Abstract
Caregiving stress is associated with negative health outcomes. Neuroendocrine functioning may be a mediator of such outcomes. The MAOA gene regulates activity of neurotransmitters involved with neuroendocrine responses to stress. Differences in polymorphisms of this gene have been shown to influence susceptibility to stress. Therefore, we examined allelic variation in MAOA-uVNTR, a functional polymorphism of MAOA, as a moderator of chronic stress effects on urinary cortisol excretion in 74 males enrolled in a case/control study of caregivers for relatives with dementia. Mixed models analysis of variance were used to examine MAOA-uVNTR genotype (3 vs 3.5/4 repeats) as a moderator of the impact of stress (caregiver versus non-caregiver) on the urinary excretion pattern (overnight, daytime, evening) of cortisol. Caregivers with MAOA-uVNTR alleles associated with less transcriptional activity (3-repeats) displayed a pattern of cortisol excretion–a decrease from overnight to daytime—that was suggestive of HPA axis blunting, as compared to noncaregivers and those caregivers with the more active alleles (3.5/4 repeats) (cortisol p<.043). Individuals with less active MAOA-uVNTR alleles who are under chronic stress may be at increased risk for exhaustion of the HPA response to such stress.
Keywords: Monoamine oxidase-A, MAOA-uVNTR, Caregiving, Stress, Cortisol
The burden associated with providing care for a loved one suffering from dementia is considerable (Schulz et al., 1995; Vitaliano et al., 2003). The caregiving role has been associated with multiple negative health outcomes (Baumgarten et al., 1992), including worse ratings of physical health, significant decreases in cellular immunity (Kiecolt-Glaser et al., 1991), higher levels of triglycerides (Vitaliano et al., 1995), and increased rates of early mortality (Schulz and Beach, 1999). Our prior research with the sample used in the present study has shown that caregivers also suffer from both increased symptoms of depression and poorer sleep quality (Brummett et al., 2005).
Life situations that promote chronic stress (e.g.,Frankenhaeuser, 1989; Ockenfels et al., 1995; Pruessner et al., 1999; Adam and Gunnar, 2001; Matthews et al., 2001; De Vente et al., 2003) have been associated with many of the symptoms often seen in chronic caregivers, e.g., physical symptoms, disturbed sleep, and symptoms of depression. This biological pattern of responding to chronic stress has been related to dysregulation of the hypothalamic-pituitary-adrenal axis (HPA) see (Tsigos and Chrousos, 2002; Kudielka and Kirschbaum, 2005; Sonnentag, 2006). A number of studies have assessed associations between stress and cortisol levels in individuals suffering from chronic work related stress, however, some of the more recent research in this area has extended to other stressors, including the chronic stress of caregiving (e.g.,Bauer et al., 2000; de Vugt et al., 2005; Gallagher-Thompson et al., 2006; McCallum et al., 2006). When summarizing the extensive body of research on chronic stress, it is apparent that many studies have shown evidence for increased levels of cortisol (HPA hyper-functioning) (e.g., Melamed et al., 1999a; De Vente et al., 2003) and others have reported decreased levels (HPA hypo-functioning) (e.g.Matthews et al., 2001; Moch et al., 2003; Sonnenschein et al., 2007), while a few have reported no association (e.g., Langelaan et al., 2006; Mommersteeg et al., 2006b; Mommersteeg et al., 2006a).
The majority of research examining the associations of chronic stress and HPA functioning have relied on measures of basal levels of circulating cortisol or the cortisol awakening response (i.e., the increase in cortisol in the 30 minutes after awakening) as an indicator of HPA response to stress. However, it has been demonstrated that cortisol follows a cycle, exhibiting the highest levels in the morning hours and then showing a decline throughout the day (Kirschbaum and Hellhammer, 1989). The relation of chronic stress and cortisol during a 24 hour period has been reported in a few studies. In the first study to take this approach, it was shown that employees with high work load, as compared to those with a low workload, had a lower than expected rise in early morning plasma cortisol levels; and failed to have the normal decrease in cortisol from morning to the afternoon (Caplan et al., 1979). VanEck et al. (VanEck and Nicolson, 1996) found no evidence for a stress-related disruption in the normal rhythm of salivary cortisol secretion in subjects who scored high versus low on a measure of perceived stress. In a study of unemployed versus employed participants, Ockenfels et al. (Ockenfels et al., 1995) reported that unemployment was associated with higher morning and lower evening levels of salivary cortisol. Finally, in a sample of participants diagnosed with chronic burnout versus healthy controls, no differences were found in the morning awakening response, nor in the daily pattern on repeated assessments of salivary cortisol (Mommersteeg et al., 2006a). Thus, findings from the existent research concerning daily HPA axis function during chronic stress remain inconsistent.
