Abstract
Objectives
Clear-cell renal cell carcinoma (RCC) is the most prevalent form of kidney cancer and is frequently associated with loss of von Hippel-Lindau (VHL) gene function, resulting in the aberrant transcriptional activation of genes that contribute to tumor growth and metastasis, including transforming growth factor-α (TGF-α), a ligand of the epidermal growth factor receptor (EGFR) tyrosine kinase. To determine the functional impact of EGFR activation on RCC, we suppressed critical components of this pathway: EGFR, Akt-1, and MEK-1.
Methods
Stable transfection of RCC cells with plasmids bearing shRNA directed against each of these genes was used to individually suppress their expression. Transfectants were characterized for growth and invasiveness in vitro and tumorigenesis in vivo.
Results
RCC cell transfectants displayed significantly reduced growth rate and matrix invasion in vitro and RCC tumor xenograft growth rate in vivo. Analysis of tumor cells that emerged after extended periods in each model showed that significant EGFR suppression was sustained, whereas Akt-1 and MEK-1 knockdown cells had escaped shRNA suppression.
Conclusions
EGFR, Akt-1, and MEK-1 are individually critical for RCC cell invasiveness in vitro and tumorigenicity in vivo, and even partial suppression of each can have a significant impact on tumor progression. The emergence of transfectants that had escaped Akt-1 and MEK-1 suppression during tumorigenicity experiments suggests that these effectors may each be more critical than EGFR for RCC tumorigenesis, consistent with results from clinical trials of EGFR inhibitors for RCC, where durable clinical responses have not been seen.
Keywords: Akt-1; EGF receptor; MEK-1; Renal cell carcinoma, VHL
1. Introduction
Renal cell carcinoma (RCC) affects approximately 100,000 individuals worldwide every year [1]. The incidence and mortality from RCC steadily increased from 1975 to 1995. In 2003, >11,900 deaths from malignancy of the kidney and renal pelvis were reported in the United States [1]. Safe and effective systemic treatment for metastatic RCC is not yet available [2], prompting increased interest in therapeutics targeting specific defects in signaling pathways implicated in RCC tumorigenesis [3,4].
Among the many pathways implicated in RCC oncogenesis is that of the epidermal growth factor receptor (EGFR), an erbB family transmembrane receptor tyrosine kinase (TK) involved in cell proliferation, motility, and survival [5]. The EGFR can be activated by several ligands, including transforming growth factor-α(TGF-α) [6]. In sporadic clear-cell RCC, frequent loss of von Hippel-Lindau (VHL) tumor suppressor gene function results in hypoxia-inducible factor (HIF)-mediated TGF-α expression and EGFR autocrine signaling [7]. Tumor hypoxia, independent of VHL loss of function, increases EGFR expression through early growth response factor 1 (Egr-1) [8].
Therapeutic approaches to blocking EGFR signaling in cancer include neutralizing monoclonal antibodies, as well as competitive antagonists of adenosine triphosphate (ATP) binding to the TK domain, administered as single agents or in combination with other chemotherapeutics [9[en]11]. Unfortunately, targeting the EGFR pathway alone has not shown widespread efficacy: gefitinib (Iressa, ZD1839), an ATP-binding antagonist, has shown little effect in glioblastomas with EGFR gene amplification and produces dramatic responses only in the relatively low percentage of patients with non[en]small-cell lung carcinoma who have activating somatic EGFR TK mutations [12,13]. Similar activating EGFR mutations have not been found in RCC [13], which may explain the low response rate of RCC to gefitinib [14]. Clinical trials of anti-EGFR antibodies in RCC also have shown a low overall objective response rate [15]. Interestingly, VHL loss may affect the efficacy of anti-EGFR antibodies; whereas a phase 2 trial of IMC-C225 (cetuximab, Erbitux) showed little response in patients with advanced RCC [16], the ability of C225 to inhibit RCC cell growth in vitro was enhanced in cells expressing VHL [17]. Other studies have shown that inhibition of the mammalian target of rapamycin (mTOR) pathway contributes synergistically to EGFR pathway inhibition for impairing RCC cell growth independent of VHL status, indicating that shared downstream effectors such as Akt-1 and MEK-1 are potentially important therapeutic targets in RCC [18,19].
