Abstract
The nuclear receptor PPARγ is a lipid sensor that regulates lipid metabolism through gene transcription. Inhibition of PPARγ activity by TNF-α is involved in pathogenesis of insulin resistance, atherosclerosis, inflammation, and cancer cachexia. PPARγ activity is regulated by TNF-α at pre-translational and post-translational levels. Activation of serine kinases including IKK, ERK, JNK and p38 may be involved in the TNF-regulation of PPARγ. Of the four kinases, IKK is a dominant signaling molecule in the TNF-regulation of PPARγ. IKK acts through at least two mechanisms: inhibition of PPARγ expression and activation of PPARγ corepressor. In this review article, literature is reviewed with a focus on the mechanisms of PPARγ inhibition by TNF-α.
The nuclear receptor PPARγ is a member of peroxisome proliferator-activated receptor (PPAR) family that includes PPARα, PPARγ, and PPARδ (PPARβ) (reviewed in (1; 2). There are two isoforms in PPARγ, PPARγ1 and PPARγ2. PPARγ1 is expressed ubiquitously and PPARγ2 is mainly expressed in adipocytes. The biological activities of PPARγ are very broad, but it is generally accepted as a master transcriptional regulator of lipid and glucose metabolism (reviewed in (1-3). Inhibition of PPARγ function by inflammatory cytokines may contribute to pathogenesis of many diseases, such as insulin resistance, atherosclerosis, inflammation, and cancer cachexia. Disorder in lipid metabolism is a common feature in many diseases. Inhibition of PPARγ function by TNF-α may represent a molecular mechanism of the lipid and glucose disorders. TNF-α is known to inhibit the ligand-dependent transcriptional activity of PPARγ. However, the mechanism remains to be fully understood (4-9). In this review, the literature is reviewed on the possible mechanisms of PPARγ regulation by TNF-α.
Inhibition of PPARγ by TNF-α
The mechanisms of TNF-inhibition of PPARγ include three models according to experimental evidence. First, PPARγ expression is reduced at mRNA level by TNF-α (5; 6). This is observed in 3T3-L1 adipocytes treated with TNF-α for 24 hours or longer. The mechanism is related to inhibition of C/EBPδ expression by TNF-α (10). C/EBPδ was shown to activate the PPARγ gene promoter through a direct protein-DNA interaction. When C/EBPδ expression is reduced by TNF-α, PPARγ gene transcription will be reduced. Second, PPARγ expression is not changed. This mechanism was demonstrated in cells transfected with a PPARγ expression vector (4; 7; 8). In the second model, the ligand-dependent transcriptional activity of PPARγ is reduced as a result of loss of DNA-binding activity of PPARγ. It was shown that the inhibition of DNA binding activity was dependent on association of NF-kB and PPARγ (7). Third, the transcriptional activity of PPARγ is inhibited by TNF-α through activation of nuclear corepressor (9). In this mechanism, the DNA-binding activity of PPARγ was not reduced by TNF-α. However, the DNA-bound PPARγ is inactivated by histone deacetylase 3 (HDAC3). All three mechanisms of inhibition are dependent on activation of IKK/NF-kB pathway as the TNF-α activity was abolished by the super repressor IkBα (Inhibitor kappa Bα) (6; 9). The activities of both PPARγ1 and PPARγ2 are inhibited by TNF-α.
Signaling pathways of TNF-α
The intracellular signaling pathways of TNF-α are activated through cell membrane receptors. Engagement of the receptors by TNF-α leads activation of many signaling pathways, such as IKK/NF-kB, MAPK (JNK, ERK, and p38) and apoptosis pathway (11; 12). NF-kB is a transcription factor that stays in the cytoplasm in the absence of activators. The inhibition is mediated by IkBα that inhibits NF-kB shuttling between the cytoplasm and nucleus (reviewed in (13)). IkBα degradation is controlled by a phosphorylation-mediated and proteasome-dependent mechanism that is initiated by activation of IKK2 (14). In the TNF-α signaling pathways, activation of IKK, ERK and JNK (c-JUN NH2 terminal kinase) were reported to inhibit the transcriptional activity of PPARγ (4; 7; 8; 15-17), but p38 was reported to enhance the function of PPARγ (18-21).
