Abstract
Coagulation factor VIII (FVIII) is secreted as a heterodimer consisting of a heavy chain (HC) and a light chain (LC), which can be expressed independently and reassociate with recovery of biological activity. Because of the size limitation of adeno-associated virus (AAV) vectors, a strategy for delivering the HC and LC separately has been developed. However, the FVIII HC is secreted 10–100-fold less efficiently than the LC. In this study, we demonstrated that the F309S mutation and enhanced B-domain glycosylations alone are not sufficient to improve FVIII HC secretion, which suggested a role of the FVIII LC in regulating HC secretion. To characterize this role of the FVIII LC, we compared FVIII HC secretion with and without the LC via post-translational protein trans-splicing. As demonstrated in vitro, ligation of the LC to the HC significantly increased HC secretion. Such HC secretion increases were also confirmed in vivo by hydrodynamic injection of FVIII intein plasmids into hemophilia A mice. Moreover, similar enhancement of HC secretion can also be observed when the LC is supplied in trans, which is probably due to the spontaneous association of the HC and the LC in the secretion pathway. In sum, enhancing the secretion of the FVIII HC polypeptide may require the proper association of the FVIII LC polypeptide in cis or in trans. These results may be helpful in designing new strategies to improve FVIII gene delivery.
INTRODUCTION
Factor VIII (FVIII) plays a critical role in the coagulation cascade by accelerating the conversion of factor X to factor Xa. Deficiency in FVIII activity is responsible for the bleeding disorder hemophilia A.1 The current treatment for hemophilia A in the developed countries is intravenous infusion of plasma-derived or recombinant FVIII protein. Despite this treatment being effective in controlling bleeding episodes, the requirement for frequent infusion, owing to the short half-life of FVIII (8–12 hours), makes it inherently costly. Gene therapy has emerged as an attractive strategy for the eventual cure of this disease.2,3 However, the progress in delivering FVIII gene using one of the most promising viral vectors, adeno-associated virus (AAV), has lagged behind the progress in delivering coagulation factor IX4–8 because of the large size of FVIII complementary DNA approaching the packaging capacity of AAV. Although a dual vector strategy delivering the FVIII heavy chain (HC) and light chain (LC) separately9–11 circumvented the packaging limitation of AAV, it exhibited a severe “chain imbalance” owing to the inefficient secretion of the FVIII HC, which rendered this approach less efficient and effective. Therefore, understanding of the causes for limited FVIII secretion is required to improve the results of this strategy.
Although FVIII and factor V share 40% sequence homology in the A and C domains, full-length FVIII protein by itself is less efficient in terms of secretion than factor V.12–14 After translation, FVIII is transported to the lumen of the endoplasmic reticulum (ER), where it associates with several protein chaperones, including immunoglobin-binding protein (BiP), calnexin, and calreticulin. The release of FVIII from BiP is an adenosine triphosphate–dependent process, which is one of the main limiting factors for efficient FVIII secretion. Calnexin and calreticulin enhance both FVIII secretion and degradation.12–14 In the ER, the signal peptide is removed and oligosaccharides are added to the FVIII protein. Any mis-folded protein is degraded in the ER, and the correctly processed FVIII molecules enter the Golgi apparatus. LMAN1(ERGIC-53) is a chaperone protein in the ER–Golgi intermediate compartment that is required for efficient FVIII and FV secretion.15 The oligosaccharide content in the B domain can also affect FVIII secretion.16 Further studies found that the inclusion of the first 226 amino acid residues in the B domain, containing six potential asparagine-linked glycosylation sites, in B-domain-deleted FVIII (BDD-FVIII) increased its secretion tenfold while maintaining the same levels of messenger RNA as BDD-FVIII.3,14,17–19 Moreover, it has been reported that the combination of certain modifications in the FVIII coding region can overcome these limitations and improve FVIII secretion 15- to 25-fold.3,14 These modifications include (i) use of BDD.FVIII to increase the steady-state messenger RNA levels, (ii) substitution of Phe at postion 309 with Ser in the A1 domain to reduce the affinity to the ER chaperone BiP and the dependence on adenosine triphosphate for secretion, and (iii) inclusion of six asparagine-linked oligosaccharides sites in BDD-FVIII to increase ER to Golgi transport.
To investigate whether the secretion of the FVIII HC requires the LC in cis, we explored an intein strategy that can ligate the LC to the HC before secretion. Inteins are the protein equivalent of introns. They are excised from a precursor protein and the N- and C-terminal sequences adjacent to an intein are rejoined with a peptide bond. Such an excise–rejoin reaction of “protein splicing”20 is directly analogous to RNA splicing of introns. However, intein-mediated protein splicing takes place post-translationally. The N- and C-terminal sequences in the precursor protein are named N-extein and C-extein, respectively. A mature polypeptide is formed when the exteins are ligated together after the inteins are removed. Although most inteins are contiguous, there exists a special category of inteins in which the precursor proteins come from two different genes.21 Those inteins are called “split inteins.” The two split intein polypeptides, through their structural complementarity, can reassemble and catalyze a protein trans-splicing reaction to produce a ligated polypeptide. The Ssp DnaB split intein was used in this study.22,23
In the current study, we determined how the FVIII LC affected the secretion of the HC. Using the novel protein trans-splicing reaction, we were able to compare the secretion of the FVIII HC with and without ligation of the LC. The results suggest that FVIII LCs are critical in maintaining the proper configuration of HCs for secretion. Overall, our findings provide valuable information on engineering FVIII protein for both protein therapy and gene therapy of hemophilia A.
