Summary
Among sensory systems, the somatic sense is exceptional in its ability to detect a wide range of chemical, mechanical, and thermal stimuli. How this sensory diversity is established during development remains largely elusive. We devised a method (BAPTISM) that uses the photoconvertible fluorescent protein Kaede to simultaneously analyze birthdate and cell fate in live zebrafish embryos. We found that trigeminal sensory ganglia are formed from early-born and late-born neurons. Early-born neurons give rise to multiple classes of sensory neurons that express different ion channels. In contrast, late-born neurons are restricted in their fate and do not form chemosensory neurons expressing the ion channel TrpA1b. Accordingly, larvae lacking early-born neurons do not respond to the TrpA1b agonist allyl isothiocyanate. These results indicate that the multimodal specification and function of trigeminal sensory ganglia depends on the timing of neurogenesis.
Keywords: neurogenesis, trigeminal sensory ganglia, Trp
Introduction
Somatosensory organs recognize a wide range of stimuli such as noxious chemicals, pressure, and temperature (for review (Julius and Basbaum, 2001)). The vertebrate somatosensory system consists of sets of sensory ganglia located in the peripheral nervous system (PNS) (for review (Lynn, 1975)). Trigeminal sensory ganglia innervate most of the head (Noden, 1980), whereas the dorsal root ganglia (DRG) flank the spinal cord and innervate the body (Swett and Woolf, 1985). Trigeminal sensory neurons project to several brainstem nuclei, whereas DRG neurons send afferent axons to the spinal cord (for review (Marmigere and Ernfors, 2007)).
The multimodal nature of the information detected by the somatosensory system is reflected in the neuronal diversity of sensory ganglia (for review (Julius and Basbaum, 2001; Marmigere and Ernfors, 2007)). Two major subpopulations of somatosensory neurons can be distinguished: the proprioceptive neurons transduce innocuous stimuli such as light touch whereas the nociceptive neurons detect potentially harmful stimuli. To cover a wide range of sensory information, nociceptive neurons express diverse channels and receptors that respond to these stimuli. For instance, TrpV1 is a heat sensing channel expressed in thermosensitive neurons (Caterina et al., 1997), whereas TrpA1 is expressed in a subset of neurons responsive to chemical irritants such as allyl isothiocyanate (mustard oil), the pungent ingredient of mustard (Bandell et al., 2004; Jordt et al., 2004). Another subset of neurons is defined by expression of the P2X3 receptor, an ATP sensor involved in the modulation of nociceptive signals (Chen et al., 1995). Thus, in contrast to other sensory systems, the somatosensory ganglia contain a diverse array of sensory neurons that are tuned to very distinct classes of stimuli. How this diversity is achieved during development is poorly understood.
Studies of the central nervous systems (CNS) in both vertebrates and invertebrates have revealed that the temporal pattern of neuron specification contributes to the generation of neuronal diversity (for review (Pearson and Doe, 2004)). During development of Drosophila melanogaster for instance, neuroblasts undergo stem cell-like divisions to generate neuronal progeny in an ordered sequence (Truman and Bate, 1988) (Pearson and Doe, 2003). Similarly, different neurons of the layered mammalian cortex form at precise developmental times (for review (McConnell, 1995)). Neurons located deep in the cortex are born before neurons that populate more superficial layers, resulting in an inside out progression of neurogenesis. In both systems, progenitors gradually lose competence to generate early-born fates. In the PNS, cell birthdating and genetic studies in mouse and chick suggest that DRG neurons derive from three waves of neurogenesis (Carr and Simpson, 1978; Frank and Sanes, 1991; Lawson and Biscoe, 1979; Ma et al., 1999; Maro et al., 2004; Marmigere and Ernfors, 2007). The second wave gives rise to the majority of proprioceptive and nociceptive neurons, whereas the first and third waves generate predominantly proprioceptive and nociceptive neurons, respectively. It is unclear if similar or different strategies are used during the diversification of trigeminal sensory neurons or how different nociceptive subsets are specified. Here we address these questions using the zebrafish trigeminal ganglia as a model system.
Similar to other vertebrates, the trigeminal sensory ganglia in zebrafish form on either side of the head, between the eye and ear (Figure 1A). The first trigeminal sensory neurons are born at around 11 hours post fertilization (hpf) and rapidly assemble into a ganglion (Knaut et al., 2005). By 24 hpf, the ganglia mediate the response to mechanical stimuli (Saint-Amant and Drapeau, 1998) and chemical irritants (Prober and Schier, unpublished), resulting in a highly stereotypic escape behavior. It has remained unclear how the different modalities within the trigeminal ganglia are generated. To address this question, we analyzed how the timing of neurogenesis regulates trigeminal sensory neuron specification. We developed a novel technology (BAPTISM) to compare neuronal birth date and specification in vivo and interfered with early or late periods of neurogenesis. Our results indicate that the full repertoire of trigeminal sensory neuron cell types and larval behaviors depends on early neurogenesis.
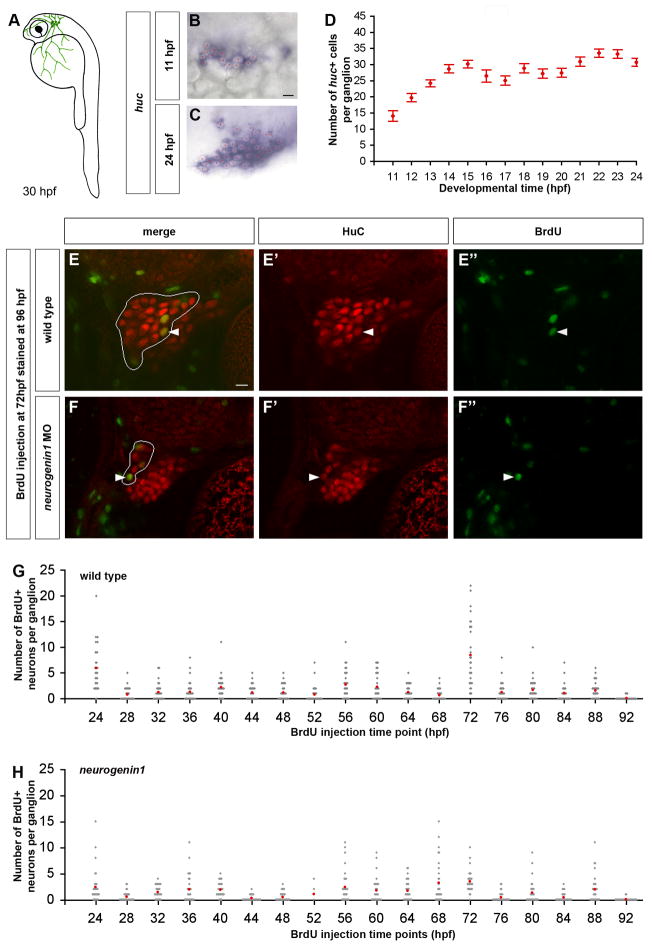
Figure 1. Continuous Neurogenesis in the Zebrafish Trigeminal Sensory Ganglia.
(A) As schematized in a 30 hpf zebrafish embryo, the trigeminal sensory ganglia (green) are located on each side of the head posterior to the eyes. Trigeminal sensory neurons innervate the head and part of the yolk. (B-D) Wild-type embryos were hybridized with a huc antisense probe to count the number of trigeminal sensory neurons per ganglion. The first neurons appear at 11 hpf forming small clusters (B). During subsequent development, the number of neurons per ganglion increases (C). Side view, anterior to the left; red asterisks label single neurons; scale bar represents 10 μm. The chart represents the average number of neurons per ganglion at different developmental stages ranging from 11 hpf to 24 hpf. An individual ganglion contains an average of 14 neurons at 11hpf and reaches an average size of 30 neurons at 24 hpf (D). Error bars indicate s.e. (n=15). (E-H) To measure the number of neurons added to the trigeminal sensory ganglion after 24 hpf, embryos were injected once with BrdU, fixed at 96 hpf and immunostained for HuC (red) and BrdU (green). Double labeled cells in the trigeminal sensory ganglia of wild-type (E) or neurogenin1 morphant (F) embryos injected with BrdU at 72 hpf are indicated with a white arrowhead. Side view, anterior to the left; white outline delimits the trigeminal sensory ganglion from the anterior lateral line; scale bar represents 10 μm. The charts represent the number of neurons per ganglion born after the BrdU injection time point in wild-type (G) and neurogenin1 morphant (H) embryos. Injections were performed at different times ranging from 24 hpf to 92 hpf. Gray dots represent individual samples and red dots represent the average number of BrdU positive trigeminal sensory neurons found per ganglion at each injection time point (n=30).