IKK is a dominant kinase in TNF-regulation of PPARγ function
Although TNF-α activates several serine kinases that are able to regulate the ligand-dependent activity of PPARγ, IKK is a dominant kinase mediating the TNF-α activity in regulation of PPARγ function (7-9). The key evidence is that TNF-α activity is completely blocked by inactivation of IKK or its downstream event. The IKK/NF-kB signaling pathway is also required for IL-1 inhibition of PPARγ function (5; 7; 8; 22).
The roles of MAPK (ERK, JNK and P38) in the regulation of PPARγ activity was known earlier than IKK. ERK and JNK was reported to inhibit PPARγ function by a direct phosphorylation of serine residues in PPARγ (4; 15-17), such as Ser112 in PPARγ2 (4). The ERK-mediated inhibition is involved in prevention of diet-induced obesity in knockout mice of tyrosine kinases-1 (Dok1) (23). However, it is controversial for JNK in the regulation of PPARγ as a study suggests that JNK is involved in activation of PPARγ in foam cells (24). Since ERK and JNK are the major kinases in the signaling pathways of EGF (epidermal growth factor) and FGF (fibroblast growth factor) (25), these kinases may play an important role in the regulation of PPARγ activity by EGF and FGF (26-28). In addition to MAPK kinases (ERK, JNK and P38), PPARγ is also phosphorylated by Protein Kinase A and C (PKA, PKC), AMP Kinase (AMPK) and glycogen synthase kinase-3 (GSK3) (see reviewed (29)).
Coactivator and corepressor for PPARγ
The transcriptional activity of PPARγ is controlled by DNA-binding activity and nuclear receptor cofactors that include corepressors and coactivators. PPARγ is a heterodimer transcription factor composed of PPARγ and retinoid X receptor (RXR), which is activated by 9-cis retinoic acid (30). The heterodimer is associated with the nuclear receptor corepressor complex in the absence of PPARγ ligand. Upon activation by a ligand, the corepressor complex is replaced by coactivators leading to transcriptional initiation of target genes. The coactivators of PPARγ include the well-established cofactors such as p300/CBP, p160 and PGC-1 (PPARγ coactivator-1) (reviewed in (31), as well as the relative new coactivators TRAP220 (Thyroid hormone Receptor-Associated Protein 220 or PBP, PPARγ-Binding Protein) (32; 33), ARA70 (Androgen Receptor-Associated protein) (34) and PRIP (PPARγ-interacting protein, ASC-2/RAP250 /TRBP/NRC) (35-38). The coactivator p160 has three isoforms: SRC-1 (steroid receptor coactivator 1, NCoA-1), SRC-2 (NCoA-2/TIF2/GRIP1) and SRC-3 (NCoA-3/pCIP/AIB-1/ACTR/RAC-3/TRAM-1) (39). The corepressor for PPARγ is a protein complex containing HDAC3 (histone deacetylase 3) and SMRT (silencing mediator for retinoic and thyroid hormone receptors) or N-CoR (nuclear corepressor). RIP140 (receptor-interacting protein) may also be a component in the corepressor complex (40-43).