Akt-1 is a serine/threonine kinase activated downstream of phosphatidylinositol 3-kinase (PI3K) that mediates various cell survival signals via mTOR, an initiator of apoptosis caspase-9, the proapoptotic member of the Bcl-2 family BAD, nuclear transcription factors NF-KB, and forkhead [20]. Elevated Akt-1 expression has been correlated with high RCC tumor grade and metastasis [21]. MEK-1 is a member of the extracellular signal-regulated kinase (ERK) serine/threonine kinase family that mediates diverse cellular processes including proliferation, survival, differentiation, and motility in normal and tumor cells. Potent small-molecule inhibitors targeting the components of the ERK pathway have been developed as anticancer drugs, including the MEK1/2 inhibitors PD184352, PD0325901, and ARRY-142886, that have reached human clinical trials [22].
To assess the roles of Akt-1 and MEK-1 as oncogenic signaling components of the EGFR pathway in VHL-negative RCC, we suppressed their expression using shRNA and characterized cell proliferation and invasiveness in vitro as well as tumorigenicity in vivo. Our results show that EGFR, Akt-1, and MEK-1 are critical but distinct determinants of oncogenesis in RCC.
2. Methods
2.1. Cell culture and RNAi gene knockdown constructs
The VHL-negative clear-cell RCC cell line 786-0 was maintained as described previously [23]. Complimentary oligonucleotides for EGFR (sense 5′-GAAGGAAACUGAAUU CAAAUU-3′), Akt-1 (sense, 5′-GACAAGGACGGGCACAUUAUU-3′), and MEK-1 (sense, 5′CCACCCUCUUGACUUCCUG-3′) siRNA target sites were inserted into the RNAi-Ready pSIREN-RetroQ vector (BD Biosciences, Palo Alto, CA). The 786-0 cells were infected with the pSIREN retroviral vector to generate stable, puromycin-selected (5 μg/ml) mass cultures producing short hairpin siRNA (shRNA) against each target gene.
2.2. Real-time PCR analysis
Total RNA was isolated from cultured 786-0 and 786-0[en]derived cell lines using TriZol (Invitrogen, Carlsbad, CA), or RNAeasy (Qiagen, Valencia, CA) and further purified via three serial phenol/chloroform extractions. cDNA was produced from purified RNA using the TaqMan Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Real-time quantitative polymerase chain reaction (RT-PCR) was performed using Universal PCR Master Mix and Assay-On-Demand Probes (Applied Biosystems) on an Applied Biosystems Prism 7000. Cells expressing nontarget shRNA were compared to those expressing target shRNA sequences using the Ct (threshold cycle) method. Relative quantification was determined by comparing the samples to β-actin, an internal control. Samples were performed in triplicate and results were analyzed using the ΔΔCt method (Applied Biosystems User Bulletin no. 2) and Microsoft Excel software.
2.3. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis
Cultured 786-0 and 786-0[en]derived cell lines were harvested with trypsin, washed with cold phosphate-buffered saline (PBS), then lysed in cold buffer containing 50 mM Tris (pH 7.4), 1% Igepal, 150 mM NaCl, 10 mM ethylenediaminetetraacetic acid (EDTA), 1 mM Na3VO4, and 1 mM NaF. Lysates were clarified by centrifugation and supernatant protein concentration was determined by BCA Protein Assay (Pierce, Rockford, IL). Equal amounts of protein were resolved by SDS-PAGE (Bio-Rad Laboratories, Hercules, CA) and transferred to Immobilon (Millipore, Bedford, MA). Membranes were probed with anti-Akt-1 monoclonal antibody (mAb) 2H10 or polyclonal anti-Akt-1 (Cell Signaling Technology, Beverly, MA), anti-MEK-1/2 mAb L38C12 (Cell Signaling), or polyclonal anti-MEK-1 (Upstate Biotech, Lake Placid, NY), anti-EGFR mAb 1F4 or polyclonal anti-EGFR (Upstate) followed by detection using enhanced chemiluminescence (ECL) Plus (Amersham Biosciences, Buckinghamshire, United Kingdom) and quantitated densitometrically (Quantity One, BioRad Laboratories, Hercules, CA).