RESULTS
F309S and glycosylation modifications alone are not sufficient to improve FVIII HC secretion
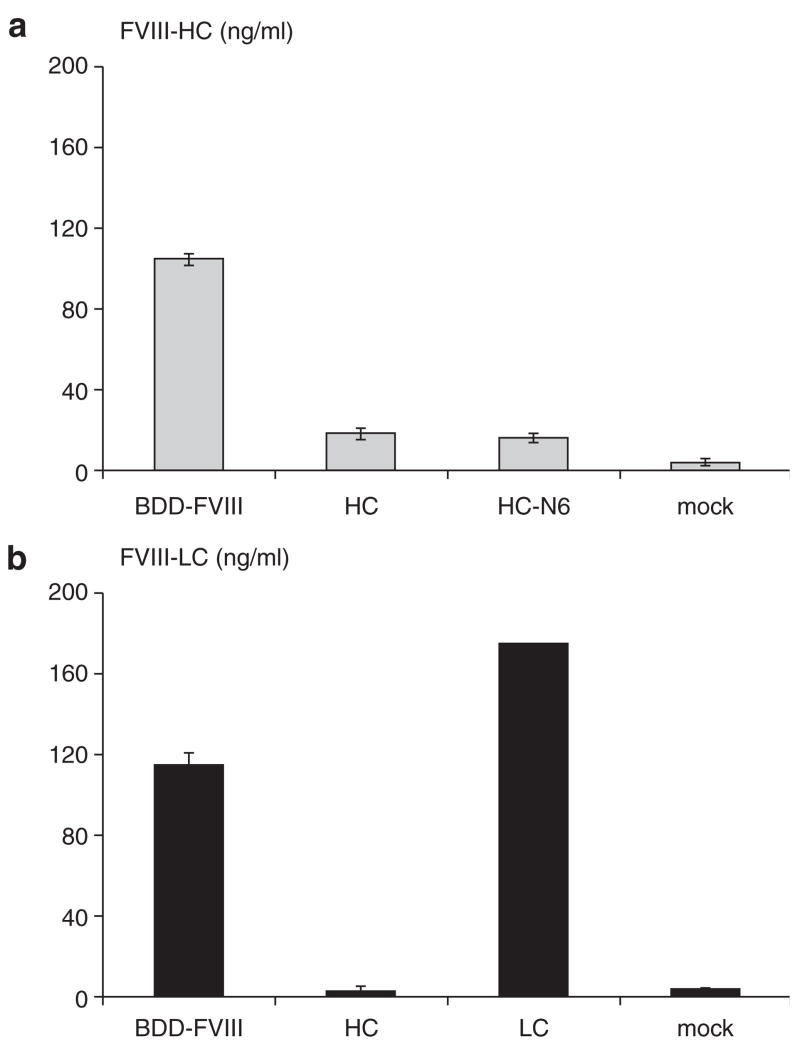
The efficiency of FVIII secretion is limited by a number of factors, which include messenger RNA instability, interaction with chaperones in the ER, and the requirement for the mannose- binding lectin LMAN1 to facilitate ER to Golgi transport.24 F309S and partial B domain sequences exhibited a dramatic enhancing effect on FVIII secretion. Because the FVIII HC is poorly secreted when expressed alone, we hypothesized that incorporating the above modifications into the HC moiety might improve the secretion efficiency of the FVIII HC. To test this hypothesis, we constructed a variety of FVIII plasmids containing different regions of the FVIII gene. As shown in Figure 1, Phe309Ser mutation and sequences of the B domain containing the six glycosylation sites were incorporated into the FVIII coding region in the expressing plasmid HC-N6. When the respective FVIII expression plasmids were transfected into 293 cells in parallel with their controls, we observed that the FVIII HC was poorly secreted into the medium 48 hours after transfection (Figure 2a and b). Surprisingly, there was no improvement in the secretion of the FVIII HC even with the Phe309Ser mutation and additional B domain sequences in the HC-N6 peptide (Figure 2a). Secretion of the modified HC in HC-N6 was similar to secretion of the unmodified HC and was approximately 5–20-fold less than secretion of BDD-FVIII. This result suggested that the missing LC might play an important role in facilitating the secretion of the HC.
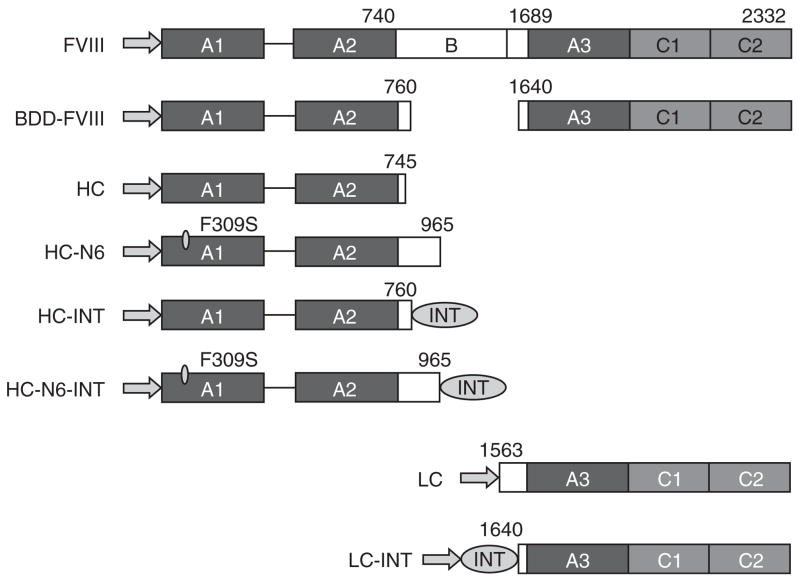
Figure 1. Schematic representation of factor VIII (FVIII) and derivatives in this study.
The arrows represent the signal peptide. The domains and the positions of amino acids relative to the full-chain FVIII peptide are identified in the figure. BDD-FVIII, B-domain-deleted FVIII; HC, heavy chain; INT, intein sequence; LC, light chain.
Figure 2. Analysis of factor VIII (FVIII) heavy chain (HC) secretion.
Plasmids expressing B-domain-deleted (BDD)-FVIII, HC, HC-N6, the light chain (LC), and mock control were transfected into 293 cells by calcium phosphate precipitation in triplicate. FVIII HC and LC secretion at 48 hours after transfection was measured. (a) Enzyme-linked immunosorbent assay (ELISA) for the HC only. (b) ELISA for the LC only. The SD is shown in the figure.