HDAC3 is required for IKK inhibition of ligand-dependent activity of PPARγ by TNF-α
TNF-α is able to inhibit PPARγ activity at two different levels (9). In the chronic (>16 hrs) treatment, TNF-α reduces expression of PPARγ in adipocytes. In the acute treatment, TNF-α inhibits the ligand-dependent activity without decreasing PPARγ expression or its DNA-binding activity. Both chronic and acute inhibition is dependent on the IKK/NF-kB pathway (6; 8; 9). Regarding the acute effect of TNF-α, NF-kB was reported to reduce the DNA-binding activity of PPARγ (7). Protein-protein interaction between NF-kB and PPARγ was proposed to mediate the inhibition, and PGC-2 was shown to be required for the NFkB-PPARγ interaction. Our data from ChIP and EMSA assays demonstrated that the DNA-binding activity of PPARγ was not changed by TNF-α in the acute treatment (9). The data is consistent in adipocytes and 293 cells. The only change induced by TNF-α was an increased association of PPARγ with HDAC3/SMRT. When this change was blocked by ssIkBα, TNF-α lost its inhibitory activity in PPARγ. These data suggest that the nuclear receptor corepressor is the target of TNF-α. Since TNF-α induces nuclear translocation of HDAC3, TNF-α may regulate the corepressor function through HDAC3. Recently, IKK has been shown to modify SMRT activity through direct phosphorylation of SMRT protein (44). If this IKK/SMRT interaction is involved in TNF-inhibition of PPARγ activity, it may not be a necessary step. The TNF-activity can be completely blocked by super suppressor IkBα (ssIkBα), which inhibits NF-kB activation without affecting of IKK activity. It is not clear if TNF-α can modify the phosphorylation status of HDAC3. If this does happen, nuclear translocation of HDAC3 is still necessary for the TNF-activity. The role of nuclear corepressor is also supported by our observation that the TNF-α inhibition was attenuated by overexpression of the nuclear receptor coactivators (Gao Z and Ye J, unpublished data). Overexpression of the coactivators is able to rescue PPARγ function from the TNF-α inhibition. The coactivator may act by antagonizing the deacetylase activity of the nuclear corepressor.
PPARγ inhibition by TNF-alpha requires nuclear translocation of HDAC3 (9)
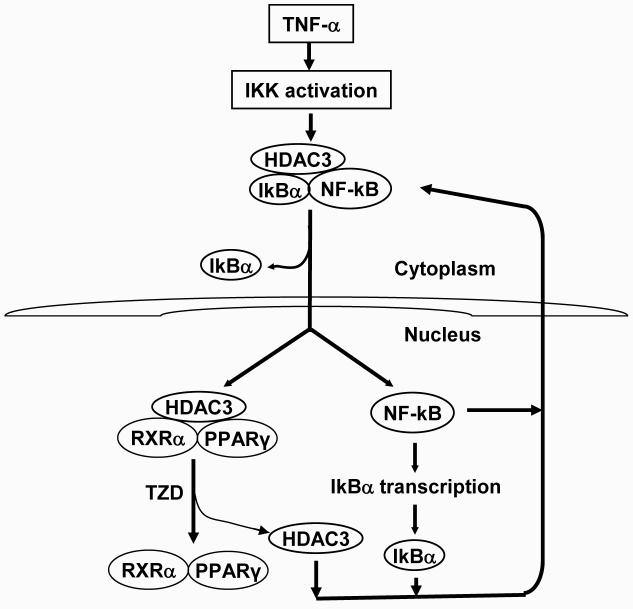
In the HDAC3 protein, there are both nuclear export signal (180-313 aa in the central portion), and the nuclear localization signal (312-428 aa in the C-terminal) (45). HDAC3 shuttles between the cytoplasm and nucleus. The shuttling is regulated by IkBα (Fig. 1) (9). When IkBα is degraded, HDAC3 enters the nucleus. When newly-synthesized IkBα is available in the nucleus, HDAC3 is bound to IkBα and exported from nucleus. This molecular model is supported by four lines of evidence: (a) Nuclear translocation of HDAC3 is coupled with IkBα degradation; (b) HDAC3 is exclusively located in the nucleus in IkBα−/− cells; (c) ssIkBα retains HDAC3 in the cytoplasm and reduces the nuclear abundance of HDAC3; (d) IkBα associates with HDAC3 through the ankyrin repeat domain (46). Since HDAC3 regulates transcription of a variety of genes (47; 48), the IkB-HDAC3 model provides a new mechanism for many phenomena observed for IkBα, such as death of newborn mice with IkBα knockout (IkBα−/−) (49), inhibition of limb development by IkBα in drosophila (50), and enhancement of transcriptional activity of other transcription factors by IkBα (46; 51). HDAC3 activity may also explain the inhibition of C/EBPδ expression by TNF-α (10).
Fig. 1. Regulation of HDAC3 cytoplasm/nucleus shuttling by IkBa.
HDAC3 is enriched in the nucleus after translocation of cytoplasmic HDAC3 into the nucleus. This change leads to inhibition of PPARγ function. The nuclear translocation of HDAC3 is initiated by degradation of IkBa, which is catalyzed by activation of IKK and required for NF-kB activation. NF-kB induces transcription and recovery of IkBa. IkBa then induces nuclear exclusion of HDAC3 and NF-kB through protein-protein association. This event leads to inhibition of NF-kB activity and reduction in HDAC3 protein abundance.
