Abstract
A survey was conducted to identify reservoirs for urban leptospirosis in the city of Salvador, Brazil. Sampling protocols were performed in the vicinity of households of severe leptospirosis cases identified during active hospital-based surveillance. Among a total of 142 captured Rattus norvegicus (Norwegian brown rat), 80.3% had a positive culture isolate from urine or kidney specimens and 68.1% had a positive serum sample by microscopic agglutination test (MAT) titre of ≥1:100. Monoclonal antibody-based typing of isolates identified that the agent carried by rats was L. interrogans serovar Copenhageni, which was the same serovar isolated from patients during hospital-based surveillance. Leptospira spp. were not isolated from 8 captured Didelphis marsupialis (Opossum), while 5/7 had a positive MAT titre against a saprophytic serogroup. R. rattus were not captured during the survey. The study findings indicate that the brown rat is a major rodent reservoir for leptospirosis in this urban setting. Furthermore, the high carriage rates of L. interrogans serovar Copenhageni in captured rats suggest that there is a significant degree of environmental contamination with this agent in the household environment of high risk areas, which in turn is a cause of transmission during urban epidemics.
Keywords: Leptospira, Leptospirosis, Rats, Poverty Areas
1. Introduction
Leptospirosis is one of the most widespread zoonoses and is caused by infection with pathogenic spirochetes of the Leptospira genus (Levett, 2001). Human patients usually present with a non-specific self-limiting febrile illness; however, 5–10% of cases develop severe forms of the disease such as Weil’s disease (which is characterized by jaundice, acute renal failure, hemorrhagic diathesis) and severe pulmonary hemorrhagic syndrome (SPHS). Case fatality from Weil’s disease and SPHS is >10% to 74%, respectively (McBride et al., 2005; Gouveia et al., 2008).
Urban outbreaks of severe leptospirosis occur annually in Brazil and are mainly associated with intense periods of high rainfall and poor living conditions (Ko et al., 1999; Romero et al., 2003; Tassinari et al., 2008). The predominant infecting serovar among patients with severe leptospirosis is L. interrogans serovar Copenhageni (Ko et al., 1999; Pereira et al., 2000; Barocchi et al., 2001; Romero et al., 2003). Serovar Copenhageni is frequently associated with rodents belonging to Rattus spp. (Tamai et al., 1988; Vinetz et al., 1996; Levett et al., 1998; Faine et al., 1999). In Brazil the brown rat (Rattus norvegicus) was implicated as a carrier of serovar Copenhageni strains in several reports (Pereira et al., 1988; Ko et al., 1999; Barocchi et al., 2001). Furthermore, a case-control study in the city of Salvador identified open sewers and sighting rats in the household environment as risk factors for severe disease (Sarkar et al., 2002). A previous survey of Leptospira carriage among rats in the city of Salvador in 1954 reported a carriage rate of 29% (Andrade et al., 1954). Over the last twelve years, studies of rodent infection in the vicinity of index cases have associated rats with human infection in both rural (Johnson et al., 2004; Ganoza et al., 2006) and urban settings (Vinetz et al., 1996; Pezzella et al., 2004; Jansen et al., 2005).
The objective of this study was to identify animals with habitats close to the homes of severe leptospirosis patients and to determine the Leptospira carrier status in captured animals. Previous studies have often relied on the use of microscopic agglutination test (MAT) to determine leptospiral prevalence and the true carriage rate may be underestimated. There are a limited number of studies on rodent carrier state using potentially more sensitive PCR assays (Vinetz et al., 1996; Sunbul et al., 2001; Pezzella et al., 2004; Priya et al., 2007). Therefore, we conducted an investigation using culture isolation, MAT, and PCR protocols to detect carriage in trapped animals.
2. Materials and Methods
2.1. Identification and definition of human cases
Patients were identified during active hospital-based surveillance in the city of Salvador, Brazil, in 1998, according to previously published protocols (Ko et al., 1999; Sarkar et al., 2002). Laboratory case confirmation was based on the criteria of a fourfold or more rise in reciprocal MAT titres or seroconversion between acute and convalescent paired serum samples or a single serum sample with a MAT titre of >800. Reservoir sampling was performed at the site of households of laboratory-confirmed cases of leptospirosis.