Neurotransmitters including serotonin, norepinephrine and dopamine, are important in the regulation of the HPA axis (Tsigos and Chrousos, 2002). Increased CNS serotonin, as induced by infusion of the precursor tryptophan, has been found to increase cortisol excretion in normal subjects (Deakin et al., 1990; Price et al., 1998). Therefore, genes that regulate activity of these neurotransmitters are viable candidates to evaluate for their potential role in moderating neuroendocrine responses to chronic stress. Monoamine oxidase A (MAOA) is a mitochondrial enzyme that degrades the neurotransmitters serotonin, norepinephrine, and dopamine. The gene that encodes MAOA is found on the X chromosome and contains a polymorphism (MAOA-uVNTR) located 1.2 kb upstream of the MAOA coding sequences (Sabol et al., 1998). In this polymorphism, consisting of a 30-base pair repeated sequence, six allele variants containing either 2-, 3-, 3.5-, 4-, 5-, or 6-repeat copies have been identified (Jacob et al., 2005). Functional studies indicate that certain alleles confer lower transcriptional efficiency than others; the 3-repeat variant conveys lower efficiency, whereas 3.5- and 4-repeat alleles result in higher efficiency (Sabol et al., 1998; Deckert et al., 1999; Denney et al., 1999). To date there is less consensus regarding the transcriptional efficiency of the other alleles (e.g., 2-, 5-, and 6-repeat).
When examining the relation between health outcomes and genetic markers, research (Moffitt et al., 2005) indicates that it is important to consider gene by environmental stress interactions. Children with a history of maltreatment, in conjunction with the presence of MAOA-uVNTR alleles that confer low activity, are more likely to develop antisocial problems (Caspi et al., 2002). Similarly, MAOA activity moderated the impact of early life experience such that low activity was associated with higher risk for conduct disorder (Foley et al., 2004) and poor mental health (Kim-Cohen et al., 2006), in children with a background of maltreatment. Of direct relevance to the present study, we have previously reported in this sample of male caregivers that those with less active MAOA alleles exhibit increased depressive symptoms and poorer sleep quality (Brummett et al., 2007). Taken altogether, these findings indicate that the MAOA gene may influence vulnerability to environmental stressors. Thus, when considering the effects of chronic environmental stressors it is important to consider potential genetic moderation. The inconsistent findings in prior research on stress and HPA axis function could stem from unmeasured genetically mediated differences in sensitivity to chronic stress.
In sum, the chronic stress associated with caregiving manifests as numerous health problems and the effect of chronic stress on neuroendocrine functioning is a likely mediator of such health outcomes. The MAOA gene regulates activity of neurotransmitters involved with the neuroendocrine response to stress, and differences in polymorphisms of this gene have been shown to influence susceptibility to chronic stress. Therefore, examination of a plausible genetic moderator, as well as the addition of an integrated measure of total cortisol excretion across a 24-hour period, may help clarify the mixed pattern of results with respect to previously demonstrated associations of chronic stress and HPA function. Based on this background, we examined the association among allelic variation (low vs high activity alleles) in the MAOA-uVNTR polymorphism, exposure to a chronic stressor, and cortisol levels in a group of 74 males who were enrolled in a case/control study of caregiving for a relative with dementia. We hypothesized that MAOA-uVNTR genotype would be a significant moderator of caregiving stress effects on urinary cortisol levels across a 24-hour period. More specifically, because it is known that increased CNS serotonin function (Deakin et al., 1990) stimulates the HPA axis to secrete cortisol; and furthermore, that acute stress leads to an increase in CNS 5-HT in animal models (Beekman et al., 2005; Samad et al., 2006; Linthorst and Reul, 2007), it is possible to hypothesize the following sequence: a) male caregivers have increased CNS 5-HT due to the stresses they experience, b) and in those with less active MAOA-uVNTR alleles CNS 5-HT is even higher, due to lower degradation; c) this combination results in a relatively high level of CNS 5-HT that would in turn be expected to cause increased HPA activation during their early caregiving weeks/months/years; and d) over time, their HPA axis may become exhausted, thereby leading to a fall in urinary cortisol during the daytime in male caregivers with less active alleles.
Methods
Patient Population
As described in Brummett et al.(Brummett et al., 2007), participants were recruited to be part of a study designed to examine the underlying biological and behavioral mechanisms whereby stressful social and physical environments might lead to health disparities. Caregivers were recruited using flyers, ads in the local media, and community outreach efforts. Non-caregiver controls were recruited by asking caregivers to nominate two to five friends who live in their neighborhood and are similar with respect to demographic factors (i.e., gender, age, and race). The study was approved by the Duke University Medical Center Institutional Review Board and all subjects gave informed consent prior to their participation in the study. Individuals who were experiencing an acute major medical or psychiatric disorder that would render them unable to fully participate in the study, or to assume the role of primary caregiver, were excluded from the study. These exclusion criteria resulted in the loss of one individual who was actively psychotic during the intake interview.
The full study sample consisted of 85 males and 259 females. Because the MAOA gene is located on the X chromosome (Xp11.23) the heterozygosity in females makes it more complicated to assign individuals to a “low” or “high” transcriptional category. Specifically, current evidence suggests that it is unclear whether levels of MAOA transcription in females is the product of one or both copies of the gene (Benjamin et al., 2000; Carrel and Willard, 2005). Therefore, as with many studies examining MAOA-uVNTR, only the 85 males were included in the present study. Eleven individuals had incomplete genetic data, leaving the present sample with 74 males (42 caregivers and 32 controls). Individuals with missing genetic data did not differ significantly from those with genetic data with regard to urinary cortisol measures.
