Abstract
Dysfunction in the neuronal nicotinic acetylcholine receptor (nAChR) system has been implicated in the pathophysiology of schizophrenia, and it has been postulated that treatments that increase nAChR activity may improve symptoms of the disorder. We investigated the effects of the acetylcholinesterase inhibitor and allosteric nAChR modulator, galantamine, on cognitive performance and clinical symptoms when added to a stable antipsychotic medication regimen in nonsmoking outpatients with schizophrenia in a double-blind, placebo-controlled, parallel-group design. Participants were randomized to receive either galantamine (n = 10) up to 32 mg/day or identical placebo (n = 10) for 8 weeks and completed a cognitive battery at baseline and week 8 and clinical scales at baseline, week 4 and week 8. The primary outcome measure was attentional performance as measured by the d’ measure in the Continuous Performance Test – Identical Pairs (CPT-IP) Version. Contrary to our hypothesis, galantamine treatment was associated with inferior performance on the CPT-IP, on the three-card Stroop task, and on the Letter-Number Span task without reordering. Galantamine had no effect on clinical symptoms. In summary, galantamine treatment, at a dose of 32 mg/day, was well tolerated but was not effective as an adjunctive treatment for cognitive deficits in stable nonsmokers with schizophrenia.
Keywords: Galantamine, Schizophrenia, Attention, Nicotine, Acetylcholine
1. INTRODUCTION
People with schizophrenia are more likely to smoke and to have a higher level of nicotine dependence than those in the general population (de Leon and Diaz, 2005). It has been suggested that people with schizophrenia use nicotine to self-medicate symptoms of their disorder that are caused from abnormal nicotinic receptor structure and function (Dalack et al., 1998; Kumari and Postma, 2005). Decreased numbers of both high- and low-affinity nicotinic acetylcholine receptor (nAChR) numbers have been described in several brain regions in those with schizophrenia compared to controls (Breese et al., 2000; Durany et al., 2000; Freedman et al., 1995). In addition, a polymorphism in the promoter region of the alpha 7 nAChR gene is associated with reduced nAChR transcription in schizophrenia (Leonard et al., 2002) and with heavy cigarette smoking in this population (De Luca et al., 2004).
Nicotine has been shown to at least partially and transiently normalize neurophysiologic deficits of schizophrenia, including sensory gating deficits, which are linked to the gene that codes for the alpha 7 nAChR (Adler et al., 1993; Adler et al., 1992; Avila et al., 2003; Olincy et al., 2003) and to improve cognitive deficits associated with schizophrenia such as attention and inhibitory processing (Barr et al., 2007; Harris et al., 2004; Jacobsen et al., 2004; Smith et al., 2002). Additionally, stimulation of presynaptic nAChRs on glutamatergic and dopaminergic neurons increases activity of these neurons in relevant brain regions (Kiba and Jayaraman, 1994; Mansvelder and McGehee, 2000; Nomikos et al., 2000; Sziraki et al., 1998), an effect that may have relevance for improving cognitive dysfunction and negative symptoms of the disorder (Carlsson and Carlsson, 1990; Ishimaru et al., 1994; Javitt and Zukin, 1991; Kim et al., 1980; Olney and Farber, 1995).
The abnormality in nicotinic signaling and possible reversal by nicotine has stimulated interest in a potential therapeutic role for agents that increase nicotinic signaling. Clinical studies of the acetylcholinesterase inhibitor, donepezil, in schizophrenia have been mixed with some reports of moderate improvements in memory (Buchanan et al., 2003; Howard et al., 2002; Lee et al., 2007a; Risch et al., 2001) or negative symptoms (Risch et al., 2007) and others showing no effect (Fagerlund et al., 2007; Freudenreich et al., 2005; Stryjer et al., 2004; Tugal et al., 2004) including a recent large well controlled trial showing no effect for donepezil augmentation on the CATIE cognitive battery or clinical symptoms (Keefe et al., 2007).
Galantamine is the weakest acetylcholinesterase inhibitor of those that are approved for the treatment of dementia of the Alzheimer’s type. Additionally, galantamine acts as an allosterically potentiating ligand (APL) at neuronal nAChRs (Maelicke and Albuquerque, 2000; Popa et al., 2006; Samochocki et al., 2003; Schrattenholz et al., 1996) and has demonstrated positive effects in animal models of schizophrenia (Csernansky et al., 2005; Deutsch et al., 2003; Hohnadel et al., 2007; Schilstrom et al., 2007; Wang et al., 2007a; Wang et al., 2007b). There have been promising case reports and small studies reporting efficacy of galantamine for the treatment of cognitive dysfunction (Bora et al., 2005; Schubert et al., 2006), negative symptoms (Rosse and Deutsch, 2002) and psychotic and disorganized symptoms of schizophrenia (Allen and McEvoy, 2002), as well as a report of no significant effect (Lee et al., 2007b).
