Abstract
Twenty years of global polio eradication efforts may soon eliminate wild-type poliovirus transmission. However, new information about poliovirus learned during this campaign, as well as the political realities of a modern world demand that universal immunity against poliomyelitis be maintained even after wild poliovirus is eradicated. Although two excellent vaccines have proven highly effective in the past, neither the live nor current inactivated products are optimal for use in the post-eradication setting. Therefore, concerted efforts are urgently needed to develop a new generation of vaccine that is risk-free and affordable and can be produced on a global scale. Here we discuss the desired properties and ways to create a new polio vaccine.
Early success of poliovirus vaccines
The discovery of vaccines against poliomyelitis in the 1950s was among the most dramatic achievements of preventive medicine [1]. Unlike most other diseases, polio afflicted children in countries with the best public health systems and hygiene, and the public was terrified by its rapid spread. As a result, significant funds were raised in a popular campaign led by the March of Dimes to support research that eventually led to preventive vaccines.
Vaccine development started in two different directions. The first was aimed at preparation of an immunogenic, inactivated vaccine, while the second sought to create attenuated strains. Formalin-inactivated poliovirus vaccine (IPV) developed by Jonas Salk [2] was first to be licensed in 1955. Mass use of this highly efficacious vaccine in the United States led to an immediate and rapid decline in polio morbidity. Many other countries followed suit, and a long process of worldwide polio decline began.
This early success of IPV might have doomed the prospects for an attenuated polio vaccine [3]. However, improper manufacturing practices at Cutter Laboratories resulted in release of insufficiently inactivated vaccine that paralyzed almost 200 recipients and contacts [4]. The incident resulted in a temporary halt of IPV use and strengthened arguments in favor of Oral Poliovirus Vaccine (OPV). Several attenuated strains were evaluated in large-scale clinical trials [5-7], and in 1963, a trivalent OPV made from Albert Sabin's strains [8] was licensed in the United States.
The attenuated vaccine had several important advantages over inactivated vaccine: lower cost, ease of administration, spread to contacts resulting in a herd effect, mucosal as well as humoral immunity [1]. All but three countries (Finland, Netherlands, Sweden) switched from IPV to exclusive use of OPV. By the mid-1960s, many countries that had implemented routine vaccination with OPV were free of epidemic poliomyelitis, as were countries that had continued using IPV.
Obstacles to eradication
By the mid-1980s poliomyelitis was declining in many regions of the world due to mass OPV immunization activities. Encouraged by the success of the smallpox eradication program, the World Health Assembly declared in 1988 that polio should be eradicated worldwide by year 2000. A global campaign was initiated and successfully stopped disease in most countries of the world. However, failure to eliminate wild virus circulation in several isolated geographic pockets forced repeated postponements of the eradication deadline. At present, there are four countries where wild poliovirus is still endemic. Political instability in regions near the Pakistan/Afghanistan border and resistance of the population to polio immunization in northern Nigeria both resulted in insufficient vaccine coverage. In northern India, exhaustive efforts to increase and maintain high vaccine coverage have been thwarted by extremely low OPV efficacy [9]. The reasons for the low efficacy are not understood; seroconversion and disease reduction rates have improved in the last two years by introducing supplementary vaccination with monovalent OPV against the predominant serotype of circulating virus [10]. These countries remain a source of continuing re-introduction of wild virus into countries where transmission had previously stopped.
Although IPV was being used primarily in just three countries, supply of this product became limited due to the demand for imported monkeys used for its manufacture in primary monkey kidney cells. In the late-1970s, a group of researchers at the National Institute for Public Health in the Netherlands created a new product, called enhanced potency IPV (eIPV) [11]. The vaccine was made from purified virus grown in large bioreactors. Further improvement was made at Pasteur-Merieux by replacing primary monkey kidney cells with Vero cells [12]. The vaccine contained more protective D-antigen and could be combined with other vaccines such as DTP, further improving its cost-efficiency and re-igniting an interest in IPV.
