Abstract
Backgound
Susceptibility loci exist for Gilles de la Tourette Syndrome (GTS), but no causative gene has been identified perhaps in part due to phenotypic heterogeneity. This study uses latent class analyses (LCA) to identify GTS subphenotypes, and assesses characteristics and heritability of the classes.
Methods
952 individuals from 222 TS families recruited for genetic studies were assessed. LCA identified a best-fit model for combinations of the diagnoses of GTS, obsessive-compulsive disorder (OCD), OC symptoms and behaviors (OCS/OCB) and attention-deficit hyperactivity disorder (ADHD) in a random sample of one sibling from each family (N=197), a replication sample randomly chosen from the remaining siblings (N=203), and in the entire sample, including all siblings and parents (N=952). Heritabilities were assessed for all categorical diagnoses and the LCA classes using a variance components approach.
Results
In this large sample of TS sib-pairs and their parents, three TS-affected groups were identified, TS + OCS/OCB (class III), TS + OCD (class IV), and TS + OCD + ADHD (class V), in addition to a minimally affected class (I) and a small chronic tics + OCD class (II). There was a preponderance of males and a younger age at onset in more comorbidly affected classes. Only the TS + OCD + ADHD class was highly heritable.
Conclusions
Our data suggest that GTS classes may represent distinct entities, with both shared and unique etiologies. In particular, TS + OCD + ADHD may represent a separate, heritable phenotype that can be used to further inform genetic studies.
INTRODUCTION
Tourette Syndrome (GTS) is a complex neurodevelopmental disorder characterized by the occurrence of multiple motor and vocal tics (1;2). The complexity of GTS is underscored by minor variants, such as chronic tics, comorbid obsessive-compulsive symptoms and behaviors (OCS/OCB), obsessive-compulsive disorder (OCD), and/or attention-deficit hyperactivity disorder (ADHD). Hypothesized shared etiologic pathways for GTS, OCD and ADHD have led to their characterization as ‘developmental basal ganglia disorders’ (3). Although clearly clinically related, the etiological relationships between GTS, OCD, and ADHD are not well defined. Genetic epidemiology studies have suggested that OCD and GTS co-segregate in families, and thus may represent alternate phenotypes of a common gene (4–6). In contrast, although family members of GTS probands consistently show higher rates of ADHD than would be expected by chance, the etiological relationship between GTS and ADHD is not well understood, with extant research suggesting that GTS and ADHD may respond to different genetic substrates (7–10). Segregation analyses further support a complex inheritance for GTS, perhaps reflecting GTS phenotypic and etiologic heterogeneity (11–14).
Despite indications of a clinical and perhaps an etiological relationship between GTS, OCD, and ADHD, joint analyses at the symptom or disorder level have not been conducted. Symptom-level factor analyses could uncover common factors that cut across standard diagnostic categories. Alternately, latent class analyses could uncover classes of individuals with varying combinations of GTS, OCD and/or ADHD. If supported by increased heritabilities, the alternate subphenotypes generated by such studies could be useful in genetic analyses. Such dissection of the phenotype is crucial, given that current data point to multiple genetic susceptibility loci when GTS is viewed as a unitary construct (15–19).
The aims of this study are to: 1) undertake a latent class analysis (LCA) of GTS using categorical diagnoses of OCS/OCB, OCD, and ADHD in a large sample of GTS affected sibpairs and their parents; and 2) characterize the resulting classes in relation to sex, age of onset of tics and class heritabilities. The goal of this study is to determine whether inclusion of OCS/OCB, OCD and ADHD comorbidities in the GTS construct refines the phenotype and can provide support to etiological discovery efforts.
METHODS & MATERIALS
Sample
The sample consisted of 952 individuals from 222 GTS families collected by the Tourette Syndrome Association International Consortium on Genetics (TSAICG) for affected sibling-pair (ASP) genetic linkage studies (18;19). Families were ascertained based on presence of GTS in at least two siblings and the availability of at least one parent. Families were excluded if the proband had mental retardation or a pervasive developmental disorder or where both parents had a known diagnosis of GTS, chronic motor or vocal tic disorder (CT), or OCD. Parents and all siblings known to have a tic disorder at the time of interview were assessed for GTS, chronic tics, OCD, OCS/OCB, and ADHD. Siblings thought to be unaffected were not routinely assessed. The study was approved by the Institutional Review Boards of the respective institutions and written informed consent was obtained. Assent was obtained for subjects younger than 13 years.
Clinical Assessments
Clinical assessments are described in detail elsewhere (18;19). All subjects were directly interviewed using a battery of structured interviews assessing tics, obsessive-compulsive symptoms, and ADHD symptoms using a clinician-reviewed self-report instrument developed by the TSAICG. Children were administered the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS) and adults were administered the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (20). Diagnoses of GTS, CT, OCD, and ADHD-inattentive (ADHD-IA), ADHD-hyperactive/impulsive (ADHD-HI), or ADHD-combined (ADHD-C) followed DSM-IV criteria. Present and worst-ever lifetime tic severity was assessed using the Yale Global Tic Severity Scale (YGTSS) (21). A diagnosis of OCS/OCB was made when symptoms were present but time criteria were not met (i.e., the subject had at least mild distress and interference, but the symptoms took up less than one hour a day). Final diagnoses for all disorders were assigned by two or more independent clinicians using a best estimate/consensus diagnosis procedure (22).
Statistical Analyses
Demographics
Differences in sex distribution, age at onset of tics and comorbid frequencies of OCD, OCS/OCB, and ADHD subtypes between GTS and CT were assessed using unpaired student t-tests for continuous measures and Pearson chi-square tets for categorical measures.
