Abstract
Monocyte infiltration is an important pathogenic event in human immunodeficiency virus type one (HIV-1) associated dementia (HAD). CXCL8 (Interleukin 8, IL-8), a CXC chemokine that elicits chemotaxis of neutrophils, has recently been found to recruit monocytes or synergistically enhance CCL2-mediated monocyte migration. In this report, we demonstrate CXCL8 levels in the cerebrospinal fluid of HAD patients are higher than HIV-1 seropositive patients without neurological impairment. The underlying mechanisms regulating CXCL8 production during disease are not completely understood. We investigated the role of HIV-1-infected and immune competent macrophages, the principal target cell and mediator of neuronal injury in HAD, in regulating astrocyte CXCL8 production. Immune-activated and HIV-1-infected human monocyte-derived-macrophages (MDM) conditioned media (MCM) induced production of CXCL8 by human astrocytes. This CXCL8 production was dependent on MDM IL-1β and TNF-α production following viral and immune activation. CXCL8 production was reduced by inhibitors for mitogen-activated protein kinases (MAPKs), including p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinases (ERK1/2). Moreover, prolonged IL-1β or TNF-α treatment activated double-stranded RNA-activated protein kinase (PKR). Inhibition of PKR prevented elevated CXCL8 production in astrocytes. We conclude that IL-1β and TNF-α, produced from HIV-1-infected and immune-competent macrophages, are critical in astrocyte CXCL8 production. Multiple protein kinases, including p38, JNK, ERK1/2, and PKR, participate in the inflammatory response of astrocytes. These observations will help to identify effective therapeutic strategies to reduce high-levels of CXCL8-mediated CNS inflammation during HAD.
Keywords: CXCL8, Astrocyte, Macrophage, Inflammation, HIV-1 Associated Dementia
1. Introduction
HIV-1 associated dementia (HAD) and HIV-associated neurocognitive impairment remain a significant burden to persons living with HIV infection (Ances and Ellis, 2007; Gray et al., 1991; Maher et al., 1997; Navia et al., 1986b). Chronic inflammation, initiated by infected or activated microglia and macrophages, has served as a primary factor for the encephalitis. The mechanisms of inflammation in HAD are not completely understood. Infiltration of monocytes and macrophages into the central nervous system (CNS) is of great importance to the pathogenesis of HAD (Budka, 1986; Masliah et al., 1992; Navia et al., 1986a; Persidsky et al., 1997; Sharer et al., 1985; Zheng and Gendelman, 1997; Zink et al., 2002). IL-1β and TNF-α, two of the most extensively investigated pro-inflammatory cytokines, are produced primarily by HIV-1-infected macrophages and microglia within the CNS and participate in the pathogenesis of HAD (Gelbard, 1999; Kaul et al., 2005; Wesselingh et al., 1993; Zhao et al., 2001). Increased IL-1β and TNF-α during HAD may amplify the inflammatory response through further recruitment of leukocytes to the site of infection by the production of chemokines, including CXCL8.
CXCL8 is primarily a neutrophil chemokine, but it also elicits chemotaxis and activation of monocytes and T cells (Baggiolini et al., 1989; Gerszten et al., 1999; Larsen et al., 1989; Yoshimura et al., 1987). CXCL8 has also been demonstrated to facilitate CCL2-mediated monocyte migration (Gouwy et al., 2008). Thus CXCL8 is one of the important chemokines that recruit or facilitate the leukocyte recruitment during inflammation. Particularly in HIV-1-infected individuals, elevated CXCL8 has been found in the serum, lung, lymphoid tissue, and plasma (Carrol et al., 2007; Denis and Ghadirian, 1994; Lane et al., 2001; Matsumoto et al., 1993). A wide variety of cells are capable of producing CXCL8, including activated microglia and astrocytes found in HIV-1 encephalitic brain tissue (D'Aversa et al., 2008; Sanders et al., 1998; Xiong et al., 2003). Astrocytes have been suggested to synthesize CXCL8 in response to IL-1β and TNF-α stimulation (Aloisi et al., 1992). However, the sources and regulatory mechanisms of CXCL8 in astrocytes remain undefined.
Inflammatory responses of astrocytes are regulated by alteration of the phosphorylation status of multiple protein kinases. Mitogen-activated protein kinases (MAPKs) comprise a family of serine/threonine kinases, which participate in signal transduction pathways that control intracellular events including acute responses to cytokines (Pearson et al., 2001). Three MAPK families, p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK1/2), are relevant to stress and inflammation. Also relevant to cellular inflammatory responses, dsRNA-dependent protein kinase (PKR) was initially characterized as a translational inhibitor in an antiviral pathway (Stark et al., 1998). Later, PKR was identified as a component of many signal transduction pathways mediated by inflammatory cytokines and stress [for review, see (Williams, 1999)]. Understanding the signaling cascades of MAPKs and PKR in astrocytes is of particular importance for the cellular response of astrocytes to stress and inflammatory stimuli.
Previously, we reported that HIV-1-infected and/or immune activated macrophages regulate SDF-1 (CXCL12) production by astrocytes through IL-1β (Peng et al., 2006). In this report, we investigated whether interactions between macrophages and astrocytes regulate CXCL8 production during inflammation. Our data demonstrates CXCL8 levels in the cerebrospinal fluid (CSF) of HAD patients are higher than HIV-1 seropositive patients without neurological impairment. Immune-activated and/or HIV-1-infected human monocyte-derived-macrophage (MDM) conditioned media (MCM) induced production of CXCL8 by human astrocytes. The observed CXCL8 production is dependent on MDM secretion of IL-1β and TNF-α as a result of viral and immune activation. We investigated the intracellular signaling pathways associated with astrocyte CXCL8 regulation and found PKR and MAPKs, including p38, JNK, and ERK1/2, were involved in astrocyte CXCL8 induction. These results help to define the interaction between activated macrophages and astrocytes, and the relevance of this interaction to the pathogenesis of HAD through the production of CXCL8.
2. Materials and methods
2.1 Reagents
Recombinant proteins, neutralizing antibodies or chemicals were obtained as follows: IL-1β, TNF-α, IL-1 receptor antagonist (IL-1RA), soluble TNF receptor 1, soluble TNF receptor 2 (R&D Systems, Minneapolis, MN), Interferon-α (IFN-α) neutralizing antibody (PBL Interferon Source, Piscataway, NJ), Interferon-β (IFN-β) neutralizing antibody (PBL Interferon Source), lipopolysaccharide (LPS, Sigma-Aldrich, St. Louis, IL). Inhibitors used were SB203580 (Cat. No. 559389) for phospho-p38, SP600125 (Cat. No. 420119) for phospho-JNK, PD98059 (Cat. No. 513000) for phospho-ERK1/2, PKR Inhibitor (Cat. No. 527450) and a negative control of PKR Inhibitor (Cat. No. 527455) for phospho-PKR (Calbiochem, San Diego, CA).
2.2 Human fetal astrocyte culture
Human fetal astrocytes were isolated from human fetal brain tissue (gestational age 13-16 weeks) from elective aborted specimens in full compliance with the University of Nebraska Medical Center (UNMC) and National Institutes of health (NIH) ethical guidelines. Human astrocytes were isolated from fetal brain tissue cortices as previously described (Zheng et al., 1999). Cells were cultured at a density of 2 × 107 cells/150 cm2 in DMEM/F12 (Invitrogen), supplemented with fetal bovine serum (FBS, 10%) (GIBCO Invitrogen Corp) and an antibiotic mixture containing penicillin, streptomycin, and neomycin (Invitrogen). Astrocyte preparations were assessed by immunocytochemical staining using an antibody against glial fibrillary acidic protein (GFAP, Dako Corp., Carpinteria, CA). This process yields a culture of >98% pure astrocytes.