2.4. Cell proliferation and branching morphogenesis assays
For proliferation assays, cells in log phase growth seeded into 96-well plates were treated with recombinant TGF-α (50 ng/ml; R&D Systems, Minneapolis, MN) in Dulbecco modifed Eagle medium (DMEM) + 0.1% fetal bovine serum (FBS), or with media alone, prior to the addition of CellTiter 96 Aqueous One Solution (20 μl/well; Promega, Madison, WI). Cells were then incubated at 37 °C for 1 h and absorbance at 492 nm was measured using a scanning spectrophotometer daily for 4 d.
For branching morphogenesis assays, cells in log phase growth were harvested, counted, resuspended in GFR Matrigel in DMEM + 10% FBS (1:1, vol/vol; BD Biosciences) and 20,000 cells were added to each well of a 96-well plate. Once the Matrigel mixture had solidified, growth media supplemented with recombinant hepatocyte growth factor (HGF; 20 ng/ml; R&D Systems) or TGF-α (100 ng/ml; R&D Systems) was added to each well and plates were incubated for 72[en]96 h at 37 °C. Phase contrast photomicrographs of representative wells were acquired using a x40 objective and branching morphogenesis, an index of cell invasiveness, was ranked on a scale from + to ++++, from least to most.
2.5. Murine tumor xenograft experiments
Cultured cells in log phase growth were harvested, washed, and suspended in Hank’s balanced salt solution (2 × 106 cells/400 μl) prior to subcutaneous injection into the right dorsolateral region of 6-wk-old male severe combined immunodeficient (SCID)/beige mice (n = 5 mice per group). The two largest dimensions of each tumor were measured weekly using calipers and tumor volume was calculated as: TV = (D)*(d2)*(π/6), where D is the larger diameter and d is the smaller diameter, using Microsoft Excel. Mice were housed, monitored, and euthanized according to National Institutes of Health Animal Care and Use Guidelines. RNA was extracted from samples of fresh tumor tissue using TriZol and RT-PCR was performed as described for cultured cell lines.
3. Results
3.1. EGFR pathway suppression inhibits RCC cell proliferation and invasion
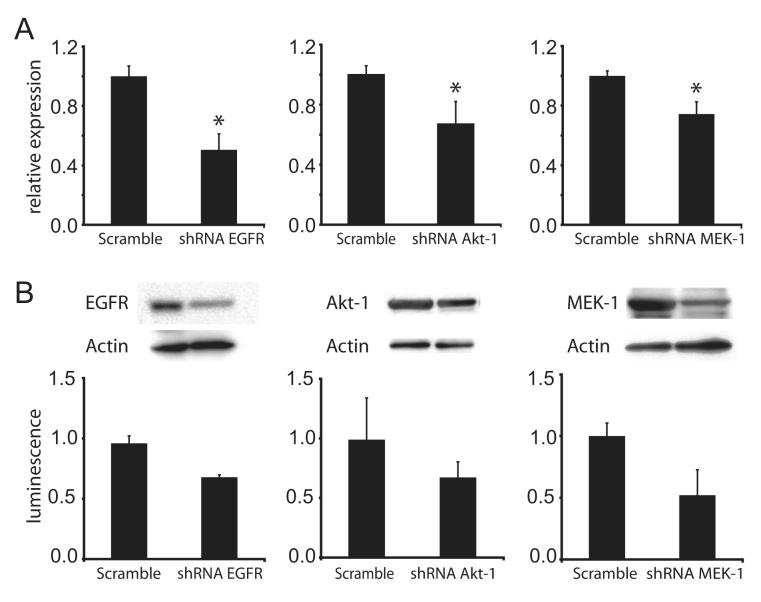
The genes encoding EGFR, Akt-1, and MEK-1 in the VHL-negative RCC cell line 786-0 were individually suppressed by deriving three separate selectionresistant mass cultures, each stably transfected with a plasmid encoding a targeted shRNA sequence. Control cultures transfected with plasmids encoding scrambled sequences with identical base composition were also derived for each target. Target gene knock-down was confirmed using quantitative RT-PCR and immunoblot analysis (Fig. 1). By RT-PCR, we found significant knock-down of EGFR, Akt-1, and MEK-1 transcripts at 50%, 35%, and 25%, respectively, of the levels present in cells harboring scrambled sequences (p < 0.05; Fig. 1 A). Quantitative immunoblot analysis revealed that expression of targeted gene products was reduced by 30%, 35%, and 45%, respectively (p < 0.05), relative to the scrambled transfectants (Fig. 1 B). These cell cultures were then further characterized for cell proliferation, extracellular matrix invasion in vitro, and tumorigenicity in vivo.
