Abstract
Adult male rats have been demonstrated to increase food intake in response to administration of drugs that interfere with oxidation of fatty acids (e.g. methyl palmoxirate and mercaptoacetate [MA]), effects that are larger in animals maintained on a high-fat diet. In contrast, while administration of MA has been reported to stimulate food intake in pre-pubertal female rats, food intake is not stimulated by MA in adult female rats. Instead, administration of MA to adult females results in changes in reproductive behavior and physiology. The present experiments were designed to examine the effects of administration of MA on food intake in adult female rats. The results demonstrated that, as previously reported, food intake was stimulated by MA in adult male rats on low-fat and high-fat diets, but food intake in was not stimulated by MA in gonadally-intact adult female rats on either low-fat or high-fat diet. Further, MA did not stimulate food intake in female rats ovariectomized as adults. However, when females were ovariectomized prior to the onset of puberty (postnatal day 25 – 28), food intake was stimulated by administration of MA in adulthood. Finally, cyclic injections of 17-β-estradiol benzoate given to females ovariectomized prior to the onset of puberty abolished the stimulatory effects of MA on food intake in adult females. Taken together, the data suggest that exposure to estrogens during the time of puberty in female rats can persistently alter adult ingestive responding to signals related to changes in energy utilization.
Animals, including rats, are sensitive to changes in the availability or utilization of energy, with signals related to decreased energy availability resulting in the initiation of food intake (Mayer 1955). For example, administration of glucoprivic drugs such as 2-deoxyglucose (2-DG), 5-thioglucose, or insulin that interfere with the availability or utilization of glucose have been demonstrated to stimulate food intake in adult male and female rats under a variety of circumstances (Smith and Epstein 1969; Houpt and Hance 1971; Panksepp, Tonge et al. 1972; Ritter and Slusser 1980). In addition, administration of lipoprivic drugs [such as mercaptoacetate (MA) or methyl palmoxirate (MP)] which interfere with the oxidation of fatty acids has been demonstrated to result in increased food intake in adult, prepubertal and neonatal male rats, with the effects of these lipoprivic drugs more pronounced in animals maintained on diets that are high in fat (Friedman and Tordoff 1986; Friedman, Tordoff et al. 1986; Langhans and Scharrer 1987; Langhans and Scharrer 1987; Swithers 1997; Scharrer 1999; Swithers, McCurley et al. 2005). Taken together, these data suggest that signals related to decreases in metabolic energy availability serve to stimulate food intake in male and female rats.
Additional work suggests that in female rats, signals related to reduced energy availability not only influence ingestive behavior, but also interact with reproductive physiology and behavior. For example, a number of studies have demonstrated that in female rats, mice and hamsters, changes in metabolic fuel availability such as those produced by food deprivation or administration of glucoprivic drugs such as 2-DG can suppress pulsatile LH release, inhibit ovulation and vaginal estrus and reduce reproductive behavior (e.g. Schneider and Wade 1989; Cagampang, Maeda et al. 1990; Wade, Schneider et al. 1991; Dickerman, Li et al. 1993; Schneider, Finnerty et al. 1995; Nagatani, Bucholtz et al. 1996; Gill and Rissman 1997; Schneider, Goldman et al. 1997; I'Anson, Starer et al. 2003). In addition, in adult female rats, reproductive status affects the behavioral consequences of administration of glucoprivic agents, such as 2-DG, on food intake (Abizaid, Jafferali et al. 2001; Abizaid and Woodside 2002). For example, while adult virgin female rats demonstrate increased food intake after administration of 2-DG, lactating females do not. Lipoprivic signals have also been demonstrated to interfere with reproductive physiology and behavior, with MP and MA having been shown to alter GnRH and LH release ovulation and vaginal estrus and sexual receptivity in female rats, mice and/or hamsters (e.g. Dickerman, Li et al. 1993; Li, Wade et al. 1994; Schneider, Hall et al. 1997; Schneider and Zhou 1999; Shahab, Sajapitak et al. 2006; Sajapitak, Iwata et al. 2008). In contrast, lipoprivic signals have not been demonstrated to stimulate food intake in female rodents. In fact, in a single published study, administration of MA to adult female rats that were ovariectomized and primed with 17-β-estradiol 3-benzoate (EB) was demonstrated to interfere with pulsatile LH release, but food intake was not stimulated (Shahab, Sajapitak et al. 2006). The failure of MA to stimulate food intake in that study was attributed to the low fat content of the maintenance diet. However, previous work in our lab had demonstrated that female rats maintained on a low-fat chow diet and tested at 30 days of age (e.g. prior to the onset of puberty), did show increases in food intake after administration of MA that were similar to those displayed by male rats of the same age (Swithers, McCurley et al. 2005). These results suggested that the failure of adult females to increase food intake following administration of MA might not depend on the fat content of the maintenance diet, but may instead reflect a developmental shift in the behavioral consequences of lipoprivic signals in adult female rats following puberty.
The present experiments were therefore designed to test whether the effects of administration of the lipoprivic drug MA on food intake in female rats are affected by the fat level of the maintenance diet, by the female’s present ovarian hormone status, and/or the female’s previous hormonal history. The effects of administration of MA on food intake were assessed in gonadally-intact adult male and female Sprague-Dawley rats maintained on high fat or standard chow diets (Experiment 1), in adult female rats following ovariectomy (OVX) and EB administration (Experiment 2), in adult female rats OVX prior to puberty (Experiment 3, which also examined the effects of glucoprivation in the same animals) and in adult female rats OVX prior to puberty and exposed to EB during the period of puberty (Experiment 4). The results suggest that exposure to estrogens during puberty may reorganize the female behavioral response to MA, such that while MA interferes with reproductive physiology and behavior in adult female rats, it does not stimulate food intake.