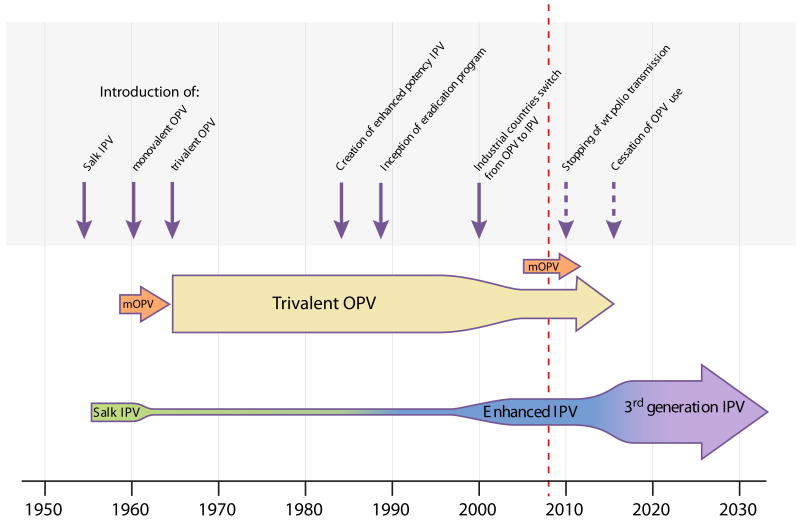
Despite great progress with polio eradication, news on the OPV front was not all good. Cases of vaccine-associated paralytic poliomyelitis (VAPP) in vaccine recipients and their contacts were reported [13]. It was a rare phenomenon, occurring at a rate of one in several hundred thousands of first-dose vaccine recipients. The genetic instability of the Sabin strains and their propensity to produce virulent derivatives after passage was well established, and VAPP was its clinical manifestation. It appeared to be an unfortunate side effect that represented an acceptable risk when assessed against the enormous benefits of OPV use. However, this risk-benefit equation changed after eradication of wild-type poliomyelitis in industrialized countries, making OPV the only source of paralytic polio. With the availability of enhanced potency IPV, the recommended vaccination schedule in these countries was changed from all-OPV to either a combination of two doses of eIPV followed by two doses of OPV [14], or IPV alone. At the beginning of the 21st century, most industrialized countries have switched to exclusive use of IPV (Figure 1).
Figure 1. Past and projected global poliovirus vaccine usage.
Vertical arrows indicate the years of major changes in vaccine product utilization. Dotted vertical arrows denote estimates of future years of possible changes to come. Horizontal arrows indicate the use of the different IPV and OPV products. The thickness of the horizontal arrows (not to scale) suggests the amount of vaccine utilized during the indicated time period.
Reverted vaccine virus with increased virulence had been previously considered unable to spread beyond immediate contacts. The first discovery of circulating vaccine-derived polioviruses (cVDPV) occurred in 2000 during investigation of a polio outbreak in the Dominican Republic and Haiti that was caused by a recombinant between a derivative of Sabin 1 strain and an unidentified enterovirus [15]. Similar outbreaks caused by cVDPV were shown to have occurred previously, although they had not been recognized, and they continue to occur [16, 17]. Cases of VAPP are restricted to single vaccine recipients and their immediate contacts, whereas cVDPV can circulate even in well-immunized communities [18] and cause multiple outbreaks of polio in areas of low population immunity. In addition, several immunodeficient individuals were identified who were persistently infected with vaccine poliovirus and excreted VDPV strains for years [19-21]. These two discoveries led to the realization that complete eradication of poliomyelitis must include eventual eradication of the live vaccine itself [22].
Cessation of OPV use was always a part of the polio eradication campaign scenario, based on cost-saving expectations as well as prevention of VAPP. The existence of VDPVs, their ability to produce outbreaks, and the demonstration that they exhibit pathogenicity similar to wild-type strains significantly changed the risk-benefit analysis associated with the endgame of the polio eradication campaign [23]. This coincided with a global shift in public perception of international security risks that was provoked by the events of September, 2001. It became obvious that the emergence of large populations of unvaccinated individuals following OPV cessation could risk re-starting a global polio pandemic caused by either VDPV, wild-type polioviruses, or chemically synthesized virus [24] re-introduced into circulation either accidentally or intentionally [25, 26]. The actual risk of re-starting polio circulation is not known, but limited experimental data suggest it could be quite serious [27]. Although mathematical modeling predicted a more optimistic scenario [28], all analyses indicate that an immunological vacuum brings a high risk of polio outbreaks. Their magnitude and our ability to contain them remain uncertain.