Fig. 1. [en] shRNA knock-down of epidermal growth factor receptor (EGFR), Akt-1, and MEK-1 expression in 786-0 renal cell carcinoma (RCC) cell lines.
(A) Abundance of EGFR, AKT-1, and MEK-1 mRNA transcripts in respective shRNA transfectants relative to scramble control shRNA transfectants as measured via real-time PCR. β-Actin was used as an internal control for normalization of cDNA content. (B) Immunoblotting of total protein lysates obtained from 786-0 cells transfected with shRNA for the indicated genes. Actin was immunoblotted to control for sample loading in the gel (glyceraldehyde phosphate dehydrogenase [GAPDH] was used for Mek-1 because of its more favorable molecular mass). Immunoblots were quantitated for luminescence as shown in the bar graph below each blot. All experiments were performed in triplicate; results shown are representative. Asterisk indicates statistical significance at p < 0.005.
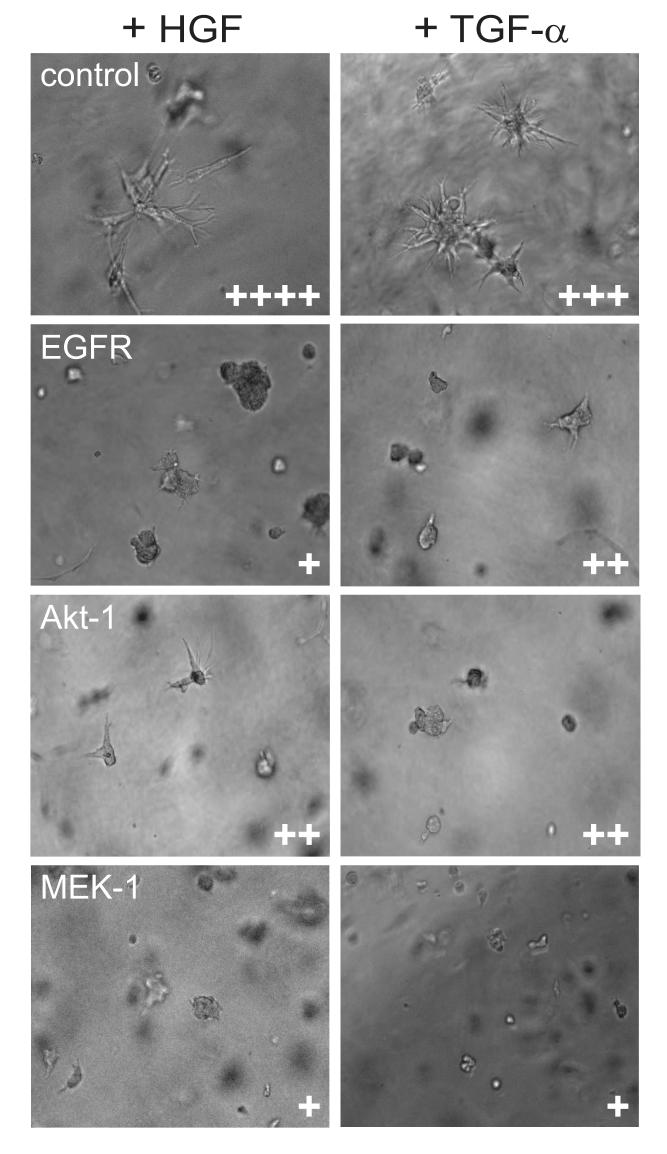
The serum-stimulated cell proliferation rate was measured in the three gene knock-down cell cultures relative to respective scramble-sequence controls. All three exhibited diminished growth compared to controls, with EGFR knock-down most severe (Fig. 2 A). The ability of the RCC cell transfectants to invade a three-dimensional extracellular matrix by branching morphogenesis was measured in response to HGF, as described previously [23], as well as the EGFR ligand TGF- . Robust HGF-α and TGF-α[en]driven branching morphogenesis was observed for 786-0 cells transfected with scrambled sequences; this was significantly reduced in EGFR, Akt-1, or MEK-1 knock-down cultures (Fig. 3). These results suggest that both Akt-1 and MEK-1 are critical effectors of matrix invasion downstream of EGFR and c-Met, the HGF receptor TK.