2.3 Monocyte cell culture, HIV-1 infection and conditioned media collection
Human monocytes were recovered from peripheral blood mononuclear cells (PBMCs) of HIV-1, 2 and hepatitis B seronegative donors after leukopheresis and counter current centrifugal elutriation (Gendelman et al., 1988). Monocytes were cultured as adherent monolayers at a density of 1.1 × 106 cells/well in 24-well plates and cultivated in Dulbecco's modified Eagles medium (DMEM, GIBCO Invitrogen Corp, Carlsbad, CA) with 10% heat-inactivated pooled human serum (Cambrex Bio Science, Walkersville, MD), 50 μg/ml gentamicin and/or 10 μg/ml ciprofloxacin (Sigma-Aldrich) and 1000 U/ml highly purified recombinant human macrophage colony stimulating factor (M-CSF) (a generous gift from Wyeth Institute, Cambridge, MA).
Seven days after plating, MDM were infected with HIV-1 at a multiplicity of infection of 0.1. On the second day, media was removed and substituted with MDM culture media that was half-exchanged every two days (Gendelman et al., 1988). Stock virus was screened for mycoplasma and endotoxin using hybridization and Limulus amebocyte lysate assays, respectively. Four to five days after infection, HIV-1-infected and uninfected MDM were treated with/without LPS (0.1 μg/ml) for 4 hours. Cells were then rinsed 2 times with DMEM to remove residual LPS and serum free DMEM/F12 media was placed onto MDM for 24 hours. Culture supernatants were obtained as macrophage-conditioned media (MCM) and subsequently stored at -80°C until assayed.
2.4 RNA extraction and TaqMan real-time RT-PCR
Total RNA was isolated with TRIzol Reagent (Invitrogen) and RNeasy Mini Kit (QIAGEN Inc., Valencia, CA). Assays-on-Demand primers for human CXCL8 (ID#, Hs00174103_m1) and human GAPDH (ID#, 4310884E) were purchased from Applied Biosystems Inc (Foster City, CA). Real-time reverse-transcription polymerase chain reaction (RT-PCR) was carried out using the one-step quantitative TaqMan Real-time RT-PCR system (Applied Biosystems Inc.). CXCL8 mRNA levels were determined and standardized with GAPDH internal control using comparative ΔΔCT method. All the primers used in the study were tested for amplification efficiencies and the results were similar.
2.5 Detection of IL-1β, TNF-α and CXCL8 by ELISA
IL-1β, TNF-α and CXCL8 concentrations from culture supernatant or CSF were determination by an in-house sandwich ELISA (Erichsen et al., 2003). Maxisorp 96-well plates (Nunc, Rochester, NY) were coated with monoclonal antibodies specific for IL-1β, TNFα and CXCL8 (R&D Systems, Minneapolis, MN) by overnight incubation at room temperature. Nonspecific binding sites were blocked with 1% BSA in PBS for 2 hours, washed, and then a standard series of diluted human recombinant IL-1β, TNF-α or CXCL8 (R&D Systems) was added to each plate along with supernatant samples of conditioned media. This was incubated for 2 hours followed by washing. Next, biotinylated goat anti-human IL-1β, TNF-α and CXCL8 antibodies (R&D Systems) were added for 1 hour followed by washing. The standards/samples were incubated with streptavidin-conjugated horseradish-peroxidase (HRP) for 30 minutes. After extensive washing with PBS containing 0.05% Tween-20, the tetramethylbenzidine (TMB) substrate for HRP was added to the plate and the reaction was stopped by the addition of 50 μl of 1 M H2SO4. Absorbance was read at 450 nm with Kinetic Vmax Microplate Reader (Molecular Devices, Sunnyvale, CA) and compared to standard values for quantification.
2.6 Western blot analysis
Cell lysates from astrocytes were prepared with M-PER Mammalian Protein Extraction Buffer (Pierce). Protein concentration was determined using the BCA Protein Assay Kit (Pierce). Protein (20 μg) was electrophoresed on 8% SDS-PAGE and transferred to an Immuno-Blot PVDF membrane (Bio-Rad). All phospho-specific antibodies and their corresponding total antibodies in this study were from Cell Signaling Technology, Inc (Beverly, MA). Loading control β-actin proteins were detected using anti-β-actin (Sigma-Aldrich) antibodies. Membranes were treated overnight with primary antibody at 4ºC followed by a horseradish peroxidase-ligand secondary anti-rabbit (Cell Signaling Technologies Inc.) or anti-mouse (Cell Signaling Technologies Inc.) antibody for 1 hour at room temperature. Antigen-antibody complexes were visualized by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ) and captured with CL-X Posure™ Film (Pierce). For data quantification the films were scanned with a CanonScan 9950F scanner; the acquired images were then analyzed on a Macintosh computer using the public domain NIH image program (http://rsb.info.nih.gov/nih-image/).
2.7 HIV-1 infected Subjects
CSF samples from twenty-three HIV-1-infected patients with advanced disease were provided by the Manhattan HIV Brain Bank, which is currently supported by the NIH (R24MH59724). Patients were accrued in accordance with Health and Human Services regulation 45CFR46. The mean CD4+ T lymphocyte count for all patients was 181 cells/mm3 and the mean viral load was 86,276 copies/ml (Roche Diagnostic Systems Inc., Somerville, NJ). All patients are under antiviral therapy. Laboratory tests included lymphocyte subset analysis, and measurement of HIV load in plasma (Table 1). At the time of evaluation, all patients were free of acute systemic or opportunistic infections. A battery of neuropsychological tests was administered to each subject. The subjects were divided into two groups: neurocognitively impaired (Memorial Sloan Kettering (Bouwman et al., 1998), MSK≥1, impaired, n=15) and neurocognitively unimpaired (MSK=0, non-impaired, n=8)(Table 1).
Table 1. Clinical characteristics of all study subjects.
| Subject/sex | Age | Ethnic group | MSK | Impaired | CD4+ T cells (cells/μl) | WBC | %Lymphs | %Monos | CSF WBC | CSF Protein | Viral Load, HIV-1 RNA (copies/ml) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/F | 63 | AF-AM | 0 | No | 44 | 3.3 | 21.7 | 6.3 | 2 | 59 | 378,334 |
| 2/M | 52 | AF-AM | 0 | No | 295 | 4.3 | 21.2 | 16.2 | 1 | 64 | <50 |
| 3/M | 31 | HISP | 0 | No | 120 | 5.1 | 39.7 | 5.0 | 3 | 55 | 429 |
| 4/M | 39 | CAUC | 0 | No | 41 | 4.8 | 14.2 | 7.2 | 1 | 35 | 143,000 |
| 5/F | 40 | HISP | 0 | No | 88 | 3.1 | 23.5 | 12.6 | 1 | 34 | 3,600 |
| 6/F | 32 | AF-AM | 0 | No | 556 | 3.8 | 64.8 | 7.1 | 4 | 44 | 116,132 |
| 7/F | 39 | CAUC | 0 | No | 188 | 2.6 | 21.2 | 9.1 | 1 | 37 | <50 |
| 8/M | 40 | AF-AM | 0 | No | 303 | 5.2 | 53 | 17 | 4 | 74 | 370,000 |
| 9/M | 52 | AF-AM | 1 | Yes | 4 | 1.7 | 15.8 | 7.8 | 0 | 76 | 117,361 |
| 10/M | 40 | AF-AM | 1 | Yes | 140 | 5.5 | 30.5 | 7.8 | 3 | 59 | 56,000 |
| 11/F | 56 | AF-AM | 1 | Yes | 155 | 4.6 | 24.6 | 17.3 | 5 | 44 | NA |
| 12/M | 50 | AF-AM | 1 | Yes | 208 | 2.6 | 43.3 | 13.2 | 9 | 83 | 13,100 |
| 13/M | 37 | HISP | 1 | Yes | 21 | 2.9 | 25 | 10.2 | 2 | 32 | NA |
| 14/F | 33 | AF-AM | 1 | Yes | 18 | 2.1 | 33.5 | 13.5 | 0 | 30 | 5,337 |
| 15/F | 40 | AF-AM | 1 | Yes | 7 | 3.4 | 14.7 | 14.1 | 0 | 28 | >75,000 |
| 16/M | 37 | AF-AM | 1 | Yes | 122 | 2.7 | 28.9 | 14.3 | 3 | 49 | 18,009 |
| 17/M | 55 | AF-AM | 1 | Yes | 849 | 9.1 | 37.9 | 9.8 | 4 | 105 | <50 |
| 18/M | 55 | AF-AM | 2 | Yes | 14 | 3.3 | 22.9 | 10.7 | 1 | 44 | 18,894 |
| 19/M | 44 | CAUC | 2 | Yes | 72 | 3.5 | 21.8 | 7.5 | 1 | 80 | 55,256 |
| 20/M | 48 | HISP | 2 | Yes | 169 | 6.5 | 40.1 | 12 | 2 | 60 | 2,592 |
| 21/M | 48 | CAUC | 2 | Yes | 536 | 6.4 | 40.4 | 7.8 | 1 | 53 | 207 |
| 22/M | 46 | HISP | 2 | Yes | 371 | 6.5 | 14.2 | 9.1 | 1 | 37 | 1,919 |
| 23/M | 42 | HISP | 3 | Yes | 84 | 3.8 | 31.5 | 10.8 | 2 | 55 | 20,655 |
Note. MSK, memorial Sloan Kettering; Abbreviations: NA, not available; ND, not detectable
2.8 Statistical tests
Data was analyzed as means ± standard deviation (SD) unless otherwise specified. The data was evaluated statistically by the analysis of variance (ANOVA), followed by the Tukey-test for paired observations. Significance was considered with a p-value less than 0.05. All experiments were performed with at least three donors to account for any donor specific differences. Assays were performed at least three times in triplicate or quadruplicate.
