Abstract
Mutations in the MAPT gene encoding tau protein lead to neurofibrillary lesion formation, neurodegeneration, and cognitive decline associated with frontotemporal lobar degeneration. While some pathogenic mutations affect MAPT introns, resulting in abnormal splicing patterns, the majority occur in the tau coding sequence leading to single amino acid changes in tau primary structure. Depending on their location within the polypeptide chain, tau missense mutations have been reported to augment aggregation propensity. To determine the mechanisms underlying mutation-associated changes in aggregation behavior, the fibrillization of recombinant pathogenic mutants R5L, G272V, P301L, V337M, and R406W prepared in a full-length four-repeat human tau background was examined in vitro as a function of time and submicromolar tau concentrations using electron microscopy assay methods. Kinetic constants for nucleation and extension phases of aggregation were then estimated by direct measurement and mathematical simulation. Results indicated that the mutants differ from each other and from wild-type tau in their aggregation propensity. G272V and P301L mutations increased the rates of both filament nucleation and extension reactions, whereas R5L and V337M increased only the nucleation phase. R406W did not differ from wild-type in any kinetic parameter. The results show that missense mutations can directly promote tau filament formation at different stages of the aggregation pathway.
Keywords: Frontotemporal lobar degeneration, tau, neurofibrillary tangle, aggregation reaction mechanism
Introduction
Frontotemporal lobar degeneration (FTLD) is a clinically and pathologically heterogeneous group of diseases associated with cognitive decline and degeneration of prefrontal and anterior temporal neocortex (Neary et al. 2005). FTLD is often familial, with ~20% of inherited cases caused by mutations in the MAPT gene located on chromosome 17 (Rademakers et al. 2004). MAPT encodes the microtubule associated protein tau, which exists in human brain as a mixture of six isoforms owing to alternative splicing of exons 2, 3, and 10 from MAPT transcripts (Fig. 1A). Tau isoforms normally bind to and function in conjunction with the microtubule cytoskeleton, but they aggregate to form filamentous inclusions in FTLD. To date more than 40 pathogenic MAPT mutations that lead to tau lesion formation and FTLD have been identified in 127 families worldwide (Rademakers et al. 2004), 34 of which change tau primary structure at one of 27 different amino-acid residues (Fig. 1A). These residues are found predominantly in the C-terminal half of tau isoforms (i.e., those encoded by exons 9 – 12), where the 31–32 residue imperfect repeats that mediate both microtubule binding (Goode et al. 2000) and tau fibrillization (Novak et al. 1993) are located. However, pathological mutations have been discovered in exon 1 as well, leading to amino acid substitutions at residue five near the N-terminus (Hayashi et al. 2002; Poorkaj et al. 2002), whereas others have been found in exon 13, which encodes the segment C-terminal to the repeat region. Each of these mutations could affect lesion formation through different mechanisms. For example, they could promote tau lesion formation directly by increasing the aggregation propensity of tau isoforms, or indirectly by altering tau-microtubule binding equilibria, levels of tau post-translational modification, or levels of tau expression. Because sequences encoded by exons 1, 9, 11, 12, and 13 affect protein segments common to all tau isoforms, mutations in these regions may affect all six tau splice variants. In contrast, mutations that change tau residues encoded by exon 10, which encodes a fourth imperfect repeat sequence, are expressed only in four-repeat (4R) tau isoforms.
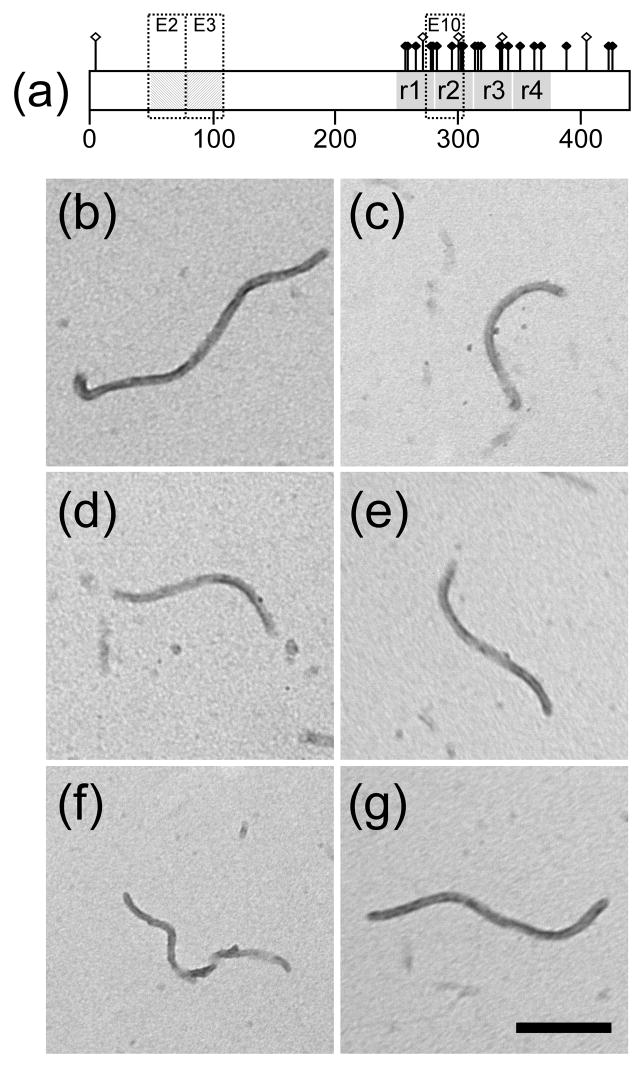
Fig. 1. Filament morphology.
(a) Distribution of 27 amino acid residues affected by pathological missense tau mutations currently tabulated at http://www.molgen.ua.ac.be/FTDMutations (Rademakers et al. 2004) and depicted on isoform 2N4R. This isoform contains alternatively spliced exons 2 and 3 (E2 and E3), each of which encodes an acidic 29-residue segment, and exon 10 (E10), which encodes an additional microtubule binding repeat sequence. Mutants R5L, G272V, P301L, V337M, and R406W modeled herein span the tau molecule and are distinguished graphically with raised hollow symbols. (b–g) Full-length wild-type 2N4R tau (b) and mutants R5L (c), G272V (d), P301L (e), V337M (f), and R406W (g) were incubated (1 μM concentration) without agitation in the presence of 100 μM Thiazine red (24 h at 37°C), spotted onto copper Formvar-carbon mesh grids, stained with 2% uranyl acetate, and viewed by transmission electron microscopy. All tau species produced unbranched filaments ~16 nm in diameter with no obvious differences in morphology or length distribution. Scale bar = 200 nm.
Previous studies support a direct effect of mutation on tau aggregation propensity, with certain tau mutants supporting increased rate (P301L, (Nacharaju et al. 1999; Gamblin et al. 2000)) and/or extent (I260V, (Grover et al. 2003); L266V, (Hogg et al. 2003); P301L and P301S (Barghorn et al. 2000; Gamblin et al. 2000; Spina et al. 2007); and Q336R, (Pickering-Brown et al. 2004)) of aggregation in vitro. Some mutations have been reported to support an increase in the numbers of filaments formed (R5H, (Hayashi et al. 2002); K257T, (Rizzini et al. 2000); G272V (Goedert et al. 1999); P301L and P301S (Goedert et al. 1999); and K369I, (Neumann et al. 2001)), suggesting that tau structure may influence the rate limiting step in fibrillization. Many of these studies were only semiquantitative and did not address the mechanism of aggregation enhancement. In addition, tau aggregation in vitro requires addition of an aggregation inducer, typically in the form of an anionic substance, which adds another layer of complexity to analysis of the reaction (Kuret et al. 2005). A more systematic study of the kinetics of the tau aggregation reaction is needed to address intrinsic tau mutant aggregation propensity and the mechanism through which the aggregation reaction may be affected.