Fig. 2. [en] shRNA knock-down of epidermal growth factor receptor (EGFR), Akt-1, and MEK-1 inhibits 786-0 renal cell carcinoma (RCC) cell proliferation.
Log phase growth of cultured 786-0 cells over time (days) transfected with plasmids bearing shRNA expression constructs (squares) targeting EGFR (A), Akt-1 (B), or MEK-1 (C) transcripts or the respective scrambled control sequence (diamonds) was measured as described in Methods. Individual points represent the mean values of triplicate samples, expressed relative to initial values, ± standard deviation. After day 0, significant inhibition occurs at all points except day 3, panel C (p < 0.01).
Fig. 3. [en] Knock-down of epidermal growth factor receptor (EGFR) and downstream target genes inhibits growth factor-stimulated branching in 786-0 renal cell carcinoma (RCC) cells.
From top to bottom, phase contrast photomicrographs (x40 objective) show branching morphogenesis by 786-0 cells transfected with shRNA expression constructs for control (scrambled sequence), EGFR, Akt-1, and MEK-1, stimulated by hepatocyte growth factor (left) or transforming growth factor α (right). Cells were cultured in Matrigel and branching was ranked on a scale from + to ++++, least to most, as indicated at the bottom right of each panel.
3.2.EGFR pathway knockdown inhibits RCC tumorigenesis in mice
The 786-0 cell cultures expressing EGFR, Akt-1, or MEK-1 knock-down constructs were injected subcutaneously into SCID/beige mice to assess the functional impact of these signaling molecules on RCC tumor onset and progression in vivo (Fig. 4). All animals injected with scrambled shRNA transfected 786-0 cells showed measurable tumor growth by 60 d after injection, whereas in contrast, none of the animals injected with knock-down cells had measurable tumors until 100 d after injection (Fig. 4). The rate of tumor progression in animals receiving EGFR and Akt-1 knock-down cell lines remained lower than that of animals receiving control cells throughout the 120-d study period (Fig. 4 A and B, respectively). The rate of tumor progression in the animals injected with the MEK-1 knock-down cell line, however, paralleled the exponential growth of the control cell-injected animals (Fig. 4 C). Consistent with prior reports [24,25], 786-0 cells transfected with a constitutive expression contruct encoding wild-type pVHL were not tumorigenic (Fig. 4 D).
Fig. 4. [en] shRNA targeting epidermal growth factor receptor (EGFR), Akt-1, or MEK-1 inhibits renal cell carcinoma (RCC) tumorigenesis in vivo.
Severe combined immunodeficient/beige mice were injected subcutaneously with von Hippel-Lindau (VHL)-negative 786-0 clear-cell RCC cell cultures stably transfected with shRNA directed against a nonsilencing scrambled sequence (squares) or EGFR (Xs, A), Akt-1 (triangles, B), and MEK-1 (circles, C) and tumor growth was measured periodically as described in Methods. Tumor growth was not observed when pVHL was expressed ectopically (diamonds, D).
An assumption in our animal study was that gene knock-down was maintained for the duration of the experiment. However, the possibility remained that a small number of cells that had lost shRNA expression could have grown as tumors with the delayed kinetics observed. To test for this escape from gene knock-down, samples were obtained from tumors at the end of the study for all groups and the target gene expression level was analyzed by quantitative RTPCR (Fig. 5). Of the three targeted genes, only EGFR showed persistent knock-down in the growing tumors (Fig. 5 A). Samples of tumors derived from Akt-1 and MEK-1 knock-down cell cultures had target gene expression levels that were indistuinguishable from control samples, indicating that the cells that had grown into tumors in these animals had lost expression of the respective shRNA construct (Fig. 5 B and C).
Figure 5. [en] Expression of shRNA targeted genes in renal cell carcinoma tumor xenografts.