Intein strategy to add the LC polypeptide in trans
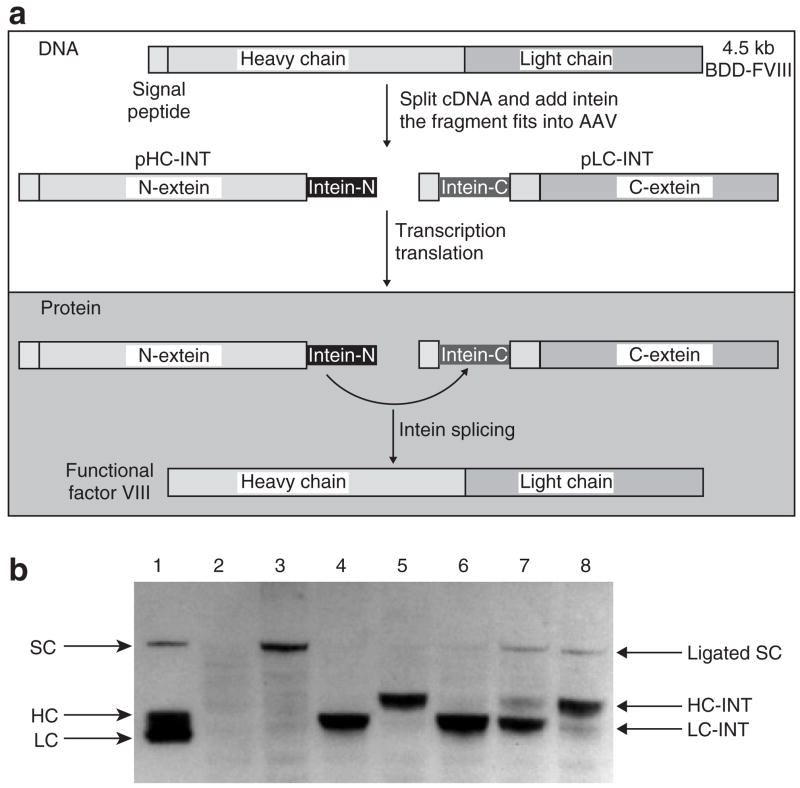
To further delineate the mechanism of FVIII secretion, we designed an intein strategy to study the effects of the addition of the LC to the HC on the secretion of FVIII molecules. An illustration of this strategy is shown in Figure 3a. In the expression plasmid HC-INT, a 153-amino-acid DnaB N-intein was added to the C-terminal of the HC (Figure 1). In the expression plasmid LC-INT, the coding region was arranged in the following configuration: the 19-amino-acid FVIII signal peptide, the 36-amino-acid DnaB C-terminal intein, and the full sequence for the FVIII LC. When these two plasmids were transfected together into 293 cells, a Western blot of the cell lysate 48 hours after transfection using polyclonal antibodies against human FVIII (Figure 3b) showed ligation products at the size of single-chain BDD-FVIII (lanes 1, 3, 7, and 8). In the cells transfected with expression plasmids for LC-INT or HC-INT alone, we observed slightly higher intracellular levels of the LC with intein than of the HC with intein (lanes 4 and 5). When these two plasmids were co-transfected at ratios of 9:1 or 1:1 of pLC-INT to pHC-INT (lanes 6 and 7), the amount of the HC with intein was considerably less than the amount of the LC polypeptide, which suggested that the majority of HC poly-peptides probably were consumed in the trans-splicing reaction. In lane 8, the LC was probably consumed close to completion, and the HC was in excess supply. Because the ligated FVIII molecules can be secreted, the intensity of the bands corresponding to the single-chain BDD-FVIII may not reflect the amount of single-chain FVIII generated through ligation. The amount of secreted FVIII was analyzed in more detail in later experiments. On the basis of consumed protein-splicing substrates, the results suggested that intein can mediate an efficient intracellular FVIII ligation reaction that resulted in the addition of the LC to the HC before FVIII secretion.
Figure 3. Intein-mediated factor VIII (FVIII) light chain ligation.
(a) An illustration of the intein strategy. The top box with the dotted line shows the DNA manipulation procedures. The bottom box with the solid line shows the protein ligation reactions. (b) Western blot of intein-mediated ligation of the heavy chain (HC) and light chain (LC). Plasmids pCMV-BDD-FVIII, pCMV-HC-INT, pCMV-LC-INT, and pCMV-HC-INT+pCMV-LC-INT at ratios of 9:1, 1:1, and 1:9 were transfected into COS cells using Lipofectamine. At 36 hours after transfection, cell lysates were prepared for Western blot analysis (4–12% gradient polyacrylamide gel electrophoresis gel). The blot was probed with a sheep anti-human FVIII polyclonal antibody (Haematologic Technologies, VT). Lanes are: 1: ReFacto FVIII protein; 2: mock transfection; 3: pCMV-BDD-FVIII; 4: pCMV-LC-INT; 5: pCMV-HC-INT; 6: pCMV-LC-INT+pCMV-HC-INT at a ratio of 9:1; 7: pCMV-LC-INT+pCMV-HC-INT at 1:1; 8: pCMV-LC-INT+pCMV-HC-INT at 1:9. cDNA, complementary DNA; SC: single-chain B-domain deleted (BDD)-FVIII protein.
LC improves HC secretion
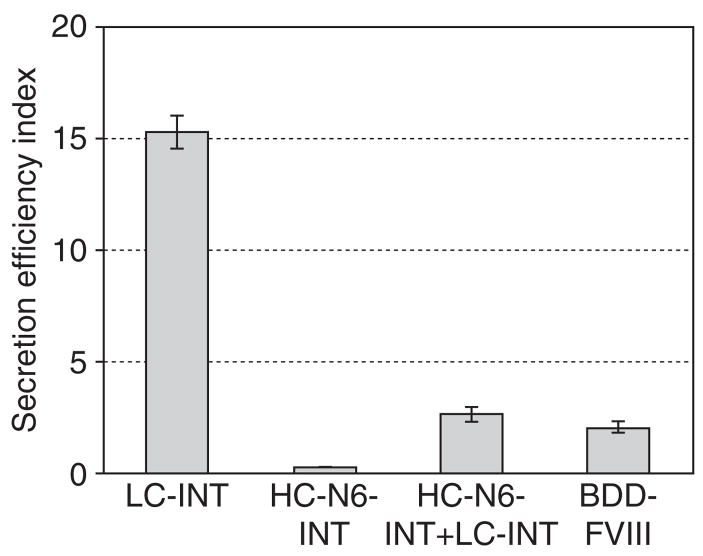
We next examined whether the addition of the LC through a trans-splicing reaction could lead to improvement in HC secretion. After the plasmids expressing HC-N6-INT and LC-INT had been co-transfected into 293 cells in parallel with positive and negative controls, FVIII antigen levels in the medium and in the cell lysates were analyzed by enzyme-linked immunosorbent assay (ELISA). We employed the ratio of secreted FVIII to intracellular FVIII as an index for secretion efficiency. As shown in Figure 4, the LC showed a much higher secretion efficiency index. The amount of the FVIII LC expressed from the LC-INT plasmid and secreted in the medium was 18 times more than that in the cytoplasm. In contrast, the HC polypeptide was scarcely secreted, even though the HC-N6-INT expression vector included six glycosylation sites and the F309S mutation. When the LC-INT expression plasmid was included in the transfection, the expressed FVIII LC would go through the same secretion pathway as the HC fragment and undergo the splicing reaction. The addition of the LC to the HC improved the secretion of the HC to the level of BDD-FVIII secretion, though not to the level of FVIII LC secretion. This result suggested that the LC was critical in promoting FVIII HC secretion and explained why the HC by itself did not secrete efficiently.
Figure 4. The effects of the light chain (LC) on the secretion efficiency index.
The plasmids pCB-LC-INT, pCB-HC-N6-INT, pCB-BDD-FVIII, and pCB-LC-INT+pCB-HC-N6-INT (1:1) were transfected into 293 cells. The amount of total FVIII antigen in the medium and inside the cells was measured by enzyme-linked immunosorbent assay at 48 hours after transfection. The secretion efficiency was defined as the ratio of factor VIII (FVIII) in the medium to FVIII in the cells and is presented as a bar graph. BDD-FVIII, B-domain-deleted FVIII; HC, heavy chain; INT, intein sequence.