Measures
Genotyping
Fresh blood samples were obtained and signed into the Center for Human Genetics DNA Bank. DNA was extracted and stored according to methods and quality checks as previously reported (Rimmler et al., 1998). An aliquot of DNA was used for MAOA genotyping. The MAOA-u VNTR region was amplified with primers: Forward-FAM-CAGCCTGACCGTGGAGAAG and Reverse-GAACGGACGCTCCATTCGGA as described before (Sabol et al., 1998). PCR products were separated by polyacrylamide gel electrophoresis and visualized on a Hitachi FMBIO IIT Multi-View Scanner. We required that each assay achieve 95% efficiency (i.e. the genotypes of at least 95% of the samples could be called with certainty) to be considered for statistical analysis.
Urinary Cortisol
Participants were asked to collect 24-hour urine samples. Samples were collected for three different time periods: 1) overnight - bedtime to waking, including first void of the morning, 2) day - entire day, typically 8:00 a.m. to 4:30 p.m., and 3) evening - end of day to bedtime. Participants refrigerated the samples and returned them following the conclusion of the final collection. Urine samples were then taken to the Pharmacology Department at Duke University Medical Center. They were frozen and stored at −70 degrees C. Later they were assayed for cortisol and creatinine levels.
Cortisol was measured by RIA using an Enzyme Immunoassay kit (Oxford Biomedical Research, Oxford MI). Inter- and intra assay coefficients of variation were less than 10% and 5% respectively. Cortisol values were corrected for any differences in volume/completeness of the 24-hour urinary output by dividing hormone levels by the concentrations of creatinine. Urine creatinine is used widely as a normalization for variation in urine volume related to fluid intake (Saude, et al., 2007; Stewart, 2008; Viardot, et al., 2005; Zuppi, et al., 2007). Prior to statistical analyses, cortisol values were log-transformed to correct for their geometric distribution. Prior to transformation, values were in units of nanograms per milligram (ng/mg) creatinine.
Statistical Analysis
Individual growth curve models (mixed, or random effects models) were used to quantify the effects of MAOA-uVNTR (high vs low activity) and caregiver stress (control vs caregiver) on repeated measures of urinary cortisol. A log-transformed, continuous measure of cortisol (in the overnight, daytime, and evening aliquots) served as the repeated outcome measure of HPA axis function. The interaction term MAOA-uVNTR X caregiver stress X time was modeled as the primary predictor variable of the hormonal outcome, and race was included as a covariate. Each model contained all main effects and the corresponding two- and three-way terms. Thus, our main analyses examined the interactive effects of MAOA-uVNTR and caregiver stress as a predictor of change in cortisol (i.e., predictor of the slope at three time points that captured hormonal excretion during a 24-hour period). The analyses were carried out using PROC MIXED in SAS version 9.0.
Results
Table 1 presents the characteristics of the present sample. On average, they were 68 years old and the majority were Caucasian. Regarding MAOA-uVNTR frequencies, 67.6% of the sample had alleles (3.5 or 4 repeats) that indicate higher transcription of MAOA; this distribution is similar to those from participants without existing psychiatric disorders from two other U.S. samples (Williams et al., 2003; Jacob et al., 2005) i.e., approximately 60% and 65%, respectively.
Table 1.
Sample Characteristics (n = 74)
| Characteristic | Total | Caregivers | Non-Caregivers |
|---|---|---|---|
| N=74 | n=42 | n =32 | |
| Age, mean (years) (SD) | 68.4 (13.9) | 70.1 (13.6) | 66.1 (14.1) |
| Race | |||
| Caucasian | 59 (79.7%) | 34 (80.9%) | 25 (78.1%) |
| African American | 15 (20.3%) | 8 (19.1%) | 7 (21.9%) |
| Cortisol, mean (SD) | |||
| Overnight | 85.4 (39.4) | 88.4 (45.0) | 81.5 (30.8) |
| Day | 95.7 (49.8) | 92.0 (52.7) | 100.5 (46.0) |
| Evening | 67.0 (30.7) | 68.6 (32.3) | 64.9 (28.8) |
| MAOA-uVNTR Frequencies* | |||
| 3 Repeat (less active) | 24 (32.4%) | 16 (38.1%) | 8 (25.0%) |
| 3.5 / 4 Repeat (more active) | 50 (67.6%) | 26 (61.9%) | 24 (75.0%) |
Note: Cortisol values are in units of nanograms per milligram (ng/mg) creatinine.
None of the individuals within the present sample were found to have the less frequently occurring alleles (i.e., 2, 5, and 6).