Population immunity must be maintained
In order to pursue a safe long-term strategy, we must maintain a high level of immunity against poliovirus. Deliberate creation of an immunologically naïve population is neither medically nor ethically acceptable. Since OPV's greatest shortcoming is its propensity to revert to virulence, several attempts were made to genetically manipulate the vaccine strains to prevent emergence of undesirable mutations. Results obtained in vitro suggest that this may indeed be feasible [29, 30]; however, the low incidence of VAPP and VDPV would require that clinical trials be conducted in millions of people to prove that increased stability in vitro would confer increased safety in humans. Clearly, this is impractical. Therefore the only realistic way to maintain worldwide immunity against poliomyelitis is to replace OPV with IPV, administered as part of a universal routine immunization program [31].
Arguments in favor of worldwide use of IPV were made in several recent publications [32, 33]. However, there are substantial financial and logistical challenges to its implementation, as well as certain scientific issues that must be addressed.
Approaches to a new IPV
IPV has demonstrated an excellent safety and efficacy record. Its relatively minor shortcomings such as poor induction of intestinal immunity and the need to administer by injection do not justify development of a new product. However, even though the current IPV could continue to be successfully used, other considerations support a proposal to develop a new generation IPV product. A stable supply of inexpensive IPV for use in low and middle-income countries will likely require substantial increases in worldwide production capacity. Scaling up the existing manufacturing base may reduce the vaccine price, but maximum cost reduction would be achieved by building production facilities in developing countries. However, ensuring containment of wild-type polioviruses [34], from which the current IPV is made, in new production facilities that lack experience and that are situated in regions with inadequate population immunity raises major concerns. Thus, development of IPV from non-pathogenic strains becomes a top priority. Below we review recent advances that make the goal of creating a new IPV feasible; it would cost less, increase efficacy, and alleviate biosecurity concerns.
Sabin IPV
Several groups have attempted to prepare IPV from the attenuated Sabin strains (sIPV) [35]. However, the antigenic properties and immunogenicity of sIPV appear different from conventional IPV [35-37] because the protective antigens of Sabin viruses are significantly less stable upon treatment with formaldehyde [38, 39]. For serotypes 2 and 3, much higher quantities of inactivated Sabin virus were needed to induce comparable levels of immunity [40], and protection against challenge in transgenic mice was significantly lower and not as broad as the one induced by conventional IPV [37]. There are four possible solutions to the problem of lower immunogenicity of Sabin IPV. First would be to increase antigen content to the level that would ensure adequate seroconversion. However, this would require growing greater quantities of virus, which would work against cost reduction, especially since yields of Sabin strains are lower than wild-type viruses. A second approach might be to stimulate immunogenicity with adjuvants. A third solution could be to explore use of alternative inactivating agents [41, 42] that do not damage antigens as much as formaldehyde. For example, beta-propiolactone, used in making rabies vaccine [43], can rapidly inactivate poliovirus without damaging its protective antigens [44], but more extensive studies of the effect of this and other inactivating agents are needed. Finally, the fourth solution would be to make IPV from strains that have an antigenic structure identical to the currently used wild-type strains, but that were rendered non-pathogenic by genetic manipulations.
Modification of the 5′ non-coding region of the viral genome
Work in this direction started some years ago for a different purpose, to make OPV more stable. One of the important determinants of neurovirulence of poliovirus is a specific RNA structural element located in the internal ribosome entry site (IRES) within the 5′-non-coding region of polio RNA. Partial disruption of this structure is associated with decreased neurovirulence. Reversion of the Sabin 3 strain to virulence is partly due to a single mutation that restores the original base pairing in the IRES. Substitution of additional weaker base pairs also reduces IRES structural stability that is not easily restored because it requires multiple mutations instead of one [30]. Since these changes do not involve the coding region of the genome, the antigenic properties of the mutant virus are identical to wild-type. This principle could be used to create more stable, attenuated polio vaccines to serve as a source for manufacture of IPV.