Tumor xenografts actively growing in male severe combined immunodeficient/beige mice as described in Figure 4 were harvested, total RNA was extracted, and real-time quantitative polymerase chain reaction was perfomed to assess shRNA target gene expression relative to a control (scramble) shRNA sequence for EGFR (A), Akt-1 (B) or MEK-1 (C). Values are mean ± standard deviation of at least three tumor samples analyzed in triplicate, exept for Akt-1, where only one tumor specimen was available for triplicate real-time polymerase chain reaction analysis (*, p < 0.007).
4. Discussion
The general oncogenic relevance of the TGF-α/EGFR pathway downstream in VHL-negative clear-cell RCC has already been demonstrated [5]. Multiple studies have also shown over-expression of the EGFR receptor in RCC compared to normal renal tissue [26,27]. Several independent studies provide evidence that certain EGFR pathway effectors are activated in clear-cell RCC. Elevated Akt-1 activation is common in RCC, especially in high-grade tumors and metastatic disease [21]. Akt-1 activation contributes to oncogenic signaling in RCC cells in vitro, as established by Sourbier et al using siRNA targeting Akt-1 in several cell lines, including 786-0 [28]. Constitutive MAPK pathway activation has also been demonstrated in RCC tumor tissue relative to surrounding normal tissue, and the level of pathway activation correlated positively with RCC tumor grade and stage [29]. These findings provided a strong impetus to assess the oncogenic contribution of each of these EGFR pathway components functionally in whole animals.
Using shRNA constructs to create stable, VHL-negative RCC cell cultures with diminished expression of EGFR, Akt-1, and MEK-1, we found that each protein contributed significantly to cell proliferation and that each was required for growth factor-induced extracellular matrix invasion and for aggressive tumor growth. Overall our results indicate that each of these signaling components is a critical oncogenic effector in RCC, at or near the level of importance of VHL and HIF-2α [24,30]. Interestingly, whereas partial knock-down of EGFR, Akt-1, or MEK-1 resulted in a significant but partial growth rate reduction, the more profound effects on extracellular matrix invasion in vitro were more closely correlated with dramatic inhibition of tumorigenesis in vivo. This relationship highlights the value of invasion assays as a predictor of tumorigenesis for RCC and demonstrates that even partial silencing of Akt-1 or MEK-1 can produce striking changes in RCC cell invasiveness amidst persistent survival and growth signaling. This is particularly relevant to targeted anticancer therapies because it suggests that complete silencing of certain genes that may cause unacceptable systemic toxicity may not be required for effective disease control.
While our study was in progress, Smith et al reported that shRNA-mediated EGFR knock-down in 786-0 cells resulted in suppression of tumor growth in vivo for 15 wk [31]. Similar cell lines and silencing techniques were used, so it remains to be determined whether our observation of delayed tumor growth starting 14[en]15 wk after injection, despite persistent EGFR knock-down in tumors, differs from theirs technically or biologically. Smith et al [31] used more cells per injection site (1 × 107 vs. 2 × 106 in our study), but used nude mice whereas we used SCID/beige mice. In our hands, VHL-negative 786-0 cell xenografts grow significantly faster in SCID/beige mice than in nude mice. Our observations raise the possibility that EGFR knock-down may delay but not completely suppress tumor growth, consistent with results of clinical trials of drugs targeting EGFR in patients with RCC, who have shown low objective response rates [32].
In contrast to results obtained by EGFR knock-down, the late-onset RCC tumor xenografts observed in mice receiving Akt-1[en] or MEK-1[en]targeted shRNA constructs displayed near normal levels of these mediators, suggesting that recovery of normal Akt-1 and MEK-1 levels was required for tumorigenesis. In a recent report, Sourbier et al showed partial regression of 786-0 RCC tumor xenografts after systemic treatment of animals with the PI3K family inhibitor LY294002 [28]. Our findings using Akt-1[en]directed shRNA build on these earlier results by demonstrating the critical role of a single downstream target of PI3K enzymes for RCC tumorigenesis.
5. Conclusions
EGFR, Akt-1, and MEK-1 appear to be individually critical for RCC cell invasiveness in vitro and tumorigenicity in vivo, and even partial suppression of these signaling mediators significantly impaired tumor growth. Despite sustained EGFR silencing, RCC tranfectants retained long-term tumorigenic potential. In contrast, the emergence of transfectants that had escaped Akt-1 and MEK-1 suppression in long-term RCC tumor studies suggests that these effectors may each outweigh EGFR in overall importance, consistent with their roles as critical nodes for several signaling pathways, and with results from clinical trials of EGFR inhibitors for RCC, where durable clinical responses have not been reported.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Support: This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
S.J.L. and J-B.L. contributed equally to this work.