2.2. Capture protocol
Animals were trapped near the households of 10 leptospirosis patients that were situated in 10 urban slum communities in Salvador. Additional trapping sessions were performed in two neighbourhoods where there were no reported cases of leptospirosis. Trapping commenced one week to three months after leptospirosis was diagnosed in the patients included in the study and was performed between May 1998 and March 1999 in collaboration with the Zoonotic Disease Control Centre (CCZ) of the Municipal Health Secretary of Salvador. Traps (20 × 20 × 60 cm Tomahawk Live Trap cages, Tomahawk Live Trap Co.) were positioned near selected homes at distances of 50 to 150 m, with at least 5 m spacing between cages. Trap placement was based on indicators of rodent activity such as refuse, open sewers, faeces and tracks. Three to eight cages were placed per household and the capture sessions ranged from 15 h (overnight) to 24 h periods. Over the course of the study, 109 capture sessions were carried out in 12 different neighbourhoods. Captured animals were transported to the Fiocruz animal facility where they were housed and given food and water ad libitum prior to euthanasia. The ethics committee of the Oswaldo Cruz Foundation (CEUA) approved the protocols used in this study.
2.3. Sample collection
Animals were identified by genus, species, and gender based on phenotypic characteristics (ears, body, tail, fur colour and sex). The rats were identified as R. norvegicus based on the following physical characteristics: a grey-brown coat with lighter coloured underparts and a tail shorter than the combined head and body length. Adults were defined as 18–26cm in body length, weighing 400–600g. Biological samples were obtained 5 to 24 hours following capture, and animals were anaesthetized with ether and ketamine. Blood samples were collected by cardiac puncture while direct puncture of the bladder was performed under aseptic conditions for urine collection (0.2–0.7 ml). One kidney from each animal was removed for culture isolation.
2.4. Culture isolation
Four tubes containing 5 ml of liquid Ellinghausen-McCullough-Johnson-Harris (EMJH) medium (Difco, USA) were inoculated with four drops of urine. In addition, one kidney from each rat was removed, macerated, and inoculated into 5 ml of liquid medium (Faine, 1982). Tissue debris was allowed to sediment for 30 min then 0.5 ml of debris-free medium was used to inoculate four tubes of liquid medium. Cultures were incubated at 28.4°C and evaluated weekly by darkfield microscopy for up to two months. Isolates were sent to the National Reference Laboratory (Oswaldo Cruz Foundation, Rio de Janeiro, Brazil) where they were typed to serovar level by the monoclonal antibody typing method (Collares-Pereira et al., 2000; Sehgal et al., 2000) with an antibody panel provided by the Royal Tropical Institute (KIT), Holland.
2.5. Microscopic agglutination test
MAT was performed as previously described (Faine, 1982), using a reference battery of 29 serovars comprising 18 pathogenic and two non-pathogenic serogroups from seven Leptospira spp. The serogroups used as live antigens were Australis, Autumnalis, Ballum, Bataviae, Canicola, Celledoni, Cynopteri, Djasiman, Grippotyphosa, Icterohaemorrhagiae, Hebdomadis, Javanica, Panama, Pomona, Pyrogenes, Sejroe, Tarassovi, Shermani, Andamana and Semaranga. Serum samples were considered positive at a reciprocal titre of ≥100.