Other ways to manipulate the IRES element have been shown to attenuate the virus. For example, the neurocytopathic phenotype of poliovirus type 1 was eliminated by replacing the entire IRES with that from human rhinovirus type 2, creating a non-pathogenic recombinant with unaltered polio antigenic structure [29]. Deletions or insertions within the 5′-untranslated region also lead to viral attenuation [45, 46]. In the latter example, movement of a critically important cis-acting replication element (cre) from an internal site in the polio genome to a location upstream of the IRES ensured that the inserted sequence is not eliminated by recombination.
Polymerase fidelity
RNA viruses manifest very high mutation rates due to the extremely low fidelity of their RNA-dependent RNA polymerase. As a result, virus populations consist of a spectrum of mutants, described as quasi-species [47]. Alteration of this population diversity results in strains with modified pathogenicity. For example, a point mutation in the polio 3D gene sequence coding for the RNA polymerase results in increased replication fidelity [48], and therefore a narrower spectrum of diversity in the viral quasi-species [49]. Such viruses manifest reduced virulence and do not invade the CNS [50]. Similarly, changes in the 3D gene that reduce replication fidelity and increase mutation rates cause “error catastrophe” and loss of virus viability. Therefore, the fidelity of viral genome replication is fine-tuned in a narrow range, and its manipulation could be used to make strains with reduced pathogenicity, but which retain the antigenic structure of their virulent progenitors.
Codon usage
Another way to genetically modify the pathogenicity of a virus without changing its protein sequences is to alter the nucleotide sequence to display a different codon set. Redundancy in the genetic code means that many amino acids are coded by more than one codon, and there is a different bias in codon usage in different organisms or even in different tissues in the same organism [51]. Therefore, viral proteins are codon-optimized for expression in a specific host. Conversely, codon de-optimization may lead to reduced expression of a protein or the entire virus. Reduction of poliovirus fitness was demonstrated by introducing rare codons into the genes coding for capsid proteins [52, 53]. There was a perfect correlation between the number of non-optimal codons and viral fitness, and by extension, virus virulence.
Codon pair bias
A similar approach was used in another study that took advantage of codon pair bias de-optimization. It is based on the observation that there are preferred combinations of neighboring codons. Therefore swapping different but synonymous codons within the same sequence results in altered pairs of codons, while leaving the overall codon usage and amino acid sequence unchanged. This manipulation resulted in a marked decrease in polioviral fitness and therefore attenuation of virulence [54]. Interestingly, the yield of infectious virus was significantly reduced while the number of virus particles remained the same. This suggests that de-optimization may create a virus that produces little infectious virus while generating sufficient amounts of antigen that could be used for making IPV. Since reversion of codon-deoptimized viruses would require many mutations, their attenuated phenotype is much more stable.
Micro RNA sequence insertion
Yet another way to create attenuated viruses with wild-type immunogenicity takes advantage of the recently discovered activity of micro RNA (miRNA). Eukaryotic cells produce short RNAs that downregulate expression of specific genes by binding and targeting their messenger RNAs for degradation or preventing their translation. By engineering short stretches of RNA into a viral genome that are complementary to miRNA prevalent in tissues that are targets of the virus (e.g. neuronal cells), virus growth and pathogenicity can be abrogated. At the same time, these viruses could be easily grown in cultured cells that do not express this particular miRNA [55].
All these novel approaches have been tested in preliminary experiments and showed promising results for developing non-pathogenic polioviruses with wild-type antigenicity. However, research is still needed to demonstrate that IPV made from such strains is feasible to produce and efficacious. It may be prudent to create strains that combine some of these approaches.
Regulatory issues should be considered early in the development of a new product. Licensing of a new vaccine to replace the existing one requires assurance of comparable efficacy. Since clinical trials in which protection serves as the efficacy endpoint are impossible, licensure decisions must be based on surrogate markers such as the ability to induce neutralizing antibodies [56]. Differences in the immunochemical structures of formalin-treated Sabin viruses and conventional IPV [37] suggest that formulating dosages and predicting protection efficacy against the spectrum of different strains of the same serotype could be problematic. Therefore, regulatory evaluation of a new vaccine that is not immunochemically different from the Salk IPV would be simplified.