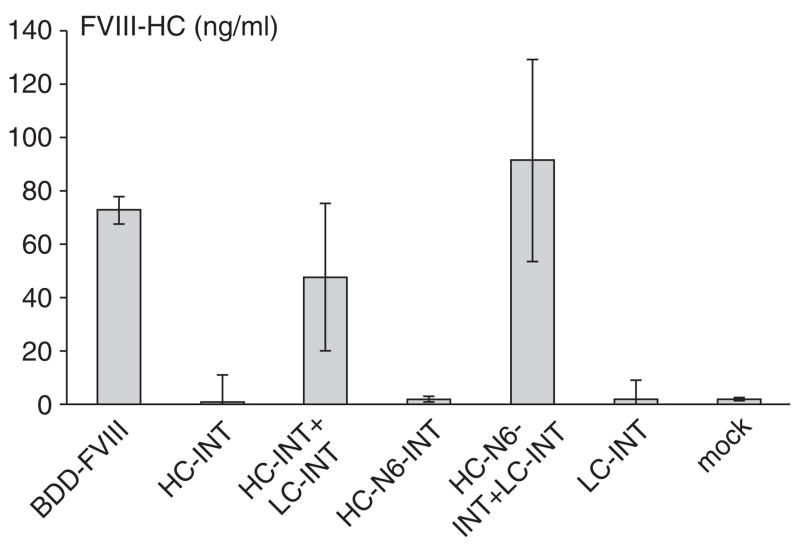
Additional evidence supporting the hypothesis that the FVIII LC facilitates HC secretion is presented in Figure 5. This experiment showed the amount of the HC secreted in medium with or without the LC in cis. HC secretion was improved when HC-INT was co-transfected with LC-INT, but still at a lower level than that of BDD-FVIII. However, additional B domain sequences with six glycosylation sites and F309S mutation had enhancing effects when the LC sequence was ligated to the modified HC. Although we did not observe a fivefold to tenfold expression as reported elsewhere,14 HC-N6 consistently outperformed BDD-FVIII in secretion in all repeated experiments.
Figure 5. The light chain (LC) facilitates heavy chain (HC) secretion in cis.
The plasmids pCB-BDD-FVIII, pCB-HC-INT, pCB-HC-INT+pCB-LC-INT (1:1), pCB-HC-N6-INT, pCB-HC-N6-INT+pCB-LC-INT (1:1), pCB-LC-INT, and mock control were transfected into 293 cells and assayed 48 hours after transfection. The secreted factor VIII (FVIII) HC was measured by enzyme-linked immunosorbent assay. The y-axes show the concentration of FVIII HC in ng/ml. Transfections were performed in quadruplicate to obtain average values. The SD is shown in the figure. BDD-FVIII, B-domain-deleted FVIII; INT, intein sequence.
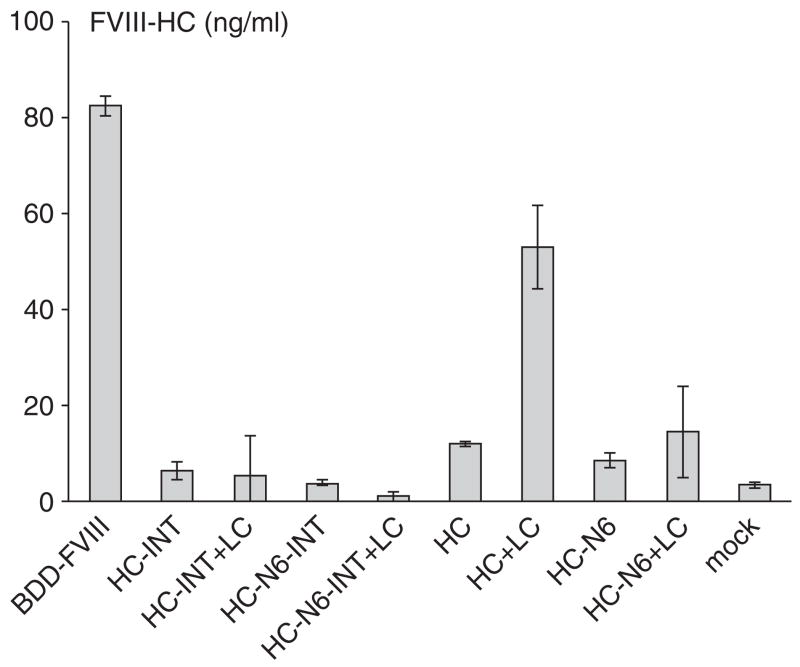
To evaluate whether the LC can enhance FVIII HC intein polypeptide secretion in trans, we constructed a plasmid pLC (Figure 1) that expresses a functional LC without intein. The LC fragment from this plasmid would not be ligated to the expressed HC intein. As shown in Figure 6, the mere presence of the LC during the secretion process had no influence on HC-INT or HC-N6-INT secretion. However, co-transfection of HC+LC, HC-N6+LC into 293 cells showed a different result (Figure 6). The LC efficiently improved HC secretion even in trans, although the level remained lower than that for BDD-FVIII. In contrast, HC-N6 secretion was improved very little in the presence of the LC. This suggested that the additional B domain sequence attached to the HC polypeptide alone had a negative effect on the interaction of the HC with the LC. Therefore, the enhancing effect of the LC on HC secretion may depend upon the substrates. Only FVIII HC molecules with proper interaction with the LC can be enhanced in secretion.
Figure 6. The light chain (LC) may affect heavy chain (HC) secretion in trans.
The plasmids pCB-FVIII, pCB-HC-INT, pCB-HC-INT+pCB-LC (1:1), pCB-HC-N6-INT, pCB-LC+pCB-HC-N6-INT (1:1), pCB-HC, pCB-HC+pCB-LC (1:1), pCB-HC-N6, pCB-LC+pCB-HC-N6 (1:1), and mock control were transfected into 293 cells and assayed 48 hours after transfection. The secreted factor VIII (FVIII) HC was measured by enzyme-linked immunosorbent assay. The y-axes show the concentration of FVIII HC in ng/ml. Transfections were performed in quadruplicate to obtain average values. The SD is shown in the figure. BDD-FVIII, B-domain-deleted FVIII; INT, intein sequence.