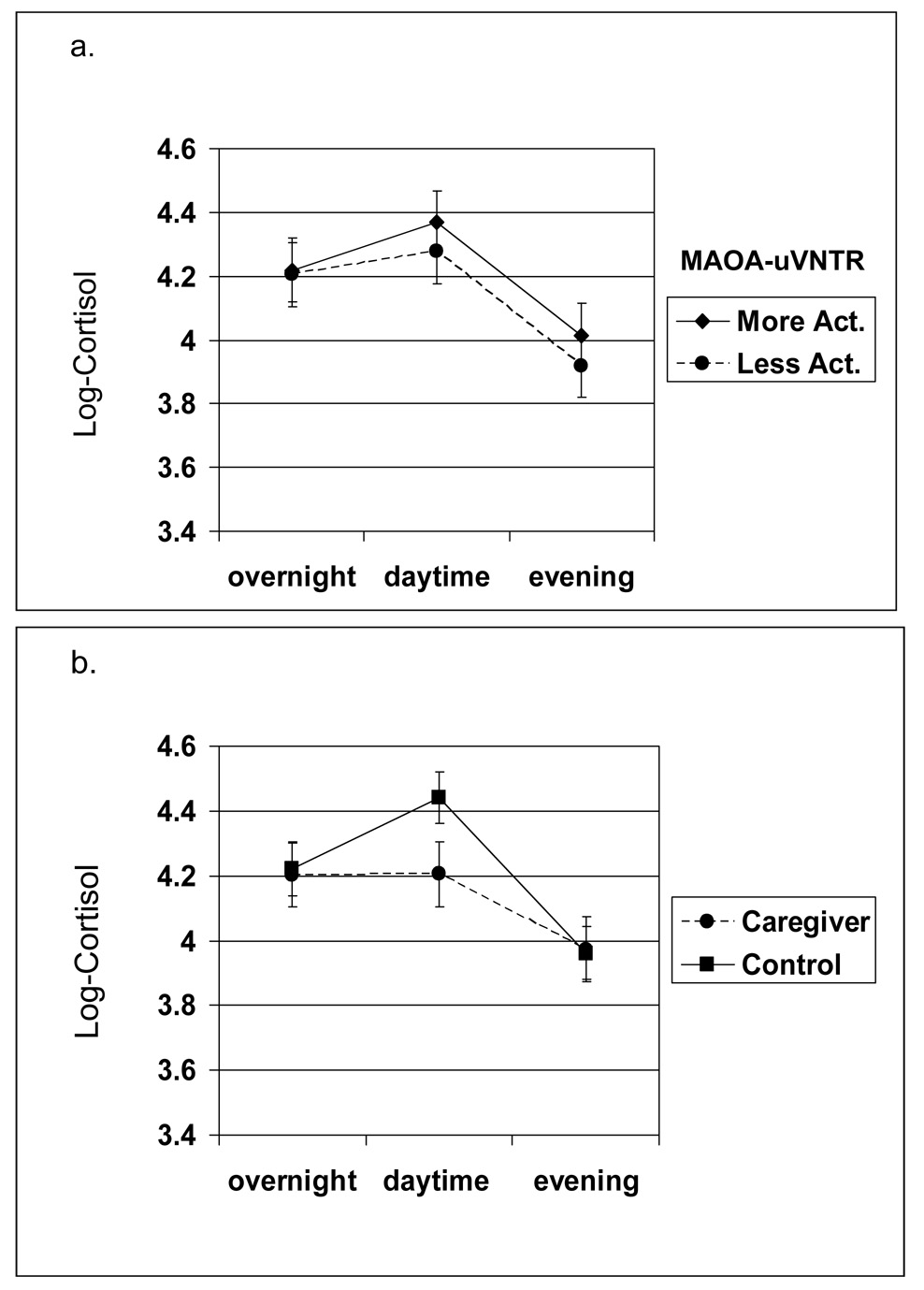
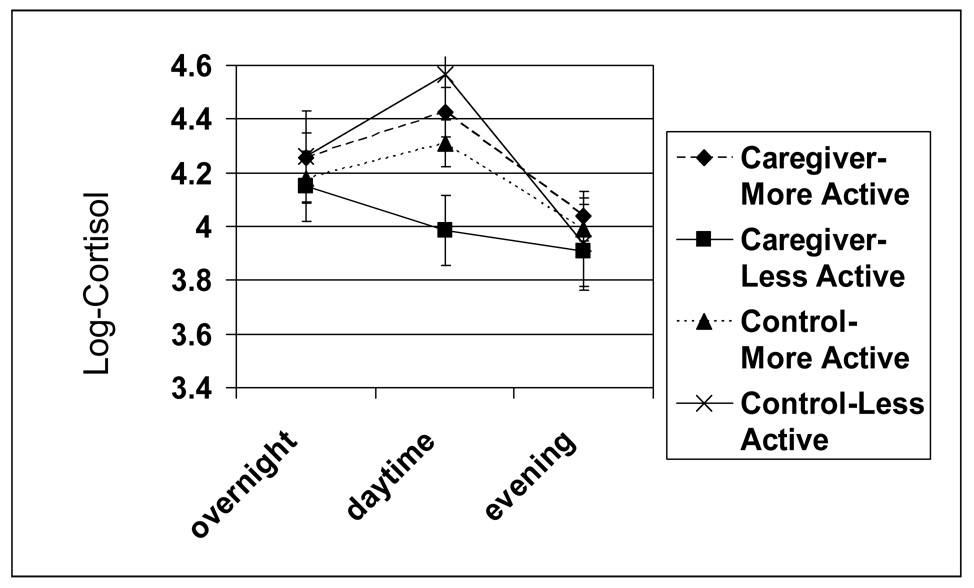
The MAOA-uVNTR X caregiver group X time interaction was statistically significant (p = .043); and the change in cortisol did not differ by MAO genotype or caregiver status (MAOA-uVNTR X time p = .827 and caregiver group X time p = .131, respectively). As shown in Figure 1, for all non-caregivers, as well as caregivers with the more active MAOA genotype, a typical pattern was found (i.e., a rise from overnight to daytime, with a decline in the evening). In contrast, as shown in Figure 2, for caregivers with the less active MAOA-uVNTR genotype, the pattern of cortisol excretion followed a somewhat linear pattern, starting out higher in the morning and then falling throughout the day till the evening—a trend suggesting HPA axis blunting.
