Abstract
Akt/mTOR signaling plays an important role in tumorigenesis and is dysregulated in many tumors, especially metastatic prostate cancers. Curcumin has been shown to effectively prevent or inhibit prostate cancer in vivo and inhibit Akt/mTOR signaling in vitro, but the mechanism(s) remains unclear. Here we show that curcumin concentration- and time-dependently inhibited the phosphorylation of Akt, mTOR, and their downstream substrates in human prostate cancer PC-3 cells, and this inhibitory effect acts downstream of PI3K and PDK1. Overexpression of constitutively activated Akt or disruption of TSC1-TSC2 complex by siRNA or gene knockout only partially restored curcumin-mediated inhibition of mTOR and downstream signaling, indicating they are not the primary effectors of curcumin-mediated inhibition of Akt/mTOR signaling. Curcumin also activated AMPK and MAP kinases, however, inhibition of these kinases failed to rescue the inhibition by curcumin. Finally, it was demonstrated that the inhibition of Akt/mTOR signaling by curcumin is resulted from calyculin A-sensitive protein phosphatase-dependent dephosphorylation. Our study reveals the profound effects of curcumin on the Akt/mTOR signaling network in PC-3 cells, and provides new mechanisms for the anti-cancer effects of curcumin.
Keywords: Curcumin, Akt, mTOR, AMPK, TSC1/TSC2, Protein phosphatase
Introduction
The phosphatidylinositol 3-kinase (PI3K)/Akt (also termed PKB, protein kinase B)/mammalian target of rapamycin (mTOR) signaling axis plays a central role in regulation of multiple critical cellular functions including stress responses, cell growth and survival, and metabolism (1). Activated PI3K converts phosphatidylinositol (PtdIns) into PtdIns(3,4)P2 (PIP2) and PtdIns(3,4,5)P3 (PIP3). Consequently, phosphotidylinositol-dependent kinase-1 (PDK1) and Akt are recruited to the cell membrane, and then Akt is phosphorylated at residues Thr308 and Ser473 by PDK1 and PDK2 (presumably mTOR complex 2), respectively (2, 3). Phosphorylated and activated, Akt phosphorylates and regulates a plethora of substrates including glycogen synthase kinase 3 (GSK3), Forkhead family transcription factors, and mTOR (4). On the other hand, The phosphatase and tensin homolog deleted on chromosome ten (PTEN) counteracts PI3K activity by dephosphorylating PIP2 and PIP3 (5).
Specifically, mTOR is a key mediator of Akt signaling, especially in oncogenic transformation. mTOR forms two functional complexes, C1 and C2, and integrates signals from nutrients, growth factors, and cellular energy status to control cell growth and proliferation by regulating protein synthesis (6). Phosphorylation of mTOR at Ser2448 by Akt (7) or S6K1 (8) and at Ser2481 by auto-phosphorylation is important for its activity (9). The activity of mTOR is negatively regulated by tuberous sclerosis complex 1 and 2 (TSC1 and TSC2, also known as hamartin and tuberin). TSC1/TSC2 complex dissociates Ras homolog enriched in brain (Rheb) from mTOR, thus inhibits mTOR activation. Akt phosphorylates TSC2 and disrupts the TSC1/TSC2 complex, leading to activation of mTOR (10). On the other hand, 5′-AMP-activated protein kinase (AMPK), which is activated by increased AMP/ATP ratio and/or tumor suppressor LKB1, inhibits mTOR activation by activating TSC1/TSC2 (11). Activated mTOR C1 phosphorylates the translation inhibitor 4E-BP1 and the ribosomal protein S6 kinase (S6K), results in initiation of protein translation (6). p70 S6K also phosphorylates and inhibits insulin receptor substrate-1 (IRS-1), forms a negative feed back regulation of PI3K/Akt signaling (12).
The PI3K/Akt/mTOR pathway is also controlled by serine/threonine protein phosphatases. Two major classes of serine/threonine protein kinases, PP2A and PP1, are extensively involved in many signaling pathways. It has been well documented that PP2A interacts with and dephosphorylates Akt in vitro and in vivo (13-15). PP2A has also been reported to dephosphorylate S6K in response to different stimuli (16, 17). Likewise, 4E-BP1 has been identified as a substrate of PP2A in vivo and in vitro (18, 19). Currently no direct evidence proves that mTOR is dephosphorylated by PP2A. However, study using adenovirus implied that mTOR activity is regulated by PP2A (20); and mTOR is also involved in the regulation of PP2A activity (21). Compare to PP2A, PP1 is less involved in Akt/mTOR signaling, possibly due to the absence of PP1 recognition sequences and docking motifs in the major components of Akt/mTOR signaling (22). Besides PP1 and PP2A, PH domain leucine-rich repeat protein phosphatase 1 and 2 (PHLPP1/2) have been identified as specific Akt S473 phosphatases (23)
In many human tumors, particularly prostate cancers, PI3K/Akt/mTOR signaling is dysregulated by various oncogenic events (24). The hormone-refractory prostate cancers are frequently characterized by inactivation of PTEN and activation of Akt/mTOR signaling. Akt activity is an important determinant of the sensitivity of prostate cancer cells to therapies (25). Thus, inhibition of PI3K/Akt/mTOR signaling provides promising strategies of prevention and therapies for prostate cancer (26, 27).
Curcumin (Diferuloylmethane), a major chemical component of turmeric (curcuma longa), possess a broad spectrum of chemopreventive and therapeutic properties against various tumors in both in vitro and in vivo models and clinical trials (28, 29). Curcumin has been shown to inhibit cell proliferation, induce apoptosis, suppress inflammation, and sensitize tumor cells to cancer therapies (30-32). The mechanism(s) underlying the anti-cancer activity of curcumin has been extensively investigated, and several signaling pathways including NFκB, AP-1, mitogen-activated protein kinases (MAPKs), and cell cycle machinery have been suggested as the targets of curcumin (31). Recently it has been reported that curcumin inhibits Akt/mTOR signaling in various tumor cells including prostate cancer cells (33-36); however, the molecular mechanism by which curcumin inhibits Akt/mTOR signaling remains unclear.
In the present study we investigated the molecular mechanism by which curcumin inhibits Akt/mTOR signaling in the androgen-independent and PTEN-null PC-3 prostate cancer cells. Our results show that curcumin concentration- and time-dependently inhibits Akt/mTOR signaling, and this inhibitory effect is primarily mediated by curcumin-activated PP2A and/or unspecified calyculin A-sensitive protein phosphatase. At the same time, curcumin also activates AMPK and MAPKs, but these kinases are less involved in curcumin-mediated inhibition of Akt/mTOR signaling.
Material and Methods
Reagents, plasmids, and cell culture
Curcumin, PI3K inhibitor Ly294002, MEK1 inhibitor PD98059, JNK inhibitor II and p38 inhibitor SB238004 were purchased from Sigma (St. Louis, MO). L-α-Phosphatidylinositol-3, 4, 5-trisphosphate, Compound C and Tautomycetin were purchased from EMD Biosciences (San Diego, CA). Akt1/PKBα protein, active PDK1 protein, Ser/Thr Phosphatase Assay Kit and okadaic acid sodium salt were purchased from Upstate (Chicago, IL). MTS assay kit was obtained from Promega (Madison, WI). [6-3H] thymidine and L-[3, 4, 5-3H] leucine were obtained from Perkin Elmer (Boston, MA). Calyculin A, siRNA against tuberin/TSC2, control scrambled siRNA, cell lysis buffer (10X) and antibodies against p-PI3K p85 (T458)/p55 (T199), p-PDK1 (S241), p-Akt (T308), p-Akt (S473), Akt, p-FoxO1 (S256), p-GSK3β (S9), p-mTOR (S2448), p-mTOR (S2481), mTOR, p-p70 S6K (T389), p-S6 ribosomal protein (S235/236), p-4E-BP1 (T37/46), p-eIF4G (S1108), Tuberin/TSC2, p-Tuberin/TSC2 (T1462), p-AMPKα (T172), p-ACC (S79), methylated and non-methylated PP2A catalytic (PP2A C) subunit were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against HA tag, PDK1 (PKB kinase), β-actin, cyclin D1 and HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Lipofectamine 2000, recombinant protein G-conjugated agarose and all cell culture materials were purchased from Invitrogen (Carlsbad, CA). All the other chemicals were of the highest grade available.