Conflicts of interest: The authors report that there are no conflicts to disclose.
Take Home Message: Clear-cell renal cell carcinoma (RCC), the most prevalent form of kidney cancer, is frequently associated with aberrant epidermal growth factor receptor (EGFR) pathway activation. The intracellular EGFR effectors, Akt-1 and MEK-1, appear to be particularly critical for RCC tumorigenicity.
References
- [1].Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- [2].Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–90. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- [3].Tibes R, Trent J, Kurzrock R. Tyrosine kinase inhibitors and the dawn of molecular cancer therapeutics. Annu Rev Pharmacol Toxicol. 2005;45:357–84. doi: 10.1146/annurev.pharmtox.45.120403.100124. [DOI] [PubMed] [Google Scholar]
- [4].Ljungberg B, Hanbury DC, Kuczyk MA, et al. Renal cell carcinoma guideline. Eur Urol. 2007;51:1502–10. doi: 10.1016/j.eururo.2007.03.035. [DOI] [PubMed] [Google Scholar]
- [5].Gunaratnam L, Morley M, Franovic A, et al. Hypoxia inducible factor activates the transforming growth factor-alpha/epidermal growth factor receptor growth stimulatory pathway in VHL(-/-) renal cell carcinoma cells. J Biol Chem. 2003;278:44966–74. doi: 10.1074/jbc.M305502200. [DOI] [PubMed] [Google Scholar]
- [6].Lockhart AC, Berlin JD. The epidermal growth factor receptor as a target for colorectal cancer therapy. Semin Oncol. 2005;32:52–60. doi: 10.1053/j.seminoncol.2004.09.036. [DOI] [PubMed] [Google Scholar]
- [7].de PN, Brychzy A, Fournier MC, et al. Role of transforming growth factor-alpha in von Hippel[en]Lindau (VHL)(-/-) clear cell renal carcinoma cell proliferation: a possible mechanism coupling VHL tumor suppressor inactivation and tumorigenesis. Proc Natl Acad Sci USA. 2001;98:1387–92. doi: 10.1073/pnas.031587498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nishi H, Nishi KH, Johnson AC. Early growth response-1 gene mediates up-regulation of epidermal growth factor receptor expression during hypoxia. Cancer Res. 2002;62:827–34. [PubMed] [Google Scholar]
- [9].Dancey JE, Freidlin B. Targeting epidermal growth factor receptor[em]are we missing the mark? Lancet. 2003;362:62–4. doi: 10.1016/S0140-6736(03)13810-X. [DOI] [PubMed] [Google Scholar]
- [10].Fischel JL, Formento P, Milano G. Epidermal growth factor receptor double targeting by a tyrosine kinase inhibitor (Iressa) and a monoclonal antibody (Cetuximab). Impact on cell growth and molecular factors. Br J Cancer. 2005;92:1063–8. doi: 10.1038/sj.bjc.6602428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zanchi C, Zuco V, Lanzi C, Supino R, Zunino F. Modulation of survival signaling pathways and persistence of the genotoxic stress as a basis for the synergistic interaction between the atypical retinoid ST1926 and the epidermal growth factor receptor inhibitor ZD1839. Cancer Res. 2005;65:2364–72. doi: 10.1158/0008-5472.CAN-04-2495. [DOI] [PubMed] [Google Scholar]
- [12].Chou TY, Chiu CH, Li LH, et al. Mutation in the tyrosine kinase domain of epidermal growth factor receptor is a predictive and prognostic factor for gefitinib treatment in patients with non-small cell lung cancer. Clin Cancer Res. 2005;11:3750–7. doi: 10.1158/1078-0432.CCR-04-1981. [DOI] [PubMed] [Google Scholar]
- [13].Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- [14].Drucker B, Bacik J, Ginsberg M, et al. Phase II trial of ZD1839 (IRESSA) in patients with advanced renal cell carcinoma. Invest New Drugs. 2003;21:341–5. doi: 10.1023/a:1025472712456. [DOI] [PubMed] [Google Scholar]
- [15].Rowinsky EK, Schwartz GH, Gollob JA, et al. Safety, pharmacokinetics, and activity of ABX-EGF, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer. J Clin Oncol. 2004;22:3003–15. doi: 10.1200/JCO.2004.11.061. [DOI] [PubMed] [Google Scholar]