For treatment of Alzheimer’s disease, a dosage of 16–24 mg/day is recommended, but up to 32 mg/day has been shown to be safe and effective (Raskind et al., 2000). Prior studies of the effects of galantamine in schizophrenia described modest effects at a dose of 16–24 mg/day (Allen and McEvoy, 2002; Bora et al., 2005; Lee et al., 2007b; Rosse and Deutsch, 2002; Schubert et al., 2006). This study aimed to test the hypothesis, in a randomized, double-blind, placebo-controlled, parallel group design, that a relatively high dose of galantamine, 32 mg/day, would be associated with improved cognitive performance and negative symptoms in stable, nonsmoking outpatients with schizophrenia. We have recently demonstrated an effect for nicotine on attention (Barr et al., 2007) and memory (Weiss et al., 2006) in nonsmokers with schizophrenia and chose to study the effect of galantamine on attention and working memory in nonsmokers with the hypothesis that those not receiving exogenous nicotinic stimulation from tobacco smoking would benefit more from galantamine than those already receiving exogenous stimulation (eg. smokers).
2. MATERIALS AND METHODS
The study protocol was approved by the appropriate Institutional Review Board. All participants provided written informed consent prior to beginning study procedures.
2.1. Participants
Participants were stable, adult outpatients with schizophrenia or schizoaffective disorder depressed type who had been on a stable dose of an antipsychotic medication for at least 8 weeks prior to enrollment. Participants were excluded who were taking an anticholinergic medication or who reported use of an illicit drug or a nicotine-containing product in the past 3 months or who had expired air carbon monoxide (CO) >9 ppm or salivary drug screen positive for cotinine, cocaine, THC, ethanol, amphetamine, or benzodiazepines at screening.
2.2. Procedures
The study took place at an urban, university-affiliated community mental health clinic. Participants were randomized to receive either galantamine or identical placebo in addition to their usual medications for 8 weeks. Randomization was performed with concealed allocation with a 1:1 ratio, in blocks of 4. The following dosing schedule was followed: 4 mg per day for 3 days; then 4 mg twice per day for 11 days; then 8 mg twice per day for 14 days; then 12 mg twice per day for 14 days; and finally 16 mg twice per day for 14 days.
2.3. Measures
The following neuropsychological tests were performed at baseline and week 8: Continuous Performance Test – Identical Pairs (CPT-IP) Version 4.0 (Biobehavioral Technologies, New York, NY, USA) to measure attention (Cornblatt et al., 1989); the Three-Card Stroop (Stoelting Co., Wood Dale, IL, USA) to measure response inhibition; Letter-Number Span (LNS) from the WAIS-III (Wechsler, 1997) to measure attention and working memory; and Grooved Pegboard (Model 32025, Lafayette Instrument Company, Lafayette, IN, USA) to measure motor speed.
The following standard clinical rating scales were performed at baseline, week 4 and week 8: Positive and Negative Syndrome Scale (PANSS), the Scale for the Assessment of Negative Symptoms (SANS), the Calgary Depression Scale for Schizophrenia (CDSS), the Abnormal Involuntary Movement Scale (AIMS), the Simpson-Angus Scale (SAS) and the Barnes Akathisia Scale.
Medication compliance was assessed weekly by self-report and pill counts. Adverse event questionnaires were performed weekly. Weekly self-report of nonsmoking status was confirmed by weekly expired air carbon monoxide (CO) measurement (Bedfont Smokerlyzer II, Bedfont Scientific, LTD, Kent, UK) of <9 ppm and by semi-quantitative salivary cotinine concentration of <10 ng/mL at baseline, week 4 and week 8 (Nicalert™, Jant Pharmacal Corporation, Endocino, CA, USA).
For an assessment of compliance, the presence or absence of galantamine in previously frozen patient serum samples was determined using high-performance liquid chromatography (HPLC) and photo-diode-array absorbance (PDA) detectors in a subset of participants. A working internal standard was created using intermediate solution and previously frozen drug-free serum. A galantamine standard was created by extracting galantamine from the pill form of the drug with a hexane containing solvent. Galantamine was found to elute at approximately 2.6 min on both the 214-nm and 230-nm chromatograms. Peaks <0.0015 A were considered to be below the detection limits for this method, and therefore clinically insignificant (Puopolo et al., 1991). Each peak >0.0015 A, with a retention time between 2.60 min and 2.67 min was examined and the presence of galantamine was confirmed by matching the retention time and the ultraviolet spectra to the galantamine standard. If no peak >0.0015 A was observed then the sample was considered negative for galantamine. This method was used to generate the qualitative presence or absence of galantamine. Quantitative analysis was not performed because pure galantamine compound was not available to create the galantamine standard.
2.4. Data Analysis
Baseline characteristics were compared by randomization status with student’s T tests and Exact tests for continuous and dichotomous variables, respectively. The data were examined for distributional properties. The random errors variable of the CPT-IP was transformed to a natural logarithm. Linear regression models were performed to evaluate the effect of medication status on change in cognitive performance, controlling for baseline scores. Point estimates and 95% confidence intervals of medication effect were calculated from these regression models. The five-factor subscale structure was used to analyze PANSS scores (Lindenmayer et al., 1994). The use of covariates was limited to medication status and baseline score due to sample size considerations. For cognitive tests and for the SANS total score, effect estimates (Cohen’s d) were also calculated. SAS Version 9.1 statistical software was used for all analyses.