Recently we showed that small-molecule inducers such as Thiazine red drive aggregation of full-length tau at submicromolar concentrations in the absence of macromolecular inducers such as heparin (Chirita et al. 2005). The reaction approximates a homogeneous nucleation scheme characterized by initial formation of an unstable dimeric nucleus, followed by filament elongation through monomer addition (Congdon et al. 2008). Under these conditions, it is possible to fit the reaction to a mathematical model and estimate the elementary rate constants for tau aggregation. Thus, the inherent aggregation propensity for mutant tau may be quantified and mechanistically localized under near physiological tau protein concentrations.
Here we examine the effects of tauopathy mutants R5L, G272V, P301L, V337M, and R406W on aggregation propensity. The results suggest that some but not all tauopathy mutants increase tau aggregation propensity, and that they can do so at both the nucleation and elongation reaction steps of the reaction.
Experimental Procedures
Materials
Recombinant polyhistidine-tagged human 2N4R tau, and mutants G272V, P301L, V337M, and R406W in 2N4R background, were prepared as described previously (Carmel et al. 1996; Gamblin et al. 2000). R5L was generated by site-directed mutagenesis (Stratagene QuikChange XL) of the pT7C-htau40 vector using the following primers: forward (5′-CTG AGC CCC TCC AGG AGT TCG AAG TGA TG-3′) and reverse (5′-CAT CAC TTC GAA CTC CTG GAG GGG CTC AG-3′). Successful mutagenesis was confirmed by DNA sequence analysis. R5L mutant tau was then expressed and purified as described for wild-type 2N4R tau (Carmel et al. 1996).
Aggregation inducer Thiazine red (Chemical Abstract Service registry number 2150-33-6) was obtained from TCI America (Portland, OR, USA). Formvar/carbon-coated copper grids (300 mesh), glutaraldehyde, and uranyl acetate were obtained from Electron Microscopy Sciences (Fort Washington, PA, USA).
Tau Fibrillization Assay
Purified tau preparations were incubated at 37°C without agitation in assembly buffer (10 mM HEPES pH 7.4, 100 mM NaCl, and 5 mM dithiothreitol) containing aggregation inducer Thiazine red (100 μM final concentration) for various times up to 24 h. Reactions were then fixed with 2% glutaraldehyde, adsorbed onto Formvar/carbon-coated copper grids, stained with 2% uranyl acetate, and examined by transmission electron microscopy as described previously (Necula and Kuret 2004b). At least three fields were captured for each reaction condition with a Tecnai G2 Spirit BioTWIN transmission electron microscope (FEI, Hillsboro, OR, USA) operated at 80 kV and 23,000 – 49,000× magnification, and tau filaments >10 nm in length were quantified with ImageJ software (National Institutes of Health). Total filament length is defined as the sum of the lengths of all resolved filaments per field and is reported ± SD.
Critical concentration determination
Kcrit values were determined from linear regression analysis of the tau concentration dependence of aggregation by inverse prediction of abscissa intercepts (Congdon et al. 2008). The accompanying standard error of the estimate, Sx, was calculated as:
| (Eq. 1) |
where C.I. is the length of the Fieller 95% confidence interval of each regression, and t0.975,n-2 is the Student’s t distribution percentage at 1- α = 0.975 and n - 2 degrees of freedom.
Dissociation Rates
Tau filaments prepared as described above for 24 h were diluted 10-fold into assembly buffer containing 100 μM Thiazine red and incubated at 37°C. Aliquots were withdrawn as a function of time up to 5 h post dilution and then assayed for filament length. The resultant disaggregation time series was fit to an exponential decay function to obtain kapp, the pseudo-first order rate constant describing the time-dependent decrease in filament length, and L0, the total filament length at time zero. The rate constant ke− was estimated from kapp, L0, and the number of filaments at time zero as described previously (Kristofferson et al. 1980; Necula and Kuret 2005). The association rate constant for elongation, ke+, was then obtained from the relationship (Congdon et al. 2008):
| (Eq. 2) |
Aggregation time series
Aliquots of tau aggregation reactions prepared as described above were removed as a function of time and assayed for filament formation by electron microscopy. Aggregation lag times, defined as the time when the tangent to the point of maximum aggregation rate intersects the abscissa of the sigmoidal curve (Evans et al. 1995), were obtained ± SE from each time series by Gompertz regression as described in (Necula and Kuret 2004a). To determine the nucleation dissociation equilibrium constant, Kn, filament length data was converted to protomer concentration, cp*, by assuming that all protein above the critical concentration formed filaments (Congdon et al. 2008), and that the resultant filaments were composed of two tau protomers per β-sheet spacing (Congdon et al. 2008). Data were then fitted to the simplified homogeneous nucleation scheme of Wegner and Engel (Wegner and Engel 1975) assuming a dimeric nucleus (Congdon et al. 2008):
| (Eq. 3) |
| (Eq. 4) |
| (Eq. 5) |
where ctotal, c1, and cp represent bulk tau, tau monomer, and tau filament concentrations, respectively. Parameter estimates were obtained by fitting experimentally determined values of ctotal, cp*, ke−, and ke+ to equations 3–5 in JACOBIAN™ modeling software (Numerica Technology, LLC, Cambridge, MA). The simulation yielded estimates of forward and reverse nucleation rate constants kn+ and kn−, with the ratio kn−/kn+ recorded as Kn.
Statistical analysis
The probability (p) of obtaining the observed results, assuming the null hypothesis, was assessed by z-test:
| (Eq. 6) |
where x1 ± Sx1 and x2 ± Sx2 are the pair of estimates ± SE being compared, z is the 1-α point of the standard normal distribution, and p is 2α. All statistical analyses were carried out using JMP 7.0 (SAS Institute, Cary, NC).
Results
Initial characterization
Missense tau mutants R5L, G272V, P301L, V337M, and R406W, were selected for analysis because their association with FTLD is well established and because all except R5L have been shown to promote neurofibrillary lesion formation in transgenic mice (Denk and Wade-Martins 2007). Furthermore, the mutations span the length of the tau molecule (Fig. 1A) to include the N-terminal projection domain (R5L), the first, second, and third microtubule-binding repeats (G272V, P301L, and V337M), and the C-terminal tail (R406W). Because P301L can be accommodated in 4R but not 3R tau isoforms, all mutants were prepared in a 2N4R background to facilitate direct comparison to each other and also wild-type 2N4R prepared by identical methods.
When incubated in the presence of Thiazine red aggregation inducer under near physiological conditions of pH, reducing conditions, and ionic strength, sub-micromolar concentrations of wild-type 2N4R form twisted ribbons (Chirita et al. 2005) with a mass-per-unit length similar to authentic brain-derived PHFs (Congdon et al. 2008). When the missense mutants were incubated under identical conditions up to 1 μM concentration, all formed filaments of similar width and morphology as wild-type 2N4R (Fig. 1B-G). Filament length distributions were also similar to wild-type 2N4R tau (data not shown). These data indicate that all five missense mutations retain the fundamental aggregation properties of wild-type tau and can be characterized at physiological bulk tau concentrations.