Latent Class Analysis (LCA)
The diagnostic categories were used in LCA included GTS, OCD and ADHD. Each subject was diagnosed with a) GTS or chronic tics; b) OCD or OCS/OCB; and c) ADHD-combined (ADHD-C), ADHD-hyperactive/impulsive (ADHD-HI), or ADHD-inattentive (ADHD-IA). MPLUS version 3.0 was used for LCA calculations using all diagnoses as categorical variables in the analysis (23). Given the sib-pair composition of the sample and potential non-independence of diagnoses, LCA was first conducted on a random sample of GTS-affected sibs with complete clinical data (n = 197), one from each family, then replicated in a second set of independent GTS-affected sibs randomly chosen from the remaining affected siblings after excluding the first sample (n = 202). Finally, LCA of the whole sample containing all parents and all siblings was completed (n = 952). This strategy allows for a qualitative comparison of LCA of the random subsets of sibs in order to minimize the tendency of sibs to produce non-random classes and sets the context for the omnibus LCA results (n=952).
Fit-Criteria for LCA
The lowest Akaike Information Criteria (AIC), Bayesian information criteria (BIC), and sample size adjusted-Bayesian information criteria (adj-BIC) were used to determine the best-fit model. For competing models, the AIC is defined by the model and the maximum likelihood estimates of the parameters: AIC = −2 × log(maximum likelihood) + 2 × number of independently adjusted parameters within the model (24). The BIC is similar to AIC except that the dimension of the model, or number of independent parameters, is multiplied by ½ × log(n). While AIC uses maximum likelihood, BIC uses the asymptotic behavior of Bayes estimators under a special class of priors to choose the appropriate dimensionality of the model (25). To assess class characteristics, sex and age at onset of tics were compared in LCA classes using a non-parametric test of trend.
Heritability Analysis
Heritability estimates and the corresponding significance levels for the resulting LCA classes were calculated using the Sequential Oligogenic Linkage Analysis Routine (SOLAR) statistical package (26). SOLAR employs a variance components approach using information from all available family members across generations and does not assume an inheritance model. The resultant heritability (h2) is based on a maximum-likelihood-based variance decomposition approach providing an estimate and a confidence interval. Although developed for quantitative traits, support for discrete traits (i.e., classes) is provided. To maximize the information content, the posterior probability for each individual belonging to each latent class is taken as the quantitative trait in this analysis. Because these posterior probabilities are not independent of one another, and because SOLAR cannot effectively correct for these dependencies, heritabilities for membership in each latent class, which are mutually exclusive, were also calculated. Age at interview and gender were controlled for in these analyses.
RESULTS
Sample Characteristics
From this sib-pair GTS sample, most affecteds (N=668) had GTS diagnoses (N=596), while a minority had CT diagnoses (N=72). Subjects with GTS had a younger age at onset of tics, and more frequent comorbid OCD and ADHD diagnoses compared to subjects with CT (Table 1).
Table 1.
Characteristics of Subjects with Gilles de la Tourette Syndrome (GTS) or Chronic Tics (CT) from 222 Tourette Sib-Pair Families.
| Total (N=668) |
CT (N= 72) |
GTS (N=596) |
Significance CT vs. GTS | |
|---|---|---|---|---|
| Sex (% males) | 67% | 53% | 69% | χ2(1) = 7.29; p = 0.007 |
| Age First Motor Tic | 6.34 (3.66) | 7.3 (3.6) | 6.3 (3.7) | Not Significant |
| Age First Phonic Tic | 7.57 (4.84) | 13.3 (11.2) | 7.4 (4.4) | 4.58 (454); p < 0.0001 |
| Age Worst Motor Tic | 10.7 (6.8) | 14 (9.3) | 10.5 (6.6) | t = 2.516; p = 0.006 |
| Age Worst Vocal Tic | 10.8 (7.1) | 15.4 (11.7) | 10.7 (6.9) | t = 2.360; p = 0.019 |
| OCD | 46% | 31% | 48% | χ2(1) = 7.71; p = 0.005 |
| Subclinical OCD | 18% | 18% | 18% | Not Significant |
| ADHD-C | 42% | 21% | 45% | χ2(1) = 14.73; p = 0.001 |
| ADHD-IA | 7% | 10% | 6% | χ2(1) = 9.65; p = 0.008 |
| ADHD-HI | 4% | 6% | 4% | Not Significant |
OCD = Obsessive-Compulsive Disorder; ADHD = Attention-Deficit Hyperactivity Disorder. C=combined subtype, IA=inattentive subtype, HI=hyperactive/impulsive subtype.
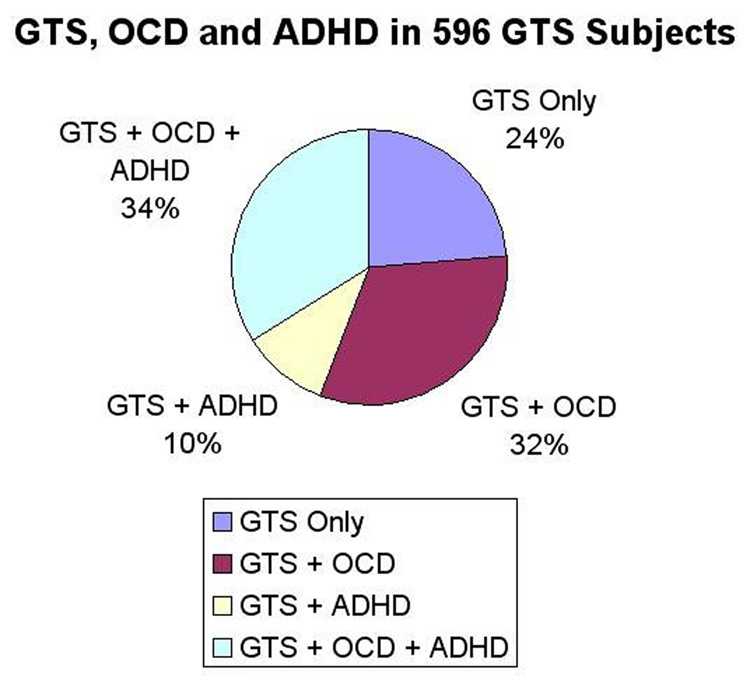
Considering only GTS subjects, 75% had either OCD or ADHD, with approximately a third of the sample having both OCD and ADHD. The other third had comorbid OCD only, and a smaller subset (10%) had comorbid ADHD only (Figure 1).