3. RESULTS
Thirty-five potential participants were screened, 23 signed informed consent and 20 met entry criteria, were randomized, took at least one dose of study medication and are included in the analysis. Of the 20 participants who enrolled in the study, 18 (9 placebo, 9 galantamine) completed 8 weeks of treatment. Data from the final visit of the two participants who discontinued study procedures prior to week 8 are included in the LOCF analysis. Baseline demographic and clinical characteristics are presented by randomization status in Table 1. There were no significant differences between the treatment groups at baseline. All participants were taking a stable dose of a second-generation antipsychotic medication. Two participants in the galantamine group were taking two different antipsychotics.
Table 1.
Baseline Demographic Characteristics
| Galantamine (n=10) |
Placebo (n=10) |
||
|---|---|---|---|
| Age | 44.3 (11.9) | 50.5 (4.7) | |
| Male/Female | 7/3 | 6/4 | |
| Ethnicity | |||
| Caucasian | 8 | 9 | |
| African-American | 2 | 1 | |
| Marital Status | |||
| Single | 10 | 9 | |
| Divorced or Separated | 0 | 1 | |
| IQ | 95.3 (12.1) | 98.9 (13.3) | |
| Years of Education | 12.9 (2.6) | 13.0 (3.6) | |
| Antipsychotic Medication* | |||
| clozapine | 2 | 3 | |
| risperidone | 0 | 4 | |
| aripiprazole | 2 | 1 | |
| olanzapine | 4 | 2 | |
| ziprasidone | 3 | 0 | |
| quetiapine | 1 | 0 | |
| CO** at Screening (ppm) | 0.9 (0.6) | 0.8 (1.0) | |
| Clinical Symptoms | |||
| PANSS Total Score | 65.4 (14.3) | 68.1 (19.5) | |
| SANS Total Score | 30.5 (16.1) | 23.3 (9.7) | |
| CDSS Total | 3.7 (3.4) | 3.9 (3.7) |
Number of participants in each group on the antisychotic medication; some participants were taking more than one antipsychotic medication.
Carbon monoxide
3.1. Cognitive Performance
3.1.1. Attention
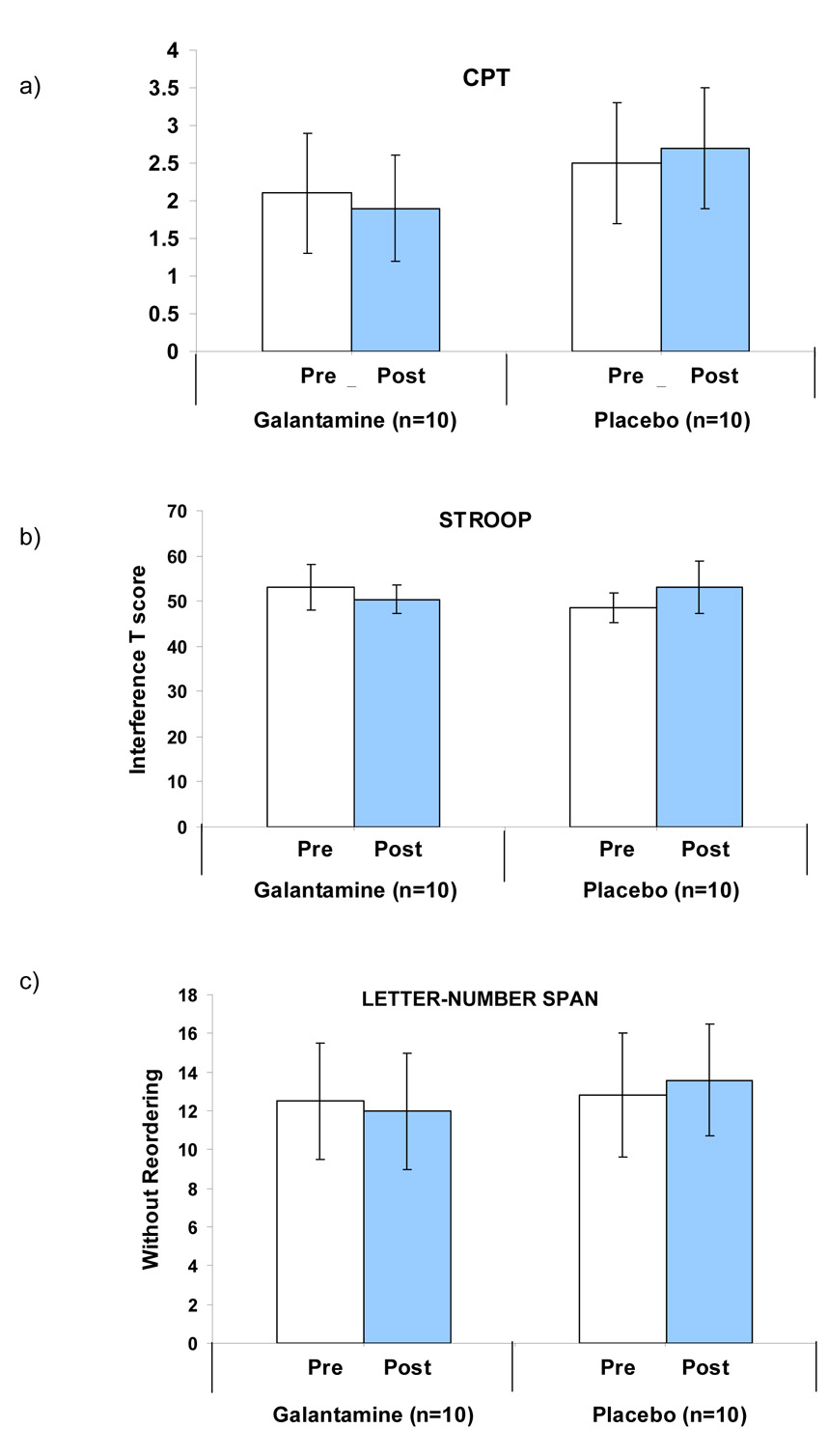
As shown in Figure 1a, galantamine treatment was associated with worsened performance on the d’ measure of the CPT-IP, the primary outcome measure, compared with placebo (β = − 10.4, p = 0.035). This is an overall measure of ability to detect signal to noise. Galantamine treatment was associated with a trend toward an increase in ln random errors compared with placebo (β = 1.7, p = 0.0740). There was no medication effect on reaction time or variability in reaction time. Data are given in Table 2.