Figure 1.
(a.) MAOA-uVNTR X Time, and (b.) Caregiver group X Time Effects on Measures of Urinary Cortisol (adjusted means and standard errors).
Figure 2.
MAOA-uVNTR X Time X Caregiver group Effects on Measures of Urinary Cortisol (adjusted means and standard errors).
The differences between the daytime level of cortisol in the caregivers with less active MAOA-uVNTR alleles, as compared to those with more active alleles was slightly greater than ½ standard deviation in magnitude (mean group difference divided by SD, Cohen’s (Cohen, 1988) d, = .54. In non-log transformed values, the mean cortisol value during the day for the caregivers with less active alleles was 75 ng/mg creatinine (SD = 48), and for those with more active alleles this value was 102 ng/mg creatinine (SD = 54). In addition, the drop in the level of cortisol from morning to daytime in the less active genotype caregivers was approximately the same size as the rise in the level of cortisol for the more active genotype caregivers, i.e., a decrease of 13 ng/mg creatinine as compared to an increase of 14 ng/mg creatinine.
In post hoc analyses we included a measure of depressive symptoms, sleep quality, perceived stress, objective and subjective burden measures as covariates in models assessing the interaction of MAOA-uVNTR x caregiver group X time as a predictor of the pattern of cortisol excretion. Depressive symptoms, perceived stress ratings, and objective and subjective burden ratings were not significantly related to the overall levels of cortisol excretion in the present sample, nor did the inclusion of these measures significantly reduce the fit of the model or alter the significance of the MAOA-uVNTR x caregiver group x time effect (p value remained = .04). Poor sleep quality was related to lower levels of cortisol excretion (p = .001), however, the inclusion of this measure did not significantly reduce the fit of the model or alter the significance of the MAOA-uVNTR x caregiver group x time effect (p value remained = .04).
Discussion
The main finding of this study is that caregivers with the less active MAOA genotype exhibited a different pattern of urinary cortisol excretion compared to controls and caregivers with a more transcriptionally efficient copy of the MAO–uVNTR genotype — a pattern that is suggestive of HPA axis dysregulation. These findings indicate that males with reduced degradation of monoamines by MAOA may be more likely to develop blunting of HPA responses to stress. To our knowledge, this is the first report of genetic moderation of effects of chronic stress on HPA axis exhaustion.
HPA dysregulation may manifest in the form of hypo- or hyper dysregulation. In the present sample, our caregivers with the less transcriptionally active MAOA-uVNTR genotype displayed a pattern consistent with hypo-functioning as indicated by their significantly lower level of daytime cortisol. HPA hypo-activity has been associated with post traumatic stress disorder (PTSD) and chronic fatigue syndrome, and hypocortisolism may be causally related to numerous chronic health conditions (Heim et al., 2000). It has been suggested that stress may follow an initial pattern of HPA hyper-activity, but the continuation of chronic stress, as would be the case for caregiving, may eventually lead to hypo-activity in certain individuals, e.g., those who are predisposed, perhaps due to genetic, personality, or other vulnerabilities (Heim et al., 2000; Sonnentag, 2006). Indeed, recent findings suggest that a polymorphism in the mineralocorticoid receptor (MR gene variant I180V) may affect bodily responses to stress (DeRijk et al., 2006). Specifically, individuals with the MR180V allele had increased salivary and plasma cortisol responses to a social stressor consisting of speech and mental arithmetic, as compared to non-carriers of the polymorphism.
The data available in the present study do not permit us to draw any final conclusions regarding the mechanism(s) whereby the less active MAOA-uNVTR alleles confer greater sensitivity to develop HPA axis blunting in men subjected to the chronic major life stress of being a caregiver for a relative with dementia. However, the present findings are in line with our our hypothesized sequence of events , i.e., that male caregivers have increased CNS 5-HT due to the stresses they experience and in those with less active MAOA-uVNTR alleles CNS 5-HT is even higher, due to lower degradation; and this combination results in a relatively high level of CNS 5-HT that would in turn be expected to cause increased HPA activation early in the caregiving process, and that over time, their HPA axis may become exhausted.