2.6. PCR detection of Leptospira genomic DNA
Within two hours of collection, all urine samples were centrifuged at 14,000 rpm for 15 minutes at 4°C with the pellet being washed in Phosphate Buffered Saline (PBS) followed by a repeat centrifugation. This washing method was necessary to remove particulate matter in the urine that complicates PCR analysis. Urine samples collected from 20 uninfected Wistar rats were used as negative controls. In order to liberate the leptospiral DNA, all samples were boiled for 15min. at 100°C. All samples were stored at −70°C until the PCR reaction was performed. PCR detection of leptospiral genomic DNA was based on two previously described techniques (Merien et al., 1992; Gravekamp et al., 1993). PCR using the A/B primer pair amplifies an expected product of 331 bp (Merien et al., 1992). The G1/G2 primer pair amplifies a 285 bp product from all Leptospira spp. except L. kirschneri, which requires primer pair B64I/B64II (563 bp product) (Gravekamp et al., 1993). The detection limits of the A/B and G1/G2 primer pairs were determined using L. interrogans serovar Copenhageni strain Fiocruz L1–130 grown in vitro and diluted in PBS or urine from Wistar rats. The leptospires where counted in a Petroff Hauser counting chamber as previously described (Faine et al., 1999) and a dilution series from 104 to 100 leptospires was used to spike the PBS or urine. Briefly, the PCR was performed using PCR mix (Life Technologies, Gibco, Grand Island NY, USA) and the relevant primer pairs under the following conditions. The A/B primer PCR conditions consisted of 1 cycle for 3 min at 94°C followed by 35 cycles of 1 min at 94°C, 1.5 min at 63°C, 2 min at 72°C, with a final elongation step of 10 min at 72°C. The PCR program for the G1/G2 and B64I/II primer sets consisted of 1 cycle for 3 min at 94°C followed by 30 cycles of 1.5 min at 94°C, 1 min at 55°C, 2 min at 72°C, with a final elongation step of 10 min at 72°C. PCR products were analysed by horizontal agarose gel electrophoresis and visualized by staining with ethidium bromide.
2.7. Statistical analysis
Calculations of statistical significance between groups were made by comparing proportions using the Chi-square test. Concordance between culture isolation, MAT, and PCR methods was calculated using Cohen’s unweighted Kappa correlation. The Epi Info 6.0 software (Centers for Disease Control and Prevention, Atlanta, USA) was used to perform the calculations.
3. Results
Active hospital-based surveillance identified 103 cases during the study period. Of them, we randomly selected ten domiciles of patients (cases) from 10 different neighbourhoods. During 109 capture sessions over a period of ten months, a total of 142 R. norvegicus and 8 D. marsupialis were captured. The average number of captured rats was 1.3 animals per capture session. Trapping sessions in control neighbourhoods did not yield captured animals, therefore the protocol was discontinued after six weeks.
Of the rats captured, 80.3% (114/142) were culture positive (Table 1). Adult male rats exhibited significantly greater renal colonization, 79.0% (49/62), compared to juveniles, 35.7% (5/14, p-value < 0.01), while carriage rates were similar among female rats, regardless of age. Serotyping of the isolates using monoclonal antibodies identified the infecting serovar as Copenhageni for all isolates tested (n = 59). None of the captured opossums were culture positive for Leptospira spp.
Table 1.
Detection of Leptospira spp. and serology of R. norvegicus captured in proximity to the urban dwellings of patients diagnosed with severe leptospirosis.
| R. norvegicus | % Positivity (No. positive/total)
|
|||
|---|---|---|---|---|
| Culturea | MATb | PCRc | ||
| Adults | Male | 79.0 (49/62) | 60.0 (30/50) | 80.0 (24/30) |
| Female | 91.1 (51/56) | 79.2 (38/48) | 83.3 (20/24) | |
| Juveniles | Male | 35.7 (5/14) | 40.0 (4/10) | 100 (4/4) |
| Female | 100 (9/9) | 85.7 (6/7) | 100 (4/4) | |
|
| ||||
| Overall (n = 142) | 80.3 (114/142)d | 68.1 (79/116)d | 83.9 (52/62) | |
Leptospires cultured from urine or kidney samples.
MAT screening criteria defined a titre of ≥1:100 as positive.
PCR positive by any method.
For one animal we lost the information about gender. This was a culture negative and MAT positive rat.