HDAC3 was reported to inhibit NF-kB p65 activity by deacetylation of p65 protein (52-55). However, it is not clear what event mediates the protein-protein association of HDAC3-p65 (52; 54). Since IkBα binds to both p65 and HDAC3, IkBα may mediate recruitment of HDAC3 by p65 (9).
In summary, there are three major mechanisms for TNF-α inhibition of PPARγ activity. One is dependent on inhibition of PPARγ gene expression. Two are related to suppression of the ligand-dependent transcriptional activity of PPARγ. The nuclear corepressor function may be required for all of the three mechanisms. TNF-α is able to enhance HDAC3 activity in the nucleus through a nuclear translocation mechanism. IkBα plays an important role in the regulation of HDAC3 shuttling between cytoplasm and nucleus. IkBα mediates IKK regulation of nuclear translocation of HDAC3. TNF-α inhibits activities of both PPARγ1 and PPARγ2.
Acknowledgements
This study is supported by NIH grant DK068036 and ADA research award to J Ye.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 2.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 3.Lazar MA. Progress in cardiovascular biology: PPAR for the course. Nat Med. 2001;7:23–24. doi: 10.1038/83301. [DOI] [PubMed] [Google Scholar]
- 4.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 5.Zhang B, Berger J, Hu E, Szalkowski D, White-Carrington S, Spiegelman BM, Moller DE. Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol Endocrinol. 1996;10:1457–1466. doi: 10.1210/mend.10.11.8923470. [DOI] [PubMed] [Google Scholar]
- 6.Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51:1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 7.Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S, Yamauchi T, Kadowaki T, Takeuchi Y, Shibuya H, Gotoh Y, Matsumoto K, Kato S. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol. 2003;5:224–230. doi: 10.1038/ncb942. [DOI] [PubMed] [Google Scholar]
- 8.Ruan H, Pownall HJ, Lodish HF. Troglitazone antagonizes TNF-alpha -induced reprogramming of adipocyte gene expression by inhibiting the transcriptional regulatory functions of NF-kB. J Biol Chem. 2003;278:28181–28192. doi: 10.1074/jbc.M303141200. [DOI] [PubMed] [Google Scholar]
- 9.Gao Z, He Q, Peng B, Chiao PJ, Ye J. Regulation of Nuclear Translocation of HDAC3 by I{kappa}B{alpha} Is Required for Tumor Necrosis Factor Inhibition of Peroxisome Proliferator-activated Receptor {gamma} Function. J Biol Chem. 2006;281:4540–4547. doi: 10.1074/jbc.M507784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M, Sugawara A, Uruno A, Takeuchi K, Ito S. Transcription suppression of peroxisome proliferator-activated receptor gamma2 gene expression by tumor necrosis factor alpha via an inhibition of CCAAT/ enhancer-binding protein delta during the early stage of adipocyte differentiation. Endocrinology. 2004;145:4948–4956. doi: 10.1210/en.2004-0180. [DOI] [PubMed] [Google Scholar]
- 11.Van Antwerp DJ, Martin SJ, Verma IM, Green DR. Inhibition of TNF-induced apoptosis by NF-kappa B. Trends Cell Biol. 1998;8:107–111. doi: 10.1016/s0962-8924(97)01215-4. [DOI] [PubMed] [Google Scholar]
- 12.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 13.Hayden MS, Ghosh S. Shared Principles in NF-[kappa]B Signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 15.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 16.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 17.Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology. 1999;140:392–397. doi: 10.1210/endo.140.1.6457. [DOI] [PubMed] [Google Scholar]
- 18.Engelman JA, Lisanti MP, Scherer PE. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J Biol Chem. 1998;273:32111–32120. doi: 10.1074/jbc.273.48.32111. [DOI] [PubMed] [Google Scholar]