In the present data, the observed variation in cortisol between caregivers with the less active MAOA-uVNTR and the other 3 groups indicates that it is the daytime period that is distinctly different. The morning rise in cortisol is captured in the daytime measurement and is at least partially responsible for part of the observed variation between groups. This is noteworthy given research in twins that has shown that the awakening response is heritable, while other daytime samples are predominantly determined by unique environmental factors (Kuppera et al., 2005). It is also relevant given the research suggesting that a smaller rise in morning rise is cortisol is associated with adverse health outcomes e.g., (Lasikiewicz, et al., 2008; Rosmond et al., 1998).
We have previously shown that caregivers with less transcriptionally active MAOA-uVNTR alleles reported significantly more problems with sleep (Brummett et al., 2007). Sleep disturbances are also reported in patients experiencing PTSD (e.g.,Kobayashi et al., 2007) and chronic fatigue syndrome (CFS)— (e.g., Van Hoof et al., 2007), conditions that have both been associated with lowered cortisol levels (e.g., Mason et al., 1986; Tsigos and Chrousos, 2002). Poorer sleep ratings were significantly correlated with lower levels of cortisol across all three time points in the present sample. When we included sleep ratings in our interaction model, sleep ratings were significantly associated with cortisol levels, however, the MAOA-uVNTR x Caregiver group x Time interaction effect was not substantially altered. However, we found that the differences in sleep between our caregivers with the less transcriptionally active MAOA-uVNTR genotype and other participants were reduced when covarying for effects of daytime cortisol level. Although speculative, it is possible that decreased HPA function in caregivers with less active alleles, as indexed by lower daytime cortisol levels, may partially account for their poorer sleep quality. Indeed, other research examining measures of salivary cortisol in middle-aged individuals has shown that blunted cortisol profiles were associated with poor sleep quality (Lasikiewicz, et al., 2008).
Certain limitations should be noted with respect to the present findings. Because the sample consisted of only 74 participants, all results should be interpreted with proper caution. This cautionary note is magnified by the limited size of the four groups that are defined by the environment by gene interaction. Thus, replication in additional samples will be required in order to determine the validity of the present results. We were unable to adequately examine the ethnicity by gene interaction due to insufficient numbers of participants in certain cells. It should also be noted that the present study only involved males and it is possible that gender differences may exist with respect to coping with the stress of caregiving, as well as with differences in HPA functioning. Therefore, the current findings may not generalize to females. Although there seems to be a consensus regarding which of the more commonly occurring MAOA-uVNTR alleles confer higher or lower transcription, there remains dispute about the transcriptional activity of the less commonly found alleles (i.e., 5 copies of VNTR). However, none of the individuals in the present sample were found to have the less frequently occurring alleles. Finally, the inclusion of other covariates, apart from race, may have altered the present findings.
In conclusion, male caregivers with less active MAOA-uVNTR alleles may be appropriate targets in future research for interventions designed to help them better manage chronic stress. From the point of view of future research on genetic moderators, the present results also highlight the importance of considering chronic stress exposure before concluding that there is no genetic effect on important phenotypes (Moffitt et al., 2005).
Acknowledgements
This research was supported by the by National Heart, Lung, and Blood Institutes grant 3P01 HL036587; the National Institutes of Mental Health grant R01MH57663; the National Institute on Aging grant R01AG19605, with co-funding by National Institute of Environmental Health Sciences; and by the Clinical Research Unit grant M01RR30l. Supported by NHLBI grant P01HL36587. The authors of this manuscript have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Vedhara K, Perks P, Wilcock GK, Lightman SL, Shanks N. Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. J Neuroimmunol. 2000;103:84–92. doi: 10.1016/s0165-5728(99)00228-3. [DOI] [PubMed] [Google Scholar]
- Baumgarten M, Battista RN, Infante-Rivard C, Hanley JA, Becker R, Gauthier S. The psychological and physical health of family members caring for an elderly person with dementia. J Clin Epidemiol. 1992;45:61–70. doi: 10.1016/0895-4356(92)90189-t. [DOI] [PubMed] [Google Scholar]
- Beekman J, Flachskamm C, Linthorst ACE. Effects of exposure to a predator on behaviour and serotonergic neurotransmission in different brain regions of C57bl/6N mice. Eur J Neurosci. 2005;21:2825–2836. doi: 10.1111/j.1460-9568.2005.04107.x. [DOI] [PubMed] [Google Scholar]