Of the 7 opossum serum samples tested by MAT, 71.4% (5/7) had agglutinating antibodies against serogroup Semaranga. Of the 116 rat serum samples tested, 68.1% (n = 79) were MAT positive. MAT highest titers were observed against serogroups Icterohaemorrhagiae (n = 75), Canicola (n = 1), and Djasiman (n = 3). Monoclonal antibody typing of one isolate from a rat with highest titer against serogroup Djasiman identified the infecting agent as serovar Copenhageni. Prevalence based on the MAT results, although higher in adult rats (69.4%), was not significantly different to that seen in juvenile rats (58.8%, Table 1). In addition, there were no significant differences in the proportions of infected male or female rats, regardless of age. When comparing the methods used in this study using Cohen’s unweighted Kappa correlation we found that there was only low agreement between culture isolation and MAT (Kappa = 0.29).
The detection limit for PCR in PBS was determined to be 10 leptospires for the A/B primer set compared to 100 leptospires in Wistar rat urine. The G1/G2 primer pair was sensitive down to one leptospire in both PBS and urine. The detection limit for the B64I/II primer set was not determined. Urine samples from 20 uninfected laboratory Wistar rats were all negative.
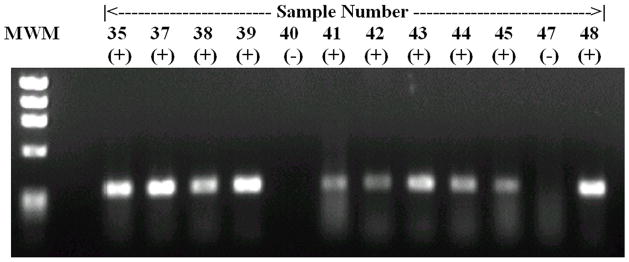
None of the samples collected from captured opossums were positive by any of the PCR assays used in this study. In culture positive animals, the sensitivity for each of the primer sets was 90% (47/52) for A/B primers and 79% (41/52) for G1/G2 primers. The overall PCR sensitivity (A/B and G1/G2 primers combined) was 48/52 (92%). One of 10 captured rats with negative cultures had a positive reaction for A/B primers. Thus, kappa coefficient of agreement between PCR (A/B and G1/G2) was 0.39. An example of PCR products in urban rats is provided in figure 1. The L. kirschneri specific B64I/II primer pair amplified a product in 11.1% (3/27) of rat urine samples; however, in two cases, amplification was also obtained for both A/B and G1/G2. Therefore, we cannot exclude a false positive B64I/II primer pair amplification in cases of other Leptospira spp. infection. In the remaining case in which there was only amplification for L. kirschneri specific prime pair, culture isolation proved negative as did the MAT, even though two L. kirschneri serovars were included in the MAT battery. Analysis of the Merien and Gravekamp PCR methods demonstrated that there was moderate agreement between the two protocols (Kappa = 0.52).
Figure 1.
This is an example of the results achieved using the PCR analysis (A/B primers) for leptospirosis in the urine of the captured animal reservoirs. The lanes have been marked as positive and negative, with each sample number representing a different animal. For this figure, we used the PhiX174 DNA/Hae III Digest DNA molecular weight marker (1353bp, 1078bp, 872bp, 603bp, 281/272bp, 194bp, 118bp, 72bp).
4. Discussion
Of the rats captured in the peridomicillary regions of severe leptospirosis patients, >80% were infected with Leptospira spp., while in neighbourhoods where there were no recorded human cases we failed to capture any animals. These later regions were notable for the higher socioeconomic status of the residents. Both rodent control and the infrastructure of the sewage system were observed to be of a higher standard in these areas and may explain the failure to trap any animals. Compared to other surveys where carriage rates ranged from 4 to 59% when based on culture isolation (Thiermann, 1977; Carter and Cordes, 1980; Hathaway and Blackmore, 1980; Pereira and Andrade, 1988; Taylor et al., 1991; Webster et al., 1995; Levett et al., 1998; Vado-Solis et al., 2002; Sharma et al., 2003; Vanasco et al., 2003; Priya et al., 2007), our results detected a much higher proportion (>80%) of leptospiral carriage among captured rats. In a survey carried out over 50 years ago in the city of Salvador, the carriage rate in rats was reported to be 29% by silver impregnation of kidney samples (Andrade and Oliveira, 1954). It is important to note that different methods were used for trapping and for determining the presence of leptospires. Therefore, it is difficult to draw firm conclusions as to whether or not the leptospiral carriage rate among rats in Salvador increased in parallel with the uncontrolled growth of urban slum (favela) populations over the same period (Ko et al., 1999; Riley et al., 2007). The differences observed could be due to study design and the detection strategies employed. Using multiple culture tubes and direct collection of urine by bladder puncture under aseptic conditions probably prevented a high level of contamination in the present work, which is a major problem that reduces the sensitivity of culture methods.