- 19.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 20.Yang TT, Xiong Q, Enslen H, Davis RJ, Chow CW. Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol Cell Biol. 2002;22:3892–3904. doi: 10.1128/MCB.22.11.3892-3904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hata K, Nishimura R, Ikeda F, Yamashita K, Matsubara T, Nokubi T, Yoneda T. Differential Roles of Smad1 and p38 Kinase in Regulation of Peroxisome Proliferator-activating Receptor gamma during Bone Morphogenetic Protein 2-induced Adipogenesis. Mol Biol Cell. 2003;14:545–555. doi: 10.1091/mbc.E02-06-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torti FM, Torti SV, Larrick JW, Ringold GM. Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor beta. J Cell Biol. 1989;108:1105–1113. doi: 10.1083/jcb.108.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosooka T, Noguchi T, Kotani K, Nakamura T, Sakaue H, Inoue H, Ogawa W, Tobimatsu K, Takazawa K, Sakai M, Matsuki Y, Hiramatsu R, Yasuda T, Lazar MA, Yamanashi Y, Kasuga M. Dok1 mediates high-fat diet-induced adipocyte hypertrophy and obesity through modulation of PPAR-[gamma] phosphorylation. Nat Med. 2008;14:188–193. doi: 10.1038/nm1706. [DOI] [PubMed] [Google Scholar]
- 24.Yin R, Dong YG, Li HL. PPARgamma phosphorylation mediated by JNK MAPK: a potential role in macrophage-derived foam cell formation. Acta Pharmacol Sin. 2006;27:1146–1152. doi: 10.1111/j.1745-7254.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 25.Porter AC, Vaillancourt RR. Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene. 1998;17:1343–1352. doi: 10.1038/sj.onc.1202171. [DOI] [PubMed] [Google Scholar]
- 26.Hauner H, Rohrig K, Petruschke T. Effects of epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) on human adipocyte development and function. Eur J Clin Invest. 1995;25:90–96. doi: 10.1111/j.1365-2362.1995.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 27.Serrero G, Mills D. Physiological role of epidermal growth factor on adipose tissue development in vivo. Proc Natl Acad Sci U S A. 1991;88:3912–3916. doi: 10.1073/pnas.88.9.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navre M, Ringold GM. Differential effects of fibroblast growth factor and tumor promoters on the initiation and maintenance of adipocyte differentiation. J Cell Biol. 1989;109:1857–1863. doi: 10.1083/jcb.109.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta. 2007;1771:952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, Tempst P, Spiegelman BM. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha. Nucleic Acids Res. 1994;22:5628–5634. doi: 10.1093/nar/22.25.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puigserver P, Spiegelman BM. Peroxisome Proliferator-Activated Receptor-gamma Coactivator 1alpha (PGC-1alpha): Transcriptional Coactivator and Metabolic Regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 32.Ge K, Guermah M, Yuan CX, Ito M, Wallberg AE, Spiegelman BM, Roeder RG. Transcription coactivator TRAP220 is required for PPAR gamma 2-stimulated adipogenesis. Nature. 2002;417:563–567. doi: 10.1038/417563a. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y, Qi C, Jain S, Rao MS, Reddy JK. Isolation and characterization of PBP, a protein that interacts with peroxisome proliferator-activated receptor. J Biol Chem. 1997;272:25500–25506. doi: 10.1074/jbc.272.41.25500. [DOI] [PubMed] [Google Scholar]
- 34.Heinlein CA, Ting HJ, Yeh S, Chang C. Identification of ARA70 as a ligand-enhanced coactivator for the peroxisome proliferator-activated receptor gamma. J Biol Chem. 1999;274:16147–16152. doi: 10.1074/jbc.274.23.16147. [DOI] [PubMed] [Google Scholar]
- 35.Lee SK, Anzick SL, Choi JE, Bubendorf L, Guan XY, Jung YK, Kallioniemi OP, Kononen J, Trent JM, Azorsa D, Jhun BH, Cheong JH, Lee YC, Meltzer PS, Lee JW. A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo. J Biol Chem. 1999;274:34283–34293. doi: 10.1074/jbc.274.48.34283. [DOI] [PubMed] [Google Scholar]
