognate protein-70 mRNAs after transient global ischemia in gerbil brain. J Cereb Blood Flow Metab. 1992;12:794–801. doi: 10.1038/jcbfm.1992.110. [DOI] [PubMed] [Google Scholar]
- Kindy MS, Bhat AN, Bhat NR. Transient ischemia stimulates glial fibrillary acid protein and vimentin gene expression in the gerbil neocortex, striatum and hippocampus. Brain Res Mol Brain Res. 1992;13:199–206. doi: 10.1016/0169-328x(92)90027-9. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13:127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Koh YH, Rehfeld K, Ganetzky B. A Drosophila model of early onset torsion dystonia suggests impairment in TGF-beta signaling. Hum Mol Genet. 2004;13:2019–2030. doi: 10.1093/hmg/ddh208. [DOI] [PubMed] [Google Scholar]
- Konakova M, Huynh DP, Yong W, Pulst SM. Cellular distribution of torsin A and torsin B in normal human brain. Arch Neurol. 2001;58:921–927. doi: 10.1001/archneur.58.6.921. [DOI] [PubMed] [Google Scholar]
- Konakova M, Pulst SM. Immunocytochemical characterization of torsin proteins in mouse brain. Brain Res. 2001;922:1–8. doi: 10.1016/s0006-8993(01)03014-1. [DOI] [PubMed] [Google Scholar]
- Kuner R, Teismann P, Trutzel A, Naim J, Richter A, Schmidt N, Bach A, Ferger B, Schneider A. TorsinA, the gene linked to early-onset dystonia, is upregulated by the dopaminergic toxin MPTP in mice. Neurosci Lett. 2004;355:126–130. doi: 10.1016/j.neulet.2003.10.069. [DOI] [PubMed] [Google Scholar]
- Kuner R, Teismann P, Trutzel A, Naim J, Richter A, Schmidt N, von Ahsen O, Bach A, Ferger B, Schneider A. TorsinA protects against oxidative stress in COS-1 and PC12 cells. Neurosci Lett. 2003;350:153–156. doi: 10.1016/s0304-3940(03)00904-2. [DOI] [PubMed] [Google Scholar]
- Kuo LT, Groves MJ, Scaravilli F, Sugden D, An SF. Neurotrophin-3 administration alters neurotrophin, neurotrophin receptor and nestin mRNA expression in rat dorsal root ganglia following axotomy. Neuroscience. 2007;147:491–507. doi: 10.1016/j.neuroscience.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Kyllerman M. Dyskinetic cerebral palsy. II. Pathogenetic risk factors and intra-uterine growth. Acta Paediatr Scand. 1982;71:551–558. doi: 10.1111/j.1651-2227.1982.tb09473.x. [DOI] [PubMed] [Google Scholar]
- LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18:60–69. doi: 10.1002/mds.10301. [DOI] [PubMed] [Google Scholar]
- LeDoux MS, Xu L, Xiao J, Ferrell B, Menkes DL, Homayouni R. Murine central and peripheral nervous system transcriptomes: comparative gene expression. Brain Res. 2006;1107:24–41. doi: 10.1016/j.brainres.2006.05.101. [DOI] [PubMed] [Google Scholar]
- Lepekhin EA, Eliasson C, Berthold CH, Berezin V, Bock E, Pekny M. Intermediate filaments regulate astrocyte motility. J Neurochem. 2001;79:617–625. doi: 10.1046/j.1471-4159.2001.00595.x. [DOI] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Kang J, Nedergaard M. Astrocyte activation of presynaptic metabotropic glutamate receptors modulates hippocampal inhibitory synaptic transmission. Neuron Glia Biol. 2004;1:307–316. doi: 10.1017/S1740925X05000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zolkiewska A, Zolkiewski M. Characterization of human torsinA and its dystonia-associated mutant form. Biochem J. 2003;374:117–122. doi: 10.1042/BJ20030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean PJ, Kawamata H, Shariff S, Hewett J, Sharma N, Ueda K, Breakefield XO, Hyman BT. TorsinA and heat shock proteins act as molecular chaperones: suppression of alpha-synuclein aggregation. J Neurochem. 2002;83:846–854. doi: 10.1046/j.1471-4159.2002.01190.x. [DOI] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. The microglial reaction in the rat dorsal hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab. 1991;11:966–973. doi: 10.1038/jcbfm.1991.162. [DOI] [PubMed] [Google Scholar]