Results
Continuous Neurogenesis in the Zebrafish Trigeminal Sensory Ganglia
The birthdate of a neuron refers to the time point at which a precursor undergoes its last division before differentiating as a neuron. HuC is expressed in differentiating neurons of vertebrates shortly after their birth (Marusich et al., 1994). To study the temporal pattern of neurogenesis in the trigeminal sensory ganglion in zebrafish, we first analyzed the expression of the zebrafish homologue of HuC (Kim et al., 1996). Huc mRNA highlighted the first differentiated trigeminal neurons at 11 hours post fertilization (hpf) on each side of the head (Figure 1B). Each ganglion contained 14 ± 2 neurons (Figure 1D). By the time the trigeminal sensory ganglia are responsive to external stimuli (24 hpf), each ganglion contained an average of 31 ± 1 neurons (Figure 1C,D). To follow the development of the trigeminal sensory ganglia at later stages, we used a transgene that expressed the fluorescent protein Kaede under the control of the huc promoter (Sato et al., 2006). We found that at 24 hpf each trigeminal sensory ganglion had an average size of 37 ± 3 neurons and by 72 hpf reached an average size of 53 ± 6 neurons (Table 1; Figure 2B, 6A; Movie 3). To gain further insights into the temporal pattern of trigeminal neurogenesis after 24 hpf, we used 5-bromo-2-deoxyuridine (BrdU) incorporation. Between 24 hpf and 92 hpf, a single injection of BrdU was given per embryo. The time points for injection were separated by 4-hr intervals, because BrdU remained available for incorporation for about 4 hrs after injection (Figure S1). At 96 hpf double labeling for BrdU and HuC was used to identify neurons that were born before or after BrdU injection (Figure 1E and Movie 1). This analysis revealed that new trigeminal neurons arose continuously from 24 hpf to 96 hpf (Figure 1G). The number of new neurons added to a single ganglion per 4-hr interval ranged from one to eight with an average of 2.03 ± 0.48 neurons. This rate of neurogenesis leads to an estimated addition of 23 ± 4 neurons per ganglion from 24 hpf to 72 hpf, consistent with the increase of HuC-expressing neurons from 37 ± 3 at 24 hpf to 53 ± 6 at 72 hpf (Table 1; Figures 2B and 6A; Movie 3). Taken together, our analysis indicates that neurogenesis in trigeminal sensory ganglia consists of an early burst shortly after 11 hpf and a continuous slower phase after 24 hpf. We refer to trigeminal sensory neurons born before and after 24 hpf as early-born and late-born neurons, respectively.
Table 1. Neurons forming the trigeminal sensory ganglia.
Neurons expressing huc:kaede, p2x3b:egfp, and/or trpa1b:egfp transgenes were counted in 24 hpf and 72 hpf embryos. The BAPTISM technique was used to distinguish early-born neurons from late-born neurons at 72 hpf. The averages reported in the table represent the number of neurons per ganglion expressing a particular transgene in wild-type embryos, embryos treated with anti-proliferation drugs, or neurogenin1 morphant embryos. The standard error and sample size are indicated.
| conditions | stage | huc:kaede | p2x3b:egfp | trpa1b:egfp |
|---|---|---|---|---|
| wildtype | 24 hpf | 37 ± 3 (n=5) | 15 ± 2 (n=11) | 20 ± 3 (n=5) |
| 72 hpf | 53 ± 6 (n=5)
early-born neurons: 35 ± 4 late-born neurons: 18 ± 3 |
17 ± 2 (n=7)
early-born neurons: 14 ± 1 late-born neurons: 3 ± 1 |
13 ± 2 (n=7)
early-born neurons: 13 ± 2 late-born neurons: 0 |
|
|
| ||||
| +anti-proliferative drugs | 72 hpf | 34 ± 3 (n=11)
early-born neurons: 32 ± 3 late-born neurons: 3 ± 1 |
17 ± 3 (n=5)
mock treated: 18 ± 1 (n=3) |
20 ± 1 (n=5)
mock treated: 24 ± 3 (n=3) |
|
| ||||
| +neurogenin1 morpholino | 72hpf | 15 ± 2 (n=8) | 8 ± 2 (n=3) | 0 (n=11) |
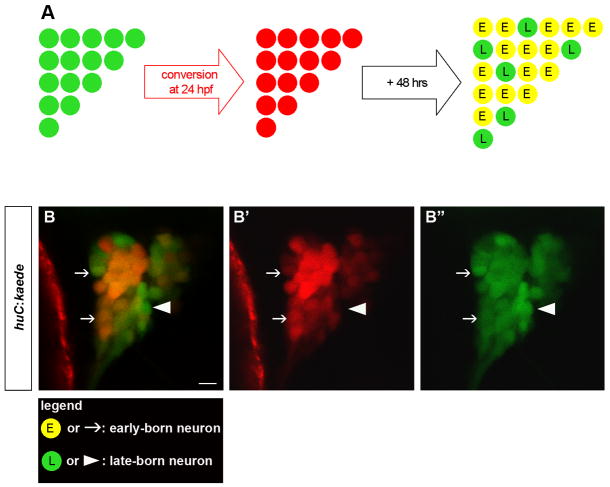
Figure 2. In Vivo Birthdating Analysis of Trigeminal Sensory Neurons Using BAPTI.
Embryos carrying the huc:kaede transgene were analyzed using the Birthdating Analysis by Photoconverted fluorescent protein Tracing In vivo method (BAPTI). (A) As schematized, the trigeminal sensory neurons initially appear green. Following illumination of 24 hpf embryos with ultraviolet light, huc:kaedegreen is converted to huc:kaedered and all neurons born prior to 24 hpf appear red. Following 48 hrs incubation, neurons born before 24 hpf retain huc:kaedered and express de novo huc:kaedegreen while neurons born after 24 hpf express only huc:kaedegreen. Early-born neurons appear red and green while late-born neurons appear green only. (B) Converted embryos were imaged at 72 hpf. Early-born neurons are identifiable by their red and green signals (arrow) while late-born neurons are identifiable by their green only signal (arrowhead). Neurons with weak (top arrow) or strong red signals (bottom arrow) were counted as early-born neurons. The entire trigeminal sensory ganglion was imaged by confocal microscopy. Note that at this plane of confocal section only one late-born neuron is present (arrowhead). Side view, anterior to the left; scale bar represents 10 μm.
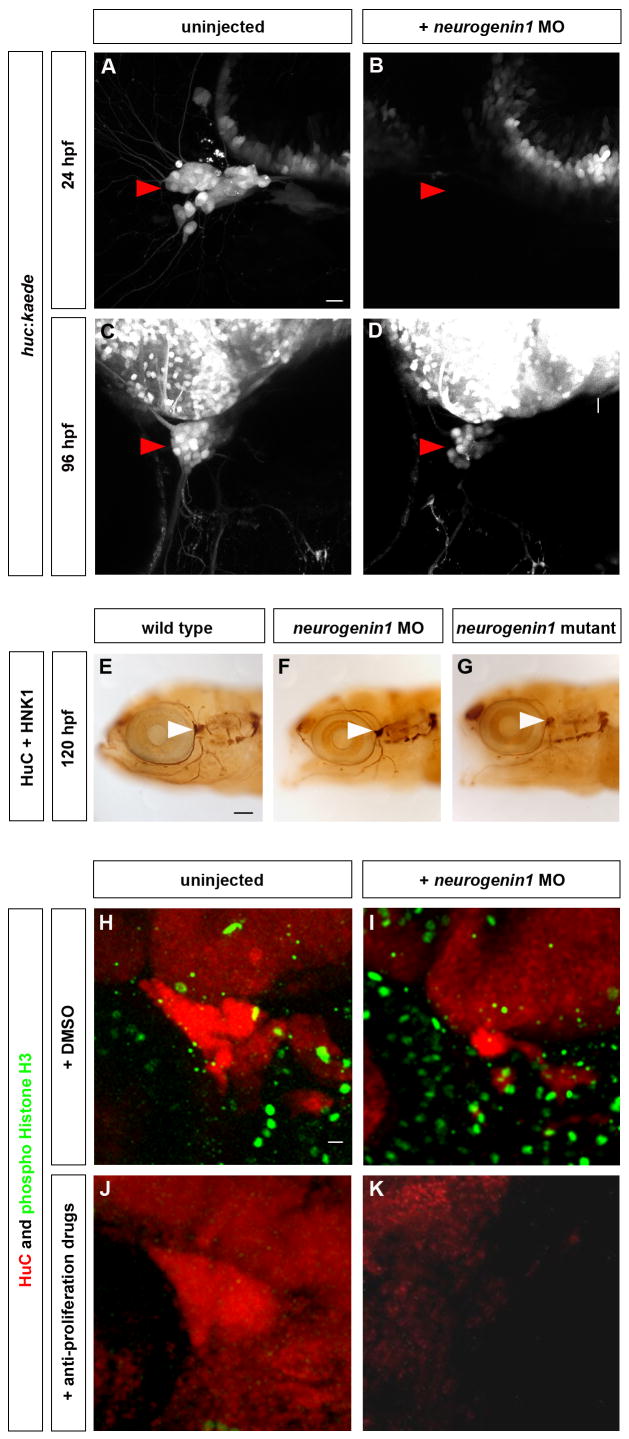
Figure 6. The Trigeminal Sensory Ganglia of neurogenin1 Mutant and Morphant Embryos Are Solely Formed from Late-Born Neurons.