Methods
Experiment 1
Subjects were adult male (350 – 400 g) and female (225–275 g) age-matched Sprague-Dawley rats (Harlan, Indianapolis) that were sexually and experimentally naive. Animals were housed individually in hanging wire cages in an animal room maintained on a 14:10 light:dark cycle with lights on at 5:30 a.m. Following arrival in the lab, animals were placed on a standard low-fat rat chow diet (Lab Diets 5001; 13 male and 13 female) or a high fat diet (12 male and 12 female) previously used in our lab (Lab Diets 5012 with 16% peanut oil and 4% starch added by weight; approximately 40% of calories from fat; Swithers, Melendez et al. 2001) for two weeks prior to testing. On the day of testing at approximately 10:00 a.m., animals were given an i.p. injection of 46 or 69 mg/kg MA (23 mg/ml) or the 0.15M NaCl control. All animals were tested once with each dose, with the order of testing counterbalanced across animals. At least 3 days separated each intake test. Estrous cyclicity in female animals was not determined in this experiment. Immediately following the injection, animals were given access to their maintenance diet (high fat or low-fat) and intake was measured after 1, 2 and 4 hr. Statistical analysis: The results from male and female rats were examined using separate 3-way Repeated measures ANOVAs with Dose and Time as within-subjects factors and Diet as a between subjects factor, with subsequent 2-Way (Dose × Time) ANOVAs performed for animals maintained on each diet as indicated. Where indicated, post-hoc differences were analyzed with Tukey’s HSD test. A p < 0.05 was taken as significant for all tests.
Experiment 2
Sexually and experimentally naïve, adult female rats (250 – 275 g at the time of surgery; Harlan, Indianapolis) were placed onto the same high fat diet used in Experiment 1 and assigned to one of three groups. All animals were anesthetized with ketamine (40 mg/kg) and xylazine (10 mg/kg), the flanks were shaved bilaterally, cleaned with iodine solution and incisions made near the junction of the last rib and vertebral column. For two of the groups, the ovary was lifted, the uterine blood vessels were ligated and the ovary removed with a scalpel. The procedure was repeated on the opposite side. The third group received a sham surgery in which the ovaries were exposed but not removed. Incisions were closed with wound clips that were removed 1 week following surgery. Butorphanol (1.0 mg/kg, s.c.) was administered post-operatively. All animals were allowed to recover for 1 week following surgery. Following recovery, one group of OVX animals received subcutaneous injections of EB (10 micrograms/0.1 ml cottonseed oil; n = 10) and one group received s.c. injections (0.1 ml) of the cottonseed oil vehicle (n=10). Sham animals received s.c. injections of the cottonseed oil vehicle (n=8). Injections were administered once every 4 days for 9 cycles to mimic cyclic variations in levels of estrogens in gonadally-intact females. The dose of EB employed was supraphysiological and was chosen to ensure that should its administration fail to produce an effect, it would not be due to the use of a subthreshold dose. Food intake tests were administered two days following the 7th EB injection and two days following the 9th EB injection; each animal was tested once following an i.p. injection of 69 mg/kg MA and once following an i.p. injection of the saline vehicle, and food intake was measured after 1, 2 and 4 hr. The order of MA and saline injection was counterbalanced across animals; intake tests were conducted at approximately 10:00 a.m. Food intake 1, 2, and 4 hr following administration of MA or saline was analyzed with a 3-Way (Dose × Treatment × Time) Repeated Measures ANOVA with Dose and Time as within-subjects factors and treatment as a between-subjects factor. Body weights on the day before the first intake test were analyzed with a One-Way ANOVA (Treatment).
Experiment 3
Thirty-three Sprague-Dawley female rats bred in our laboratory (dams and sires obtained from Harlan, Indianapolis) were weaned on P23 (day of birth = P0). OVX (n= 17) or sham OVX (n=16) was performed as described above on P25 – P28, and animals were maintained on a standard chow diet until testing. No more than 2 animals from each litter were tested in any condition. Intake testing was performed when animals were at least 70 days of age. OVX and sham OVX females were injected i.p. with 0 or 69 mg/kg MA and food intake was measured at 1, 2 and 4 hr. At least 3 days separated the intake tests, and the order of MA dosing was counterbalanced. At least 3 weeks following the MA tests, food intake was measured in the same females following administration of 200 mg/kg 2-deoxyglucose and 0.15 M NaCl (testing with 2-DG was not completed in one OVX female; sample sizes for 2-DG = 16 for both sham and OVX groups). At least 3 days separated these tests with the order of 2-DG dosing counterbalanced across animals. Separate repeated-measures ANOVAs were performed on food intake after MA and 2-DG, with Dose and Time as within-subjects variables and surgery as a between subjects variable. Because subjects were different ages at the time of testing, body weights were not analyzed.
Experiment 4
Experimentally and sexually naïve, female Sprague-Dawley rats, P20 – P21, purchased from Harlan (Indianapolis) were maintained on a standard chow diet. Bilateral OVX was performed in all animals on P27 – P28 as described above. Ten days after surgery, half of the animals received subcutaneous injections of EB every 4 days as described above (n=9) while the remaining half received s.c. injections of the cottonseed oil vehicle (n=7) every 4 days for 7 cycles; all animals also received s.c. injections of the oil vehicle two days following their respective EB or oil injections. Body weights were recorded every two days during the injections and for twelve weeks following administration of the last injection. Food intake was tested twelve weeks following the last injection after animals received i.p. administration of 69 mg/kg MA or the saline vehicle, then animals were re-tested 4 days later in the opposite condition. The order of testing was counterbalanced. Food intake after 1, 2 and 4 hr was analyzed with a 3-Way (Time × Hormone × Dose) Repeated measures ANOVA with time and dose as within-subjects factors and hormone as a between subjects factor. The amount of body weight gained from the first to the last day of hormone injections and the amount of body weight gained from the last day of hormone injections to the time of testing were analyzed with separate Two-Way Repeated-measures (Hormone × Time) ANOVAs. Body weight on the day prior to the first intake test was analyzed with a One-Way (Hormone) ANOVA.
All experiments were performed in accordance with the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Purdue University Animal Care and Use Committee.