HA-tagged Akt and AMPKα1 expressing plasmids were gifts from Dr. Kun-liang Guan (University of Michigan, Ann Arbor, MI); the constitutively activated Akt expressing plasmid (myr-HA-Akt) was a gift from Dr. Cory Abate-Shen (UMDNJ-Robert Wood Johnson Medical School, Piscataway, NJ). The dominant negative AMPKα1 was constructed by mutation of Threonine 172 to Alanine using QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and the mutation was confirmed by sequencing. Human prostate cancer PC-3 cells (ATCC, Manassas, VA) were cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum. TSC1 (-/-) and wild type MEFs were gifts from Dr. David J. Kwiatkowski (Harvard Medical School, Boston, MA) and Dr. Shengkan Victor Jin (UMDNJ-Robert Wood Johnson Medical School, Piscataway, NJ) and maintained in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% fetal bovine serum and 3.7 mg/ml sodium bicarbonate in a humidified 5% CO2 atmosphere at 37°C.
Cellular DNA synthesis, protein synthesis, and proliferation evaluations
For evaluation of DNA or protein synthesis, PC-3 cells were cultured in 24-well plates and treated with various concentrations of curcumin in FBS-free MEM medium for the indicated time. After that 1 μCi/well of [6-3H] thymidine or L-[3, 4, 5-3H] leucine were added into the cultures and incubated for 2 h. The cells were then fixed in 10% trichloroacetic acid (TCA) at room temperature for 15 min, and then washed twice with 5% TCA. The acid-insoluble material was dissolved in 2 M NaOH overnight, and then aliquots were used to determine the radioactivity using a liquid scintillation counter. For MTS cell proliferation assays, PC-3 cells were seeded in 96-well plates at a density of 5 × 103 cells/well, treated with various concentrations of curcumin for 24 h, then 20 μl of MTS reagent was added into each well and incubated for further 2 h. The optic density at 490 nm was read immediately using a μQuant microplate reader (Bio-Tek Instruments, Winooski, VT).
Transient transfection and Western blotting
Transient transfection was performed according to the protocol provided by the manufacturer, and all experiments were performed 24 hrs (48 hrs for siRNA experiments) after transfection. The cells as indicated were cultured in 6-well plates for 24 hrs followed by serum deprivation for 12 hrs, then treated with various concentrations of curcumin or chemicals in serum-free media for the indicated time. After treatment, the cells were washed with cold PBS and harvested in 1X cell lysis buffer supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN). Cell lysates were centrifuged at 4°C, 13,000 g for 10 min, and the protein concentrations in supernatants were determined by BCA protein assay (Pierce Biotechnology, Rockford, IL). Aliquots of lysates each containing 30 μg of protein were boiled in 1x SDS loading buffer and resolved by 4-15% SDS-polyacrylamide gel electrophoresis (PAGE). Proteins in gel were electro-transferred to PVDF membrane using a semi-dry transfer system. The membranes were blocked with 5% fat-free milk in phosphate-buffered saline-0.1% Tween 20 (PBST) at room temperature for 2 h, and then probed with specified primary antibodies (1:1000) in 3% bovine serum albumin in PBST overnight at 4°C. After that the blots were washed with PBST for 10 min three times, and then incubated with corresponding HRP-conjugated second antibodies (1:5000) at room temperature for 1 h. Then the blots were washed again in PBST for 10 min three times, and then were visualized by enhanced chemiluminiscence and scanned using a Gel Documentation 2000 system (Bio-Rad, Hercules, CA). Actin was blotted for each sample as loading control.
In vitro kinase assay
In vitro kinase assays were performed using either purified active PDK1 without first 52 amino acids (PDK1Δ52) or immunoprecipitated PDK1 from lysates of PC-3 cells. PC-3 cells were cultured in 10-cm dishes and treated with the indicated concentrations of curcumin for 10 min, then washed and harvested in cell lysis buffer as described above. Aliquots of lysates each containing 500 μg of proteins were pre-cleared by incubating with protein G-conjugated agarose at 4°C with agitation for 1 h, then incubated with anti-PDK1 antibody and protein G-conjugated agarose at 4°C overnight with agitation. The immunoprecipitated pellets were collected by centrifugation and washed three times with the lysis buffer, then washed twice with kinase assay buffer (25 mM Tris—HCl pH 7.5, 5 mM glycerol phosphate, 2 mM DTT, 0.1 mM Na3VO4 and 10 mM MgCl2) before using. 1 μg of purified Akt protein was incubated with either 50 ng PDK1Δ52 in the presence of the indicated concentrations of curcumin or immuno-precipitated pellets in kinase assay buffer with 1 mM ATP at 30°C for 20 min with agitation. Then the samples were boiled in 1x SDS sample loading buffer and immuno-blotted against p-Akt (T308) or PDK1.
Protein phosphatase assay
Serine/threonine phosphatase activity was determined using Malachite Green Phosphatase assay. PC-3 cells were cultured in 6-well plates and treated with various concentrations of curcumin for 10 min, and then the cells were scraped into phosphatase lysis buffer (20 mM HEPES pH7.4, 0.1% NP-40, 1mM EGTA, 30 mM β-mercaptoethanol, 0.1 mM MgCl2, 1 mM PMSF, and protease inhibitor cocktail) and sonicated on ice for three 10-sec pulses. The cell lysates were centrifuged at 2000 g at 4 °C for 5 min, and then aliquots of the supernatants were used for phosphatase assay. 5 μl of each cell lysate was diluted in 20 μl phosphatase assay buffer (50 mM Tris-HCl pH7.0, 0.1 mM EDTA), then phosphopeptide substrate K-R-pT-I-R-R was added into the mixture to a final concentration of 200 μM and incubated for 5 min. The reaction was terminated by adding 100 μl Malachite Green detection solution, 15 min later the optic density at 620nm was measured and corrected by subtracting the readings of the blank without cell lysate.
Statistical analysis
All experiments in this study were repeated at least 2 times with similar results. The values and relative percentages are presented as the mean ± SD of 4 separate samples. Statistical analysis was performed by the two-tailed Student’s t test for unpaired data, with p<0.05 considered statistically significant.
Results
Curcumin inhibited DNA/protein synthesis, cell proliferation, and Akt/mTOR signaling in PC-3 cells
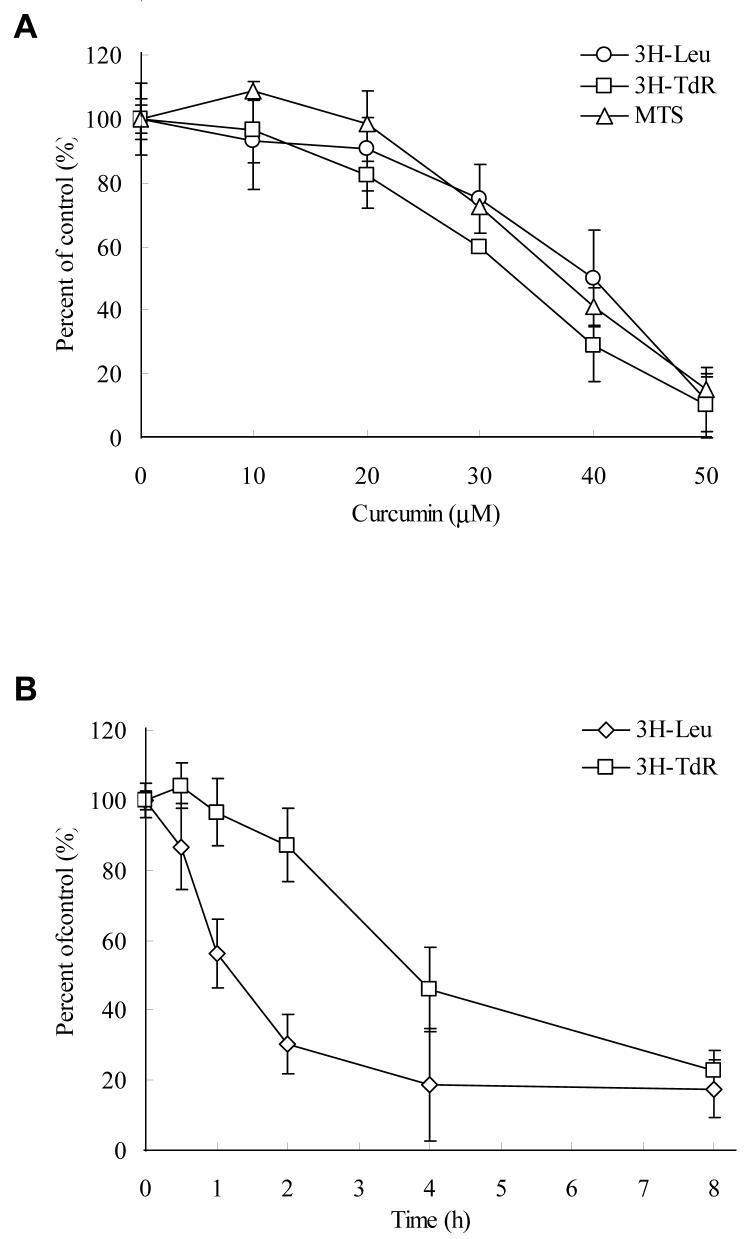
Since Akt/mTOR signaling controls protein translation and cell proliferation, we firstly determined the effects of curcumin on the DNA/protein synthesis of PC-3 cells. As indicated by 3H-TdR and 3H-Leu incorporation assays, curcumin inhibits DNA and protein synthesis in a similar concentration-dependent pattern to the inhibition of cell proliferation determined by MTS assay (fig. 1A). Moreover, the time course study indicates that the inhibition of protein synthesis occurred earlier than the inhibition of DNA synthesis (Fig. 1B).