3 Results
3.1 CXCL8 levels in CSF are elevated in HAD patients
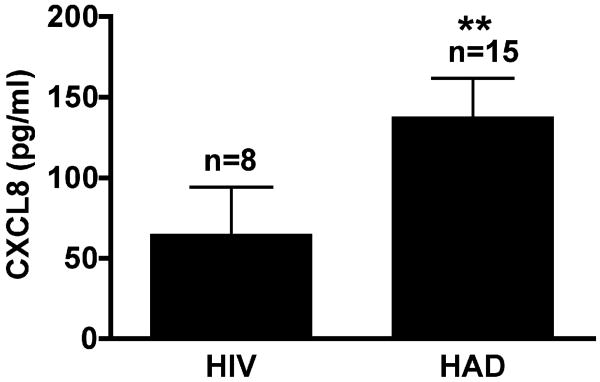
Regulation of CXCL8 in the context of HIV-1 encephalitis remains unclear. As a pilot study, we examined the CXCL8 levels by ELISA in the CSF samples of 15 HAD cases (MSK≥1(Bouwman et al., 1998), impaired, n=15) and compared the results to those obtained from HIV-1 seropositive (MSK=0, non-impaired, n=8) donors without neurological impairment (Table 1). All patients are under antiviral therapy. CXCL8 levels were higher in the CSF of the HAD group (137.7 ± 24.1 pg/ml) as compared to the HIV-1 seropositive (64.8 ± 29.5 pg/ml) individuals without neurological impairment (P < 0.01) (Fig. 1). The increase of CXCL8 in CSF was not associated with injection drug use, viral load, CD4+ cell counts, CSF proteins levels, CSF WBC or cognitive impairment scores. Although this is a pilot study with a small sample size, these data demonstrated increased CXCL8 levels in the CNS during HIVE. Considering CXCL8 has recently been demonstrated to facilitate CCL2-mediated monocyte migration in the inflammation process (Gouwy et al., 2008) and participates in HAD neuropathogenesis (Xiong et al., 2003), we aim to understand the mechanism by which CXCL8 is elevated during HIV encephalitis.
Figure 1.
CXCL8 levels in CSF of HIV-1 infected subjects. CSF samples were collected from HIV-1 infected patients with or without neurological impairment. CSF samples were assayed for CXCL8 by ELISA. Results are expressed as mean ± standard error of the mean of indicated number donors.
3.2 MDM-conditioned media (MCM) stimulates CXCL8 production in human fetal astrocytes
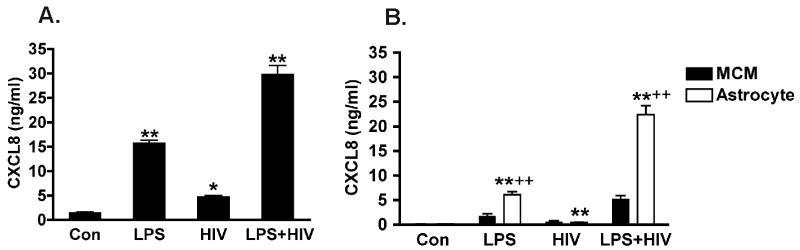
We first investigated the cell types involved in CXCL8 elevation during HIVE. HIV-1-infected and activated macrophage/microglia are central to the neuropathogenesis of HAD therefore these cells were a necessary part of our investigation. To study the effect of infection and activation of human macrophages for CXCL8 production, we used a well-characterized human MDM culture system (Gendelman et al., 1988). LPS was used to stimulate MDM to mimic their in vivo activation. We used 0.1 μg/ml LPS to stimulate MDM for 4 hours, following which the media were removed and the cultures rinsed with fresh media to remove residual LPS. CXCL8 concentration in the macrophage culture supernatants was determined 24 hours after rinsing. HIV infection induced a mild increase in CXCL8 production compared to control. LPS alone stimulated a marked increase of CXCL8 production in MDM, whereas HIV-1 infection potentiated LPS-stimulated CXCL8 production by MDM (Fig. 2A).
Figure 2.
HIV-1-infected and LPS-activated MCM stimulates CXCL8 production in astrocytes. Astrocytes were plated in 24-well plates in triplicate. MCM from control, LPS, HIV, or LPS/HIV MDM (5 days after infection) were added to and accounted for 20% volume of the total astrocyte cultures. (A) CXCL8 concentrations in MCM were determined by ELISA. (B) Astrocyte-produced CXCL8 after MCM treatment was determined by ELISA. *Denotes P < 0.05, ** Denotes P < 0.01 in comparison to control. ++ Denotes P < 0.01 compared with parallel MCM CXCL8 levels before the treatments. Results are representative of three independent experiments.
Interactions between HIV-1-infected and/or immune-activated macrophages and astrocytes may be an important component of pathogenesis of HAD. We investigated the role of astrocytes, the most abundant cell type in the CNS, in the elevation of CXCL8. We used conditioned medium from control, LPS-activated, HIV-1-infected, or LPS-activated/HIV-1-infected MDM (MCM) to treat human astrocyte cultures. The concentration of CXCL8 in the astrocyte culture supernatants was determined following (24-hour) MCM treatment. LPS-activated/HIV-1infected MCM significantly increased CXCL8 levels in astrocyte supernatants as compared to either the LPS-activated MCM treatment or the residual CXCL8 from MCM (Fig. 2B). These data suggest that HIV-1-infected and/or immune-activated MDM release factors that stimulate astrocyte CXCL8 production.
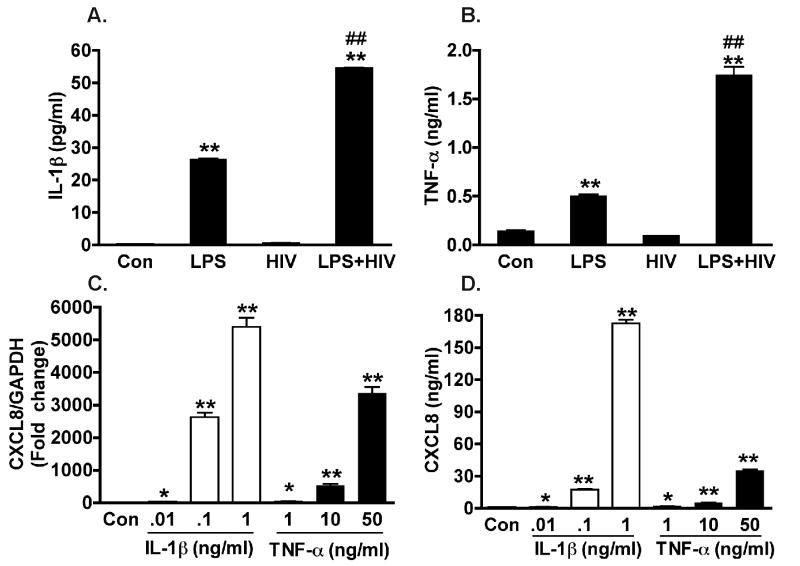
3.3 MCM-mediated astrocyte CXCL8 production is dependent on TNF-α and IL-1β
HIV-1-infected and/or immune-activated MDM have been known to produce IL-1β and TNF-α. To further investigate this, the levels of these two factors were measured in the MCM that was used to stimulate the astrocytes. While control MCM had minimal levels of IL-1β and TNF-α, following LPS activation, both IL-1β and TNF-α concentration were increased to 26 pg/ml and 0.5 ng/ml, respectively. In the LPS-activated and HIV-1-infected MCM, the levels of IL-1β and TNF-α were significantly higher (50 pg/ml and 1.7 ng/ml, respectively, Fig 3A and B). We then treated astrocytes with different doses of recombinant IL-1β and TNF-α. The addition of IL-1β (10 to 1000 pg/ml) and TNF-α (1 to 50 ng/ml) elicited a dose-dependent increase of CXCL8, at the RNA and protein levels (Fig 3C and D).