Results and Discussion
Experiment 1
Male rats
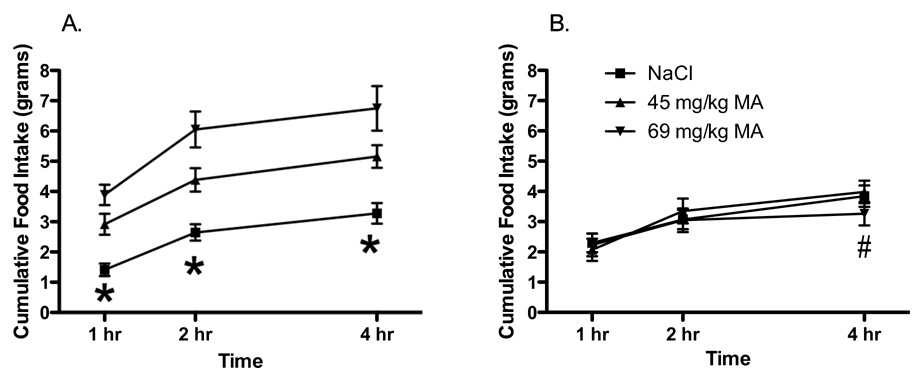
As previously demonstrated (Langhans and Scharrer 1987; Langhans and Scharrer 1987), in male rats, food intake was affected by the dose of MA (Main effect of Dose; F 2, 46 = 28.05; p < 0.05), the diet on which animals were maintained and tested (Main effect of Diet; F 1, 23 = 14.29; p < 0.05), and the elapsed time during testing (Main effect of Time; F 2, 46 = 73.45; p < 0.05; Dose × Time interaction; F 4, 92 = 3.801; p < 0.05). Adult males maintained on the high-fat diet consumed significantly more food than the males maintained on the standard chow diet (data not shown). In addition, adult males maintained on the standard chow diet, food intake was increased following administration of both doses of MA across the 4 hr of testing (Main effect of Dose; F 2, 24 = 13.20; p < 0.05; Main effect of Time; F 2, 24 = 67.01; p < 0.05; Dose × Time interaction; F 4, 48 = 2.857; p < 0.05). Similarly, males maintained on a high fat diet demonstrated increases in food intake following both doses of MA across the 4 hr of testing (Figure 1A; Main effect of Dose; F 2, 22 = 14.97; p < 0.05; Main effect of Time; F 2, 22 = 29.23; p < 0.05, No Dose × Time interaction; F 4, 44 = 1.66; p < 0.05).
Figure 1.
Adminstration of MA (45 or 69 mg/kg MA, i.p.) at time 0 produced significant increases in food intake at 1, 2 and 4 hr in gonadally-intact adult male rats (A; n= 12) maintained on a high fat diet. In contrast, in gonadally-intact female rats (B; n=12) MA did not stimulate food intake at any time point, but the highest dose significantly suppressed food intake at 4 hr.
* p < 0.05 compared to 45 and 69 mg/kg MA
# p < 0.05 compared to 0 mg/kg MA
Female rats
In adult female rats, food intake was affected by the diet on which they were maintained and tested (Main effect of Diet; F 1, 23 = 12.17; p < 0.05;) and by the elapsed time during testing (Main effect of Time; F 2, 26 = 94.19; p < 0.05). There were no significant main effects of the dose of MA (F 2, 46 =0.15; p = 0.86), but there was a significant Dose × Time × Diet interaction (F 4, 92 = 2.84; p < 0.05; all other interactions n.s.). Subsequent analyses revealed that in females maintained on the standard chow diet, there was a significant effect of Time (Main effect of time; F 2, 24 = 42.67; p < 0.05), but no significant main effects or interactions with the dose of MA. In females maintained and tested on the high fat diet, there was a significant main effect of Time (Figure 1B; Main effect of Time; F 2, 22 = 52.35 p < 0.05) and a significant Dose × Time interaction (F 4, 44 = 3.64; p < 0.05). Post-hoc tests revealed that the highest dose of MA resulted in significantly lower food intake in females on the high fat diet after 4 hr compared to the saline control.
The results from Experiment 1 demonstrate that in contrast to adult male rats, gonadally-intact adult female rats do not increase food intake following administration of MA, even when maintained on a high fat diet. One candidate mechanism that might contribute to this male-female difference in ingestive responding to lipoprivic signals is the influence of ovarian hormones. Ovarian hormones are well known to influence food intake and body weight regulation in adult female rats (e.g. Wade and Zucker 1970; Zucker 1972; Tarttelin and Gorski 1973; Blaustein and Wade 1976; Landau and Zucker 1976; Geary and Asarian 1999). In the first experiment, levels of ovarian hormones fluctuated throughout testing, since intact females were used. While the order of testing was counterbalanced across the animals, it remains possible that the observed differences in males and females may have reflected higher variability in baseline responses in the females due to the influence of endogenous hormone levels. Further, the effects of glucoprivic signals vary based on hormonal status in female rats (Abizaid, Jafferali et al. 2001). Thus, in the first experiment it is possible that the effects of administration of MA on food intake were influenced by the level of ovarian hormones at the time of testing. To control for such influences, the second experiment examined whether circulating ovarian hormones influenced ingestive behavior in response to MA in female rats by examining female rats following OVX with or without EB replacement.