Figure 1.
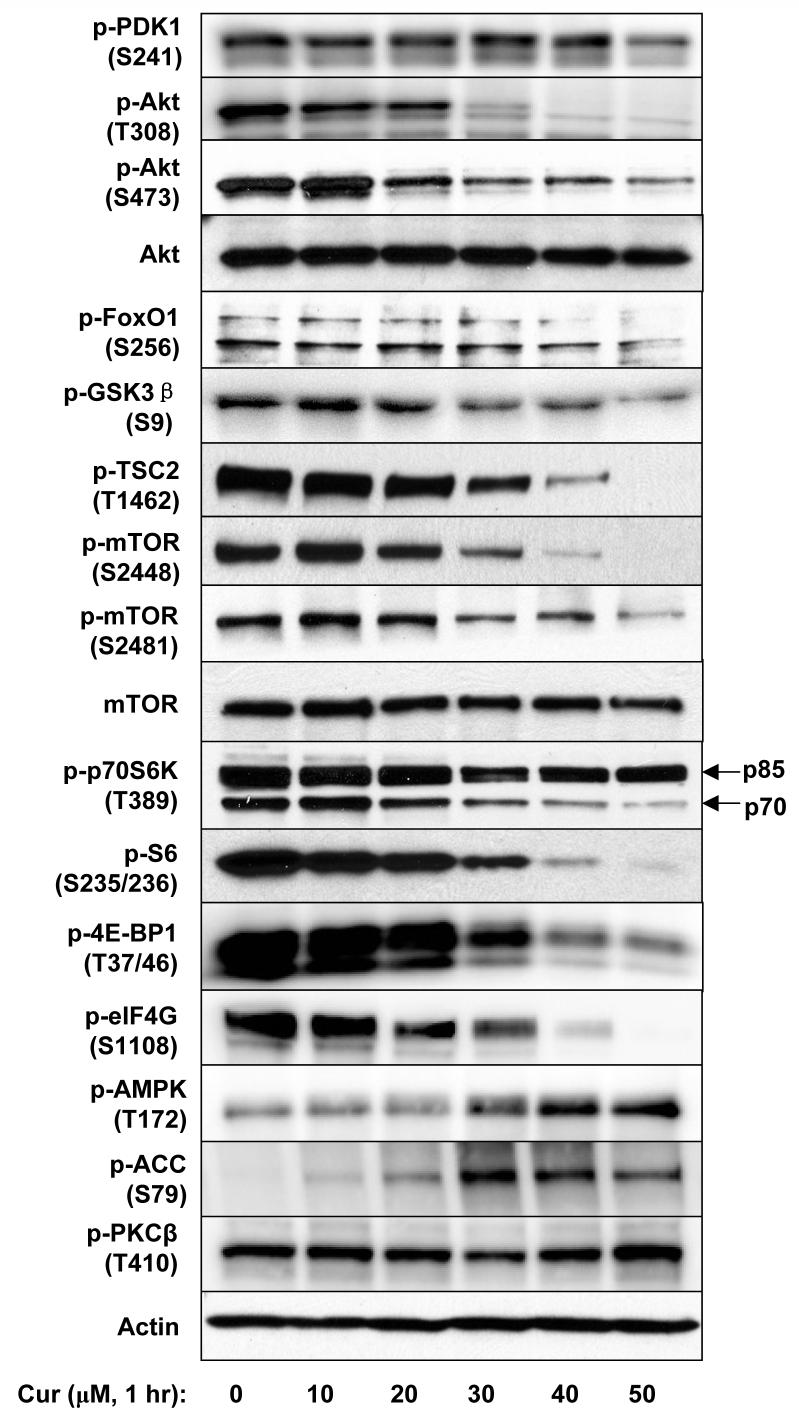
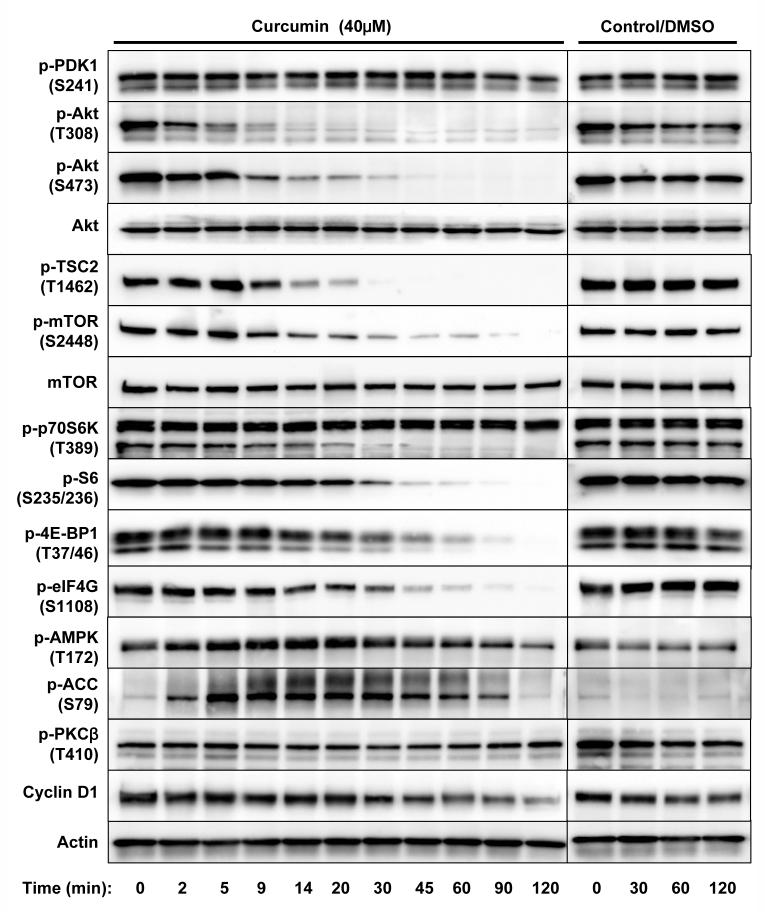
Curcumin concentration- and time-dependently inhibited cell proliferation, protein/DNA synthesis, and Akt/mTOR signaling in PC-3 cells. A, Cells were treated with various concentrations of curcumin in serum-free media for certain period then the cell viability (after 24 hrs), protein synthesis and DNA synthesis (after 8 hrs) were measured by MTS assay, 3H-Leu and 3H-TdR incorporation, respectively. B, Cells were treated with 50 μM of curcumin for indicated time, then the protein and DNA synthesis were measured by 3H-Leu and 3H-TdR incorporation, respectively. The results are presented as percentage of untreated control and each value is the mean ± SD of 4 parallel samples. C&D, Cells were treated with indicated concentrations of curcumin or DMSO (control) in serum-free media for indicated time, then harvested and immuno-blotted against indicated proteins using their phosphor-specific antibodies. Actin was blotted as a loading control. Please note that two isoforms of S6K were visualized but the p85 isoform was not affected by curcumin.