Figure 1.
Comorbidities Associated with Gilles de la Tourette Syndrome in 596 Subjects
Latent class analyses
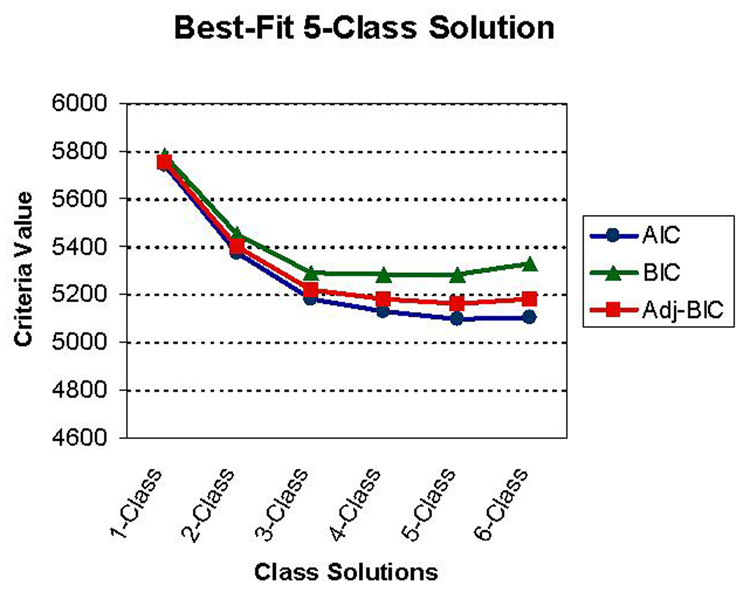
The first random sib sample consisted of 197 individuals with GTS. The best-fit three-class solution for this sample was represented by: Class I, GTS + OCS/OCB (100%); Class II, GTS + OCD (48%); and Class III, GTS + OCD (71%) + ADHD-Combined (100%). The best-fit three-class solution for the second set of random sibpairs (N = 202) was strikingly similar, and was represented by: Class I, GTS + OCS/OCB (100%); Class II, GTS + OCD (45%); and Class III, GTS + OCD (75%) + ADHD-Combined (100%). Thus, results of the LCA for each of the independent sibling samples were virtually identical, ruling out the concern that non-independence of the subjects significantly modified the class composition. Analyses of the entire sample of 952 subjects comprised of 596 GTS subjects, 72 CT subjects and 284 non-affecteds was then conducted to generate a class solution that would take into account the whole spectrum of tic disorders present in this sample. LCA produced a best-fit five-class solution, which included the three groups described above, as well as a minimal disorder group (class I) and a numerically smaller CT + OCD group (class II). The latent class analysis fit-criteria for all 952 subjects results are presented in Table 2. Fit criteria suggested that the 4-Class or 5-Class solutions were plausible. An inspection of the graphical presentation of the fit-criteria further suggests an overall analytic advantage of the 5-Class solution (Figure 2). Qualitatively, the main difference between the 5-Class and 4-Class solution was the emergence of the relatively small ‘chronic tics’ class in the 5-Class solution. Clinical criteria support a 5-class solution; a ‘chronic tics + OCD’ class, albeit a small proportion of the total, might constitute an alternate phenotype of the genetic susceptibility that is expressed as GTS in other family members. In the 5-Class solution, 30% of the entire sample had minimal symptoms (Class I), 4% were characterized as “chronic tics + OCD” (Class II), 11% were characterized as “GTS only” (class III), 31% were characterized as “GTS + OCD” (class IV) and 23% were characterized as “GTS + OCD + ADHD combined” (class V). The 5-Class solution was thus chosen as the best model (Table 3). Next, tests of trend were undertaken to examine whether sex or age at onset of subjects differentiated between classes, assuming a grading of “severity” from Class I to Class V. A higher proportion of males (z = −5.88, p < 0.001) and an earlier age at onset for motor tics (z = −4.11, p < 0.001) and phonic tics (z = −3.36, p = 0.001) were evident with increasingly comorbid classes (Table 3).
Table 2.
Latent Class Fit-Criteria in 222 GTS Sib-Pair Families and First-Degree Relatives (N = 952) Showing a Best-Fit 5-Class Solution +
| 1-Class | 2-Class | 3-Class | 4-Class | 5-Class | 6-Class | |
|---|---|---|---|---|---|---|
| Loglikelihood (HO Value) | −2865.553 | −2673.282 | −2566.854 | −2534.469 | −2508.566 | −2503.570 |
| # free parameters | 9 | 15 | 23 | 31 | 39 | 47 |
| Akaike AIC | 5745.107 | 5376.563 | 5179.707 | 5130.938 | 5095.133 | 5101.139 |
| Bayesian BIC | 5779.139 | 5449.489 | 5291.527 | 5281.651 | 5284.740 | 5329.640 |
| Sample Size Adjusted BIC | 5756.907 | 5401.850 | 5218.480 | 5183.196 | 5160.877 | 5180.370 |
| Pearson Chi-Square | 764.755 (df 119) | 506.152 (df 111) | 224.162 (df 103) | 177.285 (df 95) | 67.395 (df 88) | 56.013 (df 80) |
| p-value = 0.0000 | p-value = 0.000 | p-value = 0.000 | p-value = 0.000 | p-value = 0.950 | p-value = 0.981 | |
| Likelihood Ratio | 541.856 (df 119) | 380.391 (df 111) | 169.592 (df 103) | 104.677 (df 95) | 53.678 (df 88) | 43.685 (df 80) |
| Chi-Square | p-value = 0.000 | p-value = 0.000 | p-value = 0.000 | p-value = 0.233 | p-value = 0.999 | p-value = 0.997 |
| Entropy | 0.746 | 0.999 | 0.968 | 0.922 | 0.857 |
Best-Fit Criteria are indicated by: lowest AIC, lowest BIC, lowest sample size-adjusted BIC, less significant chi-square, highest entropy
AIC = Akaike Information Criterion; BIC = Bayesian Information Criteria
Figure 2.