Figure 3.
Levels of IL-lβ and TNF-α in LPS-stimulated and/or HIV-1-infected MCM and increased CXCL8 expression in astrocytes by IL-lβ and TNF-α. Concentrations of IL-1β (A) and TNF-α (B) in the MCM were determined by ELISA. After the stimulation with differing doses of recombinant IL-lβ (10 to 1000 pg/ml) and TNF-α (1 to 50 ng/ml) for 24 hours, CXCL8 mRNA expression (C) and protein concentrations (D) in astrocytes were determined by real time RT-PCR and ELISA, respectively. For real time RT-PCR, results were normalized with GAPDH and shown as folds change over control. * Denotes P < 0.05, ** Denotes P < 0.01 compared to control. ## Denotes P < 0.01 compared to LPS. Results are are representative of three independent experiments.
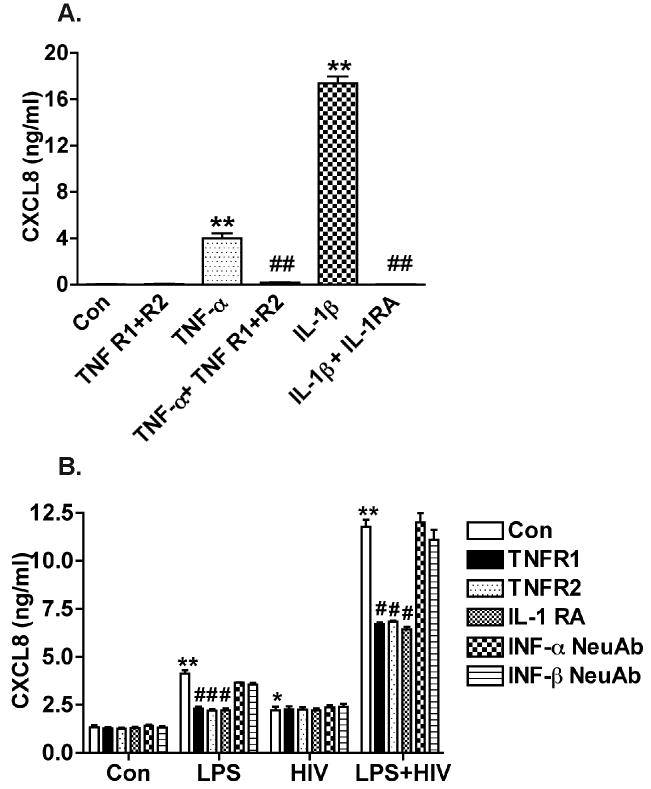
To further test whether MCM-mediated human astrocyte CXCL8 production is dependent on IL-1β and TNF-α, we pretreated astrocytes with different blocking reagents to neutralize TNF-α or antagonize IL-1β activities in MCM. Blocking reagents included soluble TNF-R1 (100 ng/ml, neutralizing TNF-α), soluble TNF-R2 (100 ng/ml, neutralizing TNF-α), IL-1 receptor antagonist (IL-1 RA) (100 ng/ml), IFN-α neutralizing antibody (1000 ng/ml), and IFN-β neutralizing antibody (1000 ng/ml). All of the reagents were found to successfully block the corresponding cytokines (Fig. 4A and data not shown). After pre-incubation with the blocking agents for one hour, MCM was added to the astrocyte cultures for 24 hours. CXCL8 production by LPS-activated or HIV-1-infected and LPS-activated MCM was significantly reduced by soluble TNF-R1, soluble TNF-R2, and IL-1 RA, but not by the interferon neutralizing antibodies (Fig. 4B). Additionally, we observed a mild increase of CXCL8 in HIV-1 MCM, an effect that could not be reduced by soluble TNF-R or IL-1 RA (Fig. 4B). Together, these results demonstrate that HIV-1 infection alone induced only a small increase of CXCL8 in astrocyte culture. However, HIV-1 infection dramatically potentiated the effect of LPS-activated macrophages on the production of CXCL8 by astrocytes through inflammatory cytokines IL-1β and TNF-α.
Figure 4.
MCM-mediated astrocyte CXCL8 production is dependent on IL-1β and TNF-α. A) Soluble receptors for TNF-α (TNF-R1 and TNFR2, 100 ng/ml), IL-1 receptor antagonist (100 ng/ml) were mixed with TNF-α (10 ng/ml) or IL-1β (1 ng/ml) as indicated for 1 hour prior to astrocytes treatment for 24 hours. ** Denotes P < 0.01 compared with control, ## Denotes P < 0.01 compared with TNF-α or IL-1 treatment. B) Soluble TNFR1, TNFR2, IL-1 receptor antagonist, or Interferon neutralizing antibody (1000 ng/ml) was mixed with MCM for 1 hour prior to the treatment for 24 hours. * Denotes P < 0.05, ** Denotes P < 0.01 compared with control. # Denotes P < 0.05, ## Denotes P < 0.01 compared with LPS or LPS+HIV-treated astrocytes. Results are representative of three independent experiments.