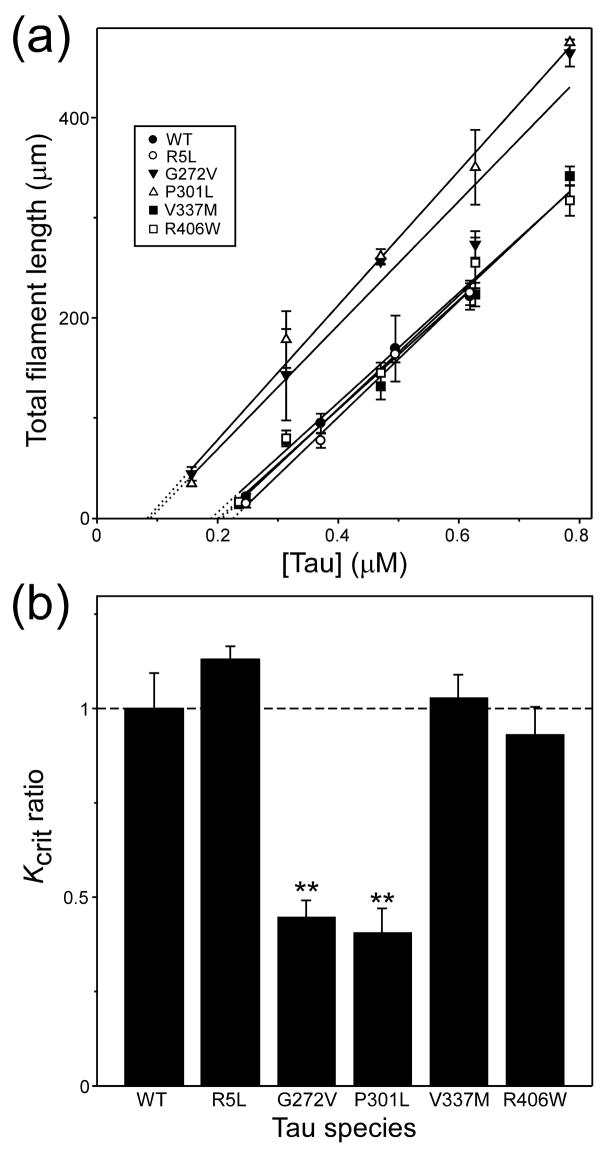
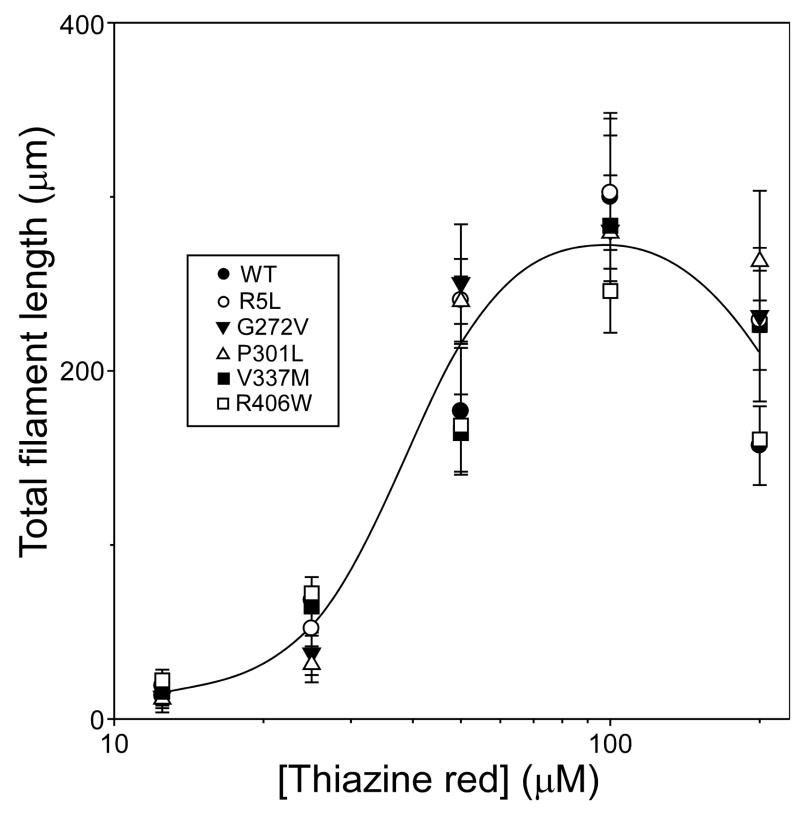
To quantify the effects of primary structure on relative aggregation propensity, the critical concentration (Kcrit) of each mutant was estimated in the presence of Thiazine red inducer. In nucleation dependent reactions, Kcrit approximates the equilibrium dissociation constant for elongation, Ke (Congdon et al. 2008). Because Kcrit also represents the highest protein monomer concentration that does not support aggregation, it can be estimated from the abscissa intercept of the tau concentration dependence of plateau fibrillization in the submicromolar concentration regime. Results showed that Kcrit values for R5L, V337M, and R406W were all ~200 nM (Fig. 2A), and did not differ from wild-type 2N4R tau at p < 0.05 (Fig. 2B; Table 1). In contrast, both G272V and P301L mutations reduced Kcrit to below 90 nM (Fig. 2B; Table 1). These data indicate that tauopathy mutants G272V and P301L lead to increased aggregation propensity by depressing the minimum concentration of tau needed to support fibril formation. However, Kcrit depression could potentially result from changes in sensitivity to Thiazine red inducer as well. To address this possibility, the concentration-effect relationship for Thiazine red mediated fibrillization was quantified for all tau preparations at constant tau supersaturation (i.e, the net difference between bulk concentration and Kcrit was held constant, so that the amount of aggregation at equilibrium was approximately the same for all tau species). Results showed that all mutants resembled wild-type 2N4R tau with respect to both the potency and efficacy of Thiazine red (Fig. 3). Together these data indicate that tauopathy mutants do not display differential sensitivity to Thiazine red, and suggest that the decreases in Kcrit observed with G272V and P301L reflect intrinsic differences in aggregation propensity relative to wild-type tau.
Fig. 2. Effect of tau mutations on critical concentration.
Wild-type 2N4R tau (●) and mutants R5L (○), G272V (▼), P301L (△), V337M (■), and R406W (□) were incubated at varying bulk concentrations in the presence of Thiazine red inducer for 24 h at 37°C, then assayed for filament formation by electron microscopy. (a) Plot of mean total filament length against bulk protein concentration, where each data point represents the mean ± SD of triplicate determinations and the solid lines represent best fit of the data points to linear regression. The abscissa intercept, which was obtained by extrapolation (dotted lines), was used to estimate critical concentration (Kcrit). (b) Replot of data from Panel (a), where each bar represents the Kcrit for examined tau species relative to wild-type 2N4R (dashed line). Both G272V and P301L tau mutants aggregated with significantly lower Kcrit values than did wild-type 2N4R. **, p < 0.01 versus wild-type 2N4R tau.
Table 1.