Fit Criteria for Latent Classes Analysis on 952 Subjects with Gilles de la Tourette Syndrome and their Relatives
Table 3.
Percentage of Individuals in 5 Classes with Gilles de la Tourette Syndrome (GTS), Chronic Tics (CT) Obsessive-Compulsive Disorder (OCD) or Attention-Deficit Hyperactivity Disorder (ADHD) Diagnoses (N=952) 1 in the 5-Class Solution.
| Classes | GTS | CT | OCD | S-OCD | ADHD-C | ADHD-HI | ADHD-IA | Total |
|---|---|---|---|---|---|---|---|---|
| Minimal Disorder (I) | 0% | 13% | 0% | 15% | 9% | 0% | 2% | 29.5% |
| Chronic Tics (II) | 0% | 100% | 53% | 17% | 29% | 9% | 15% | 3.7% |
| GTS + S-OCD (III) | 100% | 0% | 0% | 100% | 36% | 6% | 4% | 11.2% |
| GTS+OCD (IV) | 81% | 0% | 47% | 0% | 0% | 6% | 12% | 31.5% |
| GTS+OCD+ADHD (V) | 98% | 0% | 73% | 0% | 100% | 0% | 0% | 24.1% |
| Class I | Class II | Class III | Class IV | Class V | Total | |||
| Male Sex | 50% | 34% | 67% | 60% | 76%* | 61% | ||
| Age Onset Motor Tics | 9.1 (7.5) | 7.2 (3.5) | 6.4 (2.6) | 7.0 (5.1) | 5.7 (3.1)† | 6.7 (4.6) | ||
| Age Onset Vocal Tics | 11.4 (9.2) | 12.3 (8.8) | 7.3 (3.2) | 7.9 (4.7) | 6.9 (4.6)ϕ | 7.7 (4.9) | ||
| Total | 281 | 35 | 107 | 300 | 229 | 952 | ||
In Class I, for example, no subjects had GTS, 13% had CT, 15% had subclinical OCD, 9% had ADHD combined and 2% had ADHD inattentive, comprising as a whole a minimally affected class. There were a total of 29.5% × 952 = 281 subjects in class I.
S-OCD = Subclinical OCD
ADHD = Attention-Deficit Hyperactivity Disorder (C = combined, HI = Hyperactive-Impulsive, IA = Inattentive)
Non-parametric test of trend; z = −5.88, p < 0.001
Non-parametric test of trend; z = −4.11, p < 0.001
Non-parametric test of trend; z = −3.36, p = 0.001
Heritability analyses
To further explore the potential relevance of the class assignments for genetic studies, heritabilities for each of the latent classes were explored using a variance components method (SOLAR). Heritability calculations for the latent class probabilities, where each individual is assigned a probability of belonging to each class, showed that probability classes IV (GTS +OCD) (p = 0.01) and V (GTS + OCD + ADHD) (p = 7 × 10−5) were significantly heritable, while classes II (CT + OCD) and III (GTS + OCS/OCB) were not (Table 4). Heritabilities using the categorical variable of class membership showed similar results, with only classes IV and IV having statistically significant heritabilities. These analyses were done in parallel because neither analysis produces a completely reliable heritability estimate: the posterior probability analyses have the problem of non-independence of the groups, while the membership analyses have the problem that, while categorical traits are supported by SOLAR, the heritability estimates derived using categorical traits are less accurate. This is clearly shown in the heritability estimate for Class II, which shows a high heritability that is nonetheless not statistically significant because of the large standard error surrounding the estimate. However, both types of analyses gave similar results, suggesting that classes IV and V are consistently heritable. Since the data were ascertained for GTS affected status, Class I (minimal symptoms or unaffected) was not considered for heritability analyses. These results were maintained after controlling for sex and age at interview. With the aforementioned caveats, the heritabilities presented suggest that GTS, OCD, ADHD as a group are familial, and that there are likely to be shared etiological factors in their development, at least in this familial GTS sample.
Table 4.
Intrafamilial Heritabilities for GTS Latent Classes using Posterior Probalities of belonging to a Class for each Individual and Class Membership, Controlling for Sex and Age at interview.
| Heritability (SE) | p-value | Sex | Age At Interview |
|
|---|---|---|---|---|
| Probability Class I | NA | NA | NA | NA |
| Probability Class II | 0.10 (0.10) | NS | 0.02 | NS |
| Probability Class III | 0.0 (0.05) | NS | NS | 0.00007 |
| Probability Class IV | 0.10 (0.05) | 0.01 | NS | 0.008 |
| Probability Class V | 0.18 (0.05) | 0.00007 | 0.05 | 1.0 × e-15 |
| Membership Class I | NA | NA | NA | NA |
| Membership Class II | 0.49 (0.36) | NS | 0.03 | NS |
| Membership Class III | 0.0 (0.05) | NS | NS | 0.00008 |
| Membership Class IV | 0.18 (0.05) | 0.02 | NS | 0.00008 |
| Membership Class V | 0.65 (0.14) | 0.000002 | 0.05 | 6.5 × e-17 |
Class I = Minimal Symptoms; Class II = Chronic Tics; Class III = GTS + subclinical OCD; Class IV = GTS + OCD; Class V = GTS + OCD + ADHD NS = not statistically significant; NA = not applicable
DISCUSSION
This study represents the first latent class analysis of GTS and its most common comorbidities, OCD and ADHD. We identified three GTS “affected” classes, corresponding to GTS + OCS/OCB (class III; n = 107), GTS + OCD (class IV; n = 300) and GTS + OCD + ADHD (class V; n = 229). Two additional classes were also identified in the larger family sample, minimal disorder (class I; n = 281) and chronic tics (class II; n = 35). We are confident that the LCA classes derived from these analyses are reliable, as the two random sib samples and the omnibus sample replicated the same three affected classes. However, the functional meaning of the classes is somewhat less clear. One potential use of the LCA is to examine the question of whether GTS is predominantly a disorder of motor disinhibition, or should be considered in the larger context of disinhibition across functional realms (e.g., the cognitive disinhibition of OCD and the behavioral disinhibition of ADHD) for genetic and other etiological studies. Etiologically, GTS, OCD and ADHD have common putative abnormalities in the fronto-striatal systems (27–29), and common biological markers have been sought (30;31).