Next the effects of curcumin on the Akt/mTOR signaling were examined. PC-3 cells were treated with various concentrations of curcumin for 1 h, then harvested and analyzed by Western blotting. As shown in Fig.1C, curcumin inhibited the phosphorylation of Akt (T308 and S473), FoxO1 (S256), GSK3β (S9), tuberin/TSC2 (T1462), mTOR (S2448/2481), p70 S6K (T389), S6 (S235/236), 4E-BP1 (S37/46), eIF4G (S1108) in a similar concentration-dependent manner. At the same time, curcumin induced the phosphorylation of AMPKα and one of its substrates, Acetyl-CoA Carboxylase (ACC), indicating that AMPK was activated. MAPKs, including ERK1/2, JNK, and p38MAPK, were also activated by curcumin treatment (data not shown). However, the phosphorylation state of PDK1 and PKC remained unchanged.
In the following studies we focused on the Akt/mTOR signaling axis. When PC-3 cells were treated with 40 μM of curcumin, the phosphorylation of Akt at Thr308 was promptly inhibited within 5 min, followed by inhibition of phosphorylation of mTOR, Akt at Ser473, and then the other downstream targets including 4E-BP1, eIF4G, p70 S6K and S6 (Fig. 1D). In all experiments the total Akt, mTOR, 4E-BP1, p70 S6K, and S6 were also blotted and showed no significant change. Moreover, the expression of cyclin D1 was also inhibited after 1 hr of curcumin treatment (Fig. 1D), similar as reported in (32).
Curcumin acted at downstream of PI3K/PDK1
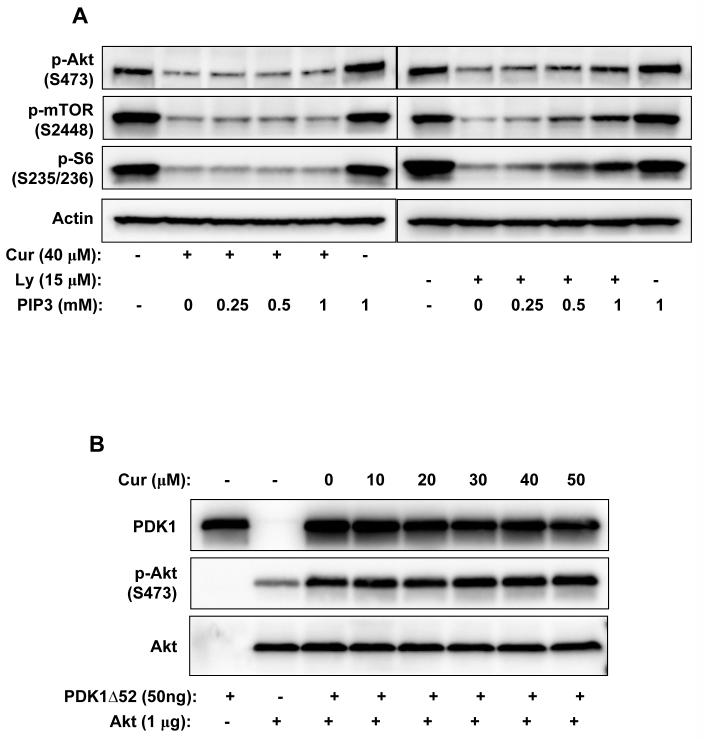
PI3K catalyzes the production of PIP3, thus activates downstream signaling including Akt/mTOR. The activity of PI3K is controlled by the binding of regulatory subunits (p85/p55/p101) to catalytic subunits (p110) and a series of phosphorylation events (37). In our experiments the phosphorylated p85/p55 was barely detectable and no change in its phosphorylation state upon curcumin treatment was observed (data not shown). The phosphorylation of PDK1 at Ser241 on the activation loop, which is necessary for PDK1 activity, was also not altered by curcumin treatment at the tested concentrations and time points (Fig. 1C&D). We further checked the effect of PIP3 on curcumin-mediated inhibition. Addition of exogenous PIP3 effectively rescued the inhibitory effects of specific PI3K inhibitor LY294002 on the downstream signaling; however, it had no effect on the curcumin-induced inhibition (Fig. 2A).
Figure 2.
A, PIP3 rescued PI3K inhibitor LY294002-mediated but not curcumin-mediated inhibition of Akt/mTOR signaling in PC-3 cells. Cells were incubated with 40μM of curcumin or 15μM of LY294002 in the presence of increasing concentrations of PIP3 for 1 hr, then harvested and the indicated proteins were blotted. B, Curcumin did not inhibit PDK1 activity towards Akt. 1 μg of purified His-tagged Akt1 protein was incubated with 50 ng of purified active PDK1 (PDK1Δ52) in the presence of indicated concentrations of curcumin in kinase assay buffer/1 mM ATP for 20 min, then phosphor-Akt T308 and PDK1 were immuno-blotted.
Since the phosphorylation of Akt at T308, which is catalyzed by PDK1, was the first one to be inhibited, we speculated that curcumin might directly inhibit PDK1 activity towards Akt. To test this hypothesis, the effect of curcumin on PDK1 activity was examined using purified His-tagged Akt1 as substrate. Purified active PDK1 without the first 52 amino acids (PDK1Δ52, Fig. 2B) or endogenous PDK1 immuno-precipitated from curcumin-treated PC-3 cells (data not shown) was used for in vitro kinase assay. However, curcumin failed to inhibit PDK1 activity both in vitro and in vivo. Moreover, the phosphorylation of PKC, which is catalyzed by PDK1, was not significantly changed by curcumin treatment (Fig. 1C&D), indicating that PDK1 is not the direct target of curcumin.
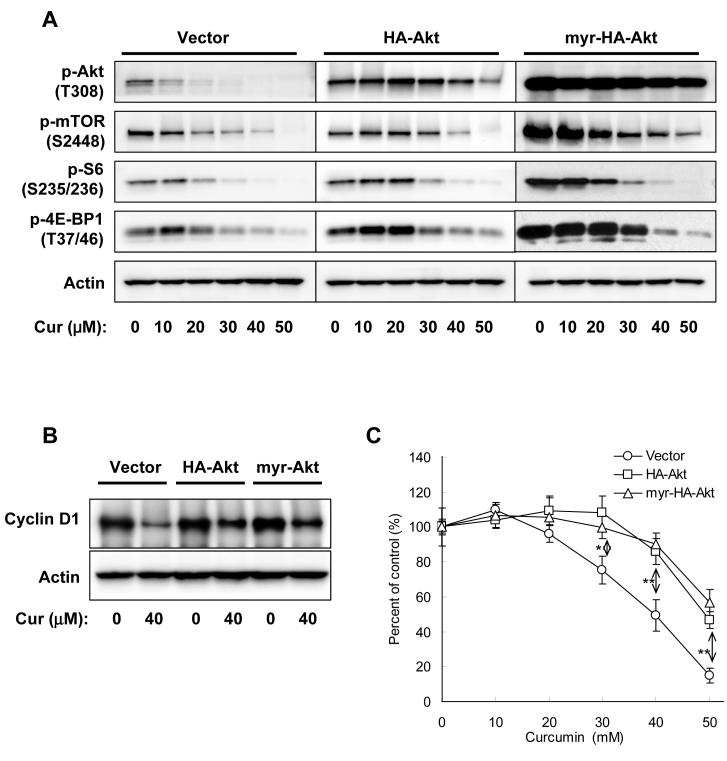
Overexpression of Akt or constitutively-activated Akt only partially restored curcumin-mediated inhibition
To assess the role of Akt in curcumin-mediated inhibition of mTOR signaling and cell proliferation, PC-3 cells were transiently transfected with plasmids encoding HA-Akt, myr-HA-Akt or empty vector. The transfected cells were treated with various concentrations of curcumin, and then the phosphorylated protein levels and cell proliferation were analyzed by Western blotting and 3H-thymidine incorporation assay. Overexpression of Akt significantly restored curcumin-mediated inhibition of Akt phosphorylation, but showed less effect on the inhibition of the phosphorylation of mTOR, 4E-BP1 and S6. Overexpression of myr-HA-Akt, which is anchored at the cell membrane by the myr group and thus constitutively activated by PDK1, resulted in highly phosphorylated Akt which could not be inhibited by curcumin, and augmented the basal phosphorylation of mTOR, 4E-BP1, and S6; but surprisingly, the phosphorylation of mTOR, 4E-BP1 and S6 was still significantly inhibited by curcumin (Fig. 3A). Similarly, overexpression of HA-Akt or myr-HA-Akt partially but significantly restored cyclin D1 level and the proliferation of PC-3 cells treated with curcumin (Fig. 3B&C). These results suggest that the inhibition of Akt phosphorylation partially contributed to curcumin-mediated inhibition of mTOR signaling and cell proliferation, but is unlikely to be the primary mechanism targeted by curcumin.
Figure 3.