The FVIII LC augmented FVIII HC secretion in vivo
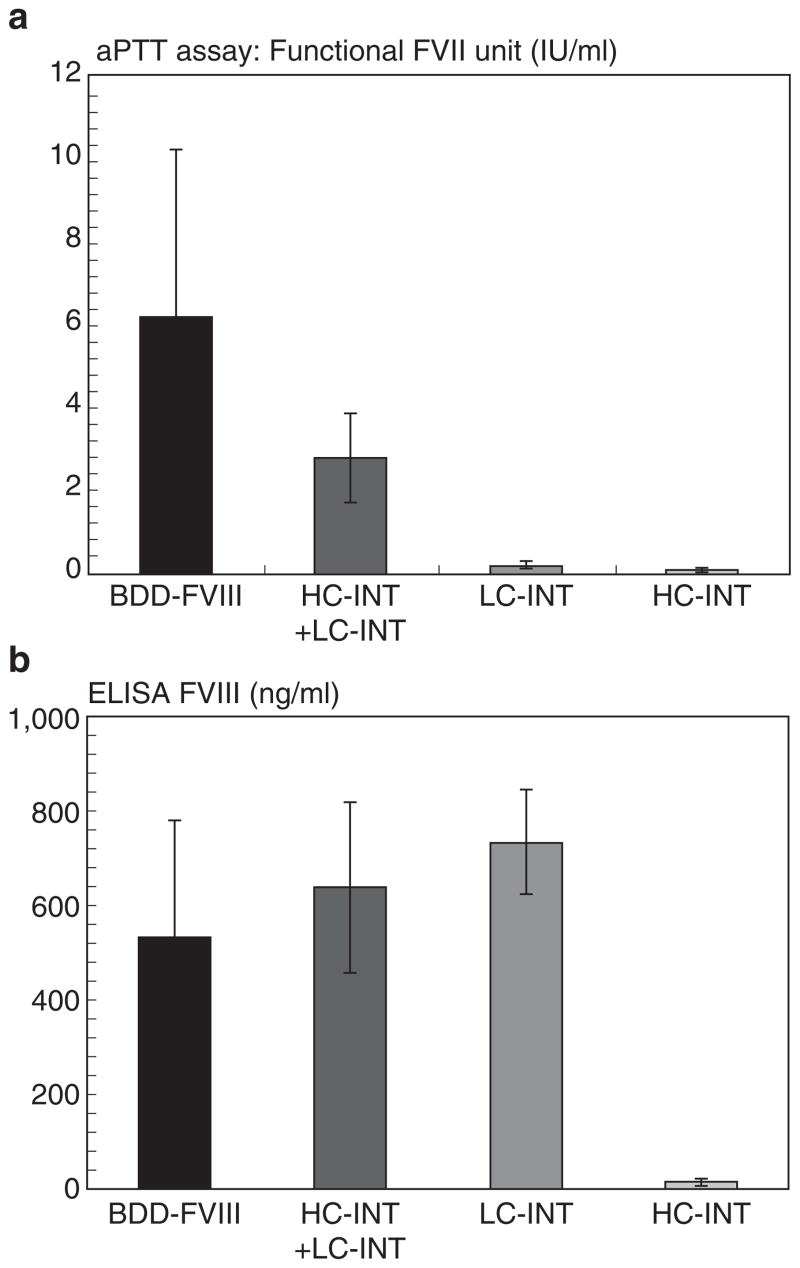
To examine whether the FVIII LC could facilitate HC secretion in vivo, expression plasmid pHC-INT, pLC-INT, or pHC-INT+ pLC-INT was introduced into the livers of hemophilia A mice by hydrodynamic injection. Plasmid pCMV-FVIII, which expresses fully functional BDD-FVIII, was included as a positive control. Twenty-four hours later, mouse plasma was collected. Expression of circulating FVIII was determined by one-stage activated partial thromboplastin time assay. The results are shown in Figure 7a. ELISA for human FVIII was performed using a sheep anti-human FVIII as capture antibody and horseradish peroxidase–conjugated polyclonal antibody as detection antibody (Figure 7b). As shown in Figure 7a, the control groups injected with pLC-INT alone or pHC-INT alone did not show any clotting activity. However, when these two plasmids were injected together, they produced a very high level of FVIII clotting activity, reaching 50% of the level in the positive control group receiving BDD-FVIII-expressing plasmid. In Figure 7b, the different levels of FVIII antigen secreted by these constructs are shown. Although the clotting activities differed dramatically among pCMV-FVIII, pHC-INT+pLC-INT, and pLC-INT (LC) injections, there was not much difference at the protein levels. This result suggests that 50% of FVIII proteins expressed though intein-mediated ligation were active and contained the FVIII HC. It confirms that restoration of the FVIII LC to the HC polypeptide can significantly increase the secretion of functional FVIII protein.
Figure 7. The light chain (LC) enhances heavy chain (HC) secretion in vivo.
Plasmids pCMV-BDD-FVIII, pCMV-HC-INT, pCMV-LC-INT, and pCMV-HC-INT+pCMV-LC-INT were administered into hemophilia A mice by hydrodynamic injection (n = 3 or 4). Each mouse received 200 μg DNA. DNA was diluted in 2.0 ml saline and injected into mice by tail vein injection. The mouse plasma was collected 24 hours after DNA administration. (a) Functional factor VIII (FVIII) units converted from activated partial thromboplastin time assay. (b) The FVIII antigen (HC and LC) in the plasma was measured by ELISA. The SD is shown in the figure. BDD-FVIII, B-domain-deleted FVIII; INT, intein sequence; LC, light chain.
DISCUSSION
FVIII is a protein that secretes inefficiently compared with similar proteins such as factor V.1,13 Despite the overall low efficiency of secretion of single-chain FVIII or B-domain-deleted FVIII protein compared with factor V, the HC and the LC polypeptides maintain a 1:1 stoichiometry. This suggests that the FVIII HC and LC must have overcome the secretion hurdle together. Owing to the limited packaging capacity of AAV vectors, splitting FVIII coding sequences into two AAV vectors is a practical approach to gene therapy.9–11 We think this approach is particularly valuable for therapy of hemophilia A using recombinant AAV vectors. Previous studies have shown that a major problem associated with this approach is chain imbalance,9–11 which is at least partially attributable to inefficient secretion of the HC not in association with the LC. Such chain imbalance not only decreases the amount of active FVIII protein in circulation but also may destabilize the host cells and induce apoptosis.25 Our results confirmed that FVIII HC secretion was almost two logs less efficient than LC secretion.10 This biologically inherent discrepancy can scarcely be solved by manipulating promoters and vector doses.