Figure 1.
Galantamine Effect on Measures of Cognitive Performance in Nonsmokers with Schizophrenia
Table 2.
Effect of Galantamine and Placebo on Neuropsychological and Clinical Symptoms
| Galantamine (n=10) |
Placebo (n=10) |
Effect Estimate* |
95% CI Effect Est. |
p |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | ||||
| Neuropsychological | |||||||||
| CPT | |||||||||
| d' | 2.1 (0.8) | 1.9 (0.7) | −0.2 (0.5) | 2.5 (0.8) | 2.7 (0.8) | 0.1 (0.4) | −10.4 | −0.9, 0.0 | 0.03 |
| Random Errors (ln) | −3.9 (1.4) | −3.6 (1.4) | 0.3 (1.2) | −2.9 (2.2) | −3.4 (2.0) | −1.6 (2.4) | 7.3 | −0.2, 3.5 | 0.07 |
| Reaction Time | 563.1 (97.7) | 550.5 (77.9) | −12.6 (61.2) | 555.7 (57.5) | 555.7 (71.5) | −0.1 (42.9) | 1.7 | −56.9, 36.0 | 0.64 |
| Reaction Time SD | 161.4 (34.6) | 158.3 (44.8) | −3.07 (28.4) | 149.9 (36.1) | 140.4 (39.4) | −9.4 (25.5) | −0.4 | −19.2, 33.7 | 0.57 |
| Stroop | |||||||||
| Interference T-score | 53.1 (5.0) | 50.4 (3.1) | −2.7 (3.7) | 48.5 (3.2) | 53.0 (5.8) | 4.5 (5.3) | −5.0 | −9.6, −0.4 | 0.04 |
| Letter-Number Span | |||||||||
| Without Reordering | 12.5 (3.0) | 12.0 (3.0) | −0.5 (1.4) | 12.8 (3.2) | 13.6 (2.9) | 0.8 (1.2) | −1.3 | −2.5, −0.1 | 0.03 |
| With Reordering | 8.8 (4.2) | 8.9 (3.4) | 0.1 (2.1) | 8.7 (2.5) | 9.4 (2.9) | 0.7 (1.1) | −0.6 | −2.1, 0.9 | 0.43 |
| Grooved Pegboard | |||||||||
| Average Pegs | 15.2 (2.8) | 15.9 (3.1) | 0.7 (1.8) | 15.8 (3.4) | 16.1 (3.4) | 0.4 (1.4) | 0.2 | −1.3, 1.8 | 0.74 |
| Clinical Symptoms | |||||||||
| PANSS | |||||||||
| Total Score | 65.4 (14.3) | 65.4 (16.3) | 0.0 (10.5) | 68.1 (19.5) | 70.4 (20.7) | 2.3 (10.9) | −2.6 | −12.9, 7.7 | 0.50 |
| SANS | |||||||||
| Total Score | 30.5 (16.1) | 26.5 (17.3) | −4.0 (9.2) | 23.3 (9.7) | 25.6 (12.3) | 2.3 (10.9) | −5.7 | −14.7, 3.3 | 0.20 |
| CDSS | |||||||||
| Total Score | 3.7 (3.4) | 3.6 (3.3) | −0.1 (2.3) | 3.9 (3.7) | 6.9 (5.2) | 3.0 (5.4) | −3.2 | −6.9, 0.5 | 0.09 |
Effect estimates presented are unstandardized coefficients (β) calculated with linear regression analyses with change from baseline to week 8 as the dependent measure, and medication status and baseline measure as independent variables.