Adjuvant use
Adjuvants can also be used to boost immunogenicity. Combination of IPV with other vaccines that contain alum demonstrated that lower doses of antigen could be protective compared to IPV alone. Adjuvants can also modulate the immune response to peripherally administered antigens to boost production of mucosal antibodies. Preliminary results with IPV showed that this could be a promising direction for future studies [57].
From the information reviewed above it is clear that there are several novel approaches to create a new generation of IPV. The final product should be inexpensive, efficacious, and safe to produce and to receive. Cooperative efforts by international public health authorities, academia and industry could serve as a paradigm for creation of other vaccines based on the same principles. Additionally, the logistics of vaccine delivery, especially in low-income countries, need to be optimized for local personnel and infrastructure. Many such studies are currently being sponsored by the World Health Organization, including a demonstration project in Yogyakarta, Indonesia, aimed at addressing operational issues of switching from OPV to IPV and determining whether IPV can prevent emergence of VDPV in tropical countries.
Future considerations
Much work is in progress to develop a medically and logistically sound scheme for worldwide use of IPV. The major challenges reside in the realm of vaccine development and evaluation, as well as in aligning resources to support this massive public health undertaking. The latter is clearly outside the scope of this review or the expertise of its authors. Nevertheless, it appears obvious that delivery of a variety of childhood vaccines can best be accomplished by combining them into a universal vaccination scheme. Therefore, the future of polio vaccination may reside within the WHO's Expanded Programme on Immunization, broadened to include IPV delivery. Ensuring that progress will occur in time to meet the global public health need will require an urgent and concerted effort by academia, industry, and public health organizations to create a new generation of IPV.
Acknowledgments
This work was supported in part by the intramural research programs of the NIAID, NIH and the FDA, DHHS. Neither author has any conflicts of interest regarding any aspect of this manuscript.
References
- 1.Robbins FC. Polio—Historical. In: Plotkin SA, Mortimer EA, editors. Vaccines. Philadephia: W B Saunders; 1988. pp. 98–114. [Google Scholar]
- 2.Salk JE, Krech U, Youngner JS, Bennett BL, Lewis LJ, Bazeley PL. Formaldehyde treatment and safety testing of experimental poliomyelitis vaccines. American journal of public health and the nation's health. 1954 May;44(5):563–70. doi: 10.2105/ajph.44.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blume SS. Lock in, the state and vaccine development: Lessons from the history of the polio vaccines. Research Policy. 2005;34:159–73. [Google Scholar]
- 4.Offit PA. The Cutter Incident: How America's First Polio Vaccine Led to the Growing Vaccine Crisis. New Haven, Conn: Yale University Press; 2005. [Google Scholar]
- 5.Plotkin SA, Koprowski H, Stokes J., Jr Clinical trials in infants of orally administered attenuated poliomyelitis viruses. Pediatrics. 1959 Jun;23(6):1041–62. [PubMed] [Google Scholar]
- 6.Melnick JL, Brennan JC. In: Monkey neurovirulence of attenuated poliovirus vaccines being used in field trials. Live Polio Virus Vaccines. PAHO, editor. Live Polio Virus Vaccines; Washington, DC: 1959. pp. 65–101. [Google Scholar]
- 7.Chumakov MP. The effect of mass peroral immunisation by live vaccines from Sabin strains on the epidemiological process of poliomyelitis. Journal of hygiene, epidemiology, microbiology, and immunology. 1960;4:287–8. [PubMed] [Google Scholar]