Rodents are generally regarded as one of the most important transmission sources of leptospirosis (Faine et al., 1999). R. norvegicus, a predominantly urban dwelling rat found in close proximity to humans, is regarded as the one of the main reservoirs for serovar Copenhageni worldwide (Faine et al., 1999), and has largely replaced R. rattus as the dominant rat in urban settings due to its more aggressive behaviour. In the current study, R. norvegicus was the only rat species captured, and R. rattus represented only 0.9% (2/229) of the rats trapped in the previous study, with the remainder identified as R. norvegicus (Andrade and Oliveira, 1954). With respect to leptospiral carriage among rats, we showed that adult males were significantly more likely to harbour pathogenic leptospires compared to juveniles (Table 1), as was reported in other studies (Thiermann, 1977; Carter and Cordes, 1980; Vanasco et al., 2003). Carter and Cordes (1980) observed that mature urban Rattus rattus are 4 times more likely to carry leptospires than immature R. rattus. This may be explained by short duration of maternal passive immunity. We do not have an explanation on why this was not observed in females.
The marsupial D. marsupialis is commonly found in urban tropical areas but its role as a leptospiral reservoir is unclear. In the present study, some (5/7) opossums had detectable agglutinating antibodies against leptospires with the highest reciprocal titres for serogroup Semaranga. This serogroup is comprised mainly of non-pathogenic strains; however, seroreactivity for this serogroup may indicate that other pathogenic serogroups should be included in the MAT reference battery. Marsupials have been reported to harbour serovars such as L. kirschneri serovar Grippotyphosa (Bharti et al., 2003), which was detected by PCR in a few rats in the present study. The three positive L. kirschneri-specific primer pair PCR products were obtained from a rat with negative MAT and culture, and two rats with positive G1/G2 (non L. kirschneri) primers. Our small sampling of opposums showed no PCR detection of L. kirshneri, nor is there any evidence of these serovars reported in severe leptospirosis patients from Salvador (Ko et al., 1999; Barocchi et al., 2001). Still, since hospital-based active surveillance is based on identification of severe cases, we cannot exclude the possibility that L. kirschneri and/or serovar Grippotyphosa are associated with milder forms of leptospirosis in Salvador.
The culture and MAT protocols used in this study were in accordance with WHO guidelines (W.H.O., 2003) and are considered sufficient for the identification of leptospirosis reservoirs (Faine et al., 1999). The methods are, however, labour intensive and require reference laboratory facilities. Furthermore, natural reservoirs may have undetectable levels of agglutinating antibodies (Vinetz et al., 1996; Sunbul et al., 2001; Priya et al., 2007). This was reflected in the present work as MAT was positive in only 74.5% (73/98) of sera collected from culture positive rats.
In this study, we were able to detect leptospiral DNA in 92% of culture positive rats tested using the A/B and G1/G2 primer pairs, confirming the suitability of PCR as a tool in epidemiology and in the identification of reservoir hosts. The sensitivity for each of the primer sets was 90% (47/52) for A/B primers and 79% (41/52) for G1/G2 primers. We do not have a clear explanation for this discrepancy since G1/G2 primers have indeed a lower detection limit under laboratory conditions. We can only speculate that other non evaluated environmental factors may be more inhibitory for G1/G2 primers performance. The detection of L. kirschneri by PCR was unexpected since it has not previously been described in cases of leptospirosis in Salvador. In addition, there was no corroborating evidence to support a L. kirschneri serovar among the infected rats. L. interrogans serovar Copenhageni is known to be the most common cause of human infection in Salvador, Brazil (Ko et al., 1999; Barocchi et al., 2001).