Besides the truly unaffected Class I, a ‘minor’ version of GTS appears as a separate class once the parents and other sibs are included in the analysis (i.e., Class II: chronic tics + OCD). In addition to chronic tics, 100% of individuals in Class II have OCD, making it plausible that in familial samples the presence of OCD is ubiquitous and may respond to common genes influencing the expression of both tics and OCD.
Class III, characterized by GTS + OCS/OCB, corresponds to the “GTS only” group previously reported in the literature, and is generally considered to be spared of major disability in relation to motor control and executive function (32). Denckla et al. (2006) reported that up to 40% of children with TS are “free of ADHD”, noting that they are also free of the motor control and executive control deficits of children with ADHD alone or GTS + ADHD. However, it was also noted that this clinical group had oculomotor control deficits in the initiation of prosaccades, regardless of their ADHD status (33). More recently, Rizzo et al. (2007) confirm that the addition of ADHD to GTS confers greater maladaptive behavior and worse cognitive functioning compared to GTS alone (34). It is important to point out that 100% of members of the “GTS only” class have OCS/OCB. While the relationship of OCS/OCB to OCD remains an area of investigation (35), OCS/OCB associated with GTS have been repeatedly observed to correspond to a specific group of OCD symptoms (36–38), and as discussed below, may represent a different phenomenological entity than OCD rather than a “forme fruste” of OCD.
Class IV is characterized by GTS + OCD and was the most numerous class in our sample, concurrent with other studies (39). GTS + OCD (Class IV) may constitute a more severe form of GTS + OCS/OCB (class III) or be a qualitatively different clinical entity. Coffey et al. (1998) set out to explore the differences between GTS, OCD and GTS + OCD. GTS + OCD was found to have higher rates of bipolar disorder, social phobia, body dysmorphic disorder and ADHD than the GTS only and OCD only groups. Since most of the increased comorbidities relate to GTS and not OCD, the authors conclude that GTS + OCD is more related to GTS and qualitatively “more severe” than either of each alone (40). The fact that OCD and OCS/OCB consistently separate into different latent classes in this sample suggests hitherto unidentified but important differences between subclinical and clinical OCD in relation to GTS. Multiple studies note OC phenomenological differences when OCD is comorbid with GTS, such as a younger age at onset of OCD, a male predominance, sensory phenomena, sexual obsessions and hoarding, repeating, and counting compulsions compared to OCD alone (41); these, however, are not the symptoms usually identified in OCS/OCB accompanying GTS (38). The heritability results, which are relatively low but positive for Class IV, and the high frequency of the GTS + OCD class in our sample continue to support a possible common genetic background for GTS and OCD as previously reported (6;7;42)
Class V, GTS + OCD + ADHD, comprises about one-third of GTS individuals in the affected classes and has many “core” GTS characteristics such as a preponderance of males, and an earlier age at onset of motor tics compared to classes III and IV. GTS + OCD + ADHD is also highly heritable in this sample (h = 0.18 ± 0.05; p = 7 × 10−5), with the caveat that the value is not overly high even in complex genetics. These data support the notion that a comorbid syndrome may be heritable in these families. Previous studies provide additional evidence for the hypothesis that GTS, OCD, and ADHD may all be part of a common disinhibition syndrome. Principal component factor analyses in four samples from three studies show that subjects with GTS can be divided into those with a “pure” or simple form consistent with class III (GTS + OCS/OCB), and those with a more complex form, consistent with classes IV (GTS + OCD) and V (GTS + OCD + ADHD) (43–45). In these studies, the “pure” form was essentially comprised of simple tics while more complex forms of tics comprised the additional forms of GTS. In the first study, the “complex” symptoms included such disinhibition syndromes as temper fits, argumentativeness, self-injurious behaviors, copralalia, and imitation, which grouped into one of four identified clusters (termed aggressive disinhibition), that was associated with comorbid ADHD in the sample (43). A related complex compulsive factor comprised of touching, picking, echolalia, and palilalia was also identified in this study, and was also found to be associated with comorbid ADHD, but not OCD. In the second study, which examined two independent samples, the “complex” cluster included symptoms related to disinhibition, reckless and impulsive behaviors, self-injury, injury to others, and coprolalia (44). Membership in the complex cluster was associated with increased tic severity, increased global impairment, need for medication treatment, family history of tics, higher rates of OCD and ADHD, and an earlier age of onset. Finally, a third study also found a “pure tics” factor with two additional more complex factors characterizing some forms of GTS: an “ADHD-aggressive” factor and a “negative affective-OCD” factor (45). A family study of GTS and ADHD has underscored the complex relationship between these two disorders. Relatives of ADHD only probands have more tics than expected, but only when they co-occurs with ADHD; vice versa, relatives of GTS only probands have more ADHD than expected, but only if concurrent with tics. OCD was more common in both groups of relatives compared to control relatives, and the presence of OCD predicted the presence of ADHD and GTS in both groups (10). Future studies that employ symptom-level factor analyses of GTS, OCD, and ADHD in combination may help to further elucidate the relationships between these disorders.
Of interest, there were only 60 subjects or about 10% of GTS subjects who had the combination GTS + ADHD, without OCD, as shown in Figure 1. These subjects did not comprise a large enough group to constitute a separate class analytically. The presence of this subgroup suggests that OCD plays a role mediating the relationship between GTS and ADHD that needs further elucidation (i.e., when GTS and ADHD are comorbid, in most instances OCD is also present).