Neurogenin1 depleted embryos develop smaller trigeminal sensory ganglia formed from late-born neurons only. (A–D) Embryos carrying the huc:kaede transgene were injected with 6 ng of neurogenin1 antisense morpholino (B,D) or uninjected (A,C). At 24 hpf, the trigeminal sensory ganglia are visible in uninjected embryos by the expression of Kaede (A,C) but no trigeminal sensory neurons are detectable in the neurogenin1 morphants at 24 hpf (B). At 96 hpf, the trigeminal sensory ganglia are visible in neurogenin1 morpholino-injected embryos (D) but contain fewer neurons than uninjected embryos (C). Side view, anterior to the left; scale bar represents 10 μm. (E–G) The morphology of the neurons of the trigeminal sensory ganglia was analyzed by immunostaining in wild-type (E), neurogenin1 morphant (F), and neurogenin1 mutant (G) embryos with HuC, a pan-neuronal marker, and HNK-1, a marker labeling the cell surface of sensory neurons. White arrowheads point at the trigeminal sensory ganglia. Side view, anterior to the left; scale bar represents 100 μm. (H-K) To determine whether the trigeminal sensory ganglia in neurogenin1 morphant embryos are partly formed from early-born neurons, embryos were treated with 2% DMSO alone (H,J) or with 20 mM hydroxyurea and 150 μM aphidicolin (I,K) at 24 hpf. HuC staining (red) labels the trigeminal sensory ganglia. Staining for the mitotic marker phospho-histone H3 (green) was used to monitor the number of proliferating cells in the whole embryos. Proliferation was not affected in mock treated embryos (H,J) but was significantly reduced in treated embryos (I,K). No trigeminal sensory neurons are detectable in the neurogenin1 morphant embryos treated with the anti-proliferative drugs (K) in contrast to the mock-treated neurogenin1morphant (I) or wild-type embryos (H, J). Side view, anterior to the left; scale bar represents 10 μm.
In Vivo Birthdating of Trigeminal Sensory Neurons
To distinguish early-born from late-born neurons in live embryos, we devised a method called BAPTI (Birthdating Analysis by Photoconverted fluorescent protein Tracing In vivo) (Figure 2A). In this method, we utilized the photoconvertible fluorescent protein Kaede. Upon exposure to ultraviolet light (405nm) Kaede is permanently cleaved, and its emission spectrum shifts from green to red (Ando et al., 2002). The converted Kaede remains stable for several days (Hatta et al., 2006; Kimura et al., 2006). To specifically label early-born trigeminal sensory neurons, Kaede was photoconverted at 24 hpf in transgenic embryos expressing Kaede under the control of ~6 kb of huc cis-regulatory region (huc:kaede) (Sato et al., 2006). This resulted in the red-fluorescent labeling of neurons born before 24 hpf. At 72 hpf, these early-born trigeminal sensory neurons were still evident by expression of the converted, red-fluorescent Kaede (huc:kaedered) (Figure 2B, white arrows). Because the huc:kaede transgene continues to be expressed in these neurons, they also expressed de novo synthesized, unconverted, green-fluorescent Kaede (huc:kaedered + huc:kaedegreen) (Figure 2B, white arrows). In contrast, neurons born after 24 hpf did not contain converted, red-fluorescent Kaede but only contained the unconverted, green-fluorescent Kaede (huc:kaedegreen) (Figure 2B, white arrowheads). Our analysis confirmed the presence of early-born and late-born neurons in the trigeminal sensory ganglia of 72 hpf zebrafish embryos. Of the 53 ± 6 neurons per ganglion present at 72 hpf (Table 1; Figure 2B; Movie 3), 35 ± 4 were born before 24 hpf (Table 1; Figure 2B, white arrows; Movie 3) whereas 18 ± 3 neurons were added after 24 hpf (Table 1; Figure 2B, white arrowheads; Movie 3). These results are consistent with the observations that each ganglion contains 37 ± 3 huc:kaede-expressing neurons at 24 hpf (Table 1, Figure 6A) and that 23 ± 4 neurons are added per ganglion based on BrdU labeling (Figure 1G). BAPTI thus supports and extends the findings obtained using BrdU and makes it possible to analyze the temporal dynamics of neurogenesis in living zebrafish embryos.
Late-Born Neurons Have Restricted Fates
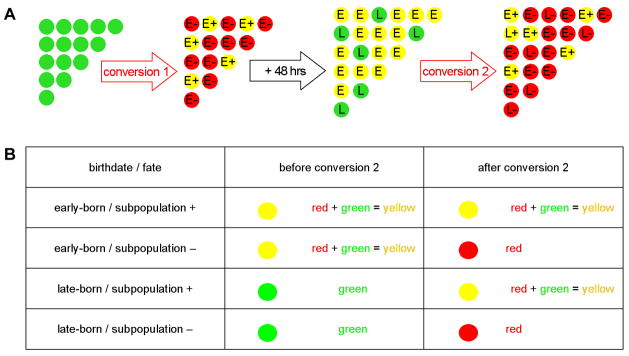
To determine whether the specification of trigeminal sensory neurons is linked to their birthdates, we assessed the fates of early-born and late-born trigeminal sensory neurons. To this end, we combined reporter transgenes with the BAPTI method (BAPTI combined with Subpopulation Markers or BAPTISM) (Figure 3A). In this approach, specific cell types within trigeminal sensory ganglia are labeled by EGFP expression under the control of cis-regulatory regions of different subpopulation markers (subpopulation:egfp). As shown in Figure 3, in embryos carrying the huc:kaede transgene as well as a subpopulation:egfp transgene, trigeminal sensory neurons will, depending on their subtype, express either huc:kaede alone or both huc:kaede and subpopulation:egfp. They will thus be uniformly green (huc:kaedegreen or huc:kaedegreen + subpopulation:egfpgreen). When early-born neurons are labeled red by photoconversion of Kaede at 24 hpf, neurons that express the subpopulation marker will be both red and green (or yellow in merged images) (huc:kaedered + subpopulation:egfpgreen). In contrast, neurons that do not express the subpopulation marker will be red but not green (huc:kaedered). When the same embryos are analyzed at 72 hpf, early-born neurons will have retained the converted, red-fluorescent Kaede but will also express unconverted, green-fluorescent Kaede (huc:kaedered + huc:kaedegreen or huc:kaedered + huc:kaedegreen + subpopulation:egfpgreen) and will thus be both red and green. By contrast, late-born neurons will at that stage only express non-converted, green Kaede (huc:kaedegreen or huc:kaedegreen + subpopulation:egfpgreen) and thus be green. To distinguish the late-born neurons that express the subpopulation marker (huc:kaedegreen + subpopulation:egfpgreen) from the ones that do not (huc:kaedegreen), a second conversion is performed at 72 hpf. Following this second conversion, both early-born and late-born neurons will contain converted, red-fluorescent Kaede (huc:kaedered) but only those neurons that also express subpopulation:egfpwill retain green fluorescence (huc:kaedered+ subpopulation:egfpgreen). Direct comparison of individual neurons before and after the second conversion will therefore reveal whether a given subpopulation marker is expressed in an early-born and/or a late-born neuron (Figure 3B). BAPTISM thus can be used to simultaneously identify in vivo both the birthdate of a neuron and its fate.