3.4 MCM-mediated CXCL8 production in astrocytes is dependent on MAPKs
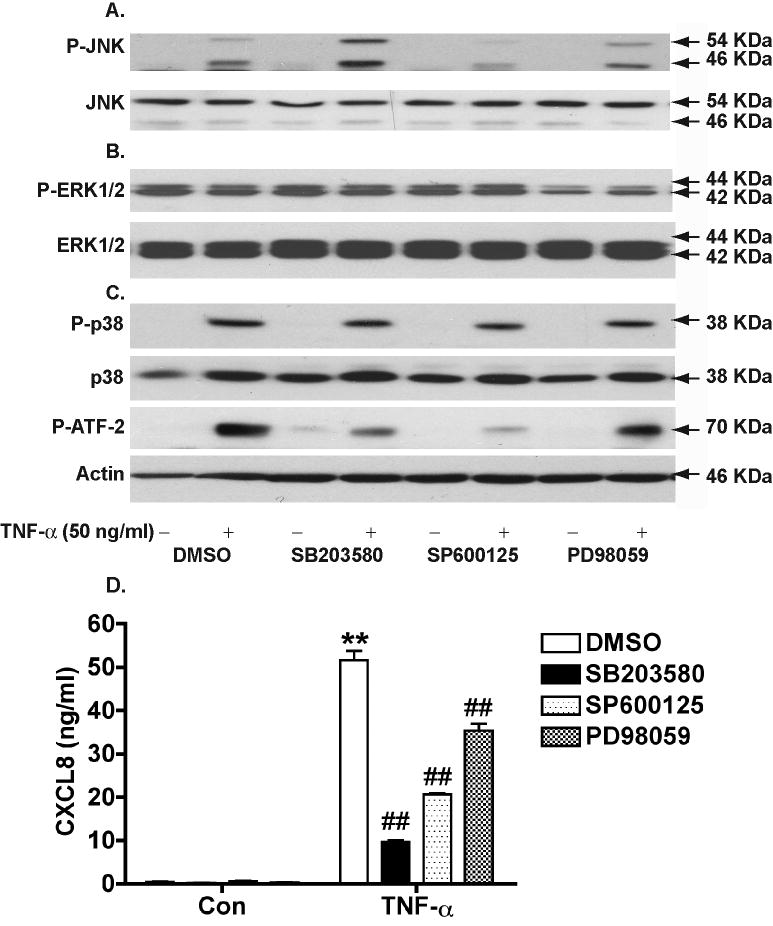
MAPK pathways are known to participate in CXCL8 gene regulation during the inflammatory process (Hoffmann et al., 2002). To identify the specific MAPK involved in CXCL8 production by astrocytes, we used a panel of MAPK inhibitors, including SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), and PD98059 (ERK1/2 inhibitor). Pre-incubation of astrocyte cultures with SP600125, prior to TNF-α treatment, reduced phosphorylation of p54 and p46 of JNK by 61% and 55%, respectively (Fig. 5A). Similarly, pre-incubation with PD98059 reduced ERK phosphorylation by 44% (Fig. 5B). These percentages of inhibition were calculated based on the densimetrical quantification of Western blotting. SB203580, surprisingly, only reduced p38 phosphorylation by 28%, which is very similar to the 25% p38 inhibition by SP600125 and the 18% inhibition by PD98059 (Fig. 5C). However, we observed that SB203580 decreased the phosphorylation of activating transcription factor 2 (ATF-2), a p38-downsteam factor, by 72%. Inhibition of ATF-2 phosphorylation was also caused by SP600125 (86%) (Fig. 5C). This was expected because JNK is also a well-known upstream kinase for ATF-2 (Gupta et al., 1995). Thus, we concluded that inhibitors for three classic MAPKs, including SP600125, SB203580 or PD98059, appear to be specific for their target kinase and do not cause significant inhibition of the phosphorylation of other kinases (Fig 5A-C). Next, we tested CXCL8 levels in astrocytes with these same inhibitors treatment. TNF-α induced a significant increase of CXCL8 levels in astrocytes, and this increase was significantly reduced by SB203580 (81% inhibition), SP600125 (60% inhibition), or PD98059 (31% inhibition) (Fig. 5D). These result suggested the MAPK pathway, particularly p38 and JNK, and to a less extent ERK1/2, are required for CXCL8 production in astrocytes as induced by TNF-α.
Figure 5.
TNF-α-mediated CXCL8 production is dependent on MAPKs. Astrocytes were pre-incubated with SB203580 (20 μM), SP600125 (20 μM), or PD98059 (20 μM) for 2 hours, and then treated with TNF-α (50 ng/ml) for 30 minutes. DMSO was as a solvent control for inhibitors. Phospho-JNK (P-JNK, Thr183/Tyr185) and total JNK (A), Phospho-ERK1/2 (P-ERK1/2, Thr202/Tyr204) and total ERK1/2 (B), Phospho-p38 (P-p38, Thr180/Tyr182), phospho-ATF-2 (Thr71), and total p38 (C) were detected by Western blotting. β-actin was used as a loading control for blots A-C. D) CXCL8 levels were determined 24 hours after the treatment by ELISA. ** Denotes P < 0.01 compared with control, ## Denotes P<0.05 compared with TNF-α treatment without inhibitors.
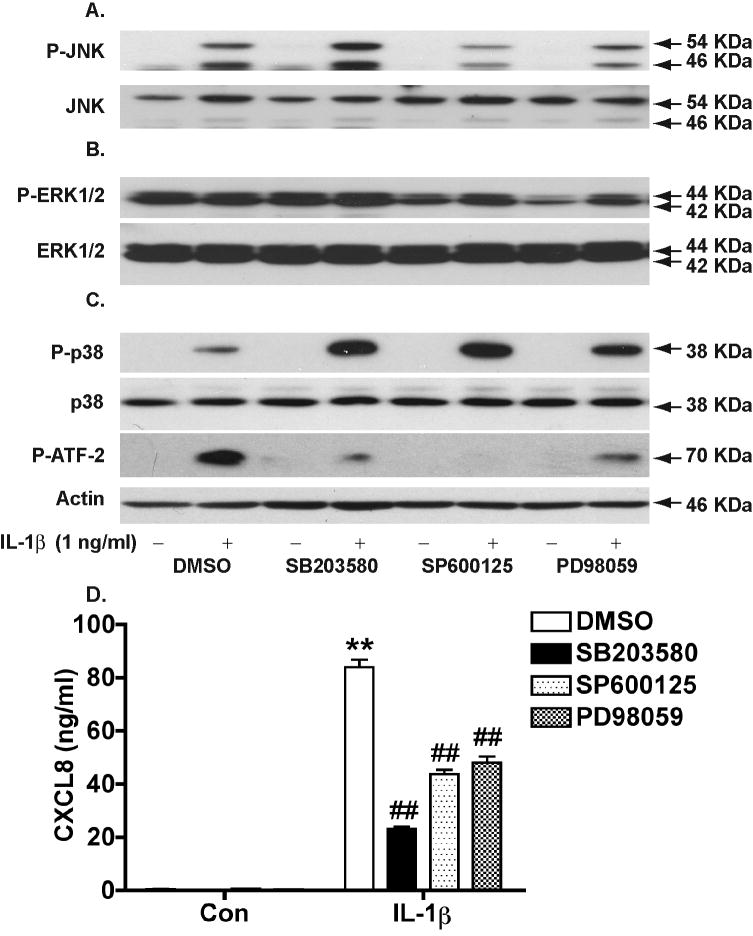
Similar to the effects of MAPK inhibitors on astrocytes treated with TNF-α, pre-incubation with MAPK inhibitors, prior to IL-1β treatment, significantly reduced each target kinase phosphorylation (Fig. 6A-C). IL-1β-induced phosphorylation of p54 and p46 JNK was reduced by 56% and 65%, respectively, when treated with SP600125 (Fig. 6A). PD98059 reduced IL-1β-stimulated phosphorylation of ERK by 35% (Fig. 6B). SB203580, surprisingly, increased IL-1β-stimulated p38 phosphorylation by 2.5 folds, which is very similar to the 2.5-fold increase of p38 by SP600125 and 2.4-fold increase of p38 by PD98059 (Fig. 6C). Inhibition of ATF-2 phosphorylation was observed following SB203580 (82%) and SP600125 (94%) preincubation and IL-1β stimulation (Fig. 6C). Accordingly, IL-1β-induced CXCL8 production in astrocytes was significantly reduced by SB203580 (73% inhibition), SP600125 (48% inhibition), or PD98059 (43% inhibition). The similar pattern of inhibition by specific MAPK inhibitors suggests a common regulation of CXCL8 by MAPK, particularly p38, whose inhibition resulted in the greatest reduction of CXCL8.
Figure 6.
IL-1β-mediated CXCL8 production is dependent on MAPKs. Astrocytes were pre-incubated with SB203580 (20 μM), SP600125 (20 μM), or PD98059 (20 μM) for 2 hours, and then treated with IL-1β (1 ng/ml) for 30 minutes. DMSO was as a solvent control for inhibitors. Phospho-JNK (P-JNK, Thr183/Tyr185) and total JNK (A), Phospho-ERK1/2 (P-ERK1/2, Thr202/Tyr204) and total ERK1/2 (B), Phospho-p38 (P-p38, Thr180/Tyr182), phospho-ATF-2 (Thr71), and total p38 (C) were detected by Western blotting. β-actin was used as a loading control for blots A-C. D) CXCL8 levels were determined 24 hours after the treatment by ELISA. ** Denotes P < 0.01 compared with control, ## Denotes P < 0.05 compared with IL-1β treatment without inhibitors.