Experiment 2 Results
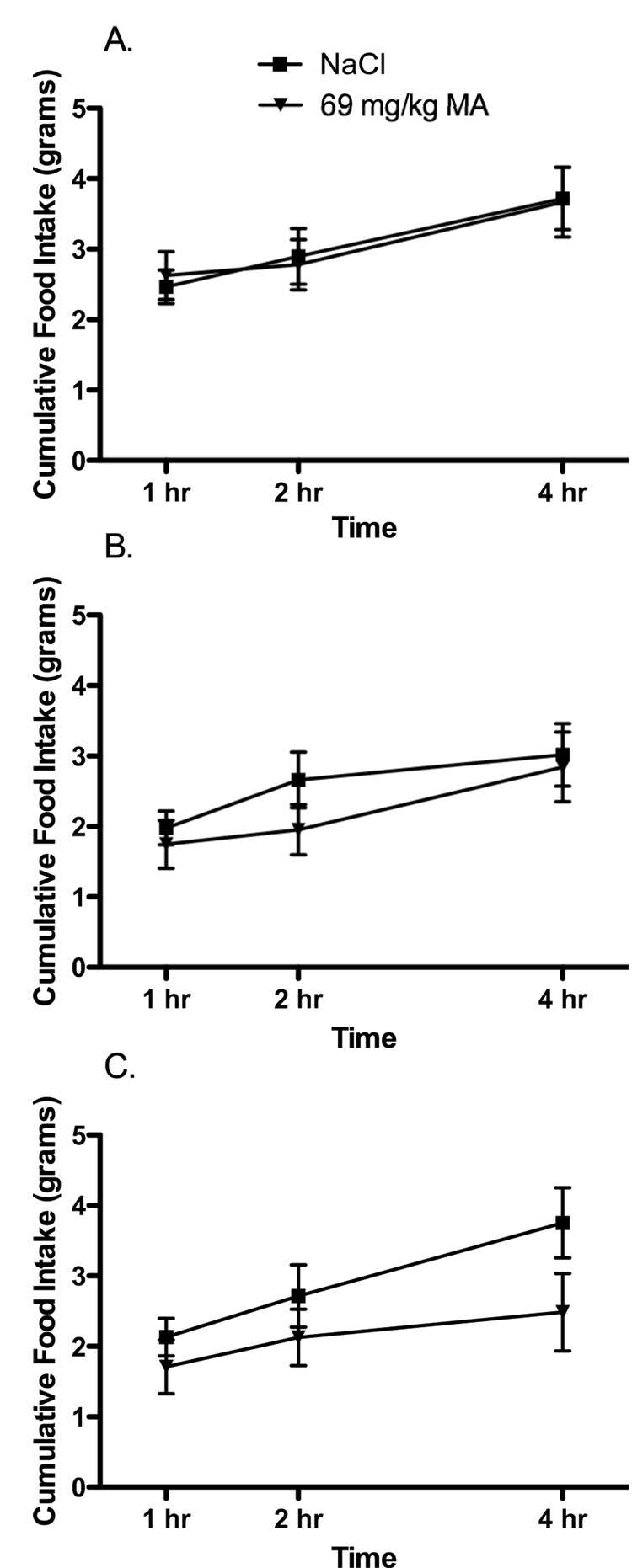
OVX females given oil injections were significantly heavier than OVX females given EB or sham females (Means ± SEM = 307 ± 4 g [OVX – Oil], 250 ± 4 g [OVX-EB] and 260 ± 5 g [Sham]; Main effect of treatment; F 2, 25 = 51.9; p < 0.05). However, during testing, there were no significant effects of the dose of MA or the hormone treatment on food intake (Figure 2; Main effect of time; F 2, 50 = 43.41; p < 0.05; all other comparisons n.s.). While these data demonstrate that MA does not stimulate food intake in adult female rats following OVX, previous work has demonstrated that rats tested on postnatal day 30 (P30) do show significant increases in food intake following administration of MA (Swithers, McCurley et al. 2005). Thus, it appears that between 30 days and adulthood, the effects of MA on ingestive behavior change in female, but not male, rats. To determine whether these changes are related to exposure to ovarian hormones during puberty, the next experiment was designed to test the effects of MA on adult female rats OVX prior to puberty. In addition, because previous work has suggested that hormonal status may affect food intake responses in female rats following administration of 2-DG (Abizaid and Woodside 2002), we also examined the effects of 2-DG on food intake in gonadally-intact females and females OVX prior to puberty.
Figure 2.
Administration of MA (69 mg/kg, i.p.) at time 0 had no effect on food intake in female rats (A) ovariectomized (OVX) and receiving cyclic injections (s.c.) of an oil vehicle[n=10]; (B) OVX females receiving cyclic injections of EB [n=10] and (C) sham OVX females receiving cyclic injections of the oil vehicle [n=8].
Experiment 3 Results
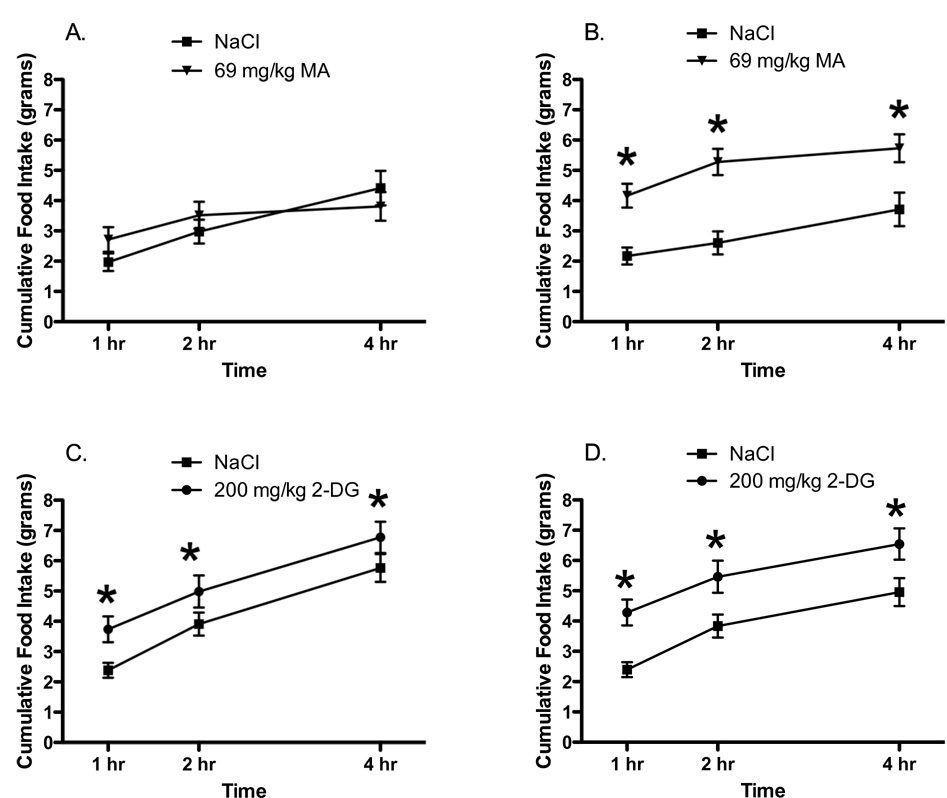
When tested as adults, food intake in female rats OVX before puberty was significantly affected by the surgery, dose of MA and time (Main effect of Dose; F 1, 31 = 7.22, p < 0.05; Dose × Surgery interaction; F 1, 31 = 4.78, p < 0.05; Main effect of Time = F 2, 62 = 89.32; p < 0.05; Dose × Time interaction; F 2, 62 = 6.89; p < 0.05). Post-hoc analyses revealed that as seen in Experiments 1 and 2, gonadally-intact females showed no increases in intake following administration of MA (Figure 3A). In contrast, in females OVX prior to 30 days of age administration of MA produced significant increases in food intake across all 4 hr of testing (Figure 3B). Further, unlike the effects of MA, administration of 2-DG resulted in increases in food intake in both gonadally-intact adult females and females OVX prior to puberty (Figures 3C and 3D; Main effect of Dose; F 1, 30 = 16.13, p < 0.05; Main effect of time; F 2, 60 = 161.4; p < 0.05; all other effects n.s). These results suggest that females not exposed to ovarian hormones after 30 days of age display significant differences in ingestive responding to MA compared to females with intact ovaries. Thus, exposure to ovarian hormones, such as typically occurs during puberty, may persistently alter ingestive responding to lipoprivic signals in female rats. To test this hypothesis, we compared the effects of administration of MA in females OVX prior to puberty and given replacement EB or oil injections.