In summary, the present study is an attempt to go beyond DSM-IV diagnoses to identify person-centered subgroups of GTS in a refinement of the phenotype for etiological studies, including genetic studies. From a clinical perspective, it will be important to recognize that each of these classes may respond to a different treatment strategies, given possible different underlying biological determinants. From a genetic perspective, our results, while exploratory, suggest that individuals with GTS can be effectively grouped into comorbid subtypes for etiological studies. That is, at least in families with multiple affected individuals, GTS is more likely to occur in the context of OCD and/or ADHD than on its own. While the classes derived from our sample were not in themselves surprising, the heritability estimates were somewhat unexpected. Previous research has suggested that GTS + OCD may represent a heritable subgroup of GTS subjects that may be useful for understanding the genetic contributions to a GTS + OCD syndrome. Concordant with recent reports (46), our results suggest that there may be a more complex subtype, the multiply comorbid, or GTS + OCD +ADHD subtype (class V), that may also be heritable, and may be caused by different or additional susceptibility loci than GTS + OCS/OCB (class III) or GTS + OCD (class IV). Whether these classes represent a continuum of clinical complexity and severity or truly discrete entities is not tested in the current manuscript; future analyses using alternate latent class mixture models or multilevel (severity) latent class models could elucidate this question. By inference, future gene discovery studies may need to consider GTS complex phenotypes in order to more reliably locate susceptibility genes, especially in linkage studies. If this assumption is true, the full expression of the disorder would consist of a multiply affected phenotype, while moderating factors, including environmental factors and partial penetrance of susceptibility genes, may lead to more simple phenotypes
Limitations
The primary limitation of this study relates to the sample composition. Because the sample consists of nuclear families ascertained for having at least two siblings with GTS, there is little phenotypic variability in the offspring generation with regard to tic diagnoses. This decrease in variability for the GTS phenotype may falsely decreaseheritability estimates for class III (GTS + subclinical OCD). Additionally, the relative lack of GTS-unaffected siblings, as well as the lack of additional generations within families, makes segregation analyses and other approaches aimed at identifying transmission patterns impractical. Additional limitations arise with regard to the heritability estimates. Although we are confident that classes IV and V are heritable as a result of our analyses, we are less confident of the precise heritability estimates. Limitations are imposed by the data structure and software, which is unable to correct for the non-independence of class probabilities (i.e., class probabilities have to add up to one). In addition, we do not have data on putative environmental contributors to GTS, OCD, and ADHD, which are ostensibly accounting for variance not explained by the genetic variance (h2). Despite these limitations, the large sample size and the completeness of the clinical data make it possible to maximize the information available from such a sample in useful and previously unexplored ways. Thus, the current analyses cannot distinguish whether the heritability findings for Class V are due to common genetic factors underlying GTS, OCD, and ADHD, or whether there are other mechanisms at play, such as common environmental factors or assortative mating between individuals with the different disorders.
Finally, the familial nature of the sample, ascertained through having two affected sibs, limits the generalizability of the GTS subtypes and may have implications for gene discovery. For example, the Class V phenotype, a more severe, complex form of the disorder may be caused by a rare high-penetrance genetic variant that is more readily found in multiplex families and would be useful for gene discovery through linkage methods. This same complex phenotype, if responsive to a highly penetrant rare genetic variant, might not be as useful in a large-scale genetic association studies that seek to locate more common susceptibility genes of minor effect.
Acknowledgements
Members of the Tourette Syndrome Association International Consortium for Genetics (TSAICG), listed alphabetically by city: D. Cath and P. Heutink, Departments of Psychiatry and Human Genetics. Free University Medical Center Amsterdam, Amsterdam, the Netherlands; M. Grados, H.S. Singer and J.T. Walkup, Departments of Psychiatry and Neurology, Johns Hopkins University School of Medicine, Baltimore, MD; C. Illmann, S. Santangelo, S. E. Stewart, J. Scharf and D.L. Pauls, Psychiatric and Neurodevelopmental Genetics Unit, Center for Human Genetic Research, Massachusetts General Hospital Harvard Medical School, Boston, MA; N.J. Cox, Departments of Medicine and Human Genetics, University of Chicago, Chicago, IL; S. Service, D. Keen-Kim, C. Sabatti and N. Freimer, Department of Psychiatry, U.C.L.A Medical School, Los Angeles, CA; M.M. Robertson, Department of Mental Health Sciences, University College London Institute of Neurology, National Hospital for Neurology and Neurosurgery Queen Square London; G.A. Rouleau, J.-B. Riviere, S. Chouinard, F. Richer, P. Lesperance and Y. Dion University of Montreal, Montreal, Quebec, Canada; R.A. King, J.R. Kidd, A.J. Pakstis, J.F. Leckman and K.K. Kidd, Child Study Center and Department of Genetics, Yale University School of Medicine, New Haven, CT; G. Gericke, Department of Biomedical Sciences, Tshwane University of Technology, Pretoria, South Africa; R. Kurlan, P. Como and, D. Palumbo, Department of Neurology, University of Rochester School of Medicine, Rochester, NY; A. Verkerk, B.A. Oostra, Department of Clinical Genetics, Erasmus University, Rotterdam, The Netherlands; W. McMahon, M. Leppert and H. Coon, Departments of Psychiatry and Human Genetics, University of Utah School of Medicine, Salt Lake City, UT; C.A. Mathews, Department of Psychiatry, University of California, San Francisco, San Francisco, CA; P. Sandor and C.L. Barr, Department of Psychiatry, The Toronto Hospital and University of Toronto, Toronto, Ontario, Canada.
The TSAICG is grateful to all the families with Gilles de la Tourette syndrome in all of the participating centers who generously agreed to be part of this study. Furthermore, the members of the Consortium are deeply indebted to the Tourette Syndrome Association and in particular to Ms. Judit Ungar, TSA president and Ms. Sue Levi-Pearl, TSA Director of Medical and Scientific Programs. Both have dedicated their professional lives to the understanding and treatment of Tourette Syndrome. Their tireless efforts to help move the research forward were critical to the success of the project. Without their support, guidance and prodding this study would not have been possible.