Summary of aggregation parameters
| aKcrit(nM) | ake−(s−1) | ake+(mM−1s−1) | Lag Time (h) | Kn (mM) | bΔGσ (Kcal/mol) | |
|---|---|---|---|---|---|---|
| 2N4R | 200 ± 15 | 0.019 ± 0.001 | 94.8 ± 12.8 | 0.54 ± 0.09 | 15.0 | 6.9 |
| R5L | 226 ± 7 | 0.021 ± 0.002 | 92.9 ± 9.9 | 0.37 ± 0.12* | 6.5 | 6.3 |
| G272V | 89 ± 8** | 0.020 ± 0.001 | 229.0 ± 22.0** | 0.10 ± 0.05** | 2.2 | 6.2 |
| P301L | 80 ± 12** | 0.019 ± 0.001 | 240.0 ± 38.0** | 0.23 ± 0.04** | 3.0 | 6.5 |
| V337M | 205 ± 12 | 0.019 ± 0.002 | 90.5 ± 9.3 | 0.33 ± 0.10* | 8.6 | 6.6 |
| R406W | 186 ± 14 | 0.016 ± 0.001 | 87.1 ± 8.5 | 0.47 ± 0.15 | 11.8 | 6.8 |
Overall constants reflecting events at both filament ends
ΔGσ = −RTlnσ, where σ = Kcrit/Kn
, p < 0.05;
, p < 0.01 versus 2N4R tau.
Fig. 3. Tau mutants share a common sensitivity to aggregation inducer Thiazine red.
Wild-type 2N4R (●) and mutants R5L (○), G272V (▼), P301L (▵), V337M (■), and R406W (□) were incubated (24 h at 37°C) at constant supersaturation (i.e., 0.5 μM above Kcrit) in the presence of varying concentrations of Thiazine red, and then assayed for filament formation by electron microscopy. Each data point represents mean total filament lengths/field from triplicate determinations, whereas the solid curve is drawn solely to aid visualization. Under these conditions, the concentration effect relationship for Thiazine red was similar for all tau species.
Effect of tau mutation on extension rates
Kcrit approximates the ratio of dissociation (ke−) and association (ke+) rate constants for filament elongation (Eq. 2). Thus decreases in Kcrit may result from decreases in ke−, corresponding to filament stabilization, from increases in ke+, reflecting a more efficient association reaction, or from both. To determine the mechanism of Kcrit depression, ke− was estimated for each tau preparation by diluting preassembled filaments in assembly buffer containing Thiazine red but no tau protein and following the time-dependent loss of filament length by electron microscopy. Loss of filament length was first order as predicted for endwise depolymerization from a Poisson-like length distribution (Kristofferson et al. 1980) (Fig. 4A). On the basis of the relationship between tau mass and filament length established for wild-type 2N4R tau (Congdon et al. 2008), the dissociation elongation constant ke− was derived from the disaggregation rate of each mutant (Table 1). Rate constant ke+ was then calculated from estimates of ke− and Kcrit for each mutant through Equation 2 (Table 1). Pairwise comparison of any mutant with wild-type 2N4R tau failed to show a significant difference in ke− at p < 0.05 (Fig. 4B). In contrast, both G272V and P301L aggregated with significantly greater ke+ values than wild-type tau. These data indicate that G272V and P301L mutations depress Kcrit by increasing the efficiency of monomer addition to nascent filament ends.
Fig. 4. Estimation of extention association and dissociation rate constants.
(a) Tau filaments prepared from wild-type 2N4R (●) and mutants R5L (○), G272V (▼), P301L (▵), V337M (■), and R406W (□) in the presence of 100 μM Thiazine red at 37°C were diluted 10-fold into assembly buffer containing Thiazine red, and the resultant disaggregation was followed as a function of time by electron microscopy. Each data point represents total filament length per field ± SD (n = 3 observations), whereas the solid lines represent best fit of the data points to linear regression. The first order decay constant kapp was estimated from each regression, and used in conjunction with measured Kcrit values to estimate ke− and ke+ as described in the Experimental Procedures section. (b) Replot of data from Panel (a), where each bar represents ke−and ke+ values for each examined tau species relative to wild-type 2N4R (dashed line). Filaments composed of mutants G272V and P301L extended significantly faster than did 2N4R tau. **, p < 0.01 versus wild-type 2N4R tau.
Effect of tau mutation on nucleation rates
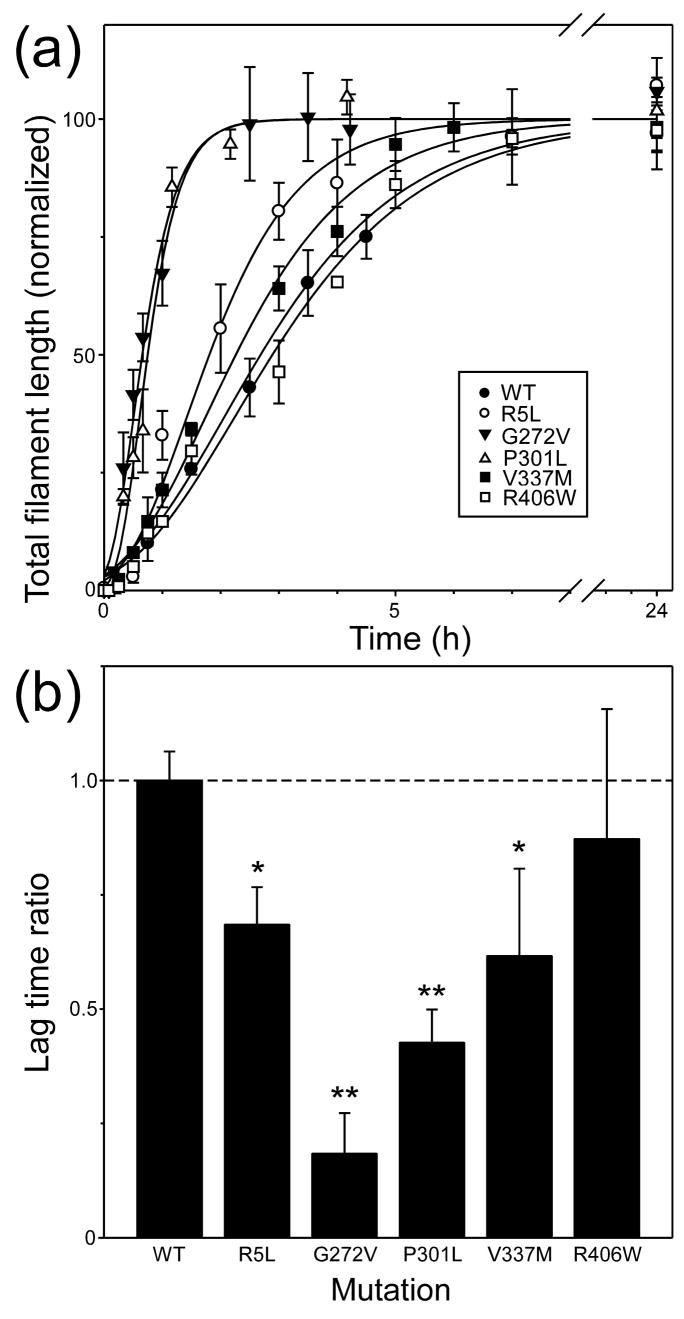
In the presence of Thiazine red inducer, tau aggregation approximates an equilibrium nucleation-elongation reaction, where assembly-competent monomer rapidly equilibrates with a thermodynamically unstable species termed the nucleus (Ferrone 1999). Once the critical nucleus cluster size is reached, subsequent additions to the nascent filament ends are energetically favorable and elongation proceeds efficiently. As a result, aggregation rate depends not only on the rate of filament elongation, but on the efficiency of the nucleation step as well. To assess the effects of primary structure on nucleation rate, the time course of aggregation was quantified for each tau construct at constant supersaturation. Under these conditions, differences in reaction rates primarily reflect differences in rates of nucleation and protein concentrations (Fesce et al. 1992). All resultant reaction progress curves were sigmoidal with lag, exponential growth, and equilibrium phases (Fig. 5A). Lag times, which vary inversely with nucleation rate (Evans et al. 1995), were obtained after fitting the data to a 3-parameter Gompertz growth function as described in Experimental Procedures. The values are summarized in Table 1 and compared graphically to wild-type 2N4R tau in Fig. 5B. The data show that all tauopathy mutants except R406W aggregated with significantly shorter lag times than wild-type 2N4R tau, suggesting that R5L, G272V, P301L, and V337M mutations can directly accelerate the nucleation phase of the aggregation reaction.