Overexpression of HA-Akt or myr-HA-Akt only partially rescued curcumin-mediated inhibition in PC-3 cells. A, Cells were transfected with equal amount of indicated plasmids for 24 hrs, serum-starved for 12 hrs, then treated with various concentrations of curcumin in serum-free media for 1 hr, and harvested and blotted against indicated proteins. B, Cells were transfected with equal amount of indicated plasmids for 24 hrs, then treated with 40 μM of curcumin in serum-free media for 4 hrs, and harvested and blotted against cyclin D1 or actin. C, Cells were transfected with equal amount of indicated plasmids for 24 hrs, and then treated with various concentrations of curcumin in serum-free media for 24 hrs, and the cell viability was determined by MTS assay. The results are presented as percentage of untreated control and each value is the mean ± SD of 4 parallel samples. The difference between vector and HA-Akt or myr-HA-Akt is verified by t test. *, p < 0.05; **, p < 0.01.
AMPK and MAPKs were activated by curcumin but not responsible for curcumin-mediated inhibition of Akt/mTOR signaling
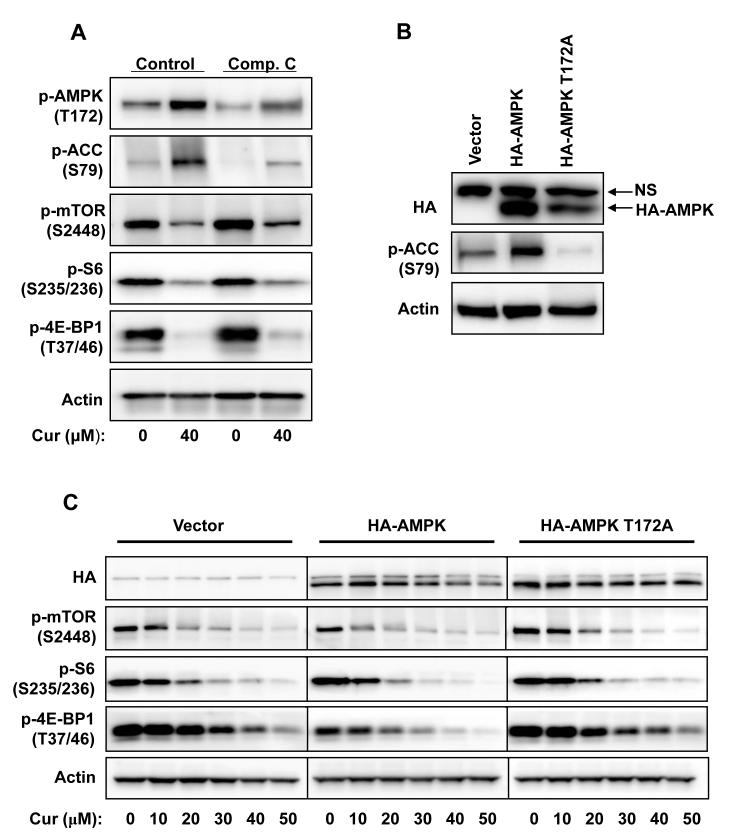
AMPK is a negative upstream regulator of mTOR (11). Indeed, we found that curcumin induced a prompt and robust phosphorylation of AMPKα at Thr172, which is required for AMPK activation. Concurrently, ACC, a substrate of AMPK, was also phosphorylated upon curcumin treatment (Fig. 1C&D). To assess the involvement of AMPK in curcumin-mediated inhibition of mTOR signaling, we firstly tested the effect of an AMPK inhibitor, compound C. As shown in Fig. 4A, pretreating the cells with Compound C inhibited the phosphorylation of ACC and AMPK; however, it showed no effect on curcumin-mediated inhibition of mTOR signaling. Then the Thr172 of AMPKα1 was mutated to Ala to construct a dominant negative form of AMPK (38), and the inhibition of cellular AMPK activity by overexpression of the AMPKα1/T172A in PC-3 cells was confirmed by inhibition of the phosphorylation of ACC (Fig. 4B). Overexpression of AMPKα1 slightly potentiated the inhibitory effect of curcumin on mTOR signaling, as indicated by decreased phosphorylation of mTOR, 4E-BP1 and S6. Nevertheless, curcumin-mediated inhibition remained unaffected (Fig. 4C). These results indicate that activation of AMPK by curcumin is not the main reason for curcumin-mediated inhibition of mTOR signaling.
Figure 4.
Activation of AMPK is not the major reason for curcumin-mediated inhibition of mTOR signaling in PC-3 cells. A, Cells were pretreated with 10 μM of compound C for 15 min then treated with 40μM of curcumin in the presence of compound C for 1 hr, then harvested and blotted against indicated proteins. B, Cells were transfected with indicated plasmids for 24 hrs, then harvested and blotted against HA, phosphor-ACC and actin. NS: Non-specific band. C, Cells were transfected with indicated plasmids for 24 hrs, serum-starved for 12 hrs and then treated with various concentrations of curcumin in serum-free media for 1 hr, and harvested and blotted against indicated proteins.
Curcumin also activated Erk1/2, JNK, and p38 in PC-3 cells. Yet again, specific inhibitors against the activated MAPK pathways had no effect on curcumin-mediated inhibition of mTOR signaling (data not shown).
Disruption of TSC1/TSC2 complex only marginally restored curcumin-mediated inhibition of mTOR signaling
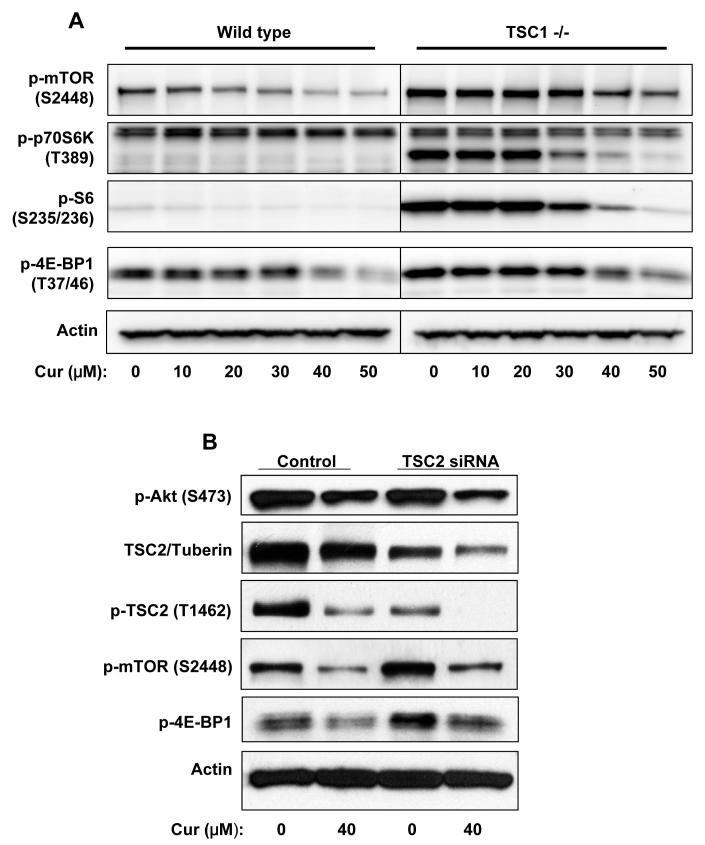
Both Akt and AMPK regulate mTOR signaling through TSC1-TSC2 complex (39). Here we checked the possible role of TSC1-TSC2 in curcumin-mediated inhibition by using TSC1 knockout MEFs or siRNA against TSC2/tuberin. TSC1(-/-) MEFs displayed remarkably elevated phosphorylation of mTOR, p70 S6K, S6, and 4E-BP1 compared to wild type MEFs. However, incubation of TSC1 (-/-) MEFs with curcumin still effectively inhibited the phosphorylation of mTOR, p70 S6K, S6, and 4E-BP1, although to a less extent due to higher basal levels (Fig. 5A). Moreover, transfection of TSC2/tuberin siRNA into PC-3 cells inhibited the expression of tuberin, mildly increased the basal phosphorylation level and only marginally counteracted curcumin-mediated inhibition (Fig. 5B), while showed no effect on the basal level or curcumin-mediated inhibition of the phosphorylation of Akt. These results suggest the existence of inhibitory mechanism(s) of mTOR signaling independent of tuberin/hamartin complex, it is to say, independent of the inhibition of Akt or the activation of AMPK.
Figure 5.