Previous studies have pointed out that the interactions between cellular chaperones and elements in the FVIII HC limit FVIII secretion.3,13–15 Although there is a difference between full-length FVIII and B-domain-deleted FVIII at the transcription level, we did not observe significant differences between the HC and LC expression vectors at the transcription level using quantitative real-time polymerase chain reaction (data not shown). In Figure 3, the Western blot analysis of HC and LC polypeptides shows that the intracellular level of the HC is about onefold to twofold lower than that of the LC. Thus, the biosynthesis of the HC is unlikely to be the main factor that causes the dramatic difference between the levels of the secreted FVIII LC and HC. The current perception is that secretion is the main hurdle to obtaining a high level of FVIII or the FVIII HC in plasma. So far it is unclear why the HC is so defective in terms of secretion. To improve FVIII secretion and solve the chain imbalance issue, we took advantage of the success of FVIII bio-engineering, which showed promise not only in improving FVIII functions, including half-life and stability, but also in reducing antigenicity and improving FVIII secretion and production.26,27 The engineered B-domain-deleted FVIII has been commercially available to hemophilia patients. Pipe’s group recently showed that the F309S modification and the addition of up to six glycosylation sites in the B domain could improve FVIII secretion by almost 20-fold.3,14 However, such modifications did not have similar effects in terms of improving FVIII HC secretion (Figure 2). One possible explanation is that the FVIII HC alone assumes a different configuration and folds improperly without the FVIII LC in the ER or the Golgi apparatus. Therefore, the FVIII HC alone does not follow the same pathway for secretion as intact single-chain FVIII molecules with the LC moiety. This leads to the intracellular retention of the FVIII HC and eventual degradation of FVIII protein. The results in Figure 4 support this hypothesis.
In this study, we successfully introduced intein as a tool to study the mechanism of FVIII secretion. As an auto-catalytic reaction, intein-mediated trans-splicing avoided any requirement for other cellular enzymes and co-factors.20 The accurate and efficient intein-mediated trans-splicing reaction allowed us to compare the secretion of the HC alone and the HC joined with the LC in the ER or the Golgi apparatus. The role of the FVIII LC in cis, along with the HC, during FVIII heavy secretion highlighted a serious defect in the expression of FVIII using the dual-vector strategy. The demonstration of intein-mediated seamless regeneration of FVIII protein in this study could offer an alternative strategy for the dual-vector approach to the delivery of the FVIII gene using small gene transfer vectors such as AAV vectors. Using this strategy, one vector could encode the first half of the FVIII gene and the second vector could encode the LC fragment. This new scheme may allow the FVIII LC to be attached to the HC polypeptide before secretion. The in vivo testing in Figure 7a using hydrodynamic injection showed 50% functional FVIII using this method, as compared with single-chain FVIII molecules, which suggested that this strategy warrants further study for human gene therapy.
It is worth noting that the LC was able to enhance HC secretion in trans (Figure 6). The co-transfection of the HC and the LC greatly improved the amount of the HC detected. An alternative explanation is that the LC may also affect the HC’s stability (half-life) and rate of synthesis. It has been reported that vWF increases FVIII half-life in vivo. Whether the LC has similar effects remain unclear, especially in vitro. The assays employed in this study demonstrated that mainly stable (ELISA) and functional FVIII was secreted (activated partial thromboplastin time assay). In contrast, HC-N6, HC-INT exhibited little or no ability to enhance secretion by the LC, which suggests that additional sequences in the HC, either native FVIII amino acids or foreign sequences, may have altered the ability of the FVIII HC to interact with the LC. This may imply that an HC molecule with enhanced interaction with the LC will be a better choice for gene therapy.
FVIII secretion follows a very delicate pathway.13 Mutations and modifications in either the HC or the LC could easily disrupt the interactions between the HC and the LC and therefore reduce the enhancing effects of the LC on HC secretion. As shown in Figure 6, the LC did not show an enhancing effect on HC-INT, HC-N6, and HC-N6-INT, as it did on the HC. One possibility is that the additional sequence after the HC, whether it is intein or the native B domain sequence, had an inhibitory effect on the interactions between the HC and the LC. The enhancing effect in cis (LC-INT+HC-N6-INT) suggested that the B domain sequence did not inhibit LC and HC interaction per se. The trans-splicing reaction may have altered the configuration of the LC and HC complex. Thus, it is not surprising that the same B domain sequences could have an inhibitory effect on secretion with the LC in trans while improving FVIII secretion with the LC in cis (Figure 6). This result demonstrated that the enhancing effects of the LC will depend on the type of HC molecules. By the same token, a single-point mutation in the LC may also be able to disrupt FVIII folding and secretion. Previous studies have shown that missense mutations in the C2 domain affected intracellular protein trafficking.13,28 Although we did not construct many mutants to study this, the point mutations in the human hemophilia A mutation database strongly support this hypothesis.29,30 In this database, point mutations are scattered almost evenly throughout the whole FVIII LC. For example, L1756V, G1760R, A1779P, S1888R, R1941Stop, and many other mutations led to less than 1% of FVIII antigen being detected in the patients. All these data point to the inevitable conclusion that a proper LC is essential for efficient FVIII secretion. Proper utilization of the enhancing effect of the LC would greatly affect the efficiency of FVIII delivery.
We demonstrated the enhancing effect of the FVIII LC on the secretion of the HC both in cis and in trans. This information will be useful for designing future strategies for split delivery of FVIII. To take advantage of either in cis and in trans enhancing effects requires both LC and HC vectors to be presented in the same cells. Thus, highly efficient AAV vectors that can carry both vectors equally into every cell will be desirable. Because the trans effect of the LC on HC secretion was at a similar level as in cis, it makes sense to emphasize novel HC or LC molecules with better enhancing effects rather than to introduce an intein, albeit small, to complicate immune responses to the gene delivery in the host tissues or organs.
MATERIALS AND METHODS
FVIII expression plasmids construction
Human FVIII complementary DNA was used in all expression constructs in this study. The expression of FVIII was directed by either a cytomegalovirus promoter (CMV) or a human β-actin promoter with a CMV enhancer (CB).31 For direct comparisons in the experiments performed in this study, all constructs were under the same promoter. The intein sequence was derived from the previously characterized Ssp DnaB split intein.22,23 The pCMV-HC-INT and pCMV-LC-INT plasmids were constructed by fusing appropriate fragments of the human FVIII complementary DNA and the intein coding sequence and inserting the fusion gene in the previously characterized pXX expression vector.32 The fusion gene of pCMV-HC-INT encodes a fusion protein consisting of the first 779 amino acids of FVIII (including its 19-amino-acid signal peptide), a 3-amino-acid Glu-Ser-Gly linker, and the first 106 amino acids of the intein. The fusion gene of pCMV-LC-INT encodes a fusion protein consisting of the 19-amino-acid signal peptide of FVIII, the last 50 amino acids of the intein, a 3-amino-acid Ser-Ile-Glu linker, and the last 693 amino acids of FVIII. The plasmids pCB-HC-INT and pCB-LC-INT were made by replacing the promoters in pCMV-HC-INT and pCMV-LC-INT with a CB promoter. The plasmids expressing human FVIII HC (pCMV-HC, pCB-HC) and LC (pCMV-LC, pCB-LC) were constructed by replacing the promoters in plasmids of pAAV-hFVIII-HC and pAAV-hFVIII-LC with a CMV promoter or a CB promoter.9,10 The plasmid pHC-N6 was constructed by replacing the HC fragment in pCB-HC or pCMV-HC with similar fragments with six glycosylation sites from F309S/226aa/N6.14 The plasmid pHC-N6-INT was similarly derived from pHC-INT.