Fig. 5. Effects of tau mutations on aggregation time course.
(a) Wild-type 2N4R (●) and mutants R5L (○), G272V (▼), P301L (▵), V337M (■), and R406W (□) were incubated (37°C) at constant supersaturation (i.e., 0.2 μM above Kcrit) in the presence of 100 μM Thiazine red, and then assayed for filament formation as a function of time. Each data point represents mean total filament lengths/field (expressed as % plateau length) calculated from triplicate electron microscopy images whereas each normalized curve represents best fit of the data points to a three parameter Gompertz growth function. The fits were used to calculate lag time as described in the Experimental Procedures section. (b) Replot of data from Panel (a), where each bar represents the lag time ± SE calculated from each Gompertz regression normalized to wild-type isoform 2N4R (dotted line). Mutants R5L, G272V, P301L, and V337M aggregated significantly faster than wild-type 2N4R when compared at constant supersaturation. *, p < 0.05; **, p < 0.01 versus wild-type 2N4R tau.
To quantify the nucleation reaction, all reaction progress curves were fit to the approximation of Wegner and Engel (Wegner and Engel 1975) as described previously for wild-type isoform 2N4R (Congdon et al. 2008). This approach simplifies the family of differential equations describing nucleation and extension of all aggregated species to just three equations (Eq. 3 – 5) that relate cp* (tau protomer concentration) and cp (tau filament concentration) to ctotal (bulk tau concentration). Length measurements were converted to molar units by assuming all tau above Kcrit was fibrillar and all tau below Kcrit was a monomer having concentration c1 (Congdon et al. 2008). The time series in molar units was then fit to equations 3 – 5 assuming a dimeric nucleus (Congdon et al. 2008) and constrained by the elongation parameters summarized in Table 1. The results confirm that tauopathy mutants R5L, G272V, P301L, and V337M increase the efficiency of the nucleation reaction, and indicate that they do so by decreasing the dissociation equilibrium constant relative to wild-type 2N4R (Table 1). In the cases of G272V and P301L, the decreases in Kn were as much as five to seven fold.
Equations 4 and 5 predict that filament number concentration (cp) should be a function of tau concentration and the four rate constants that govern the nucleation and extension phases of the aggregation reaction. Therefore, filament number is not a direct measure of any kinetic parameter, including nucleation rate. However, because both R5L and V337M display decreased Kn relative to wild-type 2N4R while their elongation rate constants remain unchanged, they would be predicted to yield more filaments relative to wild-type 2N4R tau when compared at identical tau concentrations. A simulation of this hypothetical reaction at constant supersaturation (i.e., 0.2 μM above Kcrit) using the kinetic constants summarized in Table 1 is shown in Fig. 6A. R5L and V337M were predicted to produce ~1.4 – 1.6 fold increases in filament number concentration relative to wild-type 2N4R at reaction plateau. To test this prediction, R5L, V337M, and wild-type 2N4R were aggregated under these conditions and then subjected to transmission electron microscopy to quantify filament number. The results showed that R5L and V337M yielded 1.5 ± 0.2 and 1.7 ± 0.1 fold increases in filament number relative to wild-type 2N4R tau, respectively (Fig. 6B). These data are consistent with the increases in nucleation rates predicted by mathematical simulation, and support the hypothesis that missense tauopathy mutants R5L and V337M act in part to accelerate the nucleation phase of the aggregation reaction.
Fig. 6. Mutants R5L and V337M yield increased filament number concentration.
(a) Mathematical simulation of reaction time course for R5L, V337M, and wild-type 2N4R tau at constant supersaturation (i.e., 0.2 μM above Kcrit) using equations 3 – 5 and the kinetic parameters summarized in Table 1. Each curve represents the predicted evolution of filament number concentration (cp) as function of time. The simulations predict that the decreases in Kn associated with R5L and V337M should yield increases in filament number concentration relative to 2N4R tau under these conditions. (b) R5L, V337M, and wild-type 2N4R were incubated (37°C) under identical conditions as in Panel (a) and then subjected to electron microscopy analysis at 24h. Each column represents mean total filament number/field ± S.D. (normalized to wild-type 2N4R tau) calculated from quadruplicate electron microscopy images. Consistent with mathematical simulation, R5L and V337M yielded significantly more tau filaments relative to wild-type 2N4R tau at reaction plateau. **, p < 0.01; n.s., p > 0.05.
The ratio of elongation and nucleation equilibrium constants, termed σ, provides an index of reaction cooperativity (Zhao and Moore 2003). Expressed in terms of free energy, ΔGσ represents the difference in energy that accompanies contacts formed in the elongation step relative to the nucleation step. ΔGσ varied over the narrow range of 6.2 to 6.9 Kcal/mol for wild-type and mutant tau preparations (Table 1), indicating that the free energy of nucleation was consistently less favorable than that for elongation. Overall these data indicate that tauopathy mutants can strongly and directly increase aggregation propensity at nucleation and extension steps while retaining the cooperative aggregation mechanism of wild-type tau.
Discussion
Certain tau missense mutations cause FTLD, indicating that tau dysfunction alone can induce neurofibrillary lesion formation, neurodegeneration, and cognitive decline. The findings here, along with those from previous studies, are consistent with the disease promoting activity of some missense mutations being related to augmentation of tau aggregate formation. In the case of the R5L, G272V, P301L, and V337M, aggregation was promoted directly at the level of intrinsic aggregation propensity. The largest effects on aggregation were observed with G272V and P301L mutations, which are located in exon 9 and 10 regions that encode the first and second binding repeats, respectively. As exon 10 is alternatively-spliced, P301L only affects four-repeat isoforms of tau whereas G272V affects both three- and four-repeat isoforms. Both mutants promote filament nucleation by decreasing the dissociation equilibrium constant for dimerization and support aggregation at low tau concentrations by lowering the dissociation equilibrium constant for filament extension. For both mutants, effects on the extension reaction are driven primarily by an increase in the association rate constant, ke+, governing association of tau monomer with filament ends. Therefore, the aggregation of G272V and P301L tau is predicted to be highly sensitive to changes in free cytosolic tau concentrations. Although bulk tau levels are reportedly low micromolar (Drubin et al. 1985; Khatoon et al. 1994), normal free cytosolic concentrations are well below this owing to high-affinity microtubule binding and sequestration (Ackmann et al. 2000; Makrides et al. 2004). Thus, the ability of G272V and P301L to support rapid tau aggregation at submicromolar concentrations is likely to be especially important in the early stages of disease when tau dissociation from microtubules is incomplete.