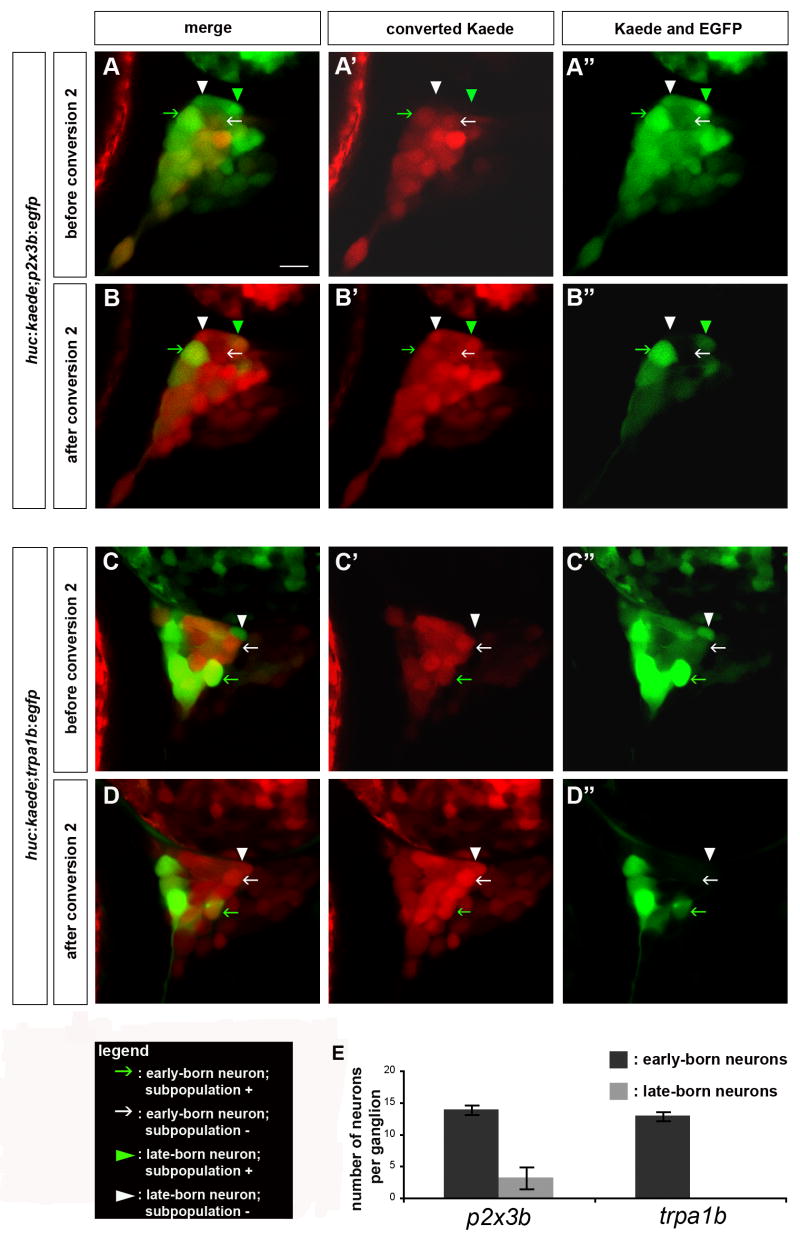
Figure 3. Simultaneous In Vivo Analysis of Trigeminal Sensory Neuron Birthdate and Fate Using BAPTISM.
Embryos carrying the huc:kaede transgene together with a subpopulation:egfp transgene were analyzed using the Birthdating Analysis by Photoconverted fluorescent protein Tracing In vivo method combined with a Subpopulation Marker (BAPTISM). (A) As schematized, the trigeminal sensory neurons initially appear all green (huc:kaedegreen or huc:kaedegreen + subpopulation:egfpgreen). Following a first conversion at 24 hpf, early-born neurons are labeled red and those neurons that express the subpopulation marker appear red and green (huc:kaedered + subpopulation:egfpgreen) whereas neurons that do not express the subpopulation marker appear red only (huc:kaedered). Following a 48 hr incubation, early-born neurons retain the converted, red-fluorescent Kaede but also express de novo unconverted green-fluorescent Kaede (huc:kaedered + huc:kaedegreen or huc:kaedered + huc:kaedegreen + subpopulation:egfpgreen). Late-born neurons express non-converted green Kaede (huc:kaedegreen or huc:kaedegreen + subpopulation:egfpgreen). Following a second conversion at 72 hpf, both early-born and late-born neurons contain red-fluorescent, converted Kaede (huc:kaedered) and only those neurons that also express the subpopulation transgene retain green fluorescence (huc:kaedered + subpopulation:egfpgreen). (B) Comparison of the signals in single neurons before and after the second conversion reveal whether a given subpopulation marker is expressed in an early-born and/or a late-born neuron. Early born neurons appear yellow before the second conversion while late-born neurons appear green only. Trigeminal neurons that express the subpopulation marker (subpopulation +) appear yellow after the second conversion while the ones that do not express it appear red only (subpopulation −).
We applied BAPTISM to investigate whether early-born and late-born neurons contribute to different subpopulations of trigeminal sensory neurons. We focused on two subpopulations of neurons: those expressing TrpA1b and those expressing P2X3b. Both genes are expressed in the trigeminal sensory ganglia of zebrafish starting at 24 hpf (Kucenas et al 2006; Prober and Schier, unpublished). We used transgenic zebrafish expressing EGFP under the control of the cis-regulatory regions of p2x3b or trpa1b. Both transgenes reflect the expression patterns of the endogenous genes (Kucenas et al 2006; Choy and Schier, unpublished). BAPTISM analysis of embryos carrying the p2x3b:egfp transgene revealed that both early-born and late-born neurons contributed to the p2x3b-expressing subpopulation (Figure 4A,B, green arrows and arrowheads; Movies 4, 5). Per ganglion, 14 ± 1 p2x3b-expressing neurons were derived from early-born neurons, and 3 ± 1 p2x3b-expressing neurons were derived from late-born neurons (Table 1; Figure 4A,B,E green arrows and arrowheads; Movies 4, 5). This indicated that both early-born and late-born neurons have the potential to form p2x3b-expressing neurons. The specification of the p2x3b-expressing subpopulation of trigeminal sensory neurons therefore appears to be determined independently of birthdate. BAPTISM analysis of trpa1b:egfp expressing embryos revealed that early-born neurons contributed to the trpa1b-expressing neurons (Figure 4C,D,E green arrows; Movies 6, 7). Per ganglion, 13 ± 2 trpa1b-expressing neurons were derived from early-born neurons (Table 1; Figure 4C,D,E, green arrows; Movies 6, 7). In contrast, none of the late-born neurons expressed trpa1b (Figure 4C,D,E white arrowheads; Movies 6, 7). Of the 132 late-born neurons analyzed in 7 huc:kaede;trpa1b:egfp embryos, none expressed trpa1b:egfp (Table 1; Figure 4C,D,E; Movies 6, 7). This indicates that trpa1b-expressing neurons are exclusively formed from early-born neurons, and that late-born neurons do not contribute to this subset of trigeminal sensory neurons. These results suggest that early-born neurons are competent to form both trpa1b-expressing and p2x3b-expressing neurons, whereas late-born neurons are restricted in their cell type specification.
Figure 4. Late-Born but not Early-Born Neurons Are Restricted in their Fate.
Embryos carrying the huc:kaede transgene together with either the p2x3b:egfp or the trpa1b:egfp transgene were analyzed using BAPTISM. (A–D) huc:kaede was first converted at 24 hpf, imaged at 72 hpf (A,C) and converted a second time (B,D). (A,B) In huc:kaede;p2x3b:egfp embryos, early-born neurons express the subpopulation marker (green arrow) or not (white arrow) and late-born neurons express the subpopulation marker (green arrowhead) or not (white arrowhead). (B,C) In huc:kaede;trpa1b:egfp embryos, early-born neurons express the subpopulation marker (green arrow) or not (white arrow). Late-born neurons do not express the subpopulation marker (white arrowhead). Side view, anterior to the left; scale bar represents 10 μm. (E) The chart represents the number of neurons per trigeminal sensory ganglion expressing the p2x3b or trpa1b subpopulation markers derived either from early-born neurons (dark gray) or late-born neurons (light gray). The error bars refer to the s.e. (n=7).
Independent Specification of Early-Born and Late-Born Neurons
The results described above reveal that early-born neurons persist in trigeminal sensory ganglia and that late-born neurons are restricted in their fate. In principle, the development of early-born neurons could be influenced by the presence of late-born neurons and vice versa. For example, the differentiation of early-born neurons might restrict the fate of late-born neurons, and late-born neurons might be required for the maintenance of early-born neurons. To test these models, we generated embryos whose trigeminal sensory ganglia contained only early-born or only late-born neurons and determined whether these ganglia expressed p2x3b and trpa1b.