- Benjamin D, Van Bakel I, Craig IW. A novel expression based approach for assessing the inactivation status of human X-linked genes. Eur J Hum Genet. 2000;8:103–108. doi: 10.1038/sj.ejhg.5200427. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Siegler IC, Rohe WM, Barefoot JC, Vitaliano PP, Surwit RS, Feinglos MN, Williams RB. Neighborhood characteristics moderate effects of caregiving on glucose functioning. Psychosom Med. 2005;67:752–758. doi: 10.1097/01.psy.0000174171.24930.11. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Krystal AD, Siegler IC, Kuhn C, Surwit RS, Züchner S, Ashley-Koch A, Barefoot JC, Williams RB. Associations of a regulatory polymorphism of the monoamine oxidase-A gene promoter (MAOA-uVNTR) with symptoms of depression and sleep quality. Psychosom Med. 2007;69:396–401. doi: 10.1097/PSY.0b013e31806d040b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan RD, Cobb S, French JRP. White collar work load and cortisol. Disruption of a circadian rhythm by job stress. J Psychosom Res. 1979;23:181–192. doi: 10.1016/0022-3999(79)90003-5. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard H. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analyses for the behavioral sciences. Hillsdale, NJ: Lawerence Erlbaum Associates; 1988. [Google Scholar]
- De Vente W, Olff M, Van Amsterdam JGC, Kamphuis JH, Emmelkamp PMG. Physiological differences between burnout patients and healthy controls: blood pressure, heart rate, and cortisol responses. Occup Environ Med. 2003;60 Suppl 1:i54–i61. doi: 10.1136/oem.60.suppl_1.i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vugt ME, Nicolson NA, Aalten P, Lousberg R, Jolle J, Verhey FRJ. Behavioral problems in dementia patients and salivary cortisol patterns in caregivers. J Neuropsychiatry Clin Neurosci. 2005;17:201–207. doi: 10.1176/jnp.17.2.201. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Pennell I, Upadhyaya AJ, Lofthouse R. A neuroendocrine study of 5HT function in depression: evidence for biological mechanisms of endogenous and psychosocial causation. Psychopharmacology. 1990;101:85–92. doi: 10.1007/BF02253723. [DOI] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Dibella DN, M M, Maffei P, Franke P, Fritze J, Maier W, Propping P, Beckmann H, Bellodi L, Lesch KP. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Human Molecular Genetics. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- Denney RM, Koch H, Craig IW. Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Human Genetics. 1999;105:542–551. doi: 10.1007/s004399900183. [DOI] [PubMed] [Google Scholar]
- DeRijk RH, Wust S, Meijer OC, Zennaro M-C, Federenko IS, Hellhammer DH, Giacchetti G, Vreugdenhil E, Zitman FG, de Kloet ER. A common polymorphism in the mineralocorticoid receptor modulates stress responsiveness. J Clin Endocrinol Metab. 2006;91:5083–5089. doi: 10.1210/jc.2006-0915. [DOI] [PubMed] [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, Silberg JI, Maes HH, Kuhn J, Riley B. Childhood adversity, monoamine oxidase A genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser M. A biopsychosocial approach to work life issues. Int J Health Serv. 1989;19:747–758. doi: 10.2190/01DY-UD40-10M3-CKY4. [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Shurgot GR, Rider K, Gray HL, McKibbin CL, Kraemer HC, Sephton SE, Thompson LW. Ethnicity, stress, and cortisol function in Hispanic and non-Hispanic white women: A preliminary study of family dementia caregivers and noncaregivers. Am J Geriatr Psychiatry. 2006;14:334–342. doi: 10.1097/01.JGP.0000206485.73618.87. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Jacob CP, Muller J, Schmidt M, Hohenberger K, Gutknecht L, Reif A, Schmidtke A, Mossner R, Lesch KP. Cluster B personality disorders are associated with allelic variation of monoamine oxidase A acitivity. Neuropsychopharmacology. 2005;4:1–8. doi: 10.1038/sj.npp.1300737. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask J, Glaser R. Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidene and a meta-analyis. Mol Psychiatry. 2006:1–11. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: An overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: A meta-analytic review. Psychophysiology. 2007 doi: 10.1111/j.1469-8986.2007.537.x. in press. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kuppera N, de Geusa EJC, van den Berga M, Kirschbaumb C, Boomsmaa DI, Willemsena G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinol. 2005;30:857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Langelaan S, Bakker AB, Schaufeli WB, van Rhenen W, van Doornen LJP. Do burned-out and work-engaged employees differ in the functioning of the hypothalamic-pituitary-adrenal axis?[see comment] Scandinavian Journal of Work, Environment & Health. 2006;32:339–348. doi: 10.5271/sjweh.1029. [DOI] [PubMed] [Google Scholar]
- Lasikiewicz N, Hendrickx H, Talbot D, Dye L. Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: Associations with sleep quality and metabolic parameters. Psychoneuroendocrinology. 2008;33:143–151. doi: 10.1016/j.psyneuen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Linthorst ACE, Reul JMHM. Stress and the brain: Solving the puzzle using microdialysis. Pharmacology Biochemistry and Behavior. 2007 doi: 10.1016/j.pbb.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary free cortisol levels in posttraumatic stress disorder patients. J Nerv Ment Dis. 1986;174:145–149. doi: 10.1097/00005053-198603000-00003. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gump BB, Owens JF. Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men. Health Psychol. 2001;20:403–410. [PubMed] [Google Scholar]