With respect to structure, both mutations affect PGGG segments located in the repeat region of several microtubule-associated proteins (Lewis et al. 1988; Olson et al. 1995); G272V alters the segment in the first repeat to 270PGVG273, whereas P301L alters the segment in the second repeat to 301LGGG304. Each segment lies adjacent to hexapeptide motifs involved in transition of the repeat region to cross-β-sheet structure (von Bergen et al. 2000). Both mutations increase local hydrophobicity, which is a major determinant of aggregation propensity (Pawar et al. 2005; Rojas Quijano et al. 2006; Tartaglia et al. 2008), and a hypothetical model linking the conformation of PGGG segments to local hexapeptide β-sheet formation has been proposed (von Bergen et al. 2001). The similarity in aggregation propensity profile – reduced Kcrit, increased ke+, and reduced lag time and Kn – are consistent with these two mutations targeting homologous segments of the repeat region.
In contrast, the R5L mutation led to increased nucleation rate but no change in extension kinetics relative to wild-type tau. The effects on nucleation are consistent with the ability of another missense mutation at the same site, R5H, to increase numbers of filaments formed at high concentrations in vitro relative to wild-type tau (Hayashi et al. 2002). As the core of the PHF is mainly composed of the repeat region of tau (Novak et al. 1993), it is perhaps surprising that an N-terminal mutation can result in increased aggregation propensity. However, conformation-sensitive antibody binding studies suggest that the N-terminus is in close proximity to or influenced by the microtubule binding repeat region (Carmel et al. 1996). In fact, the N-terminus of tau may contact the repeat domain in tau monomers (Jeganathan et al. 2006), and elimination of this interaction through truncation results in decreased aggregation rates in vitro (Gamblin et al. 2003). The change of Arg to Leu eliminates a positive charge and again introduces a hydrophobic residue, both of which are predicted to increase aggregation propensity (Pawar et al. 2005; Rojas Quijano et al. 2006; Tartaglia et al. 2008).
Mutation V337M is found in the third repeat, which composes part of the PHF core (Novak et al. 1993). Nonetheless, this region is not essential for arachidonic acid induced fibrillization in vitro (Abraha et al. 2000). V337M resembles R5L in terms of aggregation propensity, displaying an increased nucleation rate without change in Kcrit, ke−, or ke+. Consistent with these observations, a previous study showed an increase in aggregation rate but not in plateau levels in the presence of heparin (Nacharaju et al. 1999). These data are consistent with V337M favoring faster nucleation rate with little effect on the extension reaction. A similar spectrum of activity may accompany K257T, which reportedly increases filament number (Rizzini et al. 2000) but not total filament mass at reaction plateau (Grover et al. 2003).
R406W is located in the C-terminal segment flanking the repeat region. Although truncation of this region reportedly increases extent of aggregation (Abraha et al. 2000), here it was found that R406W did not differ from wild-type tau with respect to aggregation propensity. Therefore, the biological effects of R406W on aggregation are most likely indirect, and depend on differential interaction with cellular factors, such as phosphotransferases (Goedert et al. 2000; Alonso et al. 2004).
Interplay of tau mutations with cellular factors
Tau aggregation in cells is a complex process, where the intrinsic aggregation propensity of tau proteins is modulated by interactions with other factors. We have proposed that the pathway involves four principal steps that must be overcome for filamentous aggregates to accumulate in disease (Congdon et al. 2008) (Fig. 7). First, tau must dissociate from microtubules so that its free cytosolic concentration exceeds the minimal tau concentration necessary to support aggregation. Although marked decreases in microtubule assembly kinetics have been observed for many missense mutations, including G272V, P301L, V337M, and R406W (Hasegawa et al. 1998; Barghorn et al. 2000; DeTure et al. 2000; Poorkaj et al. 2002), their binding affinity for microtubules, which may more closely reflect normal tau function (Qiang et al. 2006), is only weakly affected in vitro and remains high affinity (Barghorn et al. 2000; DeTure et al. 2000). So long as the dissociation equilibrium constant for binding remains lower than the concentration of available tau binding sites on microtubules, small changes in affinity may not appreciably affect free tau concentrations. In MCF7 cells, for example, mutant tau (i.e., G272V, P301L, or R406W) microinjected at physiological concentrations colocalizes with microtubules much like wild-type tau (Bunker et al. 2006). Similarly, bulk tau levels raised by increased expression or decreased degradation of tau may have limited effects on free tau levels. R5L is associated with ~2-fold bulk tau overexpression (Poorkaj et al. 2002), whereas V337M and R406W stabilize tau against proteolytic degradation by calpain I (EC 3.4.22.52) (Yen et al. 1999). Although the magnitude of these changes may be insufficient to overcome the large excess of tubulin in cells (Drubin et al. 1985), both increased expression and decreased turnover could make more tau available for aggregation once liberated from microtubules by other means. Binding affinity is modulated by post-translational modifications such as phosphorylation (Biernat et al. 1993; Bramblett et al. 1993), which may be the primary gatekeeper controlling the amount of free tau available for aggregation. The missense tauopathy mutants examined here (R5L, G272V, P301L, V337M, and R406W) all have been reported to be better protein kinase substrates in vitro, whereas G272V, P301L, V337M, and R406W are weaker phosphoprotein phosphatase 2A (EC 3.1.3.16) binding partners than wild-type tau isoforms (Goedert et al. 2000; Alonso et al. 2004). Both of these interactions would be expected to increase occupancy of phosphorylation sites mediating tau-microtubule affinity, and to increase amounts of cytosolic tau available for aggregation (regardless of any direct affects of phosphorylation on the aggregation reaction). Overall, changes in microtubule binding affinity, expression levels, and degradation may synergize with the effects of posttranslational modifications on tau-microtubule equilibria to increase the amount of free tau available for aggregation. These interactions may be especially important for aggregation of R406W which, as shown here, does not directly modify intrinsic aggregation propensity.
Fig. 7. Effect of FTLD mutations on the fibrillization pathway.
Normal tau binds tightly to microtubules, but dissociates upon phosphorylation to form free tau, which exists as a natively disordered, assembly incompetent monomer (Ux). A conformational change to an assembly competent state accelerates polymerization (Uc). Once assembly competent species form, the rate-limiting step in tau fibrillization is formation of dimer, which represents the thermodynamic nucleus (N). Following nucleation, extension occurs through further addition of assembly competent monomers to the filament (F) ends. Tau mutations promote fibrillization at multiple points in the pathway. Reactions characterized herein are shown in green lettering, whereas those reported in the literature are shown in red lettering. See text for details.
The second step involves a conformational change to an assembly competent state (Fig. 7). This step is proposed to be a barrier to aggregation because high concentrations (i.e., up to 100 μM) of free tau alone are insufficient to support aggregation or seeding reactions in vitro (Ko et al. 2002). In contrast, tau proteins readily aggregate in the presence of anionic inducers such as Thiazine red. The effects of missense mutations on this step are not clear, but on the basis of infrared spectroscopy, neither G272V, P301L, V337M, nor R406W increases secondary structure content of tau monomers relative to wild-type constructs (von Bergen et al. 2001).
Once aggregation-competent conformations are adopted, the rate-limiting step in filament formation becomes dimerization, which is energetically disfavored at physiological tau concentrations, and therefore a third key point of control (Fig. 7). As shown here, missense mutations can directly modulate this step. Because of its dimeric nature, nucleus structure may be a key determinant of whether 3R or 4R forms of tau predominate in aggregates. For example, disulfide bond formation can favor nucleation of 3R isoforms while inhibiting nucleation of 4R isoforms (Schweers et al. 1995).