Disruption of TSC1-TSC2 complex failed to rescue curcumin-mediated inhibition of mTOR signaling. A, Wild type or TSC1 (-/-) MEFs were serum-starved for 12 hrs then incubated with various concentrations of curcumin in serum-free media for 1 hr, and harvested and blotted against indicated proteins. B, PC-3 cells were transfected with siRNA against TSC2/tuberin or scrambled control RNA for 48hrs, serum-starved for 12hrs, then treated with 40 μM of curcumin in serum-free media for 1 hr, and harvested and blotted against proteins indicated.
Curcumin-mediated inhibition of Akt/mTOR signaling is dependent on calyculin A-sensitive protein phosphatase activity
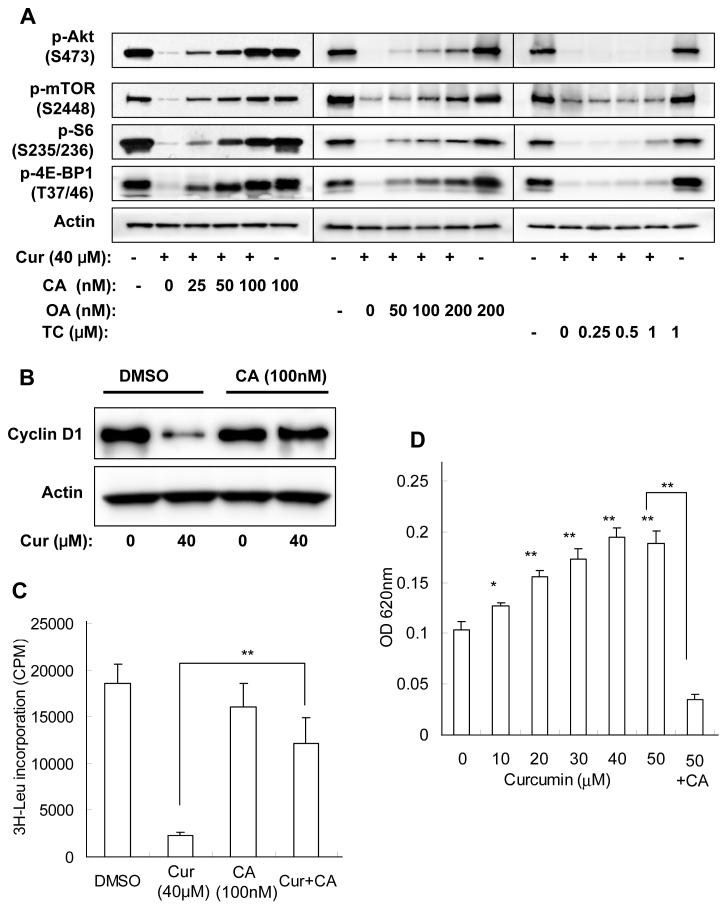
To explore the involvement of protein phosphatases in curcumin-mediated inhibition of Akt/mTOR signaling, we used three pharmacological inhibitors to inhibit different phosphatases. Calyculin A is a potent protein serine/threonine phosphatase inhibitor which inhibits both PP1 and PP2A, while okadaic acid potently inhibits PP2A but have less effect on PP1, and tautomycin preferentially inhibits PP1 activity. Treatment of PC-3 cells with calyculin A or okadaic acid induced a slight increase of basal phosphorylation level. Notably, pretreatment with calyculin A concentration-dependently reversed curcumin-mediated inhibition of the phosphorylation of Akt, mTOR, S6, and 4E-BP1, with 100 nM of calyculin A completely blocked curcumin-mediated inhibition. Okadaic acid showed a similar but much weaker effect compared to calyculin A. On the other hand, tautomycin had no effect on curcumin-mediated inhibition of Akt/mTOR signaling even at a concentration of 1 μM (Fig. 6A). The effects of calyculin A on curcumin-mediated inhibition of cyclin D1 and cell proliferation were also determined. As shown in Fig. 6B, calyculin A fully reversed the inhibition of cyclin D1 expression by curcumin. 3H-leucine incorporation assay was used for proliferation assay since MTS or 3H-TdR assays require longer treatment but prolonged incubation with calyculin A lead to cell detachment and death. Although 100 nM of calyculin A itself slightly inhibited 3H-leucine incorporation, pretreatment with calyculin A remarkably reversed curcumin-mediated inhibition (Fig. 6C). The data suggest that curcumin inhibits Akt/mTOR signaling through calyculin A-sensitive protein phosphatase(s), and restoration of Akt/mTOR phosphorylation by calyculin A reversed curcumin’s anti-proliferative effects.
Figure 6.
Curcumin-mediated inhibition of Akt/mTOR signaling in PC-3 cells is dependent on PP2A and/or unspecified calyculin A-sensitive protein phosphatase activity. A, Cells were pretreated with indicated concentrations of protein phosphatase inhibitors for 15 min, then incubated with 40 μM of curcumin in the presence of protein phosphatase inhibitors in serum-free media for 1 hr, and harvested and blotted against indicated proteins. B&C, Cells were pretreated with 100 nM of calyculin A for15 min, then incubated with 40 μM of curcumin in the presence of calyculin A for 4 hrs, and then (B) cells were harvested and blotted against cyclin D1 and actin or (C) 3H-Leu incorporation was determined as described in Material and Methods. D, Cells were treated with indicated concentrations of curcumin for 10 min, then harvested in phosphatase lysis buffer and the protein phosphatase activities in lysates were determined as described in Material and Methods. The results are expressed as mean ± SD of 4 parallel samples. *, p < 0.05; **, p < 0.01.
PP2A core enzyme consists of catalytic C and regulatory A subunits, and the C subunits is targeted to reversible methylation that regulates PP2A activity (40). However, incubation of PC-3 cells with curcumin changed neither the protein level nor the methylation state of PP2A C subunit (data not shown). Next the cellular protein phosphatase activity upon curcumin treatment was determined by Malachite Green Phosphatase assay. As shown in Fig. 6D, incubation of PC-3 cells with curcumin for 10 min concentration-dependently increased the protein phosphatase activity in the cell extract, and this curcumin-stimulated activity could be inhibited by calyculin A. Taken together, these data indicate that incubation with curcumin activated PP2A and/or unspecified calyculin A-sensitive protein phosphatase(s), and led to dephosphorylation of Akt, mTOR, and their downstream substrates.
Discussion
Curcumin has been shown to inhibit the phosphorylation and activation of Akt in PC-3 cells (41); however, the effects of curcumin on the downstream signaling of Akt have not been explored. In the present study we firstly demonstrated that curcumin also inhibited the phosphorylation of Akt substrates GSK3, FKHR1, TSC2, mTOR as well as mTOR downstream targets 4E-BP1, eIF4G, p70 S6K and S6 in a similar concentration-dependent manner as with Akt (Fig. 1C). In support of the role of Akt/mTOR signaling in the control of protein synthesis, curcumin inhibited protein synthesis and then DNA synthesis in PC-3 cells (Fig. 1A&B), and these inhibitions could be partially but significantly rescued by overexpression of Akt or by restoration of Akt/mTOR signaling by calyculin A (Fig. 3B and Fig. 6C). Cyclin D1, which is critical for cell proliferation, has been reported to be regulated by Akt/mTOR post-transcriptionally (32). In PC-3 cells the expression of cyclin D1 was also inhibited by curcumin and can be restored by overexpression of Akt or by calyculin A (Fig. 1D, Fig. 3A and Fig. 6B). These results are consistent with the important roles of Akt/mTOR signaling in cell survival and proliferation.
Curcumin has been reported to inhibit Akt/mTOR signaling in other cancer cells (33), but the underlying mechanism remains unknown. One major objective of this study is to delineate the molecular mechanism by which curcumin inhibits Akt/mTOR signaling. Firstly we examined the effect of curcumin on the p85 subunit of PI3K. The phosphorylation of p85 in PC-3 cells is barely detectable and was not affected by curcumin treatment (data not shown). LY294002, a specific PI3K inhibitor, inhibited the phosphorylation of Akt and mTOR, and this inhibition could be restored by addition of exogenous PIP3. In contrast, exogenous PIP3 failed to restore curcumin-mediated inhibition (Fig. 2A). Moreover, it has been well documented that in many cancer cells including PC-3 cells, the activation of Akt/mTOR signaling axis is less dependent on upstream signals due to loss of PTEN function (25). Actually, as reported by others (33, 42) and confirmed in our lab, curcumin also inhibited Akt/mTOR signaling and proliferation in DU145 prostate cancer cells which carry wt PTEN. Taken together, these evidences suggest that curcumin inhibits Akt/mTOR signaling at downstream of PI3K.