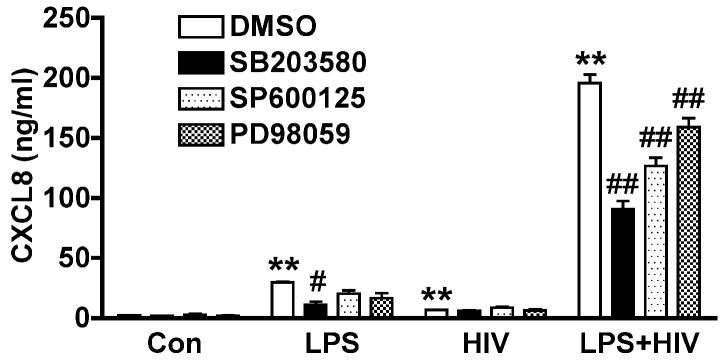
We next investigated the role of MAPKs in the MCM-mediated CXCL8 production by astrocytes. The addition of LPS-activated MCM induced CXCL8 in astrocytes, while pre-incubation of astrocytes cultures with p38 inhibitor SB203580 significantly reduced CXCL8. JNK and ERK1/2 inhibitors were also used under these conditions and while they also caused a small reduction in CXCL8 levels, it was not statistically significant. Further, LPS-activated/HIV-1-infected MCM induced CXCL8 in astrocytes and this effect was significantly reduced by SB203580 (55% inhibition), SP600125 (35% inhibition), or PD98059 (21% inhibition) (Fig. 7). The mild increase of CXCL8 in HIV MCM-treated astrocytes was not affected by the application of inhibitors, indicating HIV-1-mediated CXCL8 is independent of MAPKs. Together, these data demonstrate that HIV-infected and/or LPS-activated MCM-mediated CXCL8 production was through MAPK pathway, particularly p38 and JNK.
Figure 7.
MCM-mediated CXCL8 production is dependent on MAPKs. Astrocytes were pre-incubated with SB203580 (20 μM), SP600125 (20 μM), or PD98059 (20 μM) for 2 hours, and then treated with MCM (25% volume of culture media) for 24 hours. CXCL8 levels were determined by ELISA. ** Denotes P < 0.01 compared with control, ## Denotes P < 0.01 compared with LPS+HIV MCM-treatment without the inhibitors.
3.5 MCM-mediated CXCL8 production in astrocytes is dependent on PKR
PKR plays a major role in the cellular response to viral infection and has been found to mediate inflammatory signals in the cells of the innate immune system. The involvement of PKR in CXCL8 production in astrocytes has been demonstrated in mice (Park et al., 2006) and whether this pathway also participates in CXCL8 production in human macrophage-astrocyte interaction during HIV-1 infection remains to be determined. In this study we investigated the role of PKR in the production of CXCL8 in human astrocytes. We found that TNF-α or IL-1β treatment caused increased phosphorylation of PKR, and that this phosphorylation was ablated by the presence of an imidazolo-oxindole compound, PKR Inhibitor, which acts as a potent ATP-binding site inhibitor for PKR (Jammi et al., 2003) (Fig. 8A). Further, TNF-α or IL-1β-mediated CXCL8 production by astrocytes was also completely blocked by treatment with PKR Inhibitor (Fig 8B). Since inhibitors based on ATP-binding sites tend to have a nonspecific effect on other kinases, particularly at high concentrations, we have also tested the effect of PKR Inhibitor on MAPKs, which participate in CXCL8 regulation. The phosphorylation of JNK, ERK1/2, p38, and ATF-2 by either TNFα or IL-1β was not affected by the treatment of PKR inhibitor at 1 μM (Fig. 8C-E). These results suggest that PKR inhibitor, while not affecting MAPKs phosphorylation, plays a pivotal role in cytokine-mediated astrocyte CXCL8 production.
Figure 8.
TNF-α or IL-1β-mediated CXCL8 production is dependent on PKR phosphorylation. A) Astrocytes were pre-incubated with the PKR Inhibitor (1 μM) for two hours and then treated with TNF-α (50 ng/ml) or IL-1β (1 ng/ml) for 24 hours. Inactive analogue of the PKR Inhibitor (PKR inhibitor control, 1 μM) was used as control. A) phospho-PKR (Thr451), and total PKR were detected by Western blotting. β-actin was used as a loading control. B) CXCL8 levels were determined by ELISA. * Denotes P < 0.05, ** Denotes P < 0.01 compared to control, ## Denotes P < 0.01 compared with corresponding TNF-α or IL-1β treatment without inhibitors. C-E) Astrocytes were pre-incubated with PKR inhibitor (1 μM) for two hours and then treated with TNF-α (50 ng/ml) or IL-1β (1 ng/ml) for 30 minutes. Phospho-JNK (P-JNK, Thr183/Tyr185) and total JNK (C), Phospho-ERK1/2 (P-ERK1/2, Thr202/Tyr204) and total ERK1/2 (D), Phospho-p38 (P-p38, Thr180/Tyr182), phospho-ATF-2 (Thr71), and total p38 (E) were detected by Western blotting. β-actin was used as a loading control for blots C-E.
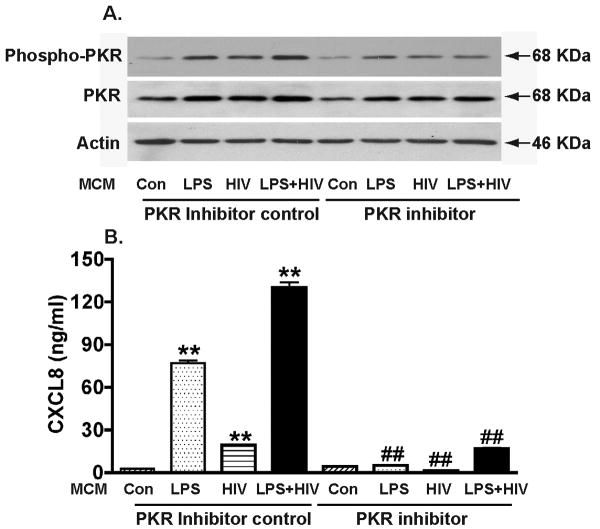
To determine the role of PKR in macrophage-mediated astrocyte CXCL8 production, we assessed the phosphorylation of PKR by MCM treatment. Notably, in astrocyte cultures, both LPS-activated and HIV-1-infected MCM induced phosphorylation of PKR, while LPS-activated/HIV-1-infected MCM induced higher levels of PKR phosphorylation, suggesting an additive effect between LPS activation and HIV-1-infection of MDM (Fig. 9A). Pre-incubation with the PKR Inhibitor reduced both LPS-activated and/or HIV-1-infected MCM-induced PKR phosphorylation. CXCL8 production, mediated by LPS-activated MCM, HIV-1-infected MCM or HIV-1 infected/LPS-activated MCM, was completely blocked by PKR Inhibitor (Fig. 9B). Together, these data demonstrated an important role for PKR in MCM, IL-1β, and TNF-α-mediated CXCL8 production in astrocytes.
Figure 9.
MCM-mediated CXCL8 production is dependent on PKR phosphorylation. A) Astrocytes were pre-incubated with the PKR Inhibitor (1 μM) for two hours and then treated with control, LPS, HIV, LPS+HIV MCM (25% volume of culture media) for 24 hours. Inactive analogue of PKR inhibitor (PKR inhibitor control, 1 μM) was used as control. A) phospho-PKR (Thr451), and total PKR were detected by Western blotting. β-actin was used as a loading control. B) CXCL8 levels were determined by ELISA. ** Denotes P < 0.01 compared to control, ## Denotes P < 0.01 compared with corresponding MCM treatment with inhibitors.
4. Discussion
Macrophages and astrocytes play an important role in the regulation of inflammatory and immune responses in HAD. One factor that participates in this regulation is chemokine CXCL8, which is produced by astrocytes in response to IL-1β and TNF-α, as previously reported (Aloisi et al., 1992). Here, we demonstrate that CXCL8 levels in the CSF of HAD patients are higher than HIV-1 seropositive patients without neurological impairment. In an attempt to determine the source and the regulation of CXCL8 within the CNS during HAD, we observed that LPS-activated and/or HIV-1-infected MCM induced CXCL8 production by human astrocytes and was dependent on MDM production of IL-1β and TNF-α. We next investigated the intracellular signaling pathways of astrocytes that are associated with CXCL8 production. We identified PKR and the MAPKs, p38, JNK, and ERK1/2 as important regulators involved in astrocyte CXCL8 production as induced by cytokines released from macrophages. In contrast, HIV-mediated CXCL8 production is independent of MAPKs but is reliant upon PKR. These findings further demonstrate that HIV-1 infected and activated macrophage, the only productively infected cells in the brain and the important cells for the pathogenesis of HAD, drive CXCL8 production following infection and activation and mediate CXCL8 production by astrocytes.