The final step in filament formation is extension. Although not rate limiting, equilibria at filament ends dictate the minimal concentration of tau required to support aggregation. In addition to promoting microtubule dissociation, the negative charge associated with phosphorylation can enhance the extension reaction by inhibiting filament dissociation (Necula and Kuret 2004c, 2005). This reaction may synergize with the effects of P301L and G272V on extension to further augment the rate of monomer association with filament ends.
Overall, the findings here are consistent with a growing literature suggesting that missense tau mutations promote tau aggregation by acting at multiple steps along a single pathway. For some mutations, these include direct effects on the rate and/or extent of aggregation. The differential activity of missense mutations on individual steps in the pathway may influence how tau misfunction leads to clinically and histopathologically distinct diseases.
Acknowledgments
We thank Ranjan Batra and Lauren Crissman for technical assistance. This work was supported by grants from the National Institutes of Health (AG14452), the Alzheimer’s Association (IIRG-05-14288), and NIH CTSA grant UL1RR025755-01 awarded to The Ohio State University.
Abbreviations used
- FTLD
frontotemporal lobar degeneration
- PHF
paired helical filament
- 3R
3-repeat tau isoforms
- 4R
4-repeat tau isoforms
- WT
wild-type tau
References
- Abraha A, Ghoshal N, Gamblin TC, Cryns V, Berry RW, Kuret J, Binder LI. C-terminal inhibition of tau assembly in vitro and in Alzheimer’s disease. J Cell Sci. 2000;113:3737–3745. doi: 10.1242/jcs.113.21.3737. [DOI] [PubMed] [Google Scholar]
- Ackmann M, Wiech H, Mandelkow E. Nonsaturable binding indicates clustering of tau on the microtubule surface in a paired helical filament-like conformation. The Journal of biological chemistry. 2000;275:30335–30343. doi: 10.1074/jbc.M002590200. [DOI] [PubMed] [Google Scholar]
- Alonso A, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. The Journal of biological chemistry. 2004;279:34873–34881. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- Barghorn S, Zheng-Fischhofer Q, Ackmann M, Biernat J, von Bergen M, Mandelkow EM, Mandelkow E. Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry. 2000;39:11714–11721. doi: 10.1021/bi000850r. [DOI] [PubMed] [Google Scholar]
- Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11:153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM. Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Bunker JM, Kamath K, Wilson L, Jordan MA, Feinstein SC. FTDP-17 mutations compromise the ability of tau to regulate microtubule dynamics in cells. The Journal of biological chemistry. 2006;281:11856–11863. doi: 10.1074/jbc.M509420200. [DOI] [PubMed] [Google Scholar]
- Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem. 1996;271:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- Chirita CN, Congdon EE, Yin H, Kuret J. Triggers of full-length tau aggregation: a role for partially folded intermediates. Biochemistry. 2005;44:5862–5872. doi: 10.1021/bi0500123. [DOI] [PubMed] [Google Scholar]
- Congdon EE, Kim S, Bonchak J, Songrug T, Matzavinos A, Kuret J. Nucleation-dependent tau filament formation: the importance of dimerization and an estimation of elementary rate constants. The Journal of biological chemistry. 2008;283:13806–13816. doi: 10.1074/jbc.M800247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk F, Wade-Martins R. Knock-out and transgenic mouse models of tauopathies. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeTure M, Ko LW, Yen S, Nacharaju P, Easson C, Lewis J, van Slegtenhorst M, Hutton M, Yen SH. Missense tau mutations identified in FTDP-17 have a small effect on tau-microtubule interactions. Brain Res. 2000;853:5–14. doi: 10.1016/s0006-8993(99)02124-1. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Feinstein SC, Shooter EM, Kirschner MW. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol. 1985;101:1799–1807. doi: 10.1083/jcb.101.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Berger EP, Cho CG, Weisgraber KH, Lansbury PT., Jr Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: implications for the pathogenesis and treatment of Alzheimer disease. Proc Natl Acad Sci USA. 1995;92:763–767. doi: 10.1073/pnas.92.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone F. Analysis of protein aggregation kinetics. Methods Enzymol. 1999;309:256–274. doi: 10.1016/s0076-6879(99)09019-9. [DOI] [PubMed] [Google Scholar]
- Fesce R, Benfenati F, Greengard P, Valtorta F. Effects of the neuronal phosphoprotein synapsin I on actin polymerization. II. Analytical interpretation of kinetic curves. The Journal of biological chemistry. 1992;267:11289–11299. [PubMed] [Google Scholar]
- Gamblin TC, Berry RW, Binder LI. Tau polymerization: role of the amino terminus. Biochemistry. 2003;42:2252–2257. doi: 10.1021/bi0272510. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, King ME, Dawson H, Vitek MP, Kuret J, Berry RW, Binder LI. In vitro polymerization of tau protein monitored by laser light scattering: method and application to the study of FTDP-17 mutants. Biochemistry. 2000;39:6136–6144. doi: 10.1021/bi000201f. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Crowther RA. Effects of frontotemporal dementia FTDP-17 mutations on heparin-induced assembly of tau filaments. FEBS Lett. 1999;450:306–311. doi: 10.1016/s0014-5793(99)00508-6. [DOI] [PubMed] [Google Scholar]
- Goedert M, Satumtira S, Jakes R, Smith MJ, Kamibayashi C, White CL, 3rd, Sontag E. Reduced binding of protein phosphatase 2A to tau protein with frontotemporal dementia and parkinsonism linked to chromosome 17 mutations. J Neurochem. 2000;75:2155–2162. doi: 10.1046/j.1471-4159.2000.0752155.x. [DOI] [PubMed] [Google Scholar]
- Goode BL, Chau M, Denis PE, Feinstein SC. Structural and functional differences between 3-repeat and 4-repeat tau isoforms. Implications for normal tau function and the onset of neurodegenetative disease. The Journal of biological chemistry. 2000;275:38182–38189. doi: 10.1074/jbc.M007489200. [DOI] [PubMed] [Google Scholar]
- Grover A, England E, Baker M, et al. A novel tau mutation in exon 9 (1260V) causes a four-repeat tauopathy. Exp Neurol. 2003;184:131–140. doi: 10.1016/s0014-4886(03)00393-5. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Smith MJ, Goedert M. Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett. 1998;437:207–210. doi: 10.1016/s0014-5793(98)01217-4. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Toyoshima Y, Hasegawa M, Umeda Y, Wakabayashi K, Tokiguchi S, Iwatsubo T, Takahashi H. Late-onset frontotemporal dementia with a novel exon 1 (Arg5His) tau gene mutation. Ann Neurol. 2002;51:525–530. doi: 10.1002/ana.10163. [DOI] [PubMed] [Google Scholar]