- 36.Caira F, Antonson P, Pelto-Huikko M, Treuter E, Gustafsson JA. Cloning and characterization of RAP250, a novel nuclear receptor coactivator. J Biol Chem. 2000;275:5308–5317. doi: 10.1074/jbc.275.8.5308. [DOI] [PubMed] [Google Scholar]
- 37.Mahajan MA, Samuels HH. A new family of nuclear receptor coregulators that integrate nuclear receptor signaling through CREB-binding protein. Mol Cell Biol. 2000;20:5048–5063. doi: 10.1128/mcb.20.14.5048-5063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko L, Cardona GR, Chin WW. Thyroid hormone receptor-binding protein, an LXXLL motif-containing protein, functions as a general coactivator. Proc Natl Acad Sci U S A. 2000;97:6212–6217. doi: 10.1073/pnas.97.11.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leo C, Chen JD. The SRC family of nuclear receptor coactivators. Gene. 2000;245:1–11. doi: 10.1016/s0378-1119(00)00024-x. [DOI] [PubMed] [Google Scholar]
- 40.Hu X, Li Y, Lazar MA. Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol Cell Biol. 2001;21:1747–1758. doi: 10.1128/MCB.21.5.1747-1758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamir I, Zhang J, Lazar MA. Stoichiometric and steric principles governing repression by nuclear hormone receptors. Genes Dev. 1997;11:835–846. doi: 10.1101/gad.11.7.835. [DOI] [PubMed] [Google Scholar]
- 42.Krogsdam AM, Nielsen CA, Neve S, Holst D, Helledie T, Thomsen B, Bendixen C, Mandrup S, Kristiansen K. Nuclear receptor corepressor-dependent repression of peroxisome-proliferator-activated receptor delta-mediated transactivation. Biochem J. 2002;363:157–165. doi: 10.1042/0264-6021:3630157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treuter E, Albrektsen T, Johansson L, Leers J, Gustafsson JA. A regulatory role for RIP140 in nuclear receptor activation. Mol Endocrinol. 1998;12:864–881. doi: 10.1210/mend.12.6.0123. [DOI] [PubMed] [Google Scholar]
- 44.Hoberg JE, Yeung F, Mayo MW. SMRT derepression by the IkappaB kinase alpha: a prerequisite to NF-kappaB transcription and survival. Mol Cell. 2004;16:245–255. doi: 10.1016/j.molcel.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Yang WM, Tsai SC, Wen YD, Fejer G, Seto E. Functional domains of histone deacetylase-3. J Biol Chem. 2002;277:9447–9454. doi: 10.1074/jbc.M105993200. [DOI] [PubMed] [Google Scholar]
- 46.Viatour P, Legrand-Poels S, van Lint C, Warnier M, Merville MP, Gielen J, Piette J, Bours V, Chariot A. Cytoplasmic IkappaBalpha increases NF-kappaB-independent transcription through binding to histone deacetylase (HDAC) 1 and HDAC3. J Biol Chem. 2003;278:46541–46548. doi: 10.1074/jbc.M306381200. [DOI] [PubMed] [Google Scholar]
- 47.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 48.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 50.Bushdid PB, Brantley DM, Yull FE, Blaeuer GL, Hoffman LH, Niswander L, Kerr LD. Inhibition of NF-kappaB activity results in disruption of the apical ectodermal ridge and aberrant limb morphogenesis. Nature. 1998;392:615–618. doi: 10.1038/33435. [DOI] [PubMed] [Google Scholar]
- 51.Espinosa L, Ingles-Esteve J, Robert-Moreno A, Bigas A. Ikappa Balpha and p65 Regulate the Cytoplasmic Shuttling of Nuclear Corepressors: Cross-talk between Notch and NFkappa B Pathways. Mol Biol Cell. 2003;14:491–502. doi: 10.1091/mbc.E02-07-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 53.Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- 54.Kiernan R, Bres V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, Jin DY, Emiliani S, Benkirane M. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem. 2003;278:2758–2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- 55.Gao Z, Chiao P, Zhang X, Zhang X, Lazar MA, Seto E, Young HA, Ye J. Coactivators and corepressors of NF-kB in IkBalpha gene promoter. J Biol Chem. 2005;280:21091–21098. doi: 10.1074/jbc.M500754200. [DOI] [PMC free article] [PubMed] [Google Scholar]