The Consortium also sincerely thanks the members of the TSA Board of Directors for their continuing support. Finally, the members of the Consortium want to thank the advisors to the collaborative group who include the following volunteer members of the TSA Scientific Advisory Board's Subcommittee for Genetics: P. Michael Conneally, Francis McMahon, John Rice, Neal Swerdlow, Peter Hollenbeck and Jonathan Mink.
The TSAICG acknowledges J. Hebebrand, B. Klug and H. Remschmidt, Department of Child and Adolescent Psychiatry, Phipps University, Marburg Germany; J.L. Weber, B.C. Hiner and M. Spindler, Center for Medical Genetics, Marshfield Medical Foundation. Marshfield, WI; and J. Jancovic, Department of Neurology, Baylor College of Medicine, Houston, TX for their assistance in collecting data from sib-pair families that were included in this genome scan. This work was supported by funds from the TSA and from NIH grant NS 40024.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
Reference List
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-IV. Washington, DC: APA; 1994. [Google Scholar]
- 2.Kurlan R, Como PG, Miller B, Palumbo D, Deeley C, Andresen EM, et al. The behavioral spectrum of tic disorders: a community-based study. Neurology. 2002;59:414–420. doi: 10.1212/wnl.59.3.414. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo D, Maughan A, Kurlan R. Hypothesis III. Tourette syndrome is only one of several causes of a developmental basal ganglia syndrome. Arch Neurol. 1997;54:475–483. doi: 10.1001/archneur.1997.00550160101023. [DOI] [PubMed] [Google Scholar]
- 4.Pauls DL, Leckman JF. The inheritance of Gilles de la Tourette's syndrome and associated behaviors. Evidence for autosomal dominant transmission. N Engl J Med. 1986;315:993–997. doi: 10.1056/NEJM198610163151604. [DOI] [PubMed] [Google Scholar]
- 5.Pauls DL, Pakstis AJ, Kurlan R, Kidd KK, Leckman JF, Cohen DJ, et al. Segregation and linkage analyses of Tourette's syndrome and related disorders. J Am Acad Child Adolesc Psychiatry. 1990;29:195–203. doi: 10.1097/00004583-199003000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Grados MA, Riddle MA, Samuels JF, Liang KY, Hoehn-Saric R, Bienvenu OJ, et al. The familial phenotype of obsessive-compulsive disorder in relation to tic disorders: the Hopkins OCD family study. Biol Psychiatry. 2001;50:559–565. doi: 10.1016/s0006-3223(01)01074-5. [DOI] [PubMed] [Google Scholar]
- 7.Pauls DL, Towbin KE, Leckman JF, Zahner GE, Cohen DJ. Gilles de la Tourette's syndrome and obsessive-compulsive disorder. Evidence supporting a genetic relationship. Arch Gen Psychiatry. 1986;43:1180–1182. doi: 10.1001/archpsyc.1986.01800120066013. [DOI] [PubMed] [Google Scholar]
- 8.Pauls DL, Leckman JF, Cohen DJ. Familial relationship between Gilles de la Tourette's syndrome, attention deficit disorder, learning disabilities, speech disorders, and stuttering. J Am Acad Child Adolesc Psychiatry. 1993;32:1044–1050. doi: 10.1097/00004583-199309000-00025. [DOI] [PubMed] [Google Scholar]
- 9.McMahon WM, Carter AS, Fredine N, Pauls DL. Children at familial risk for Tourette's disorder: Child and parent diagnoses. Am J Med Genet B Neuropsychiatr Genet. 2003;121:105–111. doi: 10.1002/ajmg.b.20065. [DOI] [PubMed] [Google Scholar]
- 10.Stewart SE, Illmann C, Geller DA, Leckman JF, King R, Pauls DL. A controlled family study of attention-deficit/hyperactivity disorder and Tourette's disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:1354–1362. doi: 10.1097/01.chi.0000251211.36868.fe. [DOI] [PubMed] [Google Scholar]
- 11.Eapen V, Pauls DL, Robertson MM. Evidence for autosomal dominant transmission in Tourette's syndrome. United Kingdom cohort study. Br J Psychiatry. 1993;162:593–596. doi: 10.1192/bjp.162.5.593. [DOI] [PubMed] [Google Scholar]
- 12.Hasstedt SJ, Leppert M, Filloux F, van de Wetering BJ, McMahon WM. Intermediate inheritance of Tourette syndrome, assuming assortative mating. Am J Hum Genet. 1995;57:682–689. [PMC free article] [PubMed] [Google Scholar]
- 13.Seuchter SA, Hebebrand J, Klug B, Knapp M, Lehmkuhl G, Poustka F, et al. Complex segregation analysis of families ascertained through Gilles de la Tourette syndrome. Genet Epidemiol. 2000;18:33–47. doi: 10.1002/(SICI)1098-2272(200001)18:1<33::AID-GEPI3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Walkup JT, LaBuda MC, Singer HS, Brown J, Riddle MA, Hurko O. Family study and segregation analysis of Tourette syndrome: evidence for a mixed model of inheritance. Am J Hum Genet. 1996;59:684–693. [PMC free article] [PubMed] [Google Scholar]
- 15.Simonic I, Nyholt DR, Gericke GS, Gordon D, Matsumoto N, Ledbetter DH, et al. Further evidence for linkage of Gilles de la Tourette syndrome (GTS) susceptibility loci on chromosomes 2p11, 8q22 and 11q23–24 in South African Afrikaners. Am J Med Genet. 2001;105:163–167. doi: 10.1002/ajmg.1192. [DOI] [PubMed] [Google Scholar]
- 16.Abelson JF, Kwan KY, O'Roak BJ, Baek DY, Stillman AA, Morgan TM, et al. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005;310:317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- 17.Merette C, Brassard A, Potvin A, Bouvier H, Rousseau F, Emond C, et al. Significant linkage for Tourette syndrome in a large French Canadian family. Am J Hum Genet. 2000;67:1008–1013. doi: 10.1086/303093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GTSAICG. A complete genome screen in sib pairs affected by Gilles de la Tourette syndrome. Am J Hum Genet. 1999;65:1428–1436. doi: 10.1086/302613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GTSAICG. Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet. 2007;80:265–272. doi: 10.1086/511052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 22.McMahon WM, Illmann CL, McGinn MM. Web-based consensus diagnosis for genetics studies of Gilles de la Tourette syndrome. Adv Neurol. 2006;99:136–143. [PubMed] [Google Scholar]