The high rates of leptospiral infection among rats and the predominance of serovar Copenhageni suggests a likely role for the rat in transmission of human leptospirosis via environmental contamination in high risk areas of Salvador. Furthermore, in a previous study we used DNA fingerprinting to show that culture isolates from 32 patients with severe leptospirosis and isolates from five captured rats were identical and were serovar Copenhageni (Barocchi et al., 2001). Recently, our group demonstrated that, in a slum community of Salvador, members of the same household of an index case of leptospirosis had a 30% prevalence of antileptospiral serum agglutinins (five times more likely to exhibit evidence of prior infection). The household clustering of Leptospira infection in slum communities indicates that the household environment and related factors are important determinants for transmission of urban leptospirosis (Maciel et al. 2008). Our present data strongly indicate the role of rats as agents of urban peridomicillary transmission of leptospirosis.
In conclusion, the results presented here support previous studies that identified R. norvegicus as a main reservoir host for serovar Copenhageni strains. R. norvegicus was identified as an important leptospiral reservoir and serovar Copenhageni was dominant. Given the increase in observed carriage rate in rats compared to an earlier study (80 versus 29%), it is possible to speculate that this rise may be involved in the increased prevalence of leptospirosis reported in urban settings. Improvements in diagnosis, both clinical and laboratorial, are likely also important in explaining this increased prevalence. This work demonstrates that leptospirosis reservoirs must be monitored constantly to minimize their impact on the transmission of leptospirosis. Although we could not rule out infection of the patients included in this study at their place of work, the data reported herein suggests that peridomicillary rats are likely to be responsible for the transmission of leptospirosis in such urban settings.
Acknowledgments
This work was supported by the Research Support Foundation for the State of Bahia (FABESB), and the National Institutes of Health (grants R01 AI052473 and D43 TW00919).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade ZA, Oliveira JC. Studies on leptospirosis in Bahia. Boletim da Fundação Gonçalo Moniz. 1954;3:1–36. [Google Scholar]
- Barocchi MA, Ko AI, Ramos FS, Faria MT, Reis MG, Riley LW. Identification of new repetitive element in Leptospira interrogans serovar copenhageni and its application to PCR-based differentiation of Leptospira serogroups. J Clin Microbiol. 2001;39:191–195. doi: 10.1128/JCM.39.1.191-195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Cordes DO. Leptospirosis and other infections of Battus rattus and Rattus norvegicus. N Z Vet J. 1980;28:45–50. doi: 10.1080/00480169.1980.34688. [DOI] [PubMed] [Google Scholar]
- Collares-Pereira M, Korver H, Cao TBV, Santos-Reis M, Bellenger E, Baranton G, Terpstra WJ. Analysis of Leptospira isolates from mainland Portugal and the Azores islands. FEMS Microbiol Lett. 2000;185:181–187. doi: 10.1111/j.1574-6968.2000.tb09059.x. [DOI] [PubMed] [Google Scholar]
- Faine S. Guidelines for the control of leptospirosis. World Health Organization; Geneva: 1982. [Google Scholar]
- Faine SB, Adler B, Bolin C, Perolat P. MediSci. 2. Melbourne, Australia: 1999. Leptospira and leptospirosis. [Google Scholar]