- 8.Sabin AB. Properties and behavior of orally administered attenuated poliovirus vaccine. Journal of the American Medical Association. 1957 Jul 13;164(11):1216–23. doi: 10.1001/jama.1957.62980110008008. [DOI] [PubMed] [Google Scholar]
- 9.Grassly NC, Fraser C, Wenger J, et al. Science. 5802. Vol. 314. New York, NY: 2006. Nov 17, New strategies for the elimination of polio from India; pp. 1150–3. [DOI] [PubMed] [Google Scholar]
- 10.Grassly NC, Wenger J, Durrani S, et al. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: a case-control study. Lancet. 2007 Apr 21;369(9570):1356–62. doi: 10.1016/S0140-6736(07)60531-5. [DOI] [PubMed] [Google Scholar]
- 11.van Wezel AL, van Steenis G, Hannik CA, Kapsenberg JG, Hofman B, Cohen H. Bereiding en toepassing in Nederland van ge inactiveerd vaccin tegen poliomyelitis anterior acuta. Nederlands Tijdschrift voor Geneeskunde. 1979;123:155–63. [PubMed] [Google Scholar]
- 12.Montagnon BJ, Fanget B, Vincent-Falquet JC. Industrial-scale production of inactivated poliovirus vaccine prepared by culture of Vero cells on microcarrier. Reviews of infectious diseases. 1984 May-Jun;6 2:S341–4. doi: 10.1093/clinids/6.supplement_2.s341. [DOI] [PubMed] [Google Scholar]
- 13.Strebel PM, Sutter RW, Cochi SL, et al. Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus-associated disease. Clin Infect Dis. 1992 Feb;14(2):568–79. doi: 10.1093/clinids/14.2.568. [DOI] [PubMed] [Google Scholar]
- 14.McBean AM, Modlin JF. Rationale for the sequential use of inactivated poliovirus vaccine and live attenuated poliovirus vaccine for routine poliomyelitis immunization in the United States. The Pediatric infectious disease journal. 1987 Oct;6(10):881–7. doi: 10.1097/00006454-198710000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Kew O, Morris-Glasgow V, Landaverde M, et al. Science. 5566. Vol. 296. New York, NY: 2002. Apr 12, Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus; pp. 356–9. [DOI] [PubMed] [Google Scholar]
- 16.Acute flaccid paralysis associated with circulating vaccine-derived poliovirus--Philippines, 2001. Mmwr. 2001 Oct 12;50(40):874–5. [PubMed] [Google Scholar]
- 17.Update on vaccine-derived polioviruses--worldwide, January 2006-August 2007. Mmwr. 2007 Sep 28;56(38):996–1001. [PubMed] [Google Scholar]
- 18.Cherkasova EA, Korotkova EA, Yakovenko ML, et al. Long-term circulation of vaccine-derived poliovirus that causes paralytic disease. Journal of virology. 2002 Jul;76(13):6791–9. doi: 10.1128/JVI.76.13.6791-6799.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labadie K, Pelletier I, Saulnier A, Martin J, Colbere-Garapin F. Poliovirus mutants excreted by a chronically infected hypogammaglobulinemic patient establish persistent infections in human intestinal cells. Virology. 2004 Jan 5;318(1):66–78. doi: 10.1016/j.virol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Martin J, Odoom K, Tuite G, et al. Long-term excretion of vaccine-derived poliovirus by a healthy child. Journal of virology. 2004 Dec;78(24):13839–47. doi: 10.1128/JVI.78.24.13839-13847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang CF, Chen HY, Jorba J, et al. Intratypic recombination among lineages of type 1 vaccine-derived poliovirus emerging during chronic infection of an immunodeficient patient. Journal of virology. 2005 Oct;79(20):12623–34. doi: 10.1128/JVI.79.20.12623-12634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowdle WR, De Gourville E, Kew OM, Pallansch MA, Wood DJ. Polio eradication: the OPV paradox. Reviews in medical virology. 2003 Sep-Oct;13(5):277–91. doi: 10.1002/rmv.401. [DOI] [PubMed] [Google Scholar]
- 23.Chumakov K, Ehrenfeld E, Wimmer E, Agol VI. Vaccination against polio should not be stopped. Nature reviews. 2007 Dec;5(12):952–8. doi: 10.1038/nrmicro1769. [DOI] [PubMed] [Google Scholar]
- 24.Cello J, Paul AV, Wimmer E. Science. 5583. Vol. 297. New York, NY: 2002. Aug 9, Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template; pp. 1016–8. [DOI] [PubMed] [Google Scholar]