- 23.Muthen LK, Muthen BO. MPLUS: Statistical Analyses with Latent Variables. Third Edition. Los Angeles, CA: 2004. [Google Scholar]
- 24.Akaike HA. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 25.Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- 26.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23:563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- 28.Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- 29.Channon S, Pratt P, Robertson MM. Executive function, memory, and learning in Tourette's syndrome. Neuropsychology. 2003;17:247–254. doi: 10.1037/0894-4105.17.2.247. [DOI] [PubMed] [Google Scholar]
- 30.Peterson BS, Leckman JF, Tucker D, Scahill L, Staib L, Zhang H, et al. Preliminary findings of antistreptococcal antibody titers and basal ganglia volumes in tic, obsessive-compulsive, and attention deficit/hyperactivity disorders. Arch Gen Psychiatry. 2000;57:364–372. doi: 10.1001/archpsyc.57.4.364. [DOI] [PubMed] [Google Scholar]
- 31.Amat JA, Bronen RA, Saluja S, Sato N, Zhu H, Gorman DA, et al. Increased number of subcortical hyperintensities on MRI in children and adolescents with Tourette's syndrome, obsessive-compulsive disorder, and attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163:1106–1108. doi: 10.1176/appi.ajp.163.6.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahone EM, Cirino PT, Cutting LE, Cerrone PM, Hagelthorn KM, Hiemenz JR, et al. Validity of the behavior rating inventory of executive function in children with ADHD and/or Tourette syndrome. Arch Clin Neuropsychol. 2002;17:643–662. [PubMed] [Google Scholar]
- 33.Denckla MB. Attention deficit hyperactivity disorder: the childhood comorbidity that most influences the disability burden in Tourette syndrome. Adv Neurol. 2006;99:17–21. [PubMed] [Google Scholar]
- 34.Rizzo R, Curatolo P, Gulisano M, Virzi M, Arpino C, Robertson MM. Disentangling the effects of Tourette syndrome and attention deficit hyperactivity disorder on cognitive and behavioral phenotypes. Brain Dev. 2007;29:413–420. doi: 10.1016/j.braindev.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Mathews CA, Jang KL, Hami S, Stein MB. The structure of obsessionality among young adults. Depress Anxiety. 2004;20:77–85. doi: 10.1002/da.20028. [DOI] [PubMed] [Google Scholar]
- 36.Mansueto CS, Keuler DJ. Tic or compulsion?: it's Tourettic OCD. Behav Modif. 2005;29:784–799. doi: 10.1177/0145445505279261. [DOI] [PubMed] [Google Scholar]
- 37.Eapen V, Robertson MM, Alsobrook JP, Pauls DL. Obsessive compulsive symptoms in Gilles de la Tourette syndrome and obsessive compulsive disorder: differences by diagnosis and family history. Am J Med Genet. 1997;74:432–438. doi: 10.1002/(sici)1096-8628(19970725)74:4<432::aid-ajmg15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 38.Hounie AG, do Rosario-Campos MC, Diniz JB, Shavitt RG, Ferrao YA, Lopes AC, et al. Obsessive-compulsive disorder in Tourette syndrome. Adv Neurol. 2006;99:22–38. [PubMed] [Google Scholar]
- 39.Termine C, Balottin U, Rossi G, Maisano F, Salini S, Di Nardo R, et al. Psychopathology in children and adolescents with Tourette's syndrome: a controlled study. Brain Dev. 2006;28:69–75. doi: 10.1016/j.braindev.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Coffey BJ, Miguel EC, Biederman J, Baer L, Rauch SL, O'Sullivan RL, et al. Tourette's disorder with and without obsessive-compulsive disorder in adults: are they different? J Nerv Ment Dis. 1998;186:201–206. doi: 10.1097/00005053-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Miguel EC, do Rosario-Campos MC, Prado HS, do VR, Rauch SL, Coffey BJ, et al. Sensory phenomena in obsessive-compulsive disorder and Tourette's disorder. J Clin Psychiatry. 2000;61:150–156. doi: 10.4088/jcp.v61n0213. [DOI] [PubMed] [Google Scholar]
- 42.Cuker A, State MW, King RA, Davis N, Ward DC. Candidate locus for Gilles de la Tourette syndrome/obsessive compulsive disorder/chronic tic disorder at 18q22. Am J Med Genet A. 2004;130:37–39. doi: 10.1002/ajmg.a.30066. [DOI] [PubMed] [Google Scholar]
- 43.Alsobrook JP, Pauls DL. A factor analysis of tic symptoms in Gilles de la Tourette's syndrome. Am J Psychiatry. 2002;159:291–296. doi: 10.1176/appi.ajp.159.2.291. [DOI] [PubMed] [Google Scholar]
- 44.Mathews CA, Jang KL, Herrera LD, Lowe TL, Budman CL, Erenberg G, et al. Tic Symptom Profiles in Subjects with Tourette Syndrome from two Genetically Isolated Populations. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Robertson MM, Cavanna AE. The Gilles de la Tourette syndrome: a principal component factor analytic study of a large pedigree. Psychiatr Genet. 2007;17:143–152. doi: 10.1097/YPG.0b013e328015b937. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Ceren JJ, Valencia-Duarte AV, Cornejo JW, Carrizosa J, Cuartas JM, Zuluaga-Espinosa NA, et al. Genetic linkage analysis of Gilles de la Tourette Syndrome in a Colombian family. Rev Neurol. 2006;42:211–216. [PubMed] [Google Scholar]