- Ganoza CA, Matthias MA, Collins-Richards D, Brouwer KC, Cunningham CB, Segura ER, Gilman RH, Gotuzzo E, Vinetz JM. Determining risk for severe leptospirosis by molecular analysis of environmental surface waters for pathogenic Leptospira. PLoS Med. 2006;3:e308. doi: 10.1371/journal.pmed.0030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia EL, Metcalfe J, de Carvalho ALF, Aires TSF, Villalobos-Bisneto JC, Queiroz A, Santos AC, Salgado K, Reis MG, Ko AI. Leptospirosis-associated severe pulmonary hemorrhage syndrome, Salvador, Brazil. Emerg Infect Dis. 2008;14:505–508. doi: 10.3201/eid1403.071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravekamp C, Van de Kemp H, Franzen M, Carrington D, Schoone GJ, Van Eys GJ, Everard CO, Hartskeerl RA, Terpstra WJ. Detection of seven species of pathogenic leptospires by PCR using two sets of primers. J Gen Microbiol. 1993;139:1691–1700. doi: 10.1099/00221287-139-8-1691. [DOI] [PubMed] [Google Scholar]
- Hathaway SC, Blackmore DK. Ecological aspects of the epidemiology of infection with leptospires of the Ballum serogroup in the black rat (Rattus rattus) and the brown rat (Rattus norvegicus) in New Zealand. Journal of Hygiene (Cambridge) 1980;87:427–436. doi: 10.1017/s0022172400069679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Schoneberg I, Frank C, Alpers K, Schneider T, Stark K. Leptospirosis in Germany, 1962–2003. Emerg Infect Dis. 2005;11:1048–1054. doi: 10.3201/eid1107.041172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Smith H, Joeph P, Gilman RH, Bautista CT, Campos KJ, Cespedes M, Klatsky P, Vidal C, Terry H, Calderon MM, Coral C, Cabrera L, Parmar PS, Vinetz JM. Environmental exposure and leptospirosis, Peru. Emerg Infect Dis. 2004;10:1016–1022. doi: 10.3201/eid1006.030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AI, Galvao Reis M, Ribeiro Dourado CM, Johnson WD, Jr, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet. 1999;354:820–825. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levett PN, Walton D, Waterman LD, Whittington CU, Mathison GE, Edwards CO. Surveillance of leptospiral carriage by feral rats in Barbados. West Indian Med J. 1998;47:15–17. [PubMed] [Google Scholar]
- Maciel EA, de Carvalho AL, Nascimento SF, de Matos RB, Gouveia EL, Reis MG, Ko AI. Household transmission of leptospira infection in urban slum communities. PLoS Negl Trop Dis. 2008;2:e154. doi: 10.1371/journal.pntd.0000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–386. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- Merien F, Amouriaux P, Perolat P, Baranton G, Saint GI. Polymerase chain reaction for detection of Leptospira spp. in clinical samples. J Clin Microbiol. 1992;30:2219–2224. doi: 10.1128/jcm.30.9.2219-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MM, Andrade J. Epidemiological aspects of leptospirosis in a slum area in the city of Rio de Janeiro, Brazil. Search for leptospires and specific antibodies in rodents. Trans R Soc Trop Med Hyg. 1988;82:768–770. doi: 10.1016/0035-9203(88)90231-3. [DOI] [PubMed] [Google Scholar]
- Pereira MM, Matsuo MGS, Bauab AR, Vasconcelos SA, Moraes ZM, Baranton G, Saint GI. A clonal subpopulation of Leptospira interrogans sensu stricto is the major cause of leptospirosis outbreaks in Brazil. J Clin Microbiol. 2000;38:450–452. doi: 10.1128/jcm.38.1.450-452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzella M, Lillini E, Sturchio E, Ierardi LA, Grassi M, Traditi F, Cristaldi M. Leptospirosis survey in wild rodents living in urban areas of Rome. Ann Ig. 2004;16:721–726. [PubMed] [Google Scholar]
- Priya CG, Hoogendijk KT, Berg M, Rathinam SR, Ahmed A, Muthukkaruppan VR, Hartskeerl RA. Field rats form a major infection source of leptospirosis in and around Madurai, India. J Postgrad Med. 2007;53:236–240. doi: 10.4103/0022-3859.37511. [DOI] [PubMed] [Google Scholar]