The importance of HIV-1-infected and immune-activated mononuclear phagocytes (MP, macrophage and microglia) in HAD is widely recognized (Gendelman, 1997; Glass et al., 1995; Koenig et al., 1986; Nath and Geiger, 1998; Strizki et al., 1996; Wiley, 1995; Zheng and Gendelman, 1997). MP contribute to the disease process by mediating brain inflammation through secretion of viral proteins or induction of cytokines that target glial cells and neurons (Merrill and Chen, 1991). Although MP produce CXCL8, our studies found that HIV-1-infected and immune-activated MP interact with astrocytes and stimulate an increase in CXCL8 levels in these cells (Fig. 2). This is consistent with a previous report that found CXCL8 expression in astrocytes in HIV-1 encephalitic tissue (Sanders et al., 1998). CXCL8 secreted by astrocytes may mediate or facilitate the extravasation of monocyte/macrophages through the blood-brain barrier. This not only leads to increased viral entry, but also activates monocytes/macrophages, which allows for higher level of viral replication. Since a recent report demonstrated that CXCL8 facilitates CCL2-mediated monocyte migration, investigation of CXCL8 regulation and its role during brain inflammation remains a significant question (Gouwy et al., 2008).
In our in vitro culture system, HIV-1 infection resulted in a minimal elevation in the production of proinflammatory cytokines IL-1β and TNF-α while inducing a modest increase of CXCL8 in both macrophages and HIV-1 MCM - treated astrocytes (Fig. 2 and 3). However, it has been well documented that macrophages in vivo are activated during HAD. This is due to the presence of inflammatory cytokines in vivo that may not be induced in our in vitro HIV-1-infected macrophages system. LPS has been used in vitro to mimic immune activation status in vivo. Recently, critical data has demonstrated that microbial products including LPS, derived from microbes in the gastrointestinal tract, were significantly increased in chronically HIV-infected individuals and provoked HIV-related systemic immune activation (Brenchley et al., 2006). As a result of viral infection and immune activation, macrophages produce and release a variety of neurotoxins. These products comprise not only viral proteins, such as gp120, gp41 and Tat, but also host cell-encoded products including proinflammatory cytokines, chemokines, glutamate, arachidonic acid and its metabolites (Kaul et al., 2005).
Among the cytokines produced by activated macrophages/microglia, IL-1β and TNF-α appear to play an important role in the pathogenesis of local inflammation and tissue damage. The effect of IL-1β and TNF-α in astrocyte CXCL8 production is likely a common scenario in other neuroinflammatory diseases such as multiple sclerosis, where elevated IL-1β, TNF-α, and CXCL8 levels have also been documented (Bartosik-Psujek and Stelmasiak, 2005; Bitsch et al., 2000; Li et al., 1993). In our report, high levels of TNF-α and IL-1β were found in the culture supernatants when LPS was used to stimulate macrophages (Fig. 3). We determined that TNF-α and IL-1β released from HIV-1-infected and activated macrophages are critical factors in up-regulating CXCL8 production by astrocytes (Fig. 4). Although the induction of CXCL8 in astrocytes was mainly driven by LPS-induced IL-1β and TNF-α in HIV-1-infected MDM, our data provides evidence that PKR seems to act as an important mediator for the potentiation effect mediated by HIV-1 infection. HIV-1-infected, LPS-activated, or HIV-1-infected/LPS-activated MCM both mediated astrocyte CXCL8 production and this effect was blocked by a specific PKR inhibitor (Fig. 8 and 9).
Significant advances in our understanding of signaling pathways regulating CXCL8 gene expression have been linked to MAPK (for review, see (Hoffmann et al., 2002)). Over-expression of dominant negative mutants of c-Jun suppressed TNF-α-stimulated CXCL8 expression (Natarajan et al., 2001). JNK and ERK1/2 activation were found to be required for TLR3-mediated CXCL8 gene induction in astrocytes (Park et al., 2006). More recently, ERK1/2, nuclear factor-κB, and activator protein-1 were identified as mechanisms for CD40 ligand-induced CXCL8 production in microglia (D'Aversa et al., 2008). We studied TNF-α and IL-1β mediated MAPKs signaling in astrocytes and found that each induced a peak phosphorylation for JNK and p38 at 30 minutes. In comparison, the peak phosphorylation for ERK is around 5-10 min (data not shown). MAPKs appeared to be important pathways for TNF-α and IL-1β-mediated CXCL8 production in astrocytes. This conclusion was reached following the use of specific inhibitors for MAPK pathways in astrocyte cultures. Significant reductions in phosphorylations of JNK and ERK were observed with JNK (SP600125) and ERK (PD98059) inhibitors, respectively, after quantification of Western blotting in the Figures 5 and 6. The p38 inhibitor SB203580 did not inhibit p38 phosphorylation, in IL-1β-treated cultures, it actually increased p38 phosphorylation. However, we observed a significant decrease in ATF-2 phosphorylation, one of downstream factors of p38, by SB203580. The inhibition of ATF-2 phosphorylation by SB203580 is consistent in both TNF-α and IL-1β-treated cultures (Fig. 5C and 6C). Together, these results suggested that SB203580 is a p38 pathway specific inhibitor, which may act by preventing substrate phosphorylation by p38, and SP600125 and PD98059 are specific for JNK and ERK, respectively. Furthermore, using these inhibitors, we found that p38, JNK, and ERK1/2 are involved in astrocyte CXCL8 production as induced by macrophage-conditioned media (Fig. 7). Accordingly, TNF-α or IL-1β-mediated CXCL8 production in astrocytes is also dependent on p38, JNK, and ERK1/2 (Fig. 5 and 6). Notably, the greatest inhibition of CXCL8 production as induced by TNF-α or IL-1β is caused by p38 pathway inhibitor, while the JNK inhibitor exhibits less inhibition with the ERK inhibitor being the least effective under these conditions. Clearly, the exact roles of downstream factors of MAPKs, including ATF-2, in the regulation of CXCL8 in astrocytes remains to be further investigated.
Activation of p38 and JNK has been reported to be dependent on PKR-dependent pathways (Goh et al., 2000). In our study, we found both macrophage-mediated and IL-1β and TNF-α-induced CXCL8 production required PKR activation (Fig. 8). It is possible that the HIV-1 virus and viral proteins such as gp120 and Tat may directly activate PKR. However, our data support a role for both inflammatory cytokines IL-1β and TNF-α and viral production from HIV-1 infected macrophage-conditioned medium in the activation of PKR and induction of CXCL8 by astrocytes. The exact mechanisms of astrocyte PKR activation by macrophages and inflammatory cytokines are still not clear and require further investigation.
In summary, HIV-1 infection potentiated LPS-induced macrophage activation; both IL-1β and TNF-α are the major factors in the MDM conditioned medium that result in increased gene expression and synthesis of CXCL8 in human astrocytes. The production of CXCL8 was found to be dependent upon the p38, JNK, ERK1/2, and PKR in astrocytes. This work has demonstrated the importance of macrophage and astrocytes interactions as well as the the regulation of CXCL8 production, which will further our understanding in the pathogenesis of HAD.