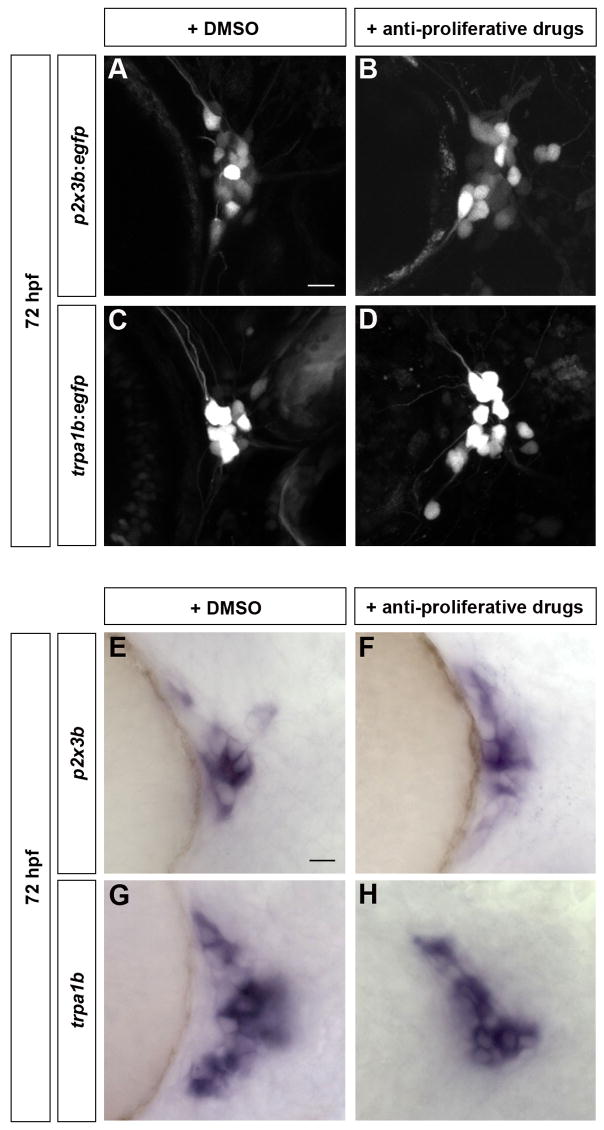
To create embryos that lacked late-born neurons, we blocked cell proliferation after 24 hpf by treating embryos with anti-proliferative drugs (Lyons et al., 2005). Phospho-histone H3 labeling indicated that treated embryos displayed a strong reduction of mitotic cells (Figure 6H,J). To further analyze the formation and survival of neurons after treatment, BAPTI was used to label neurons at 24 hpf (Figure S2). At 72 hpf, treated embryos contained 32 ± 3 early-born neurons (huc:kaedered) and only 3 ± 1 late-born neurons (huc:kaedegreen) (for a total of 34 ± 3 neurons) as opposed to 35 ± 4 early-born neurons and 18 ± 3 late-born neurons (for a total of 53 ± 6 neurons) in mock-treated embryos (Table 1; Figure S2). These results reveal that anti-proliferation treatment does not affect the survival of early-born neurons but strongly reduces the formation of late-born neurons. Trigeminal sensory ganglia consisting of early-born neurons still expressed p2x3b, p2x3b:egfp, trpa1b, and trpa1b:egfp (Figure 5B,D,F,H). Similar to untreated embryos, treated embryos contained 17 ± 3 p2x3b:egfp expressing neurons and 20 ± 1 trpa1b-GFP expressing neurons (Table 1; Figure 5A,B,C,D). In addition, we detected mRNA expression of other trigeminal subpopulation markers such as the thermally gated channel TrpV1 and the second P2X3 homologue P2X3a (Figure S3). These results indicate that late-born neurons are required neither for the maintenance nor subspecification of early-born neurons.
Figure 5. Early-Born Trigeminal Sensory Neurons Contribute to Various Subpopulations Independently of Late-Born Neurons.
Subpopulation markers were analyzed in embryos lacking late-born trigeminal sensory neurons. (A-H) Embryos were incubated from 24 hpf to 72 hpf with 2% DMSO alone (A,C,E,G) or 20 mM hydroxyurea and 150 μM aphidicolin (B,D,F,H). Embryos carrying the p2x3b:egfp (A,B) or trpa1b:egfp (C,D) transgene were imaged at 72 hpf. Wild-type embryos were fixed at 72 hpf and in situ hybridization was performed for p2x3b (E,F) and trpa1b (G,H) Side view, anterior to the left; scale bar represents 10 μm.
BAPTISM indicated that in contrast to early-born neurons, late-born neurons did not generate trpa1b-expressing neurons (Figure 4E). To test whether this restriction is imposed on the late-born neurons by the presence of early-born neurons, we specifically removed early-born neurons from the trigeminal ganglia. Zebrafish embryos that lack the transcriptional regulator neurogenin1 lack trigeminal sensory ganglia at 24 hpf (Figure 6B) (Andermann et al., 2002; Cornell and Eisen, 2002; Golling et al., 2002). We discovered, however, that neurogenin1 mutants and neurogenin1 morphants (antisense morpholino-injected embryos) formed trigeminal sensory ganglia at later stages of development (Figure 6D,F,G). In neurogenin1 depleted embryos, the first huc-expressing trigeminal sensory neurons appeared around 48 hpf (data not shown). By 96 hpf trigeminal ganglia contained fewer neurons than wild-type ganglia but formed the typical three branched pattern (Figure 6C,D,E,F,G).
To test whether the ganglia in neurogenin1 morphants were formed by late-born neurons, we performed BrdU labeling experiments. A time course of BrdU injections in neurogenin1 morphants revealed that, like in wild type, late-born neurons emerged continuously after 24 hpf (Figure 1F, H; Movie 2). To determine if delayed differentiation of early-born neurons might also contribute to the later emergence of trigeminal sensory neurons, we eliminated the late-born neurons from neurogenin1 morphants using the anti-proliferation treatment described above. No trigeminal sensory neurons were detected in treated neurogenin1 morphants at 72 hpf (Figure 6K), confirming that late neurogenesis is the major source of neurons in the absence of neurogenin.
To directly visualize the formation of late-born neurons in the absence of neurogenin1, we injected neurogenin1 morpholinos into huc:kaede embryos. Morphants had 15 ± 2 neurons per ganglion at 72 hpf, compared to 53 ± 6 neurons in wild type (Table 1; Figure 2B and data not shown; Movie 3). Consistent with late neurogenesis in neurogenin1 morphants, the number of neurons in neurogenin1 depleted embryos is comparable to the number of late-born neurons in wild-type embryos based on BrdU labeling (23 ± 4 neurons per ganglion; Figure 1G) or BAPTI (18 ± 3 neurons per ganglion; Table 1; Figure 2B; Movie 3). These results suggest that the trigeminal sensory ganglia of neurogenin1 depleted embryos are solely formed from late-born neurons.
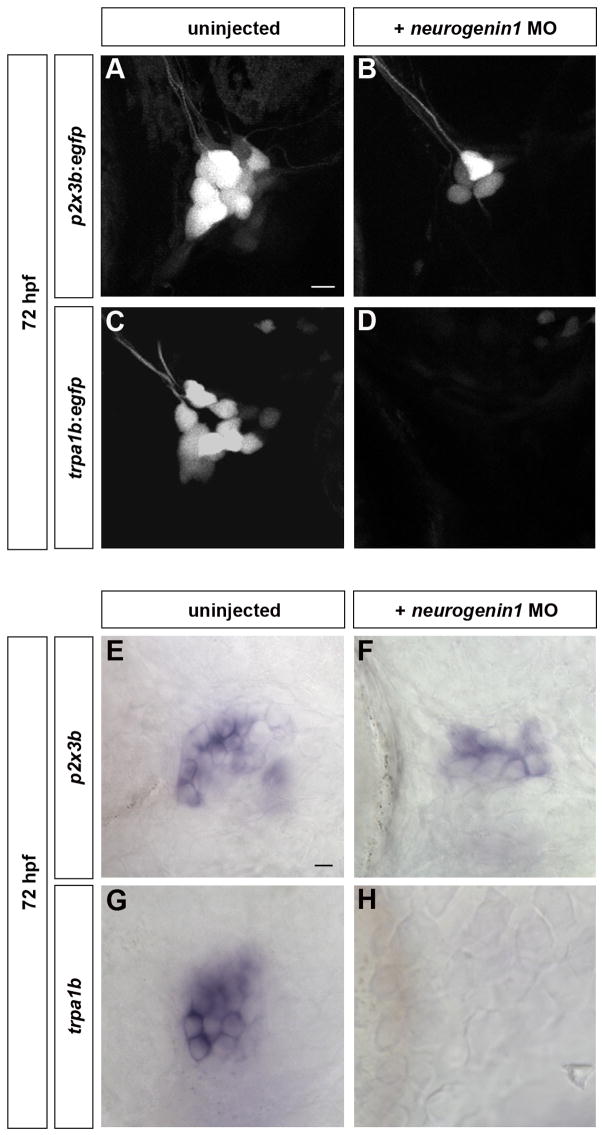
To test whether early-born neurons restrict the fate of late-born neurons, we injected neurogenin1 morpholinos into p2x3b:egfp and trpa1b:egfp embryos. Morphants had 8 ± 2 p2x3b:egfp–expressing neurons per ganglion but no trpa1b:egfp–expressing neurons could be detected (Table 1; Figure 7B,D). Neurogenin1 morphants expressed trpv1, p2x3a, and p2x3b mRNAs (Figure 7F, Figure S4) but not trpa1b (Figure 7H), consistent with our previous findings. These results indicate that the cell fate restriction of late-born trigeminal neurons can occur independently of early-born neurons.