- Hogg M, Grujic ZM, Baker M, et al. The L266V tau mutation is associated with frontotemporal dementia and Pick-like 3R and 4R tauopathy. Acta Neuropathol (Berl) 2003;106:323–336. doi: 10.1007/s00401-003-0734-x. [DOI] [PubMed] [Google Scholar]
- Jeganathan S, von Bergen M, Brutlach H, Steinhoff HJ, Mandelkow E. Global hairpin folding of tau in solution. Biochemistry. 2006;45:2283–2293. doi: 10.1021/bi0521543. [DOI] [PubMed] [Google Scholar]
- Khatoon S, Grundke-Iqbal I, Iqbal K. Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Lett. 1994;351:80–84. doi: 10.1016/0014-5793(94)00829-9. [DOI] [PubMed] [Google Scholar]
- Ko LW, DeTure M, Sahara N, Chihab R, Yen SH. Cellular models for tau filament assembly. J Mol Neurosci. 2002;19:311–316. doi: 10.1385/jmn:19:3:309. [DOI] [PubMed] [Google Scholar]
- Kristofferson D, Karr TL, Purich DL. Dynamics of linear protein polymer disassembly. The Journal of biological chemistry. 1980;255:8567–8572. [PubMed] [Google Scholar]
- Kuret J, Congdon EE, Li G, Yin H, Yu X, Zhong Q. Evaluating triggers and enhancers of tau fibrillization. Microsc Res Tech. 2005;67:141–155. doi: 10.1002/jemt.20187. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Wang DH, Cowan NJ. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science. 1988;242:936–939. doi: 10.1126/science.3142041. [DOI] [PubMed] [Google Scholar]
- Makrides V, Massie MR, Feinstein SC, Lew J. Evidence for two distinct binding sites for tau on microtubules. Proc Natl Acad Sci USA. 2004;101:6746–6751. doi: 10.1073/pnas.0400992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacharaju P, Lewis J, Easson C, Yen S, Hackett J, Hutton M, Yen SH. Accelerated filament formation from tau protein with specific FTDP-17 missense mutations. FEBS Lett. 1999;447:195–199. doi: 10.1016/s0014-5793(99)00294-x. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol. 2005;4:771–780. doi: 10.1016/S1474-4422(05)70223-4. [DOI] [PubMed] [Google Scholar]
- Necula M, Kuret J. A static laser light scattering assay for surfactant-induced tau fibrillization. Anal Biochem. 2004a;333:205–215. doi: 10.1016/j.ab.2004.05.044. [DOI] [PubMed] [Google Scholar]
- Necula M, Kuret J. Electron microscopy as a quantitative method for investigating tau fibrillization. Anal Biochem. 2004b;329:238–246. doi: 10.1016/j.ab.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Necula M, Kuret J. Pseudophosphorylation and glycation of tau protein enhance but do not trigger fibrillization in vitro. J Biol Chem. 2004c;279:49694–49703. doi: 10.1074/jbc.M405527200. [DOI] [PubMed] [Google Scholar]
- Necula M, Kuret J. Site-specific pseudophosphorylation modulates the rate of tau filament dissociation. FEBS Lett. 2005;579:1453–1457. doi: 10.1016/j.febslet.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Neumann M, Schulz-Schaeffer W, Crowther RA, Smith MJ, Spillantini MG, Goedert M, Kretzschmar HA. Pick’s disease associated with the novel Tau gene mutation K369I. Ann Neurol. 2001;50:503–513. doi: 10.1002/ana.1223. [DOI] [PubMed] [Google Scholar]
- Novak M, Kabat J, Wischik CM. Molecular characterization of the minimal protease resistant tau unit of the Alzheimer’s disease paired helical filament. EMBO J. 1993;12:365–370. doi: 10.1002/j.1460-2075.1993.tb05665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KR, McIntosh JR, Olmsted JB. Analysis of MAP 4 function in living cells using green fluorescent protein (GFP) chimeras. J Cell Biol. 1995;130:639–650. doi: 10.1083/jcb.130.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar AP, Dubay KF, Zurdo J, Chiti F, Vendruscolo M, Dobson CM. Prediction of “aggregation-prone” and “aggregation-susceptible” regions in proteins associated with neurodegenerative diseases. J Mol Biol. 2005;350:379–392. doi: 10.1016/j.jmb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pickering-Brown SM, Baker M, Nonaka T, et al. Frontotemporal dementia with Pick-type histology associated with Q336R mutation in the tau gene. Brain. 2004;127:1415–1426. doi: 10.1093/brain/awh147. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Muma NA, Zhukareva V, et al. An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann Neurol. 2002;52:511–516. doi: 10.1002/ana.10340. [DOI] [PubMed] [Google Scholar]
- Qiang L, Yu W, Andreadis A, Luo M, Baas PW. Tau protects microtubules in the axon from severing by katanin. J Neurosci. 2006;26:3120–3129. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, Cruts M, van Broeckhoven C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat. 2004;24:277–295. doi: 10.1002/humu.20086. [DOI] [PubMed] [Google Scholar]
- Rizzini C, Goedert M, Hodges JR, Smith MJ, Jakes R, Hills R, Xuereb JH, Crowther RA, Spillantini MG. Tau gene mutation K257T causes a tauopathy similar to Pick’s disease. J Neuropathol Exp Neurol. 2000;59:990–1001. doi: 10.1093/jnen/59.11.990. [DOI] [PubMed] [Google Scholar]
- Rojas Quijano FA, Morrow D, Wise BM, Brancia FL, Goux WJ. Prediction of nucleating sequences from amyloidogenic propensities of tau-related peptides. Biochemistry. 2006;45:4638–4652. doi: 10.1021/bi052226q. [DOI] [PubMed] [Google Scholar]
- Schweers O, Mandelkow EM, Biernat J, Mandelkow E. Oxidation of cysteine-322 in the repeat domain of microtubule-associated protein tau controls the in vitro assembly of paired helical filaments. Proc Natl Acad Sci USA. 1995;92:8463–8467. doi: 10.1073/pnas.92.18.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina S, Murrell JR, Yoshida H, et al. The novel Tau mutation G335S: clinical, neuropathological and molecular characterization. Acta Neuropathol. 2007;113:461–470. doi: 10.1007/s00401-006-0182-5. [DOI] [PubMed] [Google Scholar]
- Tartaglia GG, Pawar AP, Campioni S, Dobson CM, Chiti F, Vendruscolo M. Prediction of aggregation-prone regions in structured proteins. J Mol Biol. 2008;380:425–436. doi: 10.1016/j.jmb.2008.05.013. [DOI] [PubMed] [Google Scholar]
- von Bergen M, Friedhoff P, Biernat J, Heberle J, Mandelkow EM, Mandelkow E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming β structure. Proc Natl Acad Sci USA. 2000;97:5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bergen M, Barghorn S, Li L, Marx A, Biernat J, Mandelkow EM, Mandelkow E. Mutations of tau protein in frontotemporal dementia promote aggregation of paired helical filaments by enhancing local beta-structure. The Journal of biological chemistry. 2001;276:48165–48174. doi: 10.1074/jbc.M105196200. [DOI] [PubMed] [Google Scholar]
- Wegner A, Engel J. Kinetics of the cooperative association of actin to actin filaments. Biophys Chem. 1975;3:215–225. doi: 10.1016/0301-4622(75)80013-5. [DOI] [PubMed] [Google Scholar]
- Yen S, Easson C, Nacharaju P, Hutton M, Yen SH. FTDP-17 tau mutations decrease the susceptibility of tau to calpain I digestion. FEBS Lett. 1999;461:91–95. doi: 10.1016/s0014-5793(99)01427-1. [DOI] [PubMed] [Google Scholar]
- Zhao D, Moore JS. Nucleation-elongation: a mechanism for cooperative supramolecular polymerization. Org Biomol Chem. 2003;1:3471–3491. doi: 10.1039/b308788c. [DOI] [PubMed] [Google Scholar]