Figure 3.
Administration of MA (69 mg/kg, i.p.) at time 0 did not stimulate food intake in (A) gonadally-intact (sham OVX; n=16) adult female rats but did produce increased food intake in (B) adult female rats that had been OVX prior to P30, n=17). Administration of 2-DG (200 mg/kg i.p. at time 0) stimulated food intake in both (C) gonadally-intact (sham OVX) adult female rats, n= 16 and (D) adult female rats that had been OVX prior P30, n=16.
* p < 0.05 compared to NaCl
Experiment 4 Results
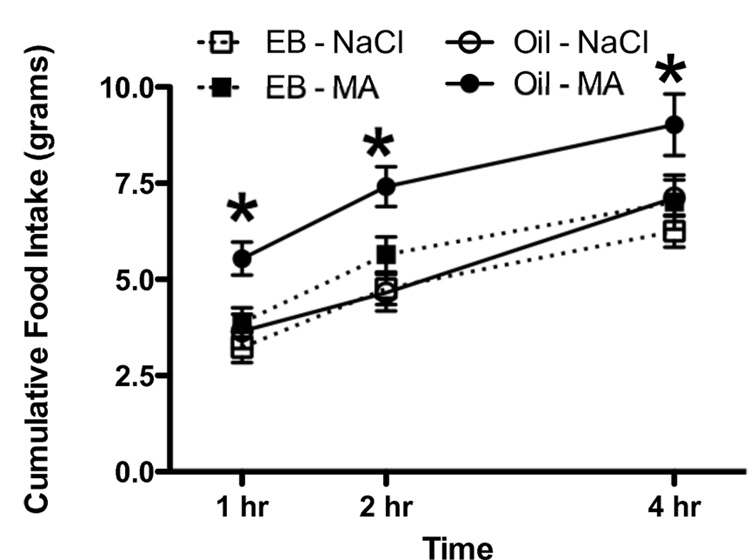
In females OVX prior to puberty, the effects of administration of MA on food intake were influenced by the administration of EB during puberty (Figure 4; Main effect of Dose; F 1, 22 = 46.5; p < 0.05; Dose × Hormone interaction; F 2, 22 = 3.45; p < 0.05, Main effect of Time; F 2, 44 = 181.5; p < 0.05). As seen in Experiment 3, females OVX prior to puberty and given the oil vehicle showed significant increases in food intake when tested with MA as adults. In contrast, in females OVX prior to puberty and given EB between P35 and P63, food intake was not stimulated by administration of MA. In addition, OVX females given EB from P35 – P63 gained significantly less weight during the injections (Main effect of Time; F 12, 168 = 807.3; p = 000000; Main effect of Hormone; F 1, 14 = 243.8, p < 0.05; Hormone × Time interaction; F 12, 168 = 134.5; p < 0.05, data not shown). After termination of the injections, the animals that had received the EB injections gained more weight than the animals that had received the oil injections (Main effect of Hormone; F 1, 14 = 9.7; p <0.05; Main effect of Time; F 41, 574 = 698.5; p < 0.05; Hormone × Time interaction; F 41, 574 = 49.9; p < 0.05, data not shown). Despite the increased weight gain following the injections, EB treated OVX animals weighed significantly less than OVX females given the oil vehicle, even twelve weeks after the termination of the injections (Means ± SEM = 295 ± 6 g [OVX – EB] and 329 ± 7 g [OVX – Oil]; Main effect of treatment; F 1, 14 = 12.8; p < 0.05).
Figure 4.
Administration of MA (69 mg/kg, i.p.) at time 0 did not stimulate food intake in adult female rats OVX prior to P30 given cyclic s.c. injections of EB (every 4 days between P35 and P63, n = 9) but did stimulate food intake in adult female rats OVX prior to P30 given cyclic s.c. injections of the oil vehicle (every 4 days between P35 and P63, n=7).
* p < 0.05 compared to Oil - NaCl
The results of these experiments indicate that in contrast to adult male rats, adult female rats do not show increased food intake in response to administration of MA, a drug that interferes with fatty acid oxidation. These results are consistent with a previous report, in which such differences in ingestive behavior were attributed to the low fat level of the diet on which the females had been maintained (Shahab, Sajapitak et al. 2006). However, the present work also demonstrated that even adult female rats maintained on a high fat diet do not show increases in food intake following administration of MA. Thus, the fat composition of the maintenance diet does not explain the significant differences in ingestive responses to administration of MA in adult male and female rats. Further, the present data (Experiment 2) indicate that levels of circulating ovarian hormones during adulthood are not directly related to failure of MA to stimulate food intake in female rats. While females OVX as adults were significantly heavier than sham OVX females or females OVX and given replacement EB, administration of MA failed to stimulate intake in any of these adult females. In contrast, the results of Experiment 3 demonstrated that when ovarian hormones are removed just prior to the onset of puberty, female rats continue to show increased intake following administration of MA when tested as adults. These data suggest that exposure to ovarian hormones may play a role in altering ingestive responses to lipoprivic signals after 30 days in female rats, with exposure to estrogens during the time of puberty likely playing a key role since administration of EB during the pubertal period abolished increases in food intake produced by administration of MA in females that were OVX prior to puberty (Experiment 4).