PANSS= Positive and Negative Affect Scale
SANS = Schedule for Assessment of Negative Symptoms, CDSS=Calgary Depression Scale for Schizophrenia
3.1.2. Executive Function/Inhibitory Control
Galantamine treatment was associated with worsened performance compared with placebo on the Stroop task, as assessed by the interference T-score, (higher T-score reflects greater inhibitory control) (β = − 5.0, p = 0.035). See Table 2 and Figure 1b.
3.1.3. Memory
Galantamine treatment was associated with worsened performance compared with placebo on the LNS task without reordering (β = − 1.3, p < 0.03; Cohen’s d = − 0.92), but had no significant effect on performance on the reordering portion of the task (β = − 0.6, p = 0.43).
3.1.4. Motor speed
There was no effect of galantamine relative to placebo on motor performance as assessed with the Grooved Pegboard task (β = 0.2, p = 0.74).
3.2. Clinical Scales
Linear regression models that included change in clinical score as the dependent variable and medication status and respective baseline clinical as independent variables revealed no significant effect of medication on PANSS, SANS and CDS total scores. The data are presented in Table 2 with confidence intervals for the point estimates. While the effect of galantamine was not significant compared with placebo, the SANS total score in the galantamine group decreased by more than 10% (baseline mean score 30.5; week 8 mean score 26.5). The effect size for galantamine on negative symptoms as assessed by SANS total score was moderate (Cohen’s d = − 0.675). An exploratory analysis was conducted for PANSS and SANS subscale scores at week 8 and for total scores at week 4; there were also no significant effects on subscale scores at week 8 or for total scores at week 4 (data not shown).
3.3. Safety and Tolerability
One participant on galantamine withdrew consent at week 6, at a dose of 24 mg/day, due to muscle cramps in feet and abdomen. One participant randomized to the placebo group discontinued study medication at week 5, stating she no longer wanted to take study medication. Both of these participants completed endpoint assessments at the time of discontinuation of study medication, and these clinical assessments, collected at study week 5 and 6, respectively, were used in the LOCF analyses for week 8 endpoint scores. One subject on placebo completed 8 weeks of study medication but did not increase her dose beyond the equivalent of 16 mg/day due to mild nausea, moderate fatigue and insomnia and increased alertness. All other participants completed the dose titration to 32 mg/day.
A total of 43 adverse events (defined here as an increase in any symptom, even if mild) were reported in the galantamine group; 46 were reported in the placebo group. The most common events (5 or more people) reported in the galantamine group were fatigue, nausea and somnolence; the most common in the placebo group were depression, fatigue, dizziness and somnolence. Repeated measures ANOVA of extrapyramidal symptom scales revealed no significant effect of medication on adverse event frequency or severity (data not shown).
For the 18 participants who completed the 8 week study, the average number of missed doses of study medication was 1.35 doses per two week interval, 1.13 in the galantamine group and 1.56 in the placebo group, by self report, confirmed by pill count. For an additional assessment of compliance, the presence or absence of galantamine in serum samples was determined in 8 of the 20 participants, 5 assigned to galantamine and 3 assigned to placebo. Galantamine was present in serum of all 5 participants randomized to galantamine and in none of the 3 assigned to placebo.
4. DISCUSSION
In this eight-week, double-blind, placebo-controlled trial of galantamine augmentation, at a maximum dose of 32 mg/day, of antipsychotic treatment in stable outpatient nonsmokers with schizophrenia, the effect of galantamine was inferior to placebo on attention as assessed by the CPT-IP d’ (signal to noise detection), on inhibitory control as assessed by the Stroop Interference T-score, and on one measure of memory, the LNS without reordering. There was also a trend for galantamine to be inferior to placebo for random errors in the CPT-IP measure of attention. Galantamine did not separate from placebo on measures of working memory or psychomotor speed, as assessed with the LNS with reordering and Grooved Pegboard. Galantamine also did not separate from placebo on clinical measures, although the study may have been underpowered to detect an effect on negative symptoms.
These findings are consistent with a recent report of a large, randomized, placebo-controlled study of donepezil for cognitive performance in schizophrenia (Keefe et al., 2007) in which few beneficial effects of donepezil were observed and placebo was superior to the cholinesterase inhibitor in some cognitive measures. The present study fails to replicate reports of beneficial effects of lower doses of galantamine (16–24 mg/day) in schizophrenia in case series (Allen and McEvoy, 2002; Bora et al., 2005; Rosse and Deutsch, 2002) and in one small, double-blind, placebo-controlled trial in patients taking risperidone (Schubert et al., 2006).