Acknowledgments
This work was supported in part by research grants by the National Institutes of Health: R01NS 41858, R21MH083525, P20 RR15635 and P01 NS043985 to JZ. We kindly acknowledge Dr. Anuja Ghorpade, Mr. Matthew Beaver, and Ms. Li Wu who provided technical support for this work. Dr. Howard E. Gendelman, Ms. Angelique Walstrom, Mr. Nathan Erdmann, Ms. Agnes Constantino, and Ms. Tess Eidem provided valuable comments and suggestions about the manuscript. Ms. Julie Ditter, Johna Belling, Robin Taylor, Myhanh Che, Na Ly and Emilie Scoggins provided outstanding administrative support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aloisi F, Care A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony- stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–2366. [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosik-Psujek H, Stelmasiak Z. The levels of chemokines CXCL8, CCL2 and CCL5 in multiple sclerosis patients are linked to the activity of the disease. Eur J Neurol. 2005;12:49–54. doi: 10.1111/j.1468-1331.2004.00951.x. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Kuhlmann T, Da Costa C, Bunkowski S, Polak T, Bruck W. Tumour necrosis factor alpha mRNA expression in early multiple sclerosis lesions: correlation with demyelinating activity and oligodendrocyte pathology. Glia. 2000;29:366–375. doi: 10.1002/(sici)1098-1136(20000215)29:4<366::aid-glia7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Bouwman FH, Skolasky RL, Hes D, Selnes OA, Glass JD, Nance-Sproson TE, Royal W, Dal Pan GJ, McArthur JC. Variable progression of HIV-associated dementia. Neurology. 1998;50:1814–1820. doi: 10.1212/wnl.50.6.1814. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Budka H. Multinucleated giant cells in brain: a hallmark of the acquired immune deficiency syndrome (AIDS) Acta Neuropathol. 1986;69:253–258. doi: 10.1007/BF00688301. [DOI] [PubMed] [Google Scholar]
- Carrol ED, Mankhambo LA, Balmer P, Nkhoma S, Banda DL, Guiver M, Jeffers G, Makwana N, Molyneux EM, Molyneux ME, Smyth RL, Hart CA. Chemokine responses are increased in HIV-infected Malawian children with invasive pneumococcal disease. J Acquir Immune Defic Syndr. 2007;44:443–450. doi: 10.1097/QAI.0b013e31802f8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aversa TG, Eugenin EA, Berman JW. CD40-CD40 ligand interactions in human microglia induce CXCL8 (interleukin-8) secretion by a mechanism dependent on activation of ERK1/2 and nuclear translocation of nuclear factor-kappaB (NFkappaB) and activator protein-1 (AP-1) J Neurosci Res. 2008;86:630–639. doi: 10.1002/jnr.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M, Ghadirian E. Dysregulation of interleukin 8, interleukin 10, and interleukin 12 release by alveolar macrophages from HIV type 1-infected subjects. AIDS Res Hum Retroviruses. 1994;10:1619–1627. doi: 10.1089/aid.1994.10.1619. [DOI] [PubMed] [Google Scholar]
- Erichsen D, Lopez AL, Peng H, Niemann D, Williams C, Bauer M, Morgello S, Cotter RL, Ryan LA, Ghorpade A, Gendelman HE, Zheng J. Neuronal injury regulates fractalkine: relevance for HIV-1 associated dementia. J Neuroimmunol. 2003;138:144–155. doi: 10.1016/s0165-5728(03)00117-6. [DOI] [PubMed] [Google Scholar]
- Gelbard HA. Neuroprotective strategies for HIV-1-associated neurologic disease. Ann N Y Acad Sci. 1999;890:312–313. doi: 10.1111/j.1749-6632.1999.tb08008.x. [DOI] [PubMed] [Google Scholar]
- Gendelman HE. The Neuropathogenesis of HIV-1-Dementia. In: Gendelman HE, Lipton SA, Epstein LG, Swindells S, editors. The neurology of AIDS. Chapman and Hall; New York: 1997. pp. 1–10. [Google Scholar]
- Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Goh KC, deVeer MJ, Williams BR. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. Embo J. 2000;19:4292–4297. doi: 10.1093/emboj/19.16.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouwy M, Struyf S, Noppen S, Schutyser E, Springael JY, Parmentier M, Proost P, Van Damme J. Synergy between co-produced CC and CXC chemokines in monocyte chemotaxis through receptor mediated events. Mol Pharmacol. 2008 doi: 10.1124/mol.108.045146. [DOI] [PubMed] [Google Scholar]
- Gray E, Haug H, Chimelli L, Geny C, Gaston A, Scaravilli E, Budka H. Prominent Cortical Atrophy with Neuronal Loss as Correlate of Human Immunodeficiency Virus Encephalopathy. Acta Neuropathol. 1991;82:229–233. doi: 10.1007/BF00294450. [DOI] [PubMed] [Google Scholar]
- Gupta S, Campbell D, Derijard B, Davis RJ. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Jammi NV, Whitby LR, Beal PA. Small molecule inhibitors of the RNA-dependent protein kinase. Biochem Biophys Res Commun. 2003;308:50–57. doi: 10.1016/s0006-291x(03)01318-4. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12 1:878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Canto MCD, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Lane BR, Lore K, Bock PJ, Andersson J, Coffey MJ, Strieter RM, Markovitz DM. Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. J Virol. 2001;75:8195–8202. doi: 10.1128/JVI.75.17.8195-8202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Li H, Newcombe J, Groome NP, Cuzner ML. Characterization and distribution of phagocytic macrophages in multiple sclerosis plaques. Neuropathol Appl Neurobiol. 1993;19:214–223. doi: 10.1111/j.1365-2990.1993.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Maher J, Choudhri S, Halliday W, Power C, Nath A. AIDS dementia complex with generalized myoclonus. Mov Disord. 1997;12:593–597. doi: 10.1002/mds.870120418. [DOI] [PubMed] [Google Scholar]
- Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Miike T, Nelson RP, Trudeau WL, Lockey RF, Yodoi J. Elevated serum levels of IL-8 in patients with HIV infection. Clin Exp Immunol. 1993;93:149–151. doi: 10.1111/j.1365-2249.1993.tb07957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE, Chen IS. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. Faseb J. 1991;5:2391–2397. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- Natarajan R, Gupta S, Fisher BJ, Ghosh S, Fowler AA., 3rd Nitric oxide suppresses IL-8 transcription by inhibiting c-Jun N-terminal kinase-induced AP-1 activation. Exp Cell Res. 2001;266:203–212. doi: 10.1006/excr.2001.5218. [DOI] [PubMed] [Google Scholar]
- Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog Neurobiol. 1998;54:19–33. doi: 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986a;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Annals of Neurology. 1986b;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–256. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–629. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE. An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J Neurovirol. 1997;3:401–416. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- Sharer LR, Cho ES, Epstein LG. Multinucleated giant cells and HTLV-III in AIDS encephalopathy. Hum Pathol. 1985;16:760. doi: 10.1016/s0046-8177(85)80245-8. [DOI] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Strizki JM, Albright AV, Sheng H, O'Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh SL, Power C, Glass JD, et al. Intracerebral cytokine messenger RNA expression in acquired immunedeficiency syndrome dementia. Ann Neurol. 1993;33:576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- Wiley CA. Quantitative neuropathologic assessment of HIV-1 encephalitis. Curr Top Microbiol Immunol. 1995;202:55–61. doi: 10.1007/978-3-642-79657-9_4. [DOI] [PubMed] [Google Scholar]
- Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- Xiong H, Boyle J, Winkelbauer M, Gorantla S, Zheng J, Ghorpade A, Persidsky Y, Carlson KA, Gendelman HE. Inhibition of long-term potentiation by interleukin-8: implications for human immunodeficiency virus-1-associated dementia. J Neurosci Res. 2003;71:600–607. doi: 10.1002/jnr.10503. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Matsushima K, Oppenheim JJ, Leonard EJ. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1) J Immunol. 1987;139:788–793. [PubMed] [Google Scholar]
- Zhao ML, Kim MO, Morgello S, Lee SC. Expression of inducible nitric oxide synthase, interleukin-1 and caspase-1 in HIV-1 encephalitis. J Neuroimmunol. 2001;115:182–191. doi: 10.1016/s0165-5728(00)00463-x. [DOI] [PubMed] [Google Scholar]
- Zheng J, Gendelman HE. The HIV-1 associated dementia complex: a metabolic encephalopathy fueled by viral replication in mononuclear phagocytes. Curr Opin Neurol. 1997;10:319–325. [PubMed] [Google Scholar]
- Zheng J, Thylin M, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng Y, Gelbard H, Shepard R, Swartz J, Gendelman H. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Zink WE, Anderson E, Boyle J, Hock L, Rodriguez-Sierra J, Xiong H, Gendelman HE, Persidsky Y. Impaired spatial cognition and synaptic potentiation in a murine model of human immunodeficiency virus type 1 encephalitis. J Neurosci. 2002;22:2096–2105. doi: 10.1523/JNEUROSCI.22-06-02096.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]