- 25.Agol VI, Chumakov K, Ehrenfeld E, Wimmer E. Don't drop current vaccine until we have new ones. Nature. 2005 Jun 16;435(7044):881. doi: 10.1038/435881b. [DOI] [PubMed] [Google Scholar]
- 26.Bompart F. Vaccination strategies for the last stages of global polio eradication. Indian pediatrics. 2005 Feb;42(2):163–9. [PubMed] [Google Scholar]
- 27.Korotkova EA, Park R, Cherkasova EA, et al. Retrospective analysis of a local cessation of vaccination against poliomyelitis: a possible scenario for the future. Journal of virology. 2003 Dec;77(23):12460–5. doi: 10.1128/JVI.77.23.12460-12465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson KM, Tebbens RJ, Pallansch MA, et al. The risks, costs, and benefits of possible future global policies for managing polioviruses. American journal of public health. 2008 Jul;98(7):1322–30. doi: 10.2105/AJPH.2007.122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gromeier M, Alexander L, Wimmer E. Proceedings of the National Academy of Sciences of the United States of America. 6. Vol. 93. 1996. Mar 19, Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants; pp. 2370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macadam AJ, Ferguson G, Stone DM, et al. Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: relevance to poliomyelitis eradication. Journal of virology. 2006 Sep;80(17):8653–63. doi: 10.1128/JVI.00370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arita I, Nakane M. Road map for polio eradication--establishing the link with millennium development goal no. 4 for child survival. Japanese journal of infectious diseases. 2008 May;61(3):169–74. [PubMed] [Google Scholar]
- 32.Bhasin VK. Problems with the oral polio vaccine. Nature medicine. 2008 Jan;14(1):9. doi: 10.1038/nm0108-9. [DOI] [PubMed] [Google Scholar]
- 33.Ehrenfeld E, Glass RI, Agol VI, et al. Immunisation against poliomyelitis: moving forward. Lancet. 2008 Apr 19;371(9621):1385–7. doi: 10.1016/S0140-6736(08)60597-8. [DOI] [PubMed] [Google Scholar]
- 34.WHO global action plan for laboratory containment of wild polioviruses. Second. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 35.Doi Y, Abe S, Yamamoto H, et al. Progress with inactivated poliovirus vaccines derived from the Sabin strains. Developments in biologicals. 2001;105:163–9. [PubMed] [Google Scholar]
- 36.Dragunsky EM, Ivanov AP, Abe S, et al. Further development of a new transgenic mouse test for the evaluation of the immunogenicity and protective properties of inactivated poliovirus vaccine. The Journal of infectious diseases. 2006 Sep 15;194(6):804–7. doi: 10.1086/506949. [DOI] [PubMed] [Google Scholar]
- 37.Dragunsky EM, Ivanov AP, Wells VR, et al. Evaluation of immunogenicity and protective properties of inactivated poliovirus vaccines: a new surrogate method for predicting vaccine efficacy. The Journal of infectious diseases. 2004 Oct 15;190(8):1404–12. doi: 10.1086/424524. [DOI] [PubMed] [Google Scholar]
- 38.Rezapkin G, Martin J, Chumakov K. Analysis of antigenic profiles of inactivated poliovirus vaccine and vaccine-derived polioviruses by block-ELISA method. Biologicals. 2005 Mar;33(1):29–39. doi: 10.1016/j.biologicals.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Tano Y, Shimizu H, Martin J, Nishimura Y, Simizu B, Miyamura T. Antigenic characterization of a formalin-inactivated poliovirus vaccine derived from live-attenuated Sabin strains. Vaccine. 2007 Oct 10;25(41):7041–6. doi: 10.1016/j.vaccine.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 40.Simizu B, Abe S, Yamamoto H, et al. Development of inactivated poliovirus vaccine derived from Sabin strains. Biologicals. 2006 Jun;34(2):151–4. doi: 10.1016/j.biologicals.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Brown F. Inactivation of viruses by aziridines. Vaccine. 2001 Nov 12;20(34):322–7. doi: 10.1016/s0264-410x(01)00342-5. [DOI] [PubMed] [Google Scholar]