At low doses, galantamine acts as an allosteric potentiator of nAChRs, increasing the probability of agonist induced nAChR channel opening, and at higher doses, galantamine acts primarily as an acetylcholinesterase (AChE) inhibitor (Samochocki et al., 2003; Samochocki et al., 2000; Schrattenholz et al., 1996). At low doses, unlikely to inhibit AChE, galantamine, like nicotine, increases burst firing activity of dopaminergic cells in the ventral tegmental area and increases dopamine levels in the pre-frontal cortex, via activation of nAChRs, an effect that is blocked by inhibitors of alpha 4 beta 2 and alpha 7 nAChRs, as well as by an N-methyl-D-aspartate receptor (NMDAR) antagonist (Schilstrom et al., 2007). Phasic cortical dopamine release has been implicated as important to cognitive performance and as a possible mechanism for a beneficial effect of galantamine on cognitive function and negative symptoms. Galantamine at concentrations of 0.1–1 microM, corresponding to cerebrospinal fluid concentrations in humans on galantamine 16–24 mg daily, appears to act as an APL, but at concentrations >1 microM galantamine does not potentiate nAChR channel opening in the presence of ACh (Schrattenholz et al., 1996) and at concentrations >10 microM, galantamine, like the selective AChE inhibitor donepezil, may inhibit nAChR-mediated currents in dopaminergic cells (Schilstrom et al., 2007). It is likely that at high doses, galantamine’s nAChR allosteric modulation effect may be masked by a stronger direct nAChR inhibitory effect. It is not known what oral doses of galantamine in humans would produce such inhibition of nAChR activity.
Our findings are limited by the small sample size, limiting our power to detect medication effects and to control for potential confounders. Smokers were excluded with the hypothesis that any medication effects would be greater in nonsmokers. It is possible, however, that nonsmokers and those who have been able to quit smoking represent a subset of patients with schizophrenia who are less responsive to nicotinic agonist treatment. Additionally, it is possible that upregulation of nAChRs by smoking may potentiate clinical effects of the allosteric potentiating ligand, galantamine. Inclusion of only nonsmokers limits generalizability of the findings to nonsmokers, currently a minority of patients with schizophrenia. The findings are also limited by measurement of cognitive function at only two time points, baseline and endpoint, when most subjects were taking 32 mg/day of galantamine, as positive clinical effects may be greater at lower doses. It is important to note two limitations with regard to SANS scores. First, baseline SANS scores were relatively low, possibly causing a floor effect. Second, SANS scores were decreased by more than 10% in the galantamine group and increased in the placebo group. While it is possible that SANS scores in each group regressed to the mean, narrowing baseline differences between groups, it is also possible that the study simply lacked the power to detect an effect of galantamine on negative symptoms.
In summary, in this double-blind, placebo-controlled trial, galantamine, 32 mg/day, had a directionally opposite effect from placebo on measures of cognitive performance and indeed significantly worsened performance on measures of attention and inhibitory processing compared with placebo, while there was no medication effect on motor speed or clinical symptoms. It is therefore possible that galantamine augmentation at this dose may worsen cognitive functioning in patients with schizophrenia. Future studies should therefore test the effects of galantamine across several levels of smoking and several doses of galantamine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am. J. Psychiatry. 1993;150(12):1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol. Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- Allen TB, McEvoy JP. Galantamine for treatment-resistant schizophrenia. Am. J. Psychiatry. 2002;159(7):1244–1245. doi: 10.1176/appi.ajp.159.7.1244. [DOI] [PubMed] [Google Scholar]
- Avila MT, Sherr JD, Hong E, Myers CS, Thaker GK. Effects of nicotine on leading saccades during smooth pursuit eye movements in smokers and nonsmokers with schizophrenia. Neuropsychopharmacology. 2003;28(12):2184–2191. doi: 10.1038/sj.npp.1300265. [DOI] [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301423. (In press; advanced online publication available 04/17/07) [DOI] [PubMed] [Google Scholar]