Tissue culture and transfection
HEK 293 and COS cells were purchased from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (HyClone, Logan, UT), penicillin (100 U/ml) and streptomycin (Invitrogen, Carlsbad, CA) (100 μg/ml) at 37 °C in a humid environment supplied with 5% CO2. Transfections were carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Alternatively, a transfection procedure using calcium phosphate precipitation was carried out as described previously.8,33 After transfection, the cells were grown for 12–24 hours in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum to minimize cell death. The cells were then maintained in OPTI-MEM (Invitrogen, Carlsbad, CA) medium for 24–72 hours before the medium was collected and the secreted FVIII antigens were analyzed. For direct comparison, a green fluorescent protein expression plasmid was also included in the transfection mix to ensure similar transfection efficiency. We used both COS cells and 293 cells for our experiments. After 293 cells were confirmed to produce similar results to COS cells, most experiments were carried out using 293 cells.
Animal procedures
Exon 16 FVIII knock-out mice were obtained from Dr. Haig Kazazian (University of Pennsylvania).8,33 All mice were housed in a specific pathogen-free environment with normal diet. All surgical procedures involving mice were in accordance with Institutional Animal Care and Use Committee guidelines under approved protocols at The Children’s Hospital of Philadelphia.
For hydrodynamic injection, animals were injected via the tail vein with 2.0 ml saline containing 200 μg column-purified plasmids in 5–10 seconds.34,35 The animals were allowed to recover before being returned to the cages. Blood was collected at 24–48 hours after hydrodynamic injection by tail clip using sodium citrate as an anticoagulant at a final concentration of 0.38% (wt/vol). The blood samples were then centrifuged at 4 °C for 10 minutes at 9,000 rpm in a microcentrifuge. The plasmas were then collected and used for FVIII assays.
Quantitative analysis of FVIII antigen
FVIII-HC and FVIII-LC antigen were determined using chain-specific ELISAs. For the human FVIII ELISA, matched-pair antibody sets for human FVIII antigen were purchased from Enzyme Research Laboratories (South Bend, IN). Both detection and capture antibodies were sheep anti-human FVIII immunoglobulin G. The linear range of this assay is from 3 to 100% reference human FVIII as determined by the manufacturer. For human HC–specific ELISA, Nunc Maxisorp (Nalge Nunc International, Rochester, NY) plates were coated with 2 μg/ml HC-specific monoclonal antibody ESH5 (American Diagnostica, Greenwich, CT). Samples and standards were diluted in OPTI-MEM (Invitrogen, Carlsbad, CA). Samples and standards (100 μl/well) were incubated at room temperature for 2 hours. After washing, a horseradish peroxidase–conjugated sheep anti-human FVIII antibody F8C-EIA-D (100 μl/well, 2 μg/ml; Affinity Biologicals, Ancaster, Canada) was added, and the plates were incubated for 2 hours at room temperature. After a final wash the antigen was detected using 2,2′-azino-bis-[3-ethylbenzthiazoline-6-sulfonic acid] substrate (Roche, Germany) and the absorbance read at 405 nm. The hFVIII-LC ELISA was performed similarly, with the following changes: the capture antibody was 2 μg/ml monoclonal antibody to human FVIII LC N55195M (Biodesign International, Saco, ME); the detection antibody was 26 μg/ml sheep antihuman FVIII antibody from Haematologic Technologies (Essex Junction, VT), followed by 2 μg/ml horseradish per-oxidase–conjugated rabbit anti-sheep immunoglobulin G (H+L) from Bio-Rad Laboratories (Hercules, CA). For all ELISAs, the standard used was Refacto (Genetics Institute, Cambridge, MA), recombinant BDD-FVIII.
Biologically active FVIII in media and plasma was measured using the activated partial thromboplastin time assay as previously described.8,9,33 Refacto (Genetics Institute, Cambridge, MA) was used as the standard.
To measure the intracellular level of FVIII antigen, the cells were harvested from tissue culture dishes. The cell pellets after centrifugation were re-suspended in phosphate-buffered saline with protease inhibitors and sonicated ten times in a Branson Sonifier (Branson Ultrasonics Corporation, Danbury, CT) under level two with 50% output. Then the samples were centrifuged for 5 minutes at 12,000g to remove cell debris. The supernatant was collected and an equal amount (i.e., 100 μ) of supernatant for each sample was used for ELISA following the procedure described above.
Western blot analysis of FVIII
To analyze intracellular FVIII antigen, cells transfected with FVIII plasmids were lysed with radioimmunoprecipitation buffer (50 mmol/l Tris–HCl pH 7.4,150 mmol/l NaCl, 1 mmol/l phenylmethylsulphonyl fluoride,1 mmol/l EDTA, 1% Triton X-100, 1% sodium dodecyl sulfate). After sonication to break the genomic DNA, an equal volume of 2× sample buffer was added and boiled for 2 minutes. The resulting samples were analyzed using 6% or 4–12% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and transferred to nitrocellulose membrane for Western blotting. After the membrane was blocked overnight in phosphate-buffered saline/10% skim milk powder/0.3% Tween-20, a sheep anti-hFVIII antibody, SAF8C (1:1,000 dilution; Enzyme Research Laboratories, IN), was added and the sample was incubated for 1 hour at room temperature. The membrane was washed, incubated with a rabbit anti-sheep antibody (1:2,000 dilution; Bio-Rad Laboratories (Hercules, CA)), and developed using an enhanced chemiluminescent substrate (Amersham-Pharmacia Biotech, Piscataway, NJ).
Acknowledgments
L.C., F.Z., J.L., X.X., X.L., R.M.C., and W.X. designed and performed the research and analyzed the data; H.L. and J.W. performed research. H.J., R.S., V.R.A., J.Z., G.F.P., Q.D., X.W., H.W., and S.W.P. contributed vital new reagents or analytic tools. H.J. and W.X. wrote the paper. We thank Marlene Webber and Junwei Sun for their help in manuscript preparation. This study was supported by NIH grant R01HL069051 and R01HL084381.