- 42.Budowsky EI. Problems and prospects for preparation of killed antiviral vaccines. Advances in virus research. 1991;39:255–90. doi: 10.1016/s0065-3527(08)60797-6. [DOI] [PubMed] [Google Scholar]
- 43.Wiktor TJ, Aaslestad HG, Kaplan MM. Immunogenicity of rabies virus inactivated by - propiolactone, acetylethyleneimine, and ionizing irradiation. Applied microbiology. 1972 May;23(5):914–8. doi: 10.1128/am.23.5.914-918.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang SD, Pye D, Cox JC. Inactivation of poliovirus with beta-propiolactone. Journal of biological standardization. 1986 Apr;14(2):103–9. doi: 10.1016/0092-1157(86)90028-4. [DOI] [PubMed] [Google Scholar]
- 45.Agol VI, Pilipenko EV, Slobodskaya OR. Modification of translational control elements as a new approach to design of attenuated picornavirus strains. Journal of biotechnology. 1996 Jan 26;44(13):119–28. doi: 10.1016/0168-1656(95)00088-7. [DOI] [PubMed] [Google Scholar]
- 46.Toyoda H, Yin J, Mueller S, Wimmer E, Cello J. Oncolytic treatment and cure of neuroblastoma by a novel attenuated poliovirus in a novel poliovirus-susceptible animal model. Cancer research. 2007 Mar 15;67(6):2857–64. doi: 10.1158/0008-5472.CAN-06-3713. [DOI] [PubMed] [Google Scholar]
- 47.Domingo E, Martin V, Perales C, Grande-Perez A, Garcia-Arriaza J, Arias A. Viruses as quasispecies: biological implications. Current topics in microbiology and immunology. 2006;299:51–82. doi: 10.1007/3-540-26397-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfeiffer JK, Kirkegaard K. A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proceedings of the National Academy of Sciences of the United States of America. 2003 Jun 10;100(12):7289–94. doi: 10.1073/pnas.1232294100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vignuzzi M, Stone JK, Arnold JJ, Cameron CE, Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006 Jan 19;439(7074):344–8. doi: 10.1038/nature04388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vignuzzi M, Wendt E, Andino R. Engineering attenuated virus vaccines by controlling replication fidelity. Nature medicine. 2008 Feb;14(2):154–61. doi: 10.1038/nm1726. [DOI] [PubMed] [Google Scholar]
- 51.Comeron JM, Aguade M. An evaluation of measures of synonymous codon usage bias. Journal of molecular evolution. 1998 Sep;47(3):268–74. doi: 10.1007/pl00006384. [DOI] [PubMed] [Google Scholar]
- 52.Burns CC, Shaw J, Campagnoli R, et al. Modulation of poliovirus replicative fitness in HeLa cells by deoptimization of synonymous codon usage in the capsid region. Journal of virology. 2006 Apr;80(7):3259–72. doi: 10.1128/JVI.80.7.3259-3272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mueller S, Papamichail D, Coleman JR, Skiena S, Wimmer E. Reduction of the rate of poliovirus protein synthesis through large-scale codon deoptimization causes attenuation of viral virulence by lowering specific infectivity. Journal of virology. 2006 Oct;80(19):9687–96. doi: 10.1128/JVI.00738-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coleman JR, Papamichail D, Skiena S, Futcher B, Wimmer E, Mueller S. Science. 5884. Vol. 320. New York, NY: 2008. Jun 27, Virus attenuation by genome-scale changes in codon pair bias; pp. 1784–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Rij RP, Andino R. The silent treatment: RNAi as a defense against virus infection in mammals. Trends in biotechnology. 2006 Apr;24(4):186–93. doi: 10.1016/j.tibtech.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008 Aug 1;47(3):401–9. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 57.Ivanov AP, Dragunsky EM, Chumakov KM. 1,25-dihydroxyvitamin d3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. The Journal of infectious diseases. 2006 Feb 15;193(4):598–600. doi: 10.1086/499970. [DOI] [PubMed] [Google Scholar]