Figure 7. Cell Fate Restriction of Late-Born Trigeminal Sensory Neurons is Independent of Early-Born Neurons.
Subpopulation markers were analyzed in embryos lacking early-born trigeminal sensory neurons. (A-D) Embryos carrying the p2x3b:egfp (A, B) or trpa1b:egfp (C, D) transgene were injected with 6ng of neurogenin1 morpholino (B, D) and imaged at 72 hpf. While both p2x3b-expressing and trpa1b-expressing neurons are visible in uninjected larvae (A,C), only p2x3b-expressing are detectable in neurogenin1 morphant larvae (B). No trpa1b-expressing neurons were detected in these larvae (D). (E–H) Wild-type (E,G) and neurogenin1 morphant (F,H) larvae were stained by in situ hybridization at 96 hpf for p2x3b (E,F) or trpa1b (G,H). No trpa1b-expressing neurons were detected in neurogenin1 morphant larvae (H). Side view, anterior to the left; scale bar represents 10 μm.
Birthdate Affects the Chemical Sensitivity of Trigeminal Sensory Ganglia
The finding that early neurogenesis is required for the formation of trpa1b-expressing neurons raised the possibility that birthdate might also underlie the function of trigeminal sensory ganglia at a behavioral level. The TrpA1b channel is required for the detection of chemical irritants such as allyl isothiocyanate in mouse (Bandell et al., 2004; Jordt et al., 2004) and zebrafish (Prober and Schier, unpublished) but is not needed for mechanosensation (Bautista et al., 2006; Kwan et al., 2006). These studies raised the hypothesis that only embryos containing early-born neurons would respond to allyl isothiocyanate. Indeed, we found that touch and allyl isothiocyanate elicited an escape response in wild-type larvae and in larvae with only early-born trigeminal sensory neurons (Table 2, Movies 8, 9). In contrast, neurogenin1-depleted embryos were completely insensitive to allyl isothiocyanate at all stages tested from 24 hpf to 192 hpf, despite the presence of late-born trigeminal sensory neurons (Table 2; Figure S5; Movie 9). Neurogenin1 mutants did not respond to touch at 24 hpf, consistent with the complete absence of trigeminal sensory neurons at this stage (Table 2; Figure 6B), but touch response became apparent around 48 hpf and was fully restored by 96 hpf, concomitant with the formation of late-born trigeminal sensory neurons (Table 2; Figures 6F,G and S5; Movie 9). These results suggest that early neurogenesis is required not only for the formation of a specific type of chemosensory trigeminal neurons but also the establishment of associated behaviors.
Table 2. Birthdate Affects the Sensory Modalities of the Trigeminal Sensory Ganglia.
Wild-type, neurogenin1 morphant, and neurogenin1 mutant zebrafish were tested for their response to touch and allyl isothiocyanate starting at 24 hpf (day 1) until 192 hpf (day 8). Touch stimuli were delivered by touching the head of the zebrafish with a glass needle. A solution of 1% allyl isothiocyanate was applied onto the head. Larvae were considered responsive if they instantaneously swam away from the source of the stimulus. Each day of development, individual zebrafish were tested five times and responsiveness was assessed as the percentage of time the animal reacted to the stimulus. The s.e. is indicated below each percentage (n=12). Wild-type and neurogenin1-depleted 96 hpf larvae were responsive to touch but only wild-type larvae were responsive to allyl isothiocyanate.
| day 1 | day 2 | day 3 | day 4 | day 5 | day 6 | day 7 | day 8 | |
|---|---|---|---|---|---|---|---|---|
|
Response to touch
| ||||||||
| wild type | 100% ± 0 | 100% ± 0 | 100% ± 0 | 100% ± 0 | 100% ± 0 | 100% ± 0 | 100% ± 0 | 100% ± 0 |
| anti-proliferation treatment | 100% ± 0 | 100% ± 0 | 100% ± 0 | n/d | n/d | n/d | n/d | n/d |
| neurogenin1 (morpholino) | 0% ± 0 | 15.00% ± 11 | 65.00% ± 31.52 | 51.67% ± 28.95 | 81.82% ± 23.24 | 100% ± 0 | 100% ± 0 | 100% ± 0 |
| neurogenin1 (mutant) | 0% ± 0 | 3.33% ± 2.25 | 41.67% ± 9.36 | 66.67% ± 6.67 | 76.67% ± 7.72 | 100% ± 0 | 100% ± 0 | 100% ± 0 |
|
| ||||||||
|
Response to mustard oil
| ||||||||
| wild type | 100% ± 0 | 100% ± 0 | 100% ± 0 | 100% ± 0 | 100% ± 0 | 100% ± 0 | 100% ± 0 | 100% ± 0 |
| anti-proliferation treatment | 100% ± 0 | 100% ± 0 | 100% ± 0 | n/d | n/d | n/d | n/d | n/d |
| neurogenin1 (morpholino) | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 |
| neurogenin1 (mutant) | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 |
Discussion
Early Neurogenesis Is Sufficient for Establishment of Multimodal Sensing in Trigeminal Sensory Ganglia
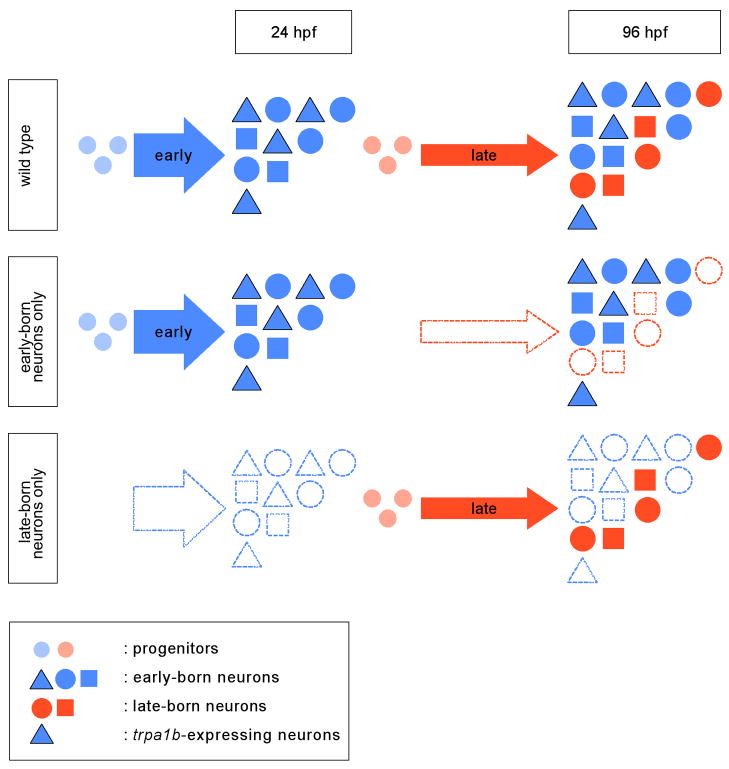
Neurogenesis of zebrafish trigeminal sensory ganglia initiates with a burst at 11 hpf, resulting in the generation of ~30 neurons by 24 hpf. At later stages trigeminal sensory ganglia grow continuously but at a slower rate to form ~55 neurons by 72 hpf. Our study reveals that a subpopulation of chemosensory neurons expressing the nociceptive channel TrpA1b forms exclusively during the early phase of zebrafish neurogenesis (Figure 8). Two lines of evidence support this conclusion. First, in vivo labeling using BAPTISM reveals that TrpA1b-expressing neurons form exclusively from early-born neurons. Second, removal of early-born neurons results in the absence of TrpA1b cells and abrogates the larval escape behavior triggered by the TrpA1b agonist allyl isothiocyanate. In contrast to TrpA1b neurons, mechanosensory and P2X3b neurons develop from both early-born and late-born neurons.
Figure 8. Model for the Role of Neurogenesis in Forming Trigeminal Sensory Ganglia in Zebrafish.
The trigeminal sensory ganglia in wild-type embryos are formed from early-born neurons (born before 24 hpf) and late-born neurons (born after 24 hpf). In 96 hpf larvae, early-born neurons (blue triangles, squares, and pentagons) are intermingled with late-born neurons (orange squares and pentagons). Early-born neurons contribute to all identified subpopulations of trigeminal sensory neurons while late-born neurons fail to form the trpa1b-expressing subpopulation (blue triangles). When proliferation is blocked after 24 hpf, embryos lack late-born neurons. The trigeminal sensory ganglia of these embryos contained all subpopulations derived from early-born neurons and consequently are able to respond to both touch and allyl isothiocyanate. neurogenin1-depleted larvae contain late-born neurons only and the trigeminal sensory ganglia of these embryos fail to form the subpopulation of trpa1b-expressing neurons derived from early-born neurons. Consequently, neurogenin1-depleted embryos are responsive to touch but not allyl isothiocyanate.