- Naismith TV, Heuser JE, Breakefield XO, Hanson PI. TorsinA in the nuclear envelope. Proc Natl Acad Sci U S A. 2004;101:7612–7617. doi: 10.1073/pnas.0308760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Nery FC, Hewett J, Niland B, Zeng J, Li Y, Wiche G, Sharma N, Breakefield XO. Program No 895.3. Abstract viewer/Itinerary planner. Society for Neuroscience; San Deigo: 2007. Partcipation of torsinA in linking the nuclear envelope to the cytoskeleton. [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- Nishino K, Nowak TS., Jr Time course and cellular distribution of hsp27 and hsp72 stress protein expression in a quantitative gerbil model of ischemic injury and tolerance: thresholds for hsp72 induction and hilar lesioning in the context of ischemic preconditioning. J Cereb Blood Flow Metab. 2004;24:167–178. doi: 10.1097/01.WCB.0000100853.67976.8B. [DOI] [PubMed] [Google Scholar]
- Nitsch C, Scotti A, Sommacal A, Kalt G. GABAergic hippocampal neurons resistant to ischemia-induced neuronal death contain the Ca2(+)-binding protein parvalbumin. Neurosci Lett. 1989;105:263–268. doi: 10.1016/0304-3940(89)90631-9. [DOI] [PubMed] [Google Scholar]
- Nowak TS., Jr Localization of 70 kDa stress protein mRNA induction in gerbil brain after ischemia. J Cereb Blood Flow Metab. 1991;11:432–439. doi: 10.1038/jcbfm.1991.84. [DOI] [PubMed] [Google Scholar]
- Nowak TS, Jr, Kiessling M. Reprogramming of gene expression after ischemia. In: Walz W, editor. Cerebral Ischemia. Molecular and Cellular Pathology. Totowa: Humana Press; 1999. pp. 145–215. [Google Scholar]
- Ogura T, Wilkinson AJ. AAA+ superfamily ATPases: common structure--diverse function. Genes Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Corey DP, Fahn S, Risch NJ, Buckler AJ, Gusella JF, Breakefield XO. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Page CE, Klein C, Hewett JW, Mineta M, Leung J, Shalish C, Bressman SB, de Leon D, Brin MF, Fahn S, Corey DP, Breakefield XO. The TOR1A (DYT1) gene family and its role in early onset torsion dystonia. Genomics. 1999;62:377–384. doi: 10.1006/geno.1999.6039. [DOI] [PubMed] [Google Scholar]
- Panickar KS, Norenberg MD. Astrocytes in cerebral ischemic injury: Morphological and general considerations. Glia. 2005;50:287–298. doi: 10.1002/glia.20181. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982a;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Levy DE, Duffy TE. Regional cerebral blood flow and glucose metabolism following transient forebrain ischemia. Ann Neurol. 1982b;11:499–509. doi: 10.1002/ana.410110510. [DOI] [PubMed] [Google Scholar]
- Riquelme R, Miralles CP, De Blas AL. Bergmann glia GABA(A) receptors concentrate on the glial processes that wrap inhibitory synapses. J Neurosci. 2002;22:10720–10730. doi: 10.1523/JNEUROSCI.22-24-10720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruohonen S, Jagodi M, Khademi M, Taskinen HS, Ojala P, Olsson T, Roytta M. Contralateral non-operated nerve to transected rat sciatic nerve shows increased expression of IL-1beta, TGF-beta1, TNF-alpha, and IL-10. J Neuroimmunol. 2002;132:11–17. doi: 10.1016/s0165-5728(02)00281-3. [DOI] [PubMed] [Google Scholar]
- Ryoke K, Ochi M, Iwata A, Uchio Y, Yamamoto S, Yamaguchi H. A conditioning lesion promotes in vivo nerve regeneration in the contralateral sciatic nerve of rats. Biochem Biophys Res Commun. 2000;267:715–718. doi: 10.1006/bbrc.1999.2017. [DOI] [PubMed] [Google Scholar]
- Sancho-Tello M, Valles S, Montoliu C, Renau-Piqueras J, Guerri C. Developmental pattern of GFAP and vimentin gene expression in rat brain and in radial glial cultures. Glia. 1995;15:157–166. doi: 10.1002/glia.440150208. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Szymas J, Hossmann KA. Immunohistochemical study of glial reaction and serum protein extravasation in relation to neuronal damage in rat hippocampus after ischemia. Neuroscience. 1990;38:527–540. doi: 10.1016/0306-4522(90)90048-9. [DOI] [PubMed] [Google Scholar]
- Sharma N, Hewitt J, Ozelius LJ, Ramesh V, McLean PJ, Breakefield XO, Hyman BT. A close association of torsinA and alpha-synuclein in Lewy bodies: a fluorescence resonance energy transfer study. Am J Pathol. 2001;159:339–344. doi: 10.1016/s0002-9440(10)61700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Liu J, Bernabeu R. Neurogenesis following brain ischemia. Brain Res Dev Brain Res. 2002;134:23–30. doi: 10.1016/s0165-3806(01)00286-3. [DOI] [PubMed] [Google Scholar]