- Bora E, Veznedaroglu B, Kayahan B. The effect of galantamine added to clozapine on cognition of five patients with schizophrenia. Clin. Neuropharmacol. 2005;28(3):139–141. doi: 10.1097/01.wnf.0000162555.68729.04. [DOI] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23(4):351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Summerfelt A, Tek C, Gold J. An open-labeled trial of adjunctive donepezil for cognitive impairments in patients with schizophrenia. Schizophr. Res. 2003;59(1):29–33. doi: 10.1016/s0920-9964(01)00387-5. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia -- Implications for schizophrenia and Parkinson's disease. TINS. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res. 1989;29(1):65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Martin M, Shah R, Bertchume A, Colvin J, Dong H. Cholinesterase inhibitors ameliorate behavioral deficits induced by MK-801 in mice. Neuropsychopharmacology. 2005;30(12):2135–2143. doi: 10.1038/sj.npp.1300761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am. J. Psychiatry. 1998;155(11):1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005;76(2–3):135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- De Luca V, Wong AH, Muller DJ, Wong GW, Tyndale RF, Kennedy JL. Evidence of association between smoking and alpha7 nicotinic receptor subunit gene in schizophrenia patients. Neuropsychopharmacology. 2004;29(8):1522–1526. doi: 10.1038/sj.npp.1300466. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Billingslea EN, Bellack AS, Mastropaolo J. Modulation of MK-801-elicited mouse popping behavior by galantamine is complex and dose-dependent. Life Sci. 2003;73(18):2355–2361. doi: 10.1016/s0024-3205(03)00642-8. [DOI] [PubMed] [Google Scholar]
- Durany N, Zochling R, Boissl KW, Paulus W, Ransmayr G, Tatschner T, Danielczyk W, Jellinger K, Deckert J, Riederer P. Human post-mortem striatal alpha4beta2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson's syndrome. Neurosci. Lett. 2000;287(2):109–112. doi: 10.1016/s0304-3940(00)01144-7. [DOI] [PubMed] [Google Scholar]
- Fagerlund B, Soholm B, Fink-Jensen A, Lublin H, Glenthoj BY. Effects of donepezil adjunctive treatment to ziprasidone on cognitive deficits in schizophrenia: a double-blind, placebo-controlled study. Clin. Neuropharmacol. 2007;30(1):3–12. doi: 10.1097/01.WNF.0000240940.67241.F6. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol. Psychiatry. 1995;38(1):22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Freudenreich O, Herz L, Deckersbach T, Evins AE, Henderson DC, Cather C, Goff DC. Added donepezil for stable schizophrenia: a double-blind, placebo-controlled trial. Psychopharmacology (Berl.) 2005;181(2):358–363. doi: 10.1007/s00213-005-2235-1. [DOI] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29(7):1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Hohnadel E, Bouchard K, Terry AV., Jr Galantamine and donepezil attenuate pharmacologically induced deficits in prepulse inhibition in rats. Neuropharmacology. 2007;52(2):542–551. doi: 10.1016/j.neuropharm.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AK, Thornton AE, Altman S, Honer WG. Donepezil for memory dysfunction in schizophrenia. J. Psychopharmacol. 2002;16(3):267–270. doi: 10.1177/026988110201600313. [DOI] [PubMed] [Google Scholar]
- Ishimaru M, Kurumaji A, Toro M. Increases in strychnine-insensitive glycine binding sites in cerebral cortex of chronic schizophrenics: Evidence for glutamate hypothesis. Biol. Psychiatry. 1994;35:84–95. doi: 10.1016/0006-3223(94)91197-5. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, D'Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol. Psychiatry. 2004;55(8):850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Javitt D, Zukin S. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Keefe R, Malhotra A, Meltzer H, Kane J, Buchanan R, Murthy A, Sovel M, Li C, Goldman R. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: Significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301499. (In press; advanced online publication available 07/11/07) [DOI] [PubMed] [Google Scholar]
- Kiba H, Jayaraman A. Nicotine induced c-fos expression in the striatum is mediated mostly by dopamine D1 receptor and is dependent on NMDA stimulation. Brain Res. Mol. Brain Res. 1994;23(1–2):1–13. doi: 10.1016/0169-328x(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kornhuber HH, Schmid-Burgk W, Holzmuller B. Low cerebrospinal glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci. Lett. 1980;20:379–382. doi: 10.1016/0304-3940(80)90178-0. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci. Biobehav. Rev. 2005;29(6):1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Lee JG, Kim YH. A 12-week, double-blind, placebo-controlled trial of donepezil as an adjunct to haloperidol for treating cognitive impairments in patients with chronic schizophrenia. J. Psychopharmacol. 2007a;21(4):421–427. doi: 10.1177/0269881106070996. [DOI] [PubMed] [Google Scholar]
- Lee SW, Lee JG, Lee BJ, Kim YH. A 12-week, double-blind, placebo-controlled trial of galantamine adjunctive treatment to conventional antipsychotics for the cognitive impairments in chronic schizophrenia. Int. Clin. Psychopharmacol. 2007b;22(2):63–68. doi: 10.1097/YIC.0b013e3280117feb. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch. Gen. Psychiatry. 2002;59(12):1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Bernstein-Hyman R, Grochowski S. A new five factor model of schizophrenia. Psychiatr. Q. 1994;65(4):299–322. doi: 10.1007/BF02354306. [DOI] [PubMed] [Google Scholar]
- Maelicke A, Albuquerque EX. Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer's disease. Eur. J. Pharmacol. 2000;393(1–3):165–170. doi: 10.1016/s0014-2999(00)00093-5. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27(2):349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Schilstrom B, Hildebrand BE, Panagis G, Grenhoff J, Svensson TH. Role of alpha7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behav. Brain Res. 2000;113(1–2):97–103. doi: 10.1016/s0166-4328(00)00204-7. [DOI] [PubMed] [Google Scholar]