The results of these studies underscore the critical links between systems mediating ingestive behavior and physiology and those subserving reproductive behavior and physiology, links that have been highlighted by a number of demonstrations of direct effects of changes in metabolic status on reproductive behavior and physiology (e.g. Schneider and Wade 1989; Cagampang, Maeda et al. 1990; Schneider and Wade 1990; Wade, Schneider et al. 1991; Dickerman, Li et al. 1993; Li, Wade et al. 1994; Wade, Schneider et al. 1996; Gill and Rissman 1997; Temple and Rissman 2000; Schneider, Buckley et al. 2002). Reproductive behavior requires energetic investment, and animals that are exhibiting reproductive behavior cannot simultaneously acquire and consume calories. Further, the energetic requirements of reproduction differ significantly in males compared to females. These differences in energetic investment associated with reproduction may help explain differential behavioral and physiological responses to different signals related to changes in energy utilization, to differences in responding in male compared to female rats, and to differences in females before and after puberty. For example, in both male and female animals, short-term suppression of reproductive physiology is produced by glucoprivation (Nagatani, Bucholtz et al. 1996). Similarly, glucoprivation stimulates food intake in male and female rats, before and after the onset of puberty (Smith and Epstein 1969; Ritter 1994; Abizaid and Woodside 2002; Swithers, McCurley et al. 2005), and these effects on food intake do not differ between gonadally-intact and OVX female rats (Experiment 3). These data are consistent with the hypothesis that low levels of glucose availability may represent a signal that energy needs are presently critical, and all resources should be immediately directed towards acquisition of food, and away from reproductive behaviors. In this context, the failure of 2-DG to stimulate food intake in lactating female rats may reflect already high levels of food intake in these females, intake that may not be subject to further increases.
In contrast to emergent nature of signals related to low glucose availability, signals related to decreases in the availability of lipids, such as those produced by administration of MA, may represent a signal related to longer-term energy availability. Responses of male and females might differ, then, because of the significantly greater long-term energy investments demanded by reproduction in female mammals compared to males. For a male animal, a signal of low long-term energy stores would be expected to stimulate food intake, and in males, lipoprivic signals have clearly been demonstrated to increase food intake. However, lipoprivation might not necessarily suppress reproductive behavior in males because both the short-term and long-term energetic consequences of reproduction are comparatively low. Thus, males faced with lipoprivic signals might continue to demonstrate near-normal sexual behavior if lipoprivation represents a longer-term energy status signal. At present, the effects of lipoprivic signals on reproductive behavior in males are not known.
In females, the meaning of lipoprivic signals appears quite different. In adult females, they are potent short-term inhibitors of reproductive physiology and behavior (e.g. Schneider and Wade 1989; Wade, Schneider et al. 1996; Schneider, Hall et al. 1997; Temple, Schneider et al. 2002). Given the significant potential energetic investment that would accrue if she were to reproduce, this short-term suppression in response to an acute signal of low fat availability would be adaptive. In contrast, for females tested before the onset of reproductive maturity, there would be no need for signals related to altered fat metabolism to modulate reproductive behavior. Instead, lipoprivation that results in increased food intake in peripubertal rats may contribute to the timing of the onset of reproductive maturity, since the onset of puberty in female rats appears to be related to nutritional status, with fat-related signals appearing to play a particularly important role. For example, vaginal opening and estrus are accelerated in female rats fed a high fat diet compared to a low-fat diet (Frisch, Hegsted et al. 1975; Frisch 1980), and the onset of puberty can be accelerated by administration of the adipose tissue-derived hormone leptin (Ahima, Dushay et al. 1997; Chehab, Mounzih et al. 1997; Gruaz, Lalaoui et al. 1998). In addition, food deprivation in females approaching puberty delays the onset of puberty as evidence by decreased uterine and ovarian weights, and the absence of pulsatile LH release (Bronson 1986). Ad lib feeding of the food-deprived females produced rapid reversal of these effects, suggesting that signals related to ongoing energy deficits may contribute to the suppressed reproductive development. For female rats, then, the role of lipoprivic signals with respect to ingestive behavior may be to contribute to reproductive maturation. Signals related to low fat stores may stimulate food intake, which leads to attainment of reproductive maturity; following maturation, these signals may continue to serve as short-term modulators of reproductive status, but no longer influence ingestive behavior.
At present, the mechanisms that underlie the developmental changes in ingestive behavior following administration of MA in female rats remain unknown, and elucidating these mechanism will require additional experimental work. The present work suggests that such changes may result from exposure to increased levels of estrogens that typically occurs during puberty in female rats. How estrogens might influence the development of neural circuitry underlying ingestive responses to lipoprivic signals is not currently clear. In adult male rats, a variety of neural structures related to control of food intake, including the nucleus of the solitary tract, the lateral parabrachial nucleus, the lateral subdivision of the central nucleus of the amygdala, and the lateral hypothalamic area (e.g. Ritter and Dinh 1994; Sergeyev, Broberger et al. 2000) have been implicated in the stimulation of food intake by administration of mercaptoacetate. While the establishment of basic pathways considered critical to regulation of food intake and body weight appears to be accomplished well before puberty, the development of such systems can be influenced by a variety of signals, including leptin (e.g. Bouret and Simerly 2007), and it is possible that estrogens may interact with leptin during puberty may influence subsequent ingestive responding in female rats. Future work will need to explore whether alterations in neural circuitry or function contribute to the developmental differences observed. In addition, the role of potential peripheral consequences of exposure to estrogens will need to be determined. Finally, experiments examining whether the peripubertal period represents a critical or sensitive phase during which exposure to estrogens produces more pronounced effects compared to other ages (e.g. Sisk and Zehr, 2005) may provide insight into how neural systems underlying control of ingestive behavior in females might be reorganized during puberty.