Different waves of neurogenesis have also been implicated in the cell type diversification of mouse DRGs, but in contrast to the zebrafish trigeminal ganglion, nociceptive DRG neurons do only derive from the second and third wave of neurogenesis. It is conceivable that these apparent differences might be based on differences in marker analysis. For example, the birthdate of TrpA1b neurons has not been determined in mouse (Ma et al., 1999; Maro et al., 2004; Marmigere and Ernfors, 2007). In addition, lineage analysis in zebrafish will be required to determine if late-born trigeminal neurons are derived from the cells analogous to the boundary cap cells responsible for the third wave of neurogenesis in the DRG (Marmigere and Ernfors, 2007).
Specific removal of late-born neurons does not affect the expression of TrpA1b, TrpV1, P2X3a and P2X3b and the response to allyl isothiocyanate and touch. These findings do not exclude the possibility that more extensive gene expression profiling might identify sensory subtypes that cannot form from early-born neurons. It is unknown how many different neuronal subtypes reside in the zebrafish trigeminal ganglion. For example, studies in mouse have shown that Trpa1 neurons are contained within the TrpV1 population, indicating that these two markers label a TrpV1+;TrpA1+ and a TrpV1+;TrpA1− subpopulation. Although similar studies have yet to be performed in zebrafish, our results indicate that early-born neurons can form a multimodal sensory ganglion independently of late-born neurons. This mode of rapid multimodal neuronal specification contrasts with the sequential patterning in systems such as the mammalian cortex. Mammalian cortical neurons are arranged in a laminar structure composed of six layers. Early-born neurons form the deep layer 6 and as development proceeds, newly born neurons populate increasingly superficial layers (for review (McConnell, 1995). Thus, each subset of mammalian cortical neurons forms during a defined time window, whereas multiple subpopulations of zebrafish trigeminal sensory neurons are generated in a short time interval. The latter strategy is well suited for the life history of zebrafish. Embryos develop externally, and larvae hatch and become free-living at about 48 hpf. Thus, functional sensory systems have to be in place early in development. This is particularly true for the trigeminal sensory ganglia and their vital function in the detection of noxious stimuli. Thus, the early neurogenesis and multimodal cell fate specification in this system allow for the rapid formation of a functional organ essential for survival in the wild.
Late Neurogenesis Contributes to Some but not All Subpopulations of Trigeminal Sensory Neurons
Our findings indicate that late-born trigeminal sensory neurons of zebrafish are restricted in their fate and do not contribute to the subpopulation of chemosensory neurons expressing TrpA1b. Even in the absence of early-born neurons, late-born neurons fail to express TrpA1b and cannot mediate the response to the TrpA1b agonist allyl isothiocyanate. The cues that restrict the fate of late-born trigeminal sensory neurons are unknown. The lack of activating cues or the presence of inhibitory signals generated by surrounding cells might restrict fate specification at later stages of neurogenesis. The latter mechanism is found in the mammalian retina where amacrine cells, an early-born fate, inhibit the formation of additional amacrine cells (Belliveau and Cepko, 1999). Our results argue against a role of early-born trigeminal neurons as a source of inhibiting cues, because the absence of these cells in neurogenin1 mutants does not alleviate the restriction of late-born neurons. Intrinsic timing mechanisms could contribute to progenitor restriction. Such a mechanism is observed in the mammalian cortex, where late cortical progenitors placed into a younger host fail to form the deep cortical neurons normally formed by early progenitors (for review (McConnell, 1995). A similar mechanism might account for the restriction of late-born trigeminal sensory neurons. Detailed characterization of trigeminal sensory neuron progenitors is needed to determine if extrinsic regulatory signals might arise from cells surrounding the trigeminal sensory neurons or if intrinsic mechanisms restrict fate specification.
Fate restriction during neurogenesis is thought to help generate the proper architecture and connectivity of neural circuits. It is conceivable that the location or connectivity of early-born and late-born trigeminal sensory neurons might reflect their functional diversification. Trigeminal sensory ganglia form a coarse topographic map along the anterior-posterior and dorsal-ventral axes (Sagasti et al., 2005). For instance, neurons whose cell bodies are located in the anterior-dorsal region of the ganglion tend to innervate the anterior-dorsal region of the head. It is possible that there is a third, proximal-distal axis to this map. Late-born neurons are generally located in deeper cell layers of the ganglion than early-born neurons (Figure S6) and TrpA1b-expressing neurons are located more superficially (Figure S6). As detectors of external sources of noxious chemicals, TrpA1b-expressing neurons would need to project into superficial layers of the skin. It is possible that the location of early-born neurons in superficial regions of the ganglia might allow or force innervation of superficial regions of the skin. In contrast, deeper neurons might project more deeply and detect internal, potentially proprioceptive stimuli. Detailed comparisons of birth dates, cell types and peripheral axon projections are required to test this model.
BAPTISM - a Novel Method to Analyze Birthdate and Fate of Neurons
Our study introduces a novel method, BAPTISM, for the in vivo analysis of the birthdate and fate of neurons. Conversion of the fluorescent protein Kaede serves as a marker to distinguish neurons born at different times. Birthdate is then correlated with fate by addition of non-convertible EGFP markers that label different neural subpopulations. BAPTISM has several advantages compared to more traditional birthdating techniques such as BrdU incorporation. First, BAPTISM can be used repeatedly throughout embryogenesis, unlike the more invasive BrdU injections that can damage cells and embryos. Second, BAPTISM labels neurons independently of their position in the cell cycle, whereas BrdU is only incorporated during S-phase. Third, BAPTISM is temporally precise, because labeling is instantaneous, whereas BrdU has to be taken up by cells and then remains available for several hours. Fourth, and most importantly, BAPTISM allows continuous in vivo observation: cells can be followed throughout development. In contrast, the visualization of BrdU-labeled cells is restricted to the single time point when the specimen is fixed. By utilizing multiple spectrally distinct fluorescent proteins, BAPTISM can be extended to follow multiple subpopulations at once. Finally, this method can be easily adapted to study additional neuronal assemblies as well as other organs.
Experimental Procedures
Zebrafish Strains
Embryos were raised at 28.5°C in water containing 0.003% 1-phenyl-2-thiourea (PTU) and staged as described by (Kimmel et al., 1995). huc:kaede;p2x3b:egfp and huc:kaede;trpa1b:egfp embryos were generated by breeding homozygous adults. neurogenin1hi1059 homozygous embryos were generated by inbreeding heterozygous adults and identified by their touch insensitivity at 24 hpf.
Whole-Mount In Situ Hybridization and Antibody Staining
The zebrafish trpv1 cDNA was generated by performing RT-PCR with Superscript II (Invitrogen) using primers based on sequence from 5′ and 3′ RACE (FirstChoice RLM-RACE, Ambion) and Ensembl exon predictions. Full-length sequence has been deposited in Genbank under accession numbers EU423314. The huc cDNA was obtained from Genbank (accession number AI959250); the p2x3a cDNA was obtained from the Seguela lab (Kucenas et al., 2006), and the p2x3b cDNA was obtained from the Voigt lab (Boue-Grabot et al., 2000). Preparation of RNA probes and in situ hybridizations were performed as described previously by (Ober and Schulte-Merker, 1999). RNA probes against trpa1b, trpv1, p2x3a, p2x3b, and huc were labeled with DIG (Roche) and detected with an anti-DIG antibody (Roche) using NBT/BCIP (Roche). Immunohistochemistry was performed as described (Trevarrow et al., 1990). Antibodies against HuC (Molecular Probe) and HNK-1 were diluted 1:500 and detected using an anti-mouse antibody conjugated to HRP (Jackson Immunolab) and the Cy3-tyramide system (NEN Life Science). Antibodies against phospho-histone H3 (Upstate) were diluted 1:250 and detected using an anti-rabbit antibody conjugated to FITC (Jackson Immunolab).
Morpholino Injections
neurogenin1 morphants were generated by injection of 6 ng of neurogenin1 morpholino (5′-cgatctccattgttgataacctta-3′) (Genetools) at the one-cell stage.