- [16].Motzer RJ, Amato R, Todd M, et al. Phase II trial of antiepidermal growth factor receptor antibody C225 in patients with advanced renal cell carcinoma. Invest New Drugs. 2003;21:99–101. doi: 10.1023/a:1022928612511. [DOI] [PubMed] [Google Scholar]
- [17].Perera AD, Kleymenova EV, Walker CL. Requirement for the von Hippel-Lindau tumor suppressor gene for functional epidermal growth factor receptor blockade by monoclonal antibody C225 in renal cell carcinoma. Clin Cancer Res. 2000;6:1518–23. [PubMed] [Google Scholar]
- [18].Gemmill RM, Zhou M, Costa L, Korch C, Bukowski RM, Drabkin HA. Synergistic growth inhibition by Iressa and rapamycin is modulated by VHL mutations in renal cell carcinoma. Br J Cancer. 2005;92:2266–77. doi: 10.1038/sj.bjc.6602646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Costa LJ, Gemmill RM, Drabkin HA. Upstream signaling inhibition enhances rapamycin effect on growth of kidney cancer cells. Urology. 2007;69:596–602. doi: 10.1016/j.urology.2007.01.053. [DOI] [PubMed] [Google Scholar]
- [20].Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–54. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- [21].Horiguchi A, Oya M, Uchida A, Marumo K, Murai M. Elevated Akt activation and its impact on clinicopathological features of renal cell carcinoma. J Urol. 2003;169:710–3. doi: 10.1097/01.ju.0000038952.59355.b2. [DOI] [PubMed] [Google Scholar]
- [22].Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38:200–11. doi: 10.1080/07853890600551037. [DOI] [PubMed] [Google Scholar]
- [23].Peruzzi B, Athauda G, Bottaro DP. The von Hippel-Lindau tumor suppressor gene product represses oncogenic beta-catenin signaling in renal carcinoma cells. Proc Natl Acad Sci USA. 2006;103:14531–6. doi: 10.1073/pnas.0606850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Iliopoulos O, Kibel A, Gray S, Kaelin WG., Jr. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1:822–6. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- [25].Gnarra JR, Zhou S, Merrill MJ, et al. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc Natl Acad Sci USA. 1996;93:10589–94. doi: 10.1073/pnas.93.20.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stumm G, Eberwein S, Rostock-Wolf S, et al. Concomitant overexpression of the EGFR and erbB-2 genes in renal cell carcinoma (RCC) is correlated with dedifferentiation and metastasis. Int J Cancer. 1996;69:17–22. doi: 10.1002/(SICI)1097-0215(19960220)69:1<17::AID-IJC4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- [27].Thomasson M, Hedman H, Guo D, Ljungberg B, Henriksson R. LRIG1 and epidermal growth factor receptor in renal cell carcinoma: a quantitative RT[en]PCR and immunohistochemical analysis. Br J Cancer. 2003;89:1285–9. doi: 10.1038/sj.bjc.6601208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sourbier C, Lindner V, Lang H, et al. The phosphoinositide 3-kinase/Akt pathway: a new target in human renal cell carcinoma therapy. Cancer Res. 2006;66:5130–42. doi: 10.1158/0008-5472.CAN-05-1469. [DOI] [PubMed] [Google Scholar]
- [29].Oka H, Chatani Y, Hoshino R, et al. Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer Res. 1995;55:4182–7. [PubMed] [Google Scholar]
- [30].Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr. Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Smith K, Gunaratnam L, Morley M, Franovic A, Mekhail K, Lee S. Silencing of epidermal growth factor receptor suppresses hypoxia-inducible factor-2-driven VHL-/- renal cancer. Cancer Res. 2005;65:5221–30. doi: 10.1158/0008-5472.CAN-05-0169. [DOI] [PubMed] [Google Scholar]
- [32].Rowinsky EK. The erbB family: targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu Rev Med. 2004;55:433–57. doi: 10.1146/annurev.med.55.091902.104433. [DOI] [PubMed] [Google Scholar]