- Olincy A, Johnson LL, Ross RG. Differential effects of cigarette smoking on performance of a smooth pursuit and a saccadic eye movement task in schizophrenia. Psychiatry Res. 2003;117(3):223–236. doi: 10.1016/s0165-1781(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch. Gen. Psychiatry. 1995;52(12):998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Popa RV, Pereira EF, Lopes C, Maelicke A, Albuquerque EX. The N-butylcarbamate derivative of galantamine acts as an allosteric potentiating ligand on alpha7 nicotinic receptors in hippocampal neurons: clinical implications for treatment of Alzheimer's disease. J. Mol. Neurosci. 2006;30(1–2):227–232. doi: 10.1385/JMN:30:1:227. [DOI] [PubMed] [Google Scholar]
- Puopolo PR, Pothier ME, Volpicelli SA, Flood JG. Single procedure for detection, confirmation, and quantification of benzodiazepines in serum by liquid chromatography with photodiode-array detection. Clin. Chem. 1991;37(5):701–706. [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54(12):2261–2268. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- Risch SC, Horner MD, McGurk SR, Palecko S, Markowitz JS, Nahas Z, DeVane CL. Double-blind donepezil-placebo crossover augmentation study of atypical antipsychotics in chronic, stable schizophrenia: a pilot study. Schizophr. Res. 2007;93(1–3):131–135. doi: 10.1016/j.schres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Risch SC, McGurk S, Horner MD, Nahas Z, Owens SD, Molloy M, Gilliard C, Christie S, Markowitz JS, DeVane CL, Mintzer J, George MS. A double-blind placebo-controlled case study of the use of donepezil to improve cognition in a schizoaffective disorder patient: functional MRI correlates. Neurocase. 2001;7(2):105–110. doi: 10.1093/neucas/7.2.105. [DOI] [PubMed] [Google Scholar]
- Rosse RB, Deutsch SI. Adjuvant galantamine administration improves negative symptoms in a patient with treatment-refractory schizophrenia. Clin. Neuropharmacol. 2002;25(5):272–275. doi: 10.1097/00002826-200209000-00010. [DOI] [PubMed] [Google Scholar]
- Samochocki M, Hoffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EF, Lubbert H, Albuquerque EX, Maelicke A. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2003;305(3):1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- Samochocki M, Zerlin M, Jostock R, Groot Kormelink PJ, Luyten WH, Albuquerque EX, Maelicke A. Galantamine is an allosterically potentiating ligand of the human alpha4/beta2 nAChR. Acta. Neurol. Scand. Suppl. 2000;176:68–73. doi: 10.1034/j.1600-0404.2000.00310.x. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32(1):43–53. doi: 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- Schrattenholz A, Pereira EF, Roth U, Weber KH, Albuquerque EX, Maelicke A. Agonist responses of neuronal nicotinic acetylcholine receptors are potentiated by a novel class of allosterically acting ligands. Mol. Pharmacol. 1996;49(1):1–6. [PubMed] [Google Scholar]
- Schubert MH, Young KA, Hicks PB. Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biol. Psychiatry. 2006;60(6):530–533. doi: 10.1016/j.biopsych.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27(3):479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Stryjer R, Strous R, Bar F, Shaked G, Shiloh R, Rozencwaig S, Grupper D, Buchman N, Kotler M, Rabey JM, Weizman A. Donepezil augmentation of clozapine monotherapy in schizophrenia patients: a double blind cross-over study. Hum. Psychopharmacol. 2004;19(5):343–346. doi: 10.1002/hup.595. [DOI] [PubMed] [Google Scholar]
- Sziraki I, Sershen H, Benuck M, Hashim A, Lajtha A. Receptor systems participating in nicotine-specific effects. Neurochem. Int. 1998;33(5):445–457. doi: 10.1016/s0197-0186(98)00049-7. [DOI] [PubMed] [Google Scholar]
- Tugal O, Yazici KM, Anil Yagcioglu AE, Gogus A. A double-blind, placebo controlled, cross-over trial of adjunctive donepezil for cognitive impairment in schizophrenia. Int. J. Neuropsychopharmacology. 2004;7(2):117–123. doi: 10.1017/S1461145703004024. [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Zhou Y, Mouri A, Mizoguchi H, Nitta A, Chen W, Nabeshima T. The allosteric potentiation of nicotinic acetylcholine receptors by galantamine ameliorates the cognitive dysfunction in beta amyloid25–35 i.c.v.-injected mice: involvement of dopaminergic systems. Neuropsychopharmacology. 2007a;32(6):1261–1271. doi: 10.1038/sj.npp.1301256. [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Zhou Y, Nitta A, Furukawa H, Nabeshima T. Synergistic effect of galantamine with risperidone on impairment of social interaction in phencyclidine-treated mice as a schizophrenic animal model. Neuropharmacology. 2007b;52(4):1179–1187. doi: 10.1016/j.neuropharm.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Weiss A, Jubelt L, Barr R, Goff D, Rigotti N, Evins A. The effect of transdermal nicotine on episodic memory performance in nonsmoking patients with schizophrenia. Neuropsychopharmacology. 2006;31:S112–S113. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale Third Edition (WAIS-III) Vol. 31. Psychological Corporation; San Antonio: 1997. pp. S112–S113. [Google Scholar]