References
- 1.Mann KG. Biochemistry and physiology of blood coagulation. Thromb Haemost. 1999;82:165–174. [PubMed] [Google Scholar]
- 2.Kaufman RJ. Advances toward gene therapy for hemophilia at the millennium. Hum Gene Ther. 1999;10:2091–2107. doi: 10.1089/10430349950017095. [DOI] [PubMed] [Google Scholar]
- 3.Pipe SW. Coagulation factors with improved properties for hemophilia gene therapy. Semin Thromb Hemost. 2004;30:227–237. doi: 10.1055/s-2004-825636. [DOI] [PubMed] [Google Scholar]
- 4.Couto LB. Preclinical gene therapy studies for hemophilia using adeno-associated virus (AAV) vectors. Semin Thromb Hemost. 2004;30:161–171. doi: 10.1055/s-2004-825630. [DOI] [PubMed] [Google Scholar]
- 5.Kay MA, High K. Gene therapy for the hemophilias [comment] Proc Natl Acad Sci USA. 1999;96:9973–9975. doi: 10.1073/pnas.96.18.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.High KA. Clinical gene transfer studies for hemophilia B. Semin Thromb Hemost. 2004;30:257–267. doi: 10.1055/s-2004-825639. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD, et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar R, Xiao W, Kazazian HH., Jr A single adeno-associated virus (AAV)-murine factor VIII vector partially corrects the hemophilia A phenotype. J Thromb Haemost. 2003;1:220–226. doi: 10.1046/j.1538-7836.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 9.Scallan CD, Liu T, Parker AE, Patarrovo-White SL, Chen H, Jiang H, et al. Phenotypic correction of a mouse model of hemophilia A using AAV2 vectors encoding the heavy and light chains of FVIII. Blood. 2003;102:3919–3926. doi: 10.1182/blood-2003-01-0222. [DOI] [PubMed] [Google Scholar]
- 10.Burton M, Nakai H, Colosi P, Cunningham J, Mitchell R, Couto L. Coexpression of factor VIII heavy and light chain adeno-associated viral vectors produces biologically active protein. Proc Natl Acad Sci USA. 1999;96:12725–12730. doi: 10.1073/pnas.96.22.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mah C, Sarkar R, Zolotukhin I, Schleissing M, Xiao X, Kazazian HH, et al. Dual vectors expressing murine factor VIII result in sustained correction of hemophilia A mice. Hum Gene Ther. 2003;14:143–152. doi: 10.1089/104303403321070838. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman RJ. Genetic engineering of factor VIII. Nature. 1989;342:207–208. doi: 10.1038/342207a0. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman RJ, Pipe SW, Tagliavacca L, Swaroop M, Moussalli M. Biosynthesis, assembly and secretion of coagulation factor VIII. Blood Coagul Fibrinolysis. 1997;8(suppl 2):S3–S14. [PubMed] [Google Scholar]
- 14.Miao HZ, Sirachainan N, Palmer L, Kucab P, Cunningham MA, Kaufman RJ, et al. Bioengineering of coagulation factor VIII for improved secretion. Blood. 2004;103:3412–3419. doi: 10.1182/blood-2003-10-3591. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham MA, Pipe SW, Zhang B, Hauri HP, Ginsburg D, Kaufman RJ. LMAN1 is a molecular chaperone for the secretion of coagulation factor VIII. J Thromb Haemost. 2003;1:2360–2367. doi: 10.1046/j.1538-7836.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 16.Dorner AJ, Wasley LC, Kaufman RJ. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J Biol Chem. 1989;264:20602–20607. [PubMed] [Google Scholar]
- 17.Kaufman RJ, Pipe SW. Can we improve on nature? “Super molecules” of factor VIII. Haemophilia. 1998;4:370–379. doi: 10.1046/j.1365-2516.1998.440370.x. [DOI] [PubMed] [Google Scholar]
- 18.Toole JJ, Pittman DD, Orr EC, Murtha P, Wasley LC, Kaufman RJ. A large region (approximately equal to 95 kDa) of human factor VIII is dispensable for in vitro procoagulant activity. Proc Natl Acad Sci USA. 1986;83:5939–5942. doi: 10.1073/pnas.83.16.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittman DD, Tomkinson KN, Kaufman RJ. Post-translational requirements for functional factor V and factor VIII secretion in mammalian cells. J Biol Chem. 1994;269:17329–17337. [PubMed] [Google Scholar]
- 20.Perler FB. The ins and outs of gene expression control. Nat Biotechnol. 2004;22:824–826. doi: 10.1038/nbt0704-824. [DOI] [PubMed] [Google Scholar]
- 21.Wu H, Hu Z, Liu XQ. Protein trans-splicing by a split intein encoded in a split DnaE gene of Synechocystis sp. PCC6803. Proc Natl Acad Sci USA. 1998;95:9226–9231. doi: 10.1073/pnas.95.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu XQ, Hu Z. A DnaB intein in Rhodothermus marinus: indication of recent intein homing across remotely related organisms. Proc Natl Acad Sci USA. 1997;94:7851–7856. doi: 10.1073/pnas.94.15.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Xu MQ, Liu XQ. Protein trans-splicing and functional mini-inteins of a cyanobacterial dnaB intein. Biochim Biophys Acta. 1998;1387:422–432. doi: 10.1016/s0167-4838(98)00157-5. [DOI] [PubMed] [Google Scholar]
- 24.Thompson AR. Structure and function of the factor VIII gene and protein. Semin Thromb Hemost. 2003;29:11–22. doi: 10.1055/s-2003-37935. [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 26.Brinkhous K, Sandberg H, Widlund L, Read M, Nichols T, Sigman J, et al. Preclinical pharmacology of albumin-free B-domain deleted recombinant factor VIII. Semin Thromb Hemost. 2002;28:269–272. doi: 10.1055/s-2002-32661. [DOI] [PubMed] [Google Scholar]
- 27.Pipe SW. The promise and challenges of bioengineered recombinant clotting factors. J Thromb Haemost. 2005;3:1692–1701. doi: 10.1111/j.1538-7836.2005.01367.x. [DOI] [PubMed] [Google Scholar]
- 28.Pipe SW, Kaufman RJ. Factor VIII C2 domain missense mutations exhibit defective trafficking of biologically functional proteins. J Biol Chem. 1996;271:25671–25676. doi: 10.1074/jbc.271.41.25671. [DOI] [PubMed] [Google Scholar]
- 29.Kemball-Cook G, Tuddenham EG, Wacey AI. The factor VIII Structure and Mutation Resource Site: HAMSTeRS version 4. Nucleic Acids Res. 1998;26:216–219. doi: 10.1093/nar/26.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannelli F, Green PM, Sommer SS, Poon M, Ludwiq M, Schwaab R, et al. Haemophilia B: database of point mutations and short additions and deletions—eighth edition. Nucleic Acids Res. 1998;26:265–268. doi: 10.1093/nar/26.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 32.Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar R, Tetreault R, Gao G, Wang L, Bell P, Chandler R, et al. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103:1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]
- 34.Song YK, Liu F, Zhang G, Liu D. Hydrodynamics-based transfection: simple and efficient method for introducing and expressing transgenes in animals by intravenous injection of DNA. Methods Enzymol. 2002;346:92–105. doi: 10.1016/s0076-6879(02)46050-8. [DOI] [PubMed] [Google Scholar]
- 35.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]