Acknowledgments
This work was supported by grant P01 HD05211 from the NIH and by a grant from the Purdue Research Foundation. We thank Dr. Robert Meisel and Dr. Kimberly Kinzig for their helpful comments on earlier versions of the manuscript. Portions of these data were presented at the 2006 Annual meeting of the International Society for Developmental Psychobiology and appear in a forthcoming book chapter authored by S.E.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abizaid A, Jafferali S, et al. Effect of metabolic fuel availability on fertility varies with reproductive state. Physiol Behav. 2001;74(1–2):77–83. doi: 10.1016/s0031-9384(01)00557-1. [DOI] [PubMed] [Google Scholar]
- Abizaid A, Woodside B. Food intake and neuronal activation after acute 2DG treatment are attenuated during lactation. Physiol Behav. 2002;75(4):483–491. doi: 10.1016/s0031-9384(02)00658-3. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Dushay J, et al. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest. 1997;99(3):391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17(2):201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Food-restricted, prepubertal, female rats: rapid recovery of luteinizing hormone pulsing with excess food, and full recovery of pubertal development with gonadotropin-releasing hormone. Endocrinology. 1986;118(6):2483–2487. doi: 10.1210/endo-118-6-2483. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Simerly RB. Development of leptin-sensitive circuits. J Neuroendocrinol. 2007;19(8):575–582. doi: 10.1111/j.1365-2826.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Maeda K, et al. Effect of food deprivation on the pulsatile LH release in the cycling and ovariectomized female rat. Horm Metab Res. 1990;22(5):269–272. doi: 10.1055/s-2007-1004900. [DOI] [PubMed] [Google Scholar]
- Chehab FF, Mounzih K, et al. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997;275(5296):88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- Dickerman RW, Li HY, et al. Decreased availability of metabolic fuels suppresses estrous behavior in Syrian hamsters. Am J Physiol. 1993;264(3 Pt 2):R568–R572. doi: 10.1152/ajpregu.1993.264.3.R568. [DOI] [PubMed] [Google Scholar]
- Friedman MI, Tordoff MG. Fatty acid oxidation and glucose utilization interact to control food intake in rats. Am J Physiol. 1986;251(5 Pt 2):R840–R845. doi: 10.1152/ajpregu.1986.251.5.R840. [DOI] [PubMed] [Google Scholar]
- Friedman MI, Tordoff MG, et al. Integrated metabolic control of food intake. Brain Res Bull. 1986;17(6):855–859. doi: 10.1016/0361-9230(86)90099-7. [DOI] [PubMed] [Google Scholar]
- Frisch RE. Pubertal adipose tissue: is it necessary for normal sexual maturation? Evidence from the rat and human female. Fed Proc. 1980;39(7):2395–2400. [PubMed] [Google Scholar]
- Frisch RE, Hegsted DM, et al. Body weight and food intake at early estrus of rats on a high-fat diet. Proc Natl Acad Sci U S A. 1975;72(10):4172–4176. doi: 10.1073/pnas.72.10.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav. 1999;67(1):141–147. doi: 10.1016/s0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- Gill CJ, Rissman EF. Female sexual behavior is inhibited by short- and long-term food restriction. Physiol Behav. 1997;61(3):387–394. doi: 10.1016/s0031-9384(96)00449-0. [DOI] [PubMed] [Google Scholar]
- Gruaz NM, Lalaoui M, et al. Chronic administration of leptin into the lateral ventricle induces sexual maturation in severely food-restricted female rats. J Neuroendocrinol. 1998;10(8):627–633. doi: 10.1046/j.1365-2826.1998.00247.x. [DOI] [PubMed] [Google Scholar]
- Houpt TR, Hance HE. Stimulation of food intake in the rabbit and rat by inhibition of glucose metabolism with 2-deoxy-D-glucose. J Comp Physiol Psychol. 1971;76(3):395–400. doi: 10.1037/h0031383. [DOI] [PubMed] [Google Scholar]
- I'Anson H, Starer CA, et al. Glucoprivic regulation of estrous cycles in the rat. Horm Behav. 2003;43(3):388–393. doi: 10.1016/s0018-506x(03)00007-2. [DOI] [PubMed] [Google Scholar]
- Landau IT, Zucker I. Estrogenic regulation of body weight in the female rat. Horm Behav. 1976;7(1):29–39. doi: 10.1016/0018-506x(76)90002-7. [DOI] [PubMed] [Google Scholar]
- Langhans W, Scharrer E. Evidence for a vagally mediated satiety signal derived from hepatic fatty acid oxidation. J Auton Nerv Syst. 1987;18(1):13–18. doi: 10.1016/0165-1838(87)90129-9. [DOI] [PubMed] [Google Scholar]
- Langhans W, Scharrer E. Role of fatty acid oxidation in control of meal pattern. Behav Neural Biol. 1987;47(1):7–16. doi: 10.1016/s0163-1047(87)90112-9. [DOI] [PubMed] [Google Scholar]
- Li HY, Wade GN, et al. Manipulations of metabolic fuel availability alter estrous behavior and neural estrogen receptor immunoreactivity in Syrian hamsters. Endocrinology. 1994;135(1):240–247. doi: 10.1210/endo.135.1.8013358. [DOI] [PubMed] [Google Scholar]
- Mayer J. Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Ann N Y Acad Sci. 1955;63(1):15–43. doi: 10.1111/j.1749-6632.1955.tb36543.x. [DOI] [PubMed] [Google Scholar]
- Nagatani S, Bucholtz DC, et al. Reduction of glucose availability suppresses pulsatile luteinizing hormone release in female and male rats. Endocrinology. 1996;137(4):1166–1170. doi: 10.1210/endo.137.4.8625885. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Tonge D, et al. Insulin and glucostatic control of feeding. J Comp Physiol Psychol. 1972;78(2):226–232. doi: 10.1037/h0032292. [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser P. 5-Thio-D-glucose causes increased feeding and hyperglycemia in the rat. Am J Physiol. 1980;238(2):E141–E144. doi: 10.1152/ajpendo.1980.238.2.E141. [DOI] [PubMed] [Google Scholar]