- Shashidharan P, Good PF, Hsu A, Perl DP, Brin MF, Olanow CW. TorsinA accumulation in Lewy bodies in sporadic Parkinson's disease. Brain Res. 2000;877:379–381. doi: 10.1016/s0006-8993(00)02702-5. [DOI] [PubMed] [Google Scholar]
- Shashidharan P, Paris N, Sandu D, Karthikeyan L, McNaught KS, Walker RH, Olanow CW. Overexpression of torsinA in PC12 cells protects against toxicity. J Neurochem. 2004;88:1019–1025. doi: 10.1046/j.1471-4159.2003.02233.x. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Waclawik AJ, August BK. The astroglial cell that guides nerve fibers from growth cone to synapse in organotypic cultures of the fetal mouse spinal cord. Synapse. 2006;59:183–200. doi: 10.1002/syn.20222. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kitagawa K, Ohtsuki T, Yagita Y, Takasawa K, Hori M, Matsumoto M. Synergistic induction of HSP40 and HSC70 in the mouse hippocampal neurons after cerebral ischemia and ischemic tolerance in gerbil hippocampus. J Neurosci Res. 2002;67:37–47. doi: 10.1002/jnr.10078. [DOI] [PubMed] [Google Scholar]
- Torres GE, Sweeney AL, Beaulieu JM, Shashidharan P, Caron MG. Effect of torsinA on membrane proteins reveals a loss of function and a dominant-negative phenotype of the dystonia-associated DeltaE-torsinA mutant. Proc Natl Acad Sci U S A. 2004;101:15650–15665. doi: 10.1073/pnas.0308088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves T, Korczyn AD. Progressive dystonia and paraparesis in cerebral palsy. Eur Neurol. 1986;25:148–153. doi: 10.1159/000116001. [DOI] [PubMed] [Google Scholar]
- Ueda M, Nowak TS., Jr Protective preconditioning by transient global ischemia in the rat: components of delayed injury progression and lasting protection distinguished by comparisons of depolarization thresholds for cell loss at long survival times. J Cereb Blood Flow Metab. 2005;25:949–958. doi: 10.1038/sj.jcbfm.9600107. [DOI] [PubMed] [Google Scholar]
- Ung L, Ignatius R, Whan-Suk H, Larkindale J, Waters E, Vierling E. The Arabidopsis ClpB/Hsp100 family of proteins: chaperones for stress and chloroplast development. The Plant Journal. 2007;49:115–127. doi: 10.1111/j.1365-313X.2006.02940.x. [DOI] [PubMed] [Google Scholar]
- Vale RD. AAA proteins. Lords of the ring. J Cell Biol. 2000;150:F13–19. doi: 10.1083/jcb.150.1.f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan A, Breakefield XO, Bhide PG. Developmental patterns of torsinA and torsinB expression. Brain Res. 2006;1073-1074:139–145. doi: 10.1016/j.brainres.2005.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RH, Good PF, Shashidharan P. TorsinA immunorectivity in inclusion bodies in trinucleotide repeat diseases. Mov Disord. 2003;18:1041–1044. doi: 10.1002/mds.10487. [DOI] [PubMed] [Google Scholar]
- Weibezahn J, Tessarz P, Schlieker C, Zahn R, Maglica Z, Lee S, Zentgraf H, Weber-Ban EU, Dougan DA, Tsai FT, Mogk A, Bukau B. Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell. 2004;119:653–665. doi: 10.1016/j.cell.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Witcher MR, Kirov SA, Harris KM. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia. 2007;55:13–23. doi: 10.1002/glia.20415. [DOI] [PubMed] [Google Scholar]
- Xiao J, Gong S, Zhao Y, LeDoux MS. Developmental expression of rat torsinA transcript and protein. Brain Res Dev Brain Res. 2004;152:47–60. doi: 10.1016/j.devbrainres.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Xue JH, Fukuyama H, Nonoguchi K, Kaneko Y, Kido T, Fukumoto M, Fujibayashi Y, Itoh K, Fujita J. Induction of Apg-1, a member of the heat shock protein 110 family, following transient forebrain ischemia in the rat brain. Biochem Biophys Res Commun. 1998;247:796–801. doi: 10.1006/bbrc.1998.8894. [DOI] [PubMed] [Google Scholar]
- Yagita Y, Kitagawa K, Ohtsuki T, Tanaka S, Hori M, Matsumoto M. Induction of the HSP110/105 family in the rat hippocampus in cerebral ischemia and ischemic tolerance. J Cereb Blood Flow Metab. 2001;21:811–819. doi: 10.1097/00004647-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Decuypere M, LeDoux MS. Abnormal motor function and dopamine neurotransmission in DYT1 DeltaGAG transgenic mice. Exp Neurol. 2008;210:719–730. doi: 10.1016/j.expneurol.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]