BrdU Birthdating Analysis
Embryos aged between 24 hpf and 92 hpf were anesthetized with Tricaine (Sigma) and immobilized on a plate of 3% agarose. 5 μL of BrdU 100mM (Sigma) was injected in the brain ventricle. Injected embryos were allowed to develop until 96hpf when they were fixed with 4% paraformaldehyde (Sigma). Embryos were permeabilized with proteinase K (30mg/mL; Sigma) and stained using antibodies against HuC (Molecular Probes) and BrdU (Becton-Dickinson). HuC was revealed by an anti-mouse IgG2κb antibody coupled to horse-radish peroxidase using the tyramide amplification system (Cy3) (NEN Life Science). Embryos were then treated for 1hr with 2M HCl to expose the incorporated BrdU. BrdU antibody was revealed by an anti-mouse IgG1 antibody coupled to HRP (Vector Laboratories) using the tyramide amplification system (FITC) (NEN Life Science). Embryos were mounted in 0.3% agarose and imaged with a Pascal confocal microscope using a 40X water immersion objective (Zeiss). Double labeling for BrdU and HuC was used to identify neurons that were born after BrdU injection. The average of neurons born after a specific time point was obtained by adding the number of double labeled neurons detected in each ganglion divided by the total number of ganglia analyzed. The average of neurons born between 24 hpf and 72 hpf was obtained by adding the number of double labeled neurons detected in each ganglion when BrdU was injected at 24, 28, 32, 36, 40, 44, 48, 52, 56, 60, 64, and 68 hpf divided by the total number of ganglia analyzed.
BAPTI and BAPTISM Methods
For BAPTI, fish homozygous for huc:kaede were used. For BAPTISM, huc:kaede;p2x3b:egfp and trpa1b:egfp; huc:kaede embryos were used. Embryos were mounted in 0.3% agarose. Kaede was converted from green to red fluorescence at 24 hpf by exposing the whole trigeminal sensory ganglion to 405nm light for one minute. Embryos were allowed to develop until 72hpf and imaged with a Pascal confocal inverted microscope using a 25x water immersion objective (Zeiss). For BAPTISM, a second conversion was performed on the whole trigeminal sensory ganglion as described above and embryos were imaged again following the second conversion.
Blocking Cell Proliferation
Wild-type and neurogenin1 morphant embryos were incubated in a mixture of 2% DMSO, 20mM hydroxyurea, and 150μM aphidicolin at 20 hpf while mock-treated embryos were incubated in 2% DMSO alone. Embryos were stained using anti-phospho histone H3 antibody (Upstate) and HuC antibody (Molecular Probes). Primary antibodies were recognized using FITC-anti-rabbit and rhodamine-anti-mouse secondary antibodies.
Behavioral Assays
Zebrafish larvae were placed into a 10 cm diameter well dish. The touch assay was carried out by poking the embryo on its face with a glass needle. Response was scored as positive if the fish escaped following touch. Allyl isothiocyanate (mustard oil) (Sigma) was diluted in DMSO (1:100) and was delivered by gently streaming liquid out of an injection needle onto the face of the larva. The flow was adjusted so that the fish would not respond to an equivalent dose of DMSO.
Supplementary Material
Acknowledgments
We thank the members of the Schier laboratory for discussion; Florian Maderspacher, Albert Pan, Jason Rihel, Nadine Vastenhouw, and Ian Woods for critical comments on the manuscript; Joon Lee for help with processing the data; and Steve Zimmerman for fish care. The HNK-1 (zn-12) antibody was obtained from the Developmental studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA. This research was supported by a predoctoral fellowship from the Canadian Institute of Health Research to SJCC, a Helen Hay Whitney Foundation fellowship to DP and NIH grant RO1 NS049319 to AFS.
Bibliography
- Andermann P, Ungos J, Raible DW. Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev Biol. 2002;251:45–58. doi: 10.1006/dbio.2002.0820. [DOI] [PubMed] [Google Scholar]
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:12651–6. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–57. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–82. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Boue-Grabot E, Akimenko MA, Seguela P. Unique functional properties of a sensory neuronal P2X ATP-gated channel from zebrafish. J Neurochem. 2000;75:1600–7. doi: 10.1046/j.1471-4159.2000.0751600.x. [DOI] [PubMed] [Google Scholar]
- Belliveau MJ, Cepko CL. Extrinsic and intrinsic factors control the genesis of amacrine and cone cells in the rat retina. Development. 1999;126:555–66. doi: 10.1242/dev.126.3.555. [DOI] [PubMed] [Google Scholar]
- Carr VM, Simpson SB., Jr Proliferative and degenerative events in the early development of chick dorsal root ganglia. II. Responses to altered peripheral fields. J Comp Neurol. 1978;182:741–55. doi: 10.1002/cne.901820411. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–31. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Eisen JS. Delta/Notch signaling promotes formation of zebrafish neural crest by repressing Neurogenin 1 function. Development. 2002;129:2639–48. doi: 10.1242/dev.129.11.2639. [DOI] [PubMed] [Google Scholar]
- Frank E, Sanes JR. Lineage of neurons and glia in chick dorsal root ganglia: analysis in vivo with a recombinant retrovirus. Development. 1991;111:895–908. doi: 10.1242/dev.111.4.895. [DOI] [PubMed] [Google Scholar]
- Golling G, Amsterdam A, Sun Z, Antonelli M, Maldonado E, Chen W, Burgess S, Haldi M, Artzt K, Farrington S, et al. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat Genet. 2002;31:135–40. doi: 10.1038/ng896. [DOI] [PubMed] [Google Scholar]
- Hatta K, Tsujii H, Omura T. Cell tracking using a photoconvertible fluorescent protein. Nat Protoc. 2006;1:960–7. doi: 10.1038/nprot.2006.96. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–5. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kim CH, Ueshima E, Muraoka O, Tanaka H, Yeo SY, Huh TL, Miki N. Zebrafish elav/HuC homologue as a very early neuronal marker. Neurosci Lett. 1996;216:109–12. doi: 10.1016/0304-3940(96)13021-4. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26:5684–97. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaut H, Blader P, Strahle U, Schier AF. Assembly of trigeminal sensory ganglia by chemokine signaling. Neuron. 2005;47:653–66. doi: 10.1016/j.neuron.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Kucenas S, Soto F, Cox JA, Voigt MM. Selective labeling of central and peripheral sensory neurons in the developing zebrafish using P2X(3) receptor subunit transgenes. Neuroscience. 2006;138:641–52. doi: 10.1016/j.neuroscience.2005.11.058. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–89. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Biscoe TJ. Development of mouse dorsal root ganglia: an autoradiographic and quantitative study. J Neurocytol. 1979;8:265–74. doi: 10.1007/BF01236122. [DOI] [PubMed] [Google Scholar]
- Lynn B. Somatosensory receptors and their CNS connections. Annu Rev Physiol. 1975;37:105–27. doi: 10.1146/annurev.ph.37.030175.000541. [DOI] [PubMed] [Google Scholar]
- Lyons DA, Pogoda HM, Voas MG, Woods IG, Diamond B, Nix R, Arana N, Jacobs J, Talbot WS. erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr Biol. 2005;15:513–24. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–28. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–27. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- Maro GS, Vermeren M, Voiculescu O, Melton L, Cohen J, Charnay P, Topilko P. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci. 2004;7:930–8. doi: 10.1038/nn1299. [DOI] [PubMed] [Google Scholar]
- Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25:143–55. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- McConnell SK. Constructing the cerebral cortex: neurogenesis and fate determination. Neuron. 1995;15:761–8. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Noden DM. Somatotopic and functional organization of the avian trigeminal ganglion: an HRP analysis in the hatchling chick. J Comp Neurol. 1980;190:405–28. doi: 10.1002/cne.901900302. [DOI] [PubMed] [Google Scholar]
- Ober EA, Schulte-Merker S. Signals from the yolk cell induce mesoderm, neuroectoderm, the trunk organizer, and the notochord in zebrafish. Dev Biol. 1999;215:167–81. doi: 10.1006/dbio.1999.9455. [DOI] [PubMed] [Google Scholar]
- Pearson BJ, Doe CQ. Regulation of neuroblast competence in Drosophila. Nature. 2003;425:624–8. doi: 10.1038/nature01910. [DOI] [PubMed] [Google Scholar]
- Pearson BJ, Doe CQ. Specification of temporal identity in the developing nervous system. Annu Rev Cell Dev Biol. 2004;20:619–47. doi: 10.1146/annurev.cellbio.19.111301.115142. [DOI] [PubMed] [Google Scholar]
- Sagasti A, Guido MR, Raible DW, Schier AF. Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Curr Biol. 2005;15:804–14. doi: 10.1016/j.cub.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol. 1998;37:622–32. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sato T, Takahoko M, Okamoto H. HuC:Kaede, a useful tool to label neural morphologies in networks in vivo. Genesis. 2006;44:136–42. doi: 10.1002/gene.20196. [DOI] [PubMed] [Google Scholar]
- Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol. 1985;231:66–77. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:669–79. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol. 1988;125:145–57. doi: 10.1016/0012-1606(88)90067-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.