- McCallum TJ, Sorocco KH, Fritsch T. Mental health and diurnal salivary cortisol patterns among African American and European American female dementia family caregivers. Am J Geriatr Psychiatry. 2006;14:684–693. doi: 10.1097/01.JGP.0000225109.85406.89. [DOI] [PubMed] [Google Scholar]
- Melamed S, Ugarten U, Shirom A, Kahana L, Lerman Y, Froom P. Chronic burnout, somatic arousal and elevated salivary cortisol levels. J Psychosom Res. 1999a;46:591–598. doi: 10.1016/s0022-3999(99)00007-0. [DOI] [PubMed] [Google Scholar]
- Melamed S, Ugarten U, Shirom A, Kahana L, Lerman Y, Froom P. Chronic burnout, somatic arousal and elevated salivary cortisol levels. J Psychosom Res. 1999b;46:591–598. doi: 10.1016/s0022-3999(99)00007-0. [DOI] [PubMed] [Google Scholar]
- Moch SL, Panz VR, Joffe BI, Havlik I, Moch JD. Longitudinal changes in pituitary-adrenal hormones in South African women with burnout. Endocrine. 2003;21:267–272. doi: 10.1385/ENDO:21:3:267. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Mommersteeg PMC, Heijnen CJ, Verbraak MJPM, van Doornen LJP. Clinical burnout is not reflected in the cortisol awakening response, the day-curve or the response to a low-dose dexamethasone suppression test. Psychoneuroendocrinology. 2006a;31:216–225. doi: 10.1016/j.psyneuen.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Mommersteeg PMC, Heijnen CJ, Kavelaars A, van Doornen LJP. Immune and endocrine function in burnout syndrome. Psychosom Med. 2006b;68:879–886. doi: 10.1097/01.psy.0000239247.47581.0c. [DOI] [PubMed] [Google Scholar]
- Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AA. Effect of chronic stress associated with unemployment on salivary cortisol: overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosom Med. 1995;57:460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- Price LH, Malison RT, McDougle CJ, Pelton GH, Heninger GR. The neurobiology of tryptophan depletion in depression: effects of intravenous tryptophan infusion. Bio Psychiatry. 1998;43:339–347. doi: 10.1016/s0006-3223(97)00284-9. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Rimmler J, McDowell JG, Slotterback BD, Haynes CS, Menold MM, Rogala A, Speer MC, Gilbert JR, Hauser ER, Vance JM, Pericak-Vance MA. Development of a data coordinating center (DCC): Data quality control for complex disease studies. Am J Hum Genet. 1998;63:240. [Google Scholar]
- Rosmond R, Dallman MF, Bjorntorp P. Stress-related cortisol secretion in men: relationships with absominal obesity and endocrine, metabolic and haemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Human Genetics. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Samad N, Perveen T, Haider S, Haleem MA, Haleem DJ. Inhibition of restraint-induced neuroendocrine and serotonergic responses by buspirone in rats. Pharmacological Reports. 2006;58 [PubMed] [Google Scholar]
- Saude EJ, Adamko D, Rowe BH, Marrie T, Sykes BD. Variation of metabolites in normal human urine Metabolomics. 2007;3:439–451. [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Schulz R, O'Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: Prevalence, correlates, and causes. The Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- Sonnenschein M, Mommersteeg PMC, Houtveen JH, Sorbi MJ, Schaufeli WB, van Doornen LJP. Exhaustion and endocrine functioning in clinical burnout: An in-depth study using the experience sampling method. Bio Psychiatry. 2007;75:176–184. doi: 10.1016/j.biopsycho.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Sonnentag S. Burnout and function of the hypothalamus-pituitary-axis--there are no simple answers. Scand J Work Environ Health. 2006;32:333–337. doi: 10.5271/sjweh.1028. [DOI] [PubMed] [Google Scholar]
- Stewart POM. The Adrenal Cortex. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, editors. Williams Textbook of Endocrinology. Philadelphia, PA: Elsevier; 2008. pp. 445–540. [Google Scholar]
- Tsigos C, Chrousos G. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:856–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Van Hoof E, De Becker P, Lapp C, Cluydts R, De Meirieir K. Defining the occurrence and influence of alpha-delta sleep in chronic fatigue syndrome. Am J Med Sci. 2007;333:78–84. doi: 10.1097/00000441-200702000-00003. [DOI] [PubMed] [Google Scholar]
- VanEck MM, Nicolson NA. Perceived stress and salivary cortisol in daily life. Ann Behav Med. 1996;16:221–227. [Google Scholar]
- Viardot A, Huber P, Puder JJ, Zulewski H, Keller U, Muller B. Reproducibility of nighttime salivary cortisol and its use in the diagnosis of hypercortisolism compared with urinaryt free cortisol and overnight dexamethasone suppression test. J Clin End Met. 2005;90:5730–5736. doi: 10.1210/jc.2004-2264. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Russo J, Niaura R. Plasma lipids and their relationships with psychosocial factors in older adults. Journal of Gerontology: Psychological Sciences. 1995;50B:18–24. doi: 10.1093/geronb/50b.1.p18. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Jianping Z, Scanlan JM. Is caregiving hazardous to one's physical health? A meta-analysis. Psychological Bulletin. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Russo J, Young HM, Becker J, Maiuro RD. The screen for caregiver burden. The Gerontologist. 1991;31:76–73. doi: 10.1093/geront/31.1.76. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Zuppi C, Messana I, Forni F, Rossi C, Pennacchiettei L, Ferrari F, Giardina B. 1H NMR spectra of normal urines: references ranges of the major metaboites. Clinica Chimica Acta. 2007;265:85–97. doi: 10.1016/s0009-8981(97)00110-1. [DOI] [PubMed] [Google Scholar]