As shown in fig. 1D, the phosphorylation of Akt at Thr308 was the first to be inhibited. This led to the hypothesis that curcumin could directly inhibit PDK1-mediated phosphorylation of Akt and led to the inhibition of downstream signaling. Phosphorylation of PDK1 at Ser241 is necessary for its activity, though may not be the major regulatory factor (43). However, curcumin did not inhibit the phosphorylation of PDK1 S241 (Fig. 1C&D). Moreover, curcumin failed to inhibit the kinase activity of PDK1 to Akt both in vitro and in vivo (Fig. 2B and data not shown), suggesting that PDK1 is not the direct target of curcumin. Similar observations have been reported that Akt/mTOR signaling can be inhibited independent of PI3K/PDK1 (19).
Next we examined the role of Akt in curcumin-mediated inhibition. Overexpression of Akt or the constitutively activated myr-Akt increased the basal level of phosphorylated Akt, mTOR and downstream molecules. However, curcumin still effectively inhibited mTOR and downstream signaling, though to a less extent which is possibly due to the increased basal phosphorylation level (Fig. 3A). These results, especially curcumin inhibited Akt downstream signaling even though the phosphorylation of myr-Akt was not inhibited at all, strongly suggest the existence of inhibitory mechanism which is independent of inhibition of Akt.
Coincidentally, AMPK was activated by curcumin in a time course comparable to the inhibition of Akt phosphorylation (Fig. 1C&D). Overexpression of AMPK in PC-3 cells slightly potentiated the inhibition of mTOR signaling by curcumin, but neither pharmacological inhibitor nor dominant negative overexpression showed significant restoration of curcumin-mediated inhibition (Fig. 4). Although curcumin-activated AMPK is not the major reason for curcumin-mediated inhibition of Akt/mTOR signaling, how curcumin activates AMPK and its physiological significance deserve further investigation in the future.
TSC1/TSC2 complex inhibits mTOR activity by activating the GTPase activity of Rheb, and both Akt and AMPK converged at TSC1/TSC2 to regulate mTOR activity (39). Consistent with the incompetence of constitutive activation of Akt or inhibition of AMPK to rescue mTOR signaling, disruption of the function of TSC1/TSC2 complex only marginally rescued curcumin-mediated inhibition (Fig. 5). Knockout of TSC1 in MEFs led to hyperphosphorylation of mTOR, 4E-BP1, p70 S6K, and S6; nonetheless, curcumin effectively inhibited the phosphorylation with a similar concentration-dependency to that in wild type MEFs (Fig. 5A). It is notable that curcumin effectively inhibited mTOR signaling in the non-cancerous MEFs, though to a less extent than in PC-3 cells, suggesting curcumin-mediated inhibition of Akt/mTOR signaling is independent on PTEN status. Likewise, knockdown of TSC2 in PC-3 cells by siRNA mildly increased the basal phosphorylation level of mTOR and 4E-BP1, but the phosphorylation could still be inhibited by curcumin (Fig. 5B).
Multiple feed back loops exist in the regulation of Akt/mTOR signaling. Importantly, p70 S6K phosphorylates and inhibits IRS-1, resulting in a negative feed back to Akt/mTOR signaling (12). By this mechanism, inhibition of mTOR signaling often leads to activation of Akt and tumor cells could gain resistance to mTOR inhibitors (44). However, in PC-3 cells curcumin inhibited both Akt and mTOR similarly (Fig. 1C). Furthermore, the inhibition of Akt phosphorylation at Thr308 occurred much earlier than the inhibition of phosphorylation of Akt at Ser473, mTOR and other downstream components (Fig. 1D). Based on these observations, it is unlikely that curcumin inhibited Akt/mTOR axis by directly inhibiting mTOR.
MAPKs, especially p38, have been reported to be involved in the inhibition of Akt signaling (13). Curcumin activated Erk1/2, JNK, and p38 in PC-3 cells, but the involvement of MAPKs in the inhibition of Akt/mTOR signaling by curcumin was ruled out by the failure of specific inhibitors to restore Akt/mTOR phosphorylation (data not shown).
Having excluded the inhibition/activation of upstream kinases from the major inhibitory mechanism, we turned to explore the possible involvement of protein phosphatases, specifically serine/threonine protein phosphatase since the phosphorylation and dephosphorylation that regulates the components of Akt/mTOR signaling pathway mainly occur at threonine or serine. PP1 and PP2A account for the majority of serine/threonine protein phosphatase activity in most cells (45). The PP1 inhibitor tautomycin exhibited only a very weak restoration of Akt/mTOR phosphorylation at a concentration much higher than that required for inhibition of PP1 (Fig. 6A). On the other hand, calyculin A fully reversed curcumin-mediated dephosphorylation of Akt, mTOR, S6, and 4E-BP1. Similar result was observed for the expression of cyclin D1 (fig. 6B). Moreover, calyculin A effectively rescued the curcumin-mediated inhibition of 3H-leucine incorporation in PC-3 cells (fig. 6C). The effect of okadaic acid was less potent but still significant, suggesting that curcumin-mediated inhibition of Akt/mTOR signaling and cell proliferation is dependent on PP2A and/or unspecified calyculin A-sensitive protein phosphatases.
Curcumin has been found to activate Src homology 2 domain-containing tyrosine phosphatase 2 (SHP2) in brain microglia (46). In another study, curcumin was shown to up-regulate MKP5 to repress inflammatory responses in prostate cells (47). Here we found that curcumin also activated serine/threonine protein phosphatase activity in PC-3 cells (Fig. 6D). The activities of protein phosphatases are subjected to multiple levels of regulation, however, the exact mechanisms is still largely unknown (48). As an example, PP2A holoenzyme, which has a diversity of substrates, is composed of a core heterodimmer of catalytic (C) and scaffold (A) subunits and a wide variety of regulatory (B) subunits. The specific activities against certain substrates are regulated by different combinations of subunits and their phosphorylation or methylation status (14, 49). Curcumin showed no significant effect on the methylation status of C subunit (data not shown); however, it did activate serine/threnione protein phosphatases activity in PC-3 cells.
Contrasting to more than 300 serine/threonine kinases in the human genome, only less than 30 serine/threonine phosphatases were identified to the date (48), and new protein phosphatases are being identified (23). Our experimental results support the involvement of PP2A and/or unspecified calyculin A-sensitive protein phosphatases in curcumin-mediated inhibition of Akt/mTOR signaling and proliferation; however, further investigation is required to identify the specific phosphatases activated by curcumin.
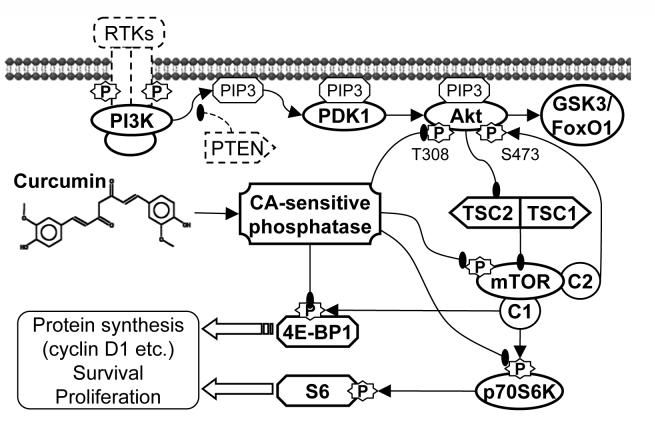
As summarized in fig. 7, Curcumin activated PP2A or unspecified calyculin A-sensitive protein phosphatase activity towards Akt, mTOR and possible their downstream molecules, leading to the inhibition of Akt/mTOR signaling and the expression of proliferation-essential proteins such as cyclin D1, finally inhibited the cell survival and proliferation. Our study systematically dissected the effects of curcumin on the Akt/mTOR signaling in PC-3 cells, revealed the importance of Akt/mTOR inhibition for the anti-proliferative activity of curcumin, and shed new light on the mechanisms of curcumin’s anti-cancer activities.
Figure 7.