- Ritter S. Multiple metabolic controls of feeding. Appetite. 1994;23(2):199. doi: 10.1006/appe.1994.1052. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT. 2-Mercaptoacetate and 2-deoxy-D-glucose induce Fos-like immunoreactivity in rat brain. Brain Res. 1994;641(1):111–120. doi: 10.1016/0006-8993(94)91822-8. [DOI] [PubMed] [Google Scholar]
- Sajapitak S, Iwata K, et al. Central lipoprivation-induced suppression of LH pulses is mediated by paraventricular catecholaminergic inputs in female rats. Endocrinology. 2008 doi: 10.1210/en.2008-0016. [DOI] [PubMed] [Google Scholar]
- Scharrer E. Control of food intake by fatty acid oxidation and ketogenesis. Nutrition. 1999;15(9):704–714. doi: 10.1016/s0899-9007(99)00125-2. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Buckley CA, et al. Metabolic signals, hormones and neuropeptides involved in control of energy balance and reproductive success in hamsters. Eur J Neurosci. 2002;16(3):377–379. doi: 10.1046/j.1460-9568.2002.02118.x. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Finnerty BC, et al. Glucoprivic treatments that induce anestrus, but do not affect food intake, increase FOS-like immunoreactivity in the area postrema and nucleus of the solitary tract in Syrian hamsters. Brain Res. 1995;698(1–2):107–113. doi: 10.1016/0006-8993(95)00860-s. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Goldman MD, et al. Central vs. peripheral metabolic control of estrous cycles in Syrian hamsters. II. Glucoprivation. Am J Physiol. 1997;272(1 Pt 2):R406–R412. doi: 10.1152/ajpregu.1997.272.1.R406. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Hall AJ, et al. Central vs. peripheral metabolic control of estrous cycles in Syrian hamsters. I. Lipoprivation. Am J Physiol. 1997;272(1 Pt 2):R400–R405. doi: 10.1152/ajpregu.1997.272.1.R400. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Wade GN. Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science. 1989;244(4910):1326–1328. doi: 10.1126/science.2734610. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Wade GN. Decreased availability of metabolic fuels induces anestrus in golden hamsters. Am J Physiol. 1990;258(3 Pt 2):R750–R755. doi: 10.1152/ajpregu.1990.258.3.R750. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Zhou D. Interactive effects of central leptin and peripheral fuel oxidation on estrous cyclicity. Am J Physiol. 1999;277(4 Pt 2):R1020–R1024. doi: 10.1152/ajpregu.1999.277.4.R1020. [DOI] [PubMed] [Google Scholar]
- Shahab M, Sajapitak S, et al. Acute lipoprivation suppresses pulsatile luteinizing hormone secretion without affecting food intake in female rats. J Reprod Dev. 2006;52(6):763–772. doi: 10.1262/jrd.18066. [DOI] [PubMed] [Google Scholar]
- Sergeyev V, Broberger C, et al. Effect of 2-mercaptoacetate and 2-deoxy-D-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus. Neuroreport. 2000;11(1):117–121. doi: 10.1097/00001756-200001170-00023. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Smith GP, Epstein AN. Increased feeding in response to decreased glucose utilization in the rat and monkey. Am J Physiol. 1969;217(4):1083–1087. doi: 10.1152/ajplegacy.1969.217.4.1083. [DOI] [PubMed] [Google Scholar]
- Swithers SE. Development of independent ingestive responding to blockade of fatty acid oxidation in rats. Am J Physiol. 1997;273(5 Pt 2):R1649–R1656. doi: 10.1152/ajpregu.1997.273.5.R1649. [DOI] [PubMed] [Google Scholar]
- Swithers SE, McCurley M, et al. Differential effects of lipoprivation and food deprivation on chow and milk intake in 25- and 30-day-old rats. Appetite. 2005;45(1):86–93. doi: 10.1016/j.appet.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Melendez RI, et al. Metabolic and behavioral responses in pre-weanling rats following alteration of maternal diet. Physiol Behav. 2001;72(1–2):147–157. doi: 10.1016/s0031-9384(00)00385-1. [DOI] [PubMed] [Google Scholar]
- Tarttelin MF, Gorski RA. The effects of ovarian steroids on food and water intake and body weight in the female rat. Acta Endocrinol (Copenh) 1973;72(3):551–568. doi: 10.1530/acta.0.0720551. [DOI] [PubMed] [Google Scholar]
- Temple JL, Rissman EF. Brief refeeding restores reproductive readiness in food-restricted female musk shrews (Suncus murinus) Horm Behav. 2000;38(1):21–28. doi: 10.1006/hbeh.2000.1596. [DOI] [PubMed] [Google Scholar]
- Temple JL, Schneider JE, et al. Mating behavior is controlled by acute changes in metabolic fuels. Am J Physiol Regul Integr Comp Physiol. 2002;282(3):R782–R790. doi: 10.1152/ajpregu.00383.2001. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE, et al. Insulin-induced anestrus in Syrian hamsters. Am J Physiol. 1991;260(1 Pt 2):R148–R152. doi: 10.1152/ajpregu.1991.260.1.R148. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE, et al. Control of fertility by metabolic cues. Am J Physiol. 1996;270(1 Pt 1):E1–E19. doi: 10.1152/ajpendo.1996.270.1.E1. [DOI] [PubMed] [Google Scholar]
- Wade GN, Zucker I. Development of hormonal control over food intake and body weight in female rats. J Comp Physiol Psychol. 1970;70(2):213–220. doi: 10.1037/h0028713. [DOI] [PubMed] [Google Scholar]
- Zucker I. Body weight and age as factors determining estrogen responsiveness in the rat feeding system. Behav Biol. 1972;7(4):527–542. doi: 10.1016/s0091-6773(72)80215-3. [DOI] [PubMed] [Google Scholar]