- Riley LW, Ko AI, Unger A, Reis MG. Slum health: diseases of neglected populations. BMC Int Health Hum Rights. 2007;7:2. doi: 10.1186/1472-698X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero EC, Bernardo CC, Yasuda PH. Human leptospirosis: a twenty-nine-year serological study in Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2003;45:245–248. doi: 10.1590/s0036-46652003000500002. [DOI] [PubMed] [Google Scholar]
- Sarkar U, Nascimento SF, Barbosa R, Martins R, Nuevo H, Kalafanos I, Grunstein I, Flannery B, Dias J, Riley LW, Reis MG, Ko AI. Population-based case-control investigation of risk factors for leptospirosis during an urban epidemic. Am J Trop Med Hyg. 2002;66:605–610. doi: 10.4269/ajtmh.2002.66.605. [DOI] [PubMed] [Google Scholar]
- Sehgal SC, Vijayachari P, Smythe LD, Norris M, Symonds M, Dohnt M, Korver H, v dKH, Hartskeerl RA, Terpstra WJ. Lai-like leptospira from the Andaman Islands. Indian J Med Res. 2000;112:135–139. [PubMed] [Google Scholar]
- Sharma S, Vijayachari P, Sugunan AP, Sehgal SC. Leptospiral carrier state and seroprevalence among animal population--a cross-sectional sample survey in Andaman and Nicobar Islands. Epidemiol Infect. 2003;131:985–989. doi: 10.1017/s095026880300880x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunbul M, Esen S, Leblebicioglu H, Hokelek M, Pekbay A, Eroglu C. Rattus norvegicus acting as reservoir of Leptospira interrogans in the Middle Black Sea region of Turkey, as evidenced by PCR and presence of serum antibodies to Leptospira strain. Scand J Infect Dis. 2001;33:896–898. doi: 10.1080/00365540110076796. [DOI] [PubMed] [Google Scholar]
- Tamai T, Sada E, Kobayashi Y. Restriction endonuclease DNA analysis of Leptospira interrogans serovars Icterohaemorrhagiae and Copenhageni. Microbiol Immunol. 1988;32:887–894. doi: 10.1111/j.1348-0421.1988.tb01450.x. [DOI] [PubMed] [Google Scholar]
- Tassinari WS, Pellegrini DC, Sa CB, Reis RB, Ko AI, Carvalho MS. Detection and modelling of case clusters for urban leptospirosis. Trop Med Int Health. 2008;13:503–512. doi: 10.1111/j.1365-3156.2008.02028.x. [DOI] [PubMed] [Google Scholar]
- Taylor KD, Turner LH, Everard JD. Leptospires in Rattus spp. on Barbados. J Trop Med Hyg. 1991;94:102–103. [PubMed] [Google Scholar]
- Thiermann AB. Incidence of leptospirosis in the detroit rat population. Am J Trop Med Hyg. 1977;26:970–974. doi: 10.4269/ajtmh.1977.26.970. [DOI] [PubMed] [Google Scholar]
- Vado-Solis I, Cardenas-Marrufo MF, Jimenez-Delgadillo B, Alzina-Lopez A, Laviada-Molina H, Suarez-Solis V, Zavala-Velazquez JE. Clinical-epidemiological study of leptospirosis in humans and reservoirs in Yucatan, Mexico. Rev Inst Med Trop Sao Paulo. 2002;44:335–340. doi: 10.1590/s0036-46652002000600008. [DOI] [PubMed] [Google Scholar]
- Vanasco NB, Sequeira MD, Sequeira G, Tarabla HD. Associations between leptospiral infection and seropositivity in rodents and environmental characteristics in Argentina. Prev Vet Med. 2003;60:227–235. doi: 10.1016/s0167-5877(03)00144-2. [DOI] [PubMed] [Google Scholar]
- Vinetz JM, Glass GE, Flexner CE, Mueller P, Kaslow DC. Sporadic urban leptospirosis. Ann Intern Med. 1996;125:794–798. doi: 10.7326/0003-4819-125-10-199611150-00002. [DOI] [PubMed] [Google Scholar]
- Malta, editor. W.H.O. Human leptospirosis: guidance for diagnosis, surveillance and control. World Health Organization; 2003. [Google Scholar]
- Webster JP, Ellis WA, Macdonald DW. Prevalence of Leptospira spp. in wild brown rats (Rattus norvegicus) on UK farms. Epidemiol Infect. 1995;114:195–201. doi: 10.1017/s0950268800052043. [DOI] [PMC free article] [PubMed] [Google Scholar]