Summary of the mechanisms by which curcumin inhibits Akt/mTOR signaling and cell survival/proliferation in PC-3 prostate cancer cells. Curcumin activated PP2A and/or unspecified calyculin A-sensitive protein phosphatase activities towards Akt, mTOR, led to the dephosphorylation of Akt/mTOR and their downstream substrates GSK3, FoxO1, p70S6K and 4E-BP1, and finally inhibited the expression of proteins that are essential for cell survival and proliferation. Curcumin also activated MAPKs and AMPK; however these kinases did not play important roles in the curcumin-mediated inhibition of Akt/mTOR signaling and cell proliferation.
Acknowledgement
This work was supported by National Institutes of Health Grant R01 CA118947 (to A.-N. T. K.). We gratefully thank Dr. Kun-liang Guan for HA-Akt and AMPK expressing plasmids, Dr. Cory Abate-Shen for myr-HA-Akt expressing plasmids, and Dr. David J. Kwiatkowski and Dr. Shengkan Victor Jin for the TSC1 (-/-) MEFs.
Abbreviations
- PI3K
Phosphatidylinositol 3-kinase
- mTOR
mammalian target of rapamycin
- PDK1
phosphotidylinositol-dependent kinase-1
- GSK3
glycogen synthase kinase 3
- PTEN
phosphatase and tensin homolog deleted on chromosome ten
- TSC1 and TSC2
Tuberous sclerosis 1 and 2
- AMPK
5′-AMP-activated protein kinase
- IRS-1
insulin receptor substrate-1
- PP2A and PP1
protein phosphatase 2A and 1
- MAPKs
mitogen-activated protein kinases
Footnotes
This work was supported by National Institutes of Health Grant R01 CA118947 (to A.-N. T. Kong).
The authors claim no conflict of interest.
References
- 1.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 2.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 3.Belham C, Wu S, Avruch J. Intracellular signalling: PDK1--a kinase at the hub of things. Curr Biol. 1999;9:R93–6. doi: 10.1016/s0960-9822(99)80058-x. [DOI] [PubMed] [Google Scholar]
- 4.Toker A, Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res. 2006;66:3963–6. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- 5.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–92. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 6.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–22. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 7.Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344(Pt 2):427–31. [PMC free article] [PubMed] [Google Scholar]
- 8.Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem. 2005;280:26089–93. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- 9.Peterson RT, Beal PA, Comb MJ, Schreiber SL. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem. 2000;275:7416–23. doi: 10.1074/jbc.275.10.7416. [DOI] [PubMed] [Google Scholar]
- 10.Dan HC, Sun M, Yang L, et al. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364–70. doi: 10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- 11.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuluaga S, Alvarez-Barrientos A, Gutierrez-Uzquiza A, Benito M, Nebreda AR, Porras A. Negative regulation of Akt activity by p38alpha MAP kinase in cardiomyocytes involves membrane localization of PP2A through interaction with caveolin-1. Cell Signal. 2007;19:62–74. doi: 10.1016/j.cellsig.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Van Kanegan MJ, Adams DG, Wadzinski BE, Strack S. Distinct protein phosphatase 2A heterotrimers modulate growth factor signaling to extracellular signal-regulated kinases and Akt. J Biol Chem. 2005;280:36029–36. doi: 10.1074/jbc.M506986200. [DOI] [PubMed] [Google Scholar]
- 15.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA. 2000;97:10832–7. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petritsch C, Beug H, Balmain A, Oft M. TGF-beta inhibits p70 S6 kinase via protein phosphatase 2A to induce G(1) arrest. Genes Dev. 2000;14:3093–101. doi: 10.1101/gad.854200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc Natl Acad Sci USA. 1999;96:4438–42. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanahoshi M, Nishiuma T, Tsujishita Y, et al. Regulation of protein phosphatase 2A catalytic activity by alpha4 protein and its yeast homolog Tap42. Biochem Biophys Res Commun. 1998;251:520–6. doi: 10.1006/bbrc.1998.9493. [DOI] [PubMed] [Google Scholar]
- 19.Guan L, Song K, Pysz MA, et al. Protein Kinase C-mediated Down-regulation of Cyclin D1 Involves Activation of the Translational Repressor 4E-BP1 via a Phosphoinositide 3-Kinase/Akt-independent, Protein Phosphatase 2A-dependent Mechanism in Intestinal Epithelial Cells. J Biol Chem. 2007;282:14213–25. doi: 10.1074/jbc.M610513200. [DOI] [PubMed] [Google Scholar]
- 20.O’Shea C, Klupsch K, Choi S, et al. Adenoviral proteins mimic nutrient/growth signals to activate the mTOR pathway for viral replication. Embo J. 2005;24:1211–21. doi: 10.1038/sj.emboj.7600597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nien WL, Dauphinee SM, Moffat LD, Too CK. Overexpression of the mTOR alpha4 phosphoprotein activates protein phosphatase 2A and increases Stat1alpha binding to PIAS1. Mol Cell Endocrinol. 2007;263:10–7. doi: 10.1016/j.mce.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Meiselbach H, Sticht H, Enz R. Structural analysis of the protein phosphatase 1 docking motif: molecular description of binding specificities identifies interacting proteins. Chem Biol. 2006;13:49–59. doi: 10.1016/j.chembiol.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–31. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 25.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–74. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 26.Grunwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002;62:6141–5. [PubMed] [Google Scholar]
- 27.deGraffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin Cancer Res. 2004;10:8059–67. doi: 10.1158/1078-0432.CCR-04-0035. [DOI] [PubMed] [Google Scholar]
- 28.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–68. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Khor TO, Keum YS, Lin W, et al. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res. 2006;66:613–21. doi: 10.1158/0008-5472.CAN-05-2708. [DOI] [PubMed] [Google Scholar]
- 30.Karunagaran D, Rashmi R, Kumar TR. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets. 2005;5:117–29. doi: 10.2174/1568009053202081. [DOI] [PubMed] [Google Scholar]
- 31.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8:E443–9. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1) Cell Cycle. 2007;6:2953–61. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- 33.Beevers CS, Li F, Liu L, Huang S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int J Cancer. 2006;119:757–64. doi: 10.1002/ijc.21932. [DOI] [PubMed] [Google Scholar]
- 34.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal B, Kondo Y. Evidence That Curcumin Suppresses the Growth of Malignant Gliomas In Vitro and In Vivo Through Induction of Autophagy: Role of Akt and ERK Signaling Pathways. Mol Pharmacol. 2007 doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–96. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Xu C, Keum YS, Reddy B, Conney A, Kong AN. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with beta-phenylethyl isothiocyanate and curcumin. Carcinogenesis. 2006;27:475–82. doi: 10.1093/carcin/bgi272. [DOI] [PubMed] [Google Scholar]
- 37.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–7. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 38.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–8. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwiatkowski DJ, Manning BD.Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways Hum Mol Genet 200514 Spec No. 2:R251–8. [DOI] [PubMed] [Google Scholar]
- 40.Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol. 2000;60:1225–35. doi: 10.1016/s0006-2952(00)00424-x. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhary LR, Hruska KA. Inhibition of cell survival signal protein kinase B/Akt by curcumin in human prostate cancer cells. J Cell Biochem. 2003;89:1–5. doi: 10.1002/jcb.10495. [DOI] [PubMed] [Google Scholar]
- 42.Deeb D, Jiang H, Gao X, et al. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J Pharmacol Exp Ther. 2007;321:616–25. doi: 10.1124/jpet.106.117721. [DOI] [PubMed] [Google Scholar]
- 43.Currie RA, Walker KS, Gray A, et al. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J. 1999;337(Pt 3):575–83. [PMC free article] [PubMed] [Google Scholar]
- 44.Kurmasheva RT, Huang S, Houghton PJ. Predicted mechanisms of resistance to mTOR inhibitors. Br J Cancer. 2006;95:955–60. doi: 10.1038/sj.bjc.6603353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Depaoli-Roach AA, Park IK, Cerovsky V, et al. Serine/threonine protein phosphatases in the control of cell function. Adv Enzyme Regul. 1994;34:199–224. doi: 10.1016/0065-2571(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 46.Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171:6072–9. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- 47.Nonn L, Duong D, Peehl DM. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis. 2007;28:1188–96. doi: 10.1093/carcin/bgl241. [DOI] [PubMed] [Google Scholar]
- 48.Goldberg Y. Protein phosphatase 2A: who shall regulate the regulator? Biochem Pharmacol. 1999;57:321–8. doi: 10.1016/s0006-2952(98)00245-7. [DOI] [PubMed] [Google Scholar]
- 49.Schonthal AH. Role of PP2A in intracellular signal transduction pathways. Front Biosci. 1998;3:D1262–73. doi: 10.2741/A361. [DOI] [